Abstract
This site study was conducted in a chemical laboratory to evaluate nanomaterial emissions from 20–30-nm-diameter bundles of single-walled carbon nanotubes (CNTs) during product development activities. Direct-reading instruments were used to monitor the tasks in real time, and airborne particles were collected using various methods to characterize released nanomaterials using electron microscopy and elemental carbon (EC) analyses. CNT clusters and a few high-aspect-ratio particles were identified as being released from some activities. The EC concentration (0.87 μg/m3) at the source of probe sonication was found to be higher than other activities including weighing, mixing, centrifugation, coating, and cutting. Various sampling methods all indicated different levels of CNTs from the activities; however, the sonication process was found to release the highest amounts of CNTs. It can be cautiously concluded that the task of probe sonication possibly released nanomaterials into the laboratory and posed a risk of surface contamination. Based on these results, the sonication of CNT suspension should be covered or conducted inside a ventilated enclosure with proper filtration or a glovebox to minimize the potential of exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbon nanotubes (CNTs) are commonly used nanomaterials in laboratories for research and development activities. CNTs are also widely used in electronics, medicine, optics, and other fields of material sciences (Piccinno et al. 2012). The work practices in research laboratories can vary greatly between activities, and between researchers. More data regarding exposure to CNTs are needed to better understand the various scenarios of possible emission due to the toxicological concerns regarding high-aspect-ratio nanoparticles (Schulte et al. 2012). Various types of CNTs have been evaluated in the scientific publications regarding their toxicity and associated physico-chemical properties. In general, some CNTs could induce lung inflammation, bronchiolar epithelial hyperplasia, fibrosis, and genotoxicity based on their studies of in vivo or in vitro exposure of mice or human lung epithelial cells to studied CNTs (Porter et al. 2010; Wang et al. 2011; Porter et al. 2013; Toyokuni 2013; Siegrist et al. 2014). Single-walled CNTs have been shown to be capable of inducing malignant transformation of human lung epithelial cells (Wang et al. 2011). These studies suggested the potential carcinogenicity of CNTs, but their test dosages were usually higher than the exposure levels monitored at general CNT facilities. The results from toxicological studies should not be generalized to humans since no incidence related to exposure to CNTs has been reported so far. Recently, one type of multi-walled CNTs (CNT 7) was classified by the International Agency for Research on Cancer (IARC) as a 2B carcinogen, “possibly carcinogenic to humans” (Grosse et al. 2014; IARC 2017; Kuempel et al. 2016).
The toxicity of CNTs can be affected by the level of purity, surface area, functionalization, fiber length, fiber thickness, agglomerate status, and chemical composition (Muller et al. 2005; Carrero-Sanchez et al. 2006; Tian et al. 2006; Wick et al. 2007; Kostarelos 2008; Poland et al. 2008; Shinde and Tsai 2016). It is not yet possible to conclusively determine the harmfulness of various types of CNTs. Due to the uncertainty of CNT toxicology, it becomes more important to identify possible material releases and to minimize exposure as much as possible.
The US National Institute for Occupational Safety and Health (NIOSH) has proposed a recommended exposure limit (REL) of 1 μg/m3 for CNT/CNF measured as elemental carbon (EC) as a respirable mass 8-h time-weighted average (TWA) concentration (NIOSH 2013). The British Standards Institute (BSI) recommended a “benchmark” CNT occupational exposure limit (OEL) of 0.01 fibers/cm3, as measured by scanning or transmission electron microscopy (BSI 2007). Some manufacturers have also developed suggested OELs for their products. For example, Bayer established an OEL of 0.05 mg/m3 for Baytubes® (multi-walled CNTs) (Bayer MaterialScience 2010).
Information regarding CNT emissions in working environments is still limited (Methner et al. 2010; Heitbrink and Lo 2015; Heitbrink et al. 2015). The goal of this study is to assess nanomaterial emissions from product development activities in a laboratory and contribute additional understanding about potential CNT exposure and effective control measures.
Materials and methods
Laboratory processes
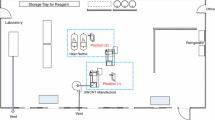
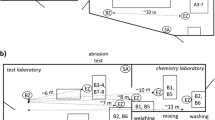
Tasks within an academic research laboratory were evaluated for nanomaterial emissions during laboratory-scale research and development activities. The researchers in this laboratory used single-walled CNTs to develop innovative coating materials on a bench-top scale. The CNTs were in the form of a nonwoven mesh of fiber bundles that are micrometers long and 20–30 nm in diameter. Each bundle was composed of tightly packed fibrils made of individual CNTs. The preparation of the CNT suspension began with weighing few micrograms of CNTs, mixing them with solvents, and dispersing CNTs throughout the suspension by sonication and centrifugation. Because two different solvents were used to disperse CNTs, suspensions A and B were named to distinguish the suspensions. The laboratory used two different methods to deposit CNTs on substrates: dip coating and spin coating. For dip coating, the substrate was dipped into the prepared suspension A, while spin coating involved dispensing drops of suspension B on top of the substrate and spreading out the suspension drops on the surface using a spinning machine. Following coating, the CNT-coated substrates were moved to a hot plate for drying inside a fume hood. Table 1 summarizes the processes required to prepare CNT-coated substrates as well as the equipment used for each activity. Following the completion of drying, the prepared CNT-coated substrates were cut using scissors inside a ventilated enclosure.
The task of weighing CNT powders was performed in a 5-panel enclosed analytical balance with sliding side panels on a laboratory table. After weigh-out, the CNTs were transferred into a beaker; solvents dispensed from wash bottles were then carefully added to the beaker for initial mixing. The CNT suspension was further mixed in a closed vial with bath sonication (Model FS30H, Fisher Scientific, Waltham, Massachusetts) for 20 min and later in a beaker with probe sonication (Model 550, Sonic Dismembrator, Fisher Scientific, Waltham, Massachusetts) for different processing times, depending on the coating process that would follow. To cool down the suspension heated by high-energy sonication, the beaker containing the CNT suspension was put inside a larger beaker filled with cold water. The probe sonication process usually was paused every 20 min to allow the cooling water to be replaced to keep the CNT suspension at desired temperature range. The preparation of the CNT suspensions with probe sonication required 40 min for suspension A and 60 min for suspension B. The last step in preparing the CNT suspension was to put the suspension in a closed vial and use a tabletop centrifuge for final preparation.
The laboratory used two methods to produce CNT-coated substrates. Substrates with uniform CNT coating were obtained by dipping clean substrates (5 cm × 5 cm) into the prepared CNT suspension for a short time and a layer of CNT deposited on the substrate (plastic sheets in this case). Spin coating was used to generate multiple layers of CNT coating on substrates. The task of spin coating was performed inside a conventional fume hood (Kewaunee Scientific Corporation, Statesvill, NC). A pipette was used to dispense drops of the CNT suspension on top of the substrate fixed to the spin equipment (Model P6712, Specialty Coating System Inc., Indianapolis, IN). A drop of the CNT suspension took 80 s of spinning to spread into a single layer of CNTs across the substrate. The final step of the process was to dry the CNT-coated substrates on hot plates inside a fume hood.
Particle emission measurements
A variety of direct-reading instruments were used to identify emission sources by monitoring airborne aerosol concentrations from the laboratory activities and to evaluate particle concentrations at the source and in the personal breathing zone (PBZ). Instruments that allowed the characterization of particles across a broad size range (5.6−20,000 nm) were used to evaluate primary and agglomerated nanoparticles in the laboratory environment. For this study, two sets of identical direct-reading instruments—including the aerodynamic particle sizer (APS) spectrometer, fast mobility particle sizer (FMPS) spectrometer, condensation particle counter (CPC), and DustTrak aerosol monitor—were used to monitor particle release from the laboratory activities: one set for the emission source and the other in the close proximity of the PBZ. These instruments were mounted on mobile carts to allow their conductive plastic sampling ports to be positioned at preferred locations (i.e., sources and PBZ) during various tasks (listed in Table 1).
The APS spectrometer (Model 3321, TSI, Inc., Shoreview, Minnesota) provides real-time size distributions of larger particles ranging from 0.5 to 20 μm. It is useful to detect nanoparticle agglomerates during nanomaterial handling. To monitor small airborne particles less than 0.5 μm in this field study, the FMPS spectrometer (Model 3091,TSI, Inc., Shoreview, Minnesota) was chosen because of its fast response rate (1 s) and high sampling flow rates (10 L/min) to minimize diffusion losses of ultrafine nanoparticles. The FMPS can measure size distributions of particles from 5.6 to 560 nm in real-time mode. A hand-held CPC (Model 3007, TSI, Inc., Shoreview, Minnesota) was used to provide instantaneous total number counts of particles from 0.01 to 1.0 μm in size. Additionally, the DustTrak (Model 8533, TSI, Inc., Shoreview, Minnesota) that can simultaneously measure both mass and selected size fractions of particles in a wide size range, from 0.1 to 15 μm, was used.
Aerosol sampling
In parallel to the real-time aerosol monitoring, task-based filter samples were collected at the production source and in the PBZ for the duration of the task being evaluated. Various approaches were taken to collect aerosol samples including three filter-based samples, i.e., (NIOSH NMAM methods 5040 and 7402, and a grid-attached diffusion method), and an electrostatic deposition of particles onto grids for electron microscope. To determine the background air quality, area samples were collected separately in the general area of the laboratory during weighing, sonication, spin coating, and substrate cutting.
Mass/filter-based air samples were collected according to NIOSH NMAM method 5040 using air sampling pumps (Universal XR Model PCXR4, SKC Inc., Eighty Four, Pennsylvania) and analyzed for EC. All pumps were calibrated before and after sampling. The pumps operated at flow rates of approximately 4.0 L/min. To determine the airborne EC mass concentration, air samples were collected on 25-mm-diameter, open-face quartz fiber filters and analyzed for EC according to NIOSH NMAM 5040 using evolved gas analysis by thermal-optical analyzer (NIOSH 2003). All sample results for the 25-mm cassettes are based on an effective sampling area of 3.46 cm2.
Alongside each mass-based air sample by method 5040, an additional sample following NIOSH NMAM method 7402 was collected on a 25-mm-diameter, open-face mixed cellulose ester filter; these samples were analyzed to identify CNT fibers using transmission electron microscopy (TEM) (NIOSH 1994). Three 3-mm copper TEM grids from each sample were examined at low magnification to determine loading and preparation quality. The counting protocol involved counting CNTs on up to 40 grid openings or 100 CNT structures. TEM analysis provides an indication of the relative abundance of nanostructures per volume of air, as well as other characteristics such as size, shape, and degree of agglomeration.
A filter sampler with a TEM grid-attached on polycarbonate filter with a tiny adhesive piece was used side by side with other samplers at source to collect airborne particles at a flow rate of 0.3 L/min. TEM copper grids (400 mesh with SiO2 film coating, SPI, West Chester, Pennsylvania) and 25-mm-diameter polycarbonate membrane filters (0.2-μm pore size, Millipore, Billerica, Massachusetts) were used together to deposit particles for analysis (Tsai et al. 2009).
In addition to filter-based sampling, a hand-held electrostatic precipitator particle sampler (ESPnano Model 100, Spokane, Washington) was used to collect representative samples of airborne particles on its built-in TEM grids to be analyzed by TEM. In this study, the samples from ESPnano were collected in parallel with the air filter samples at the source for the monitored tasks.
Control measures
As summarized in Table 1, the preparation of the CNT suspension was conducted on the bench top without engineering controls in place. Conventional chemical fume hoods were used for the tasks of spin coating and substrate drying during the process of preparing CNT-coated substrates. The spin coating equipment (Model P6712, Specialty Coating System Inc., Indianapolis, Indiana) was located on the right-hand side inside the fume hood. The fume hood was connected to the building exhaust system and had a constant exhaust flow. The fume hood had two movable vertical sashes to adjust the size of the hood face opening. During spin coating, the right sash was kept at the indicated design height, while the left sash, which was kept at a lower position, provided less open-face area. This arrangement created an opening of 55 cm on the right front and 40 cm on the left. The total width of the fume hood was 210 cm. A similar fume hood with a single sash was used for the task of substrate drying.
The post-processing of CNT-coated substrates (i.e., substrate cutting) was performed in a ventilated enclosure (Xpert Nano Enclosure, Labconco Corporation, Kansas City, Missouri). The enclosure had a constant face area—85 cm wide and 22 cm high—and exhausted contaminated air through the building exhaust system.
To evaluate the fume hood and the ventilated enclosure used as engineering controls in the study laboratory, hood face velocities were measured using a hotwire anemometer (VelociCalc plus Model 8386, TSI, Inc., Shoreview, Minnesota). These measurements were made by placing the anemometer perpendicular to the hood or enclosure faces and recording the face velocities. For the laboratory fume hood, air velocity measurements were made at eight equally spaced locations across the middle of the hood face for each side. Overall, 16 measurements (8 on each side) were collected for evaluating containment and hood exhaust air flow rate. For the ventilated enclosure, only eight equally spaced air velocity measurements were made across the enclosure face.
Results and discussion
Results from the study showed different numbers of particle release from the various processes of CNT preparation and use as listed in Table 1.
Probe sonication
The direct-reading instruments measuring in close proximity to the PBZ did not identify any major particle emissions from the probe sonication process as shown in Fig. 1a–b, but the measurements at the sonicating source as seen in Fig. 1b, indicated that the probe sonication process could generate micrometer-sized particles and cause work surface contamination. The increased concentrations at the source were detected by the CPC for larger particles but not by the FMPS, indicating that the particles generated by the high-energy sonication were around micrometer sizes (> 0.6 μm). Since relatively low and stable particle concentrations were measured in the PBZ, worker exposure to these micrometer-sized contaminants was not evident.
During the sonication process, airborne particles were collected using an ESPnano electrostatic precipitator, two mass-based filter samplers, and a TEM grid-attached filter sampler (Fig. 2a). The sampling tube of the ESPnano was located close to the top of the beaker with other samplers during real-time measurement, and contaminants of escaped CNTs were observed on the ESPnano sampling tube after sonication (Fig. 2b). The contaminants on the outer surface of ESPnano tube were collected on TEM grids by direct contact and analyzed using TEM as shown in Fig. 3a, b for large and small clusters, respectively. CNT clusters were confirmed to be released during sonication, and the released CNTs contaminated the surface of ESPnano sampler at the source. The sonicated CNT suspension was analyzed to identify the suspended CNTs. A drop of suspension was deposited on a grid, dried, and analyzed using TEM. CNT fibers in a thick layer were observed as shown in Fig. 3c.
The released CNTs, with varying morphology, were found in collected samples, and images analyzed by TEM are shown in Fig. 3d–f for the source location and Fig. 3g for the PBZ location. Submicrometer- and micrometer-sized CNT clusters were identified in airborne particles collected by the ESPnano (Fig. 3d). Both straight and curved CNT fibers were seen tangled with other materials (Figs. 3a–d); these findings are similar to other study (Dahm et al. 2012). Single CNT fibers were also found as marked in Fig. 3e, collected by a TEM grid-attached filter sampler. In addition to CNT fibers, hollow cylinder-shaped particles were found, as seen in Fig. 3f. Most of the hollow particles appeared to be shorter than the one shown in Fig. 3f. Airborne particles in the PBZ collected using NIOSH method 7402 were found to contain CNT fibers in mixed clusters as seen in Fig. 3g. Particles collected at source using NIOSH 7402 method during cutting CNT substrate was found to contain high-aspect-ratio particles as seen in Fig. 3h.
Other spherical or irregular shapes of nonfiber-structured particles were also found in the aerosol samples collected by the precipitator and TEM grid-attached filter (images not included). Using NIOSH NMAM 7402, the authors did not find CNTs on the quartz fiber filter samples for TEM analysis from the source samples and found only one CNT on the sample collected from the PBZ (Fig. 3g). The other two samplers, ESPnano and TEM grid-attached filter sampler, did collect multiple single or mixed CNT fibers and clusters. In summary, high-aspect-ratio particles and CNT fibers were found in many airborne particle samples, specifically during sonication at the source location. Previous studies have also reported the release of CNTs from the sonication process; however, information is limited regarding the type of sonication and location of sampling (Johnson et al. 2010; Methner et al. 2010). We have confirmed a detectable release of CNTs measured within 8–11 cm (3–4 in.) distance at the sonication source.
The corresponding results of EC concentrations for three processes are summarized in Table 2. According to the tests for the sonication process, higher EC concentrations were found for suspension B at the production source (0.87 and 1.04 μg/m3) than background (0.46 and 0.79 μg/m3) and the PBZ (0.53 μg/m3). The highest EC concentration (1.81 μg/m3) was found in the PBZ for the test of suspension A, but the EC collected at the source was lower than the limit of detection (LOD) (0.2 μg/sample). It has been reported that the mean observed EC concentrations due to ambient air pollution in the USA are 0.60 ± 0.72 μg/m3 (Yu et al. 2004). A previous study has shown that electrical motors with carbon brushes can be a source of particulate emissions of EC (Heitbrink and Collingwood 2005). Therefore, the EC concentrations measured for the probe sonication process could show some concentrations including sonication room background. Overall, due to the findings of CNT clusters and fibers from aerosol sampling and some slightly elevated EC concentrations, it appears that suspending CNTs in the solvents with high-energy sonication equipment and no engineering controls poses a risk for worker exposure both through inhalation and through possible dermal exposure from surface contamination. This contamination would be more significant for a similar task at the mass production scale.
Spin coating
Spin coating of multiple layers on the substrate was evaluated to assess the potential for exposure during this task. For every layer, the operator dispensed a fixed amount of CNT suspension (0.4 mL in this study) on the spinning substrate and waited 80 s to allow the suspension to be spread uniformly on the substrate. The CNT suspension for the next layer was then added after the coating process of the previous layer was finished. Ten layers of coating were applied during this process evaluation. The sampling location at the source was above the turn table of the spinning equipment inside the fume hood.
During process monitoring, all real-time instruments showed relatively stable particle concentrations with the exception of the FMPS data in the PBZ. Although the overall concentrations measured by the FMPS were low, the variability of the measurements was high and increased during the spin coating process (Fig. 4). The particle concentrations increased gradually at the late stage of the spin coating process around 10:39 am. The elevated particle concentrations could have been generated by the frequent movements of the operator or from the room ventilation system. No EC was detected in the room or in the PBZ. The EC concentration at the source was around 1.78 μg/m3 (Table 2). The TEM analysis showed that no carbon nanotubes were identified associated with spin coating process.
Substrate cutting
The task of substrate cutting by hand-operated scissors was performed in a ventilated enclosure (Table 1). According to the real-time monitoring data, this process did not generate measurable contaminants in the PBZ. The filter sample collected at the source, however, showed that carbon fibers were generated from cutting the substrate (Fig. 3h). Analysis of the filter samples showed that EC concentrations were below limit of detection (LOD) at the source or background locations, but 2.63 μg/m3 was measured in the PBZ (Table 2). TEM analysis indicated that carbon fibers were released at the source during cutting of CNT-coated substrates, but real-time measurements (calibrated with spheres and not with fibers) for the process showed no major particle releases at the source or in the PBZ. This high PBZ concentration did not correlate with CNTs because no CNTs were found on the samples collected using NIOSH 7402 method. Therefore, the PBZ EC may have been contributed by the room and ambient air.
Air flow around fume hood and ventilated enclosure
Both the fume hood and the ventilated enclosure were evaluated in the as-used condition. Face velocities were measured for the fume hood and for the ventilated enclosure. For the fume hood, the face velocity was highly variable across the hood opening due to the uneven sash heights and the blockage caused by equipment and supplies inside the hood. The overall average face velocity was 171 cm/s (337 ft/min). The average face velocity at the right opening was ~ 20% higher than that at the left opening. The fume hood exhaust flow rate was estimated at 1.73 m3/s (3660 ft3/min). The ventilated enclosure, unlike the fume hood, maintained a uniform face velocity of approximately 25.5 cm/s (50 ft/min) with an estimated exhaust flow rate of 0.048 m3/s (101 ft3/min).
The fume hood provided good emission control for the spin coating process. No EC or CNTs were detected in the PBZ or in the room for those activities performed inside the fume hood. However, it was operated at an average face velocity of around 171 cm/s (or 337 ft/min) which is much higher than consensus recommendations (typically 80–120 ft/min) (AIHA 2003; SEFA 2006; ACGIH 2016). This high face velocity would cause intense turbulence when the operator stands in front of the hood, and such turbulence can cause the release of contaminants manipulated inside the hood (Tsai et al. 2009). A detectable exposure to the operator will likely occur when a large amount of CNTs are in use and manipulated by an operator under similar conditions to those in this study.
The ventilated enclosure provided good control for the manual cutting of the CNT-coated substrate. This process is a low-energy, manual process and is less likely to generate large quantities of particles than a powered cutting process. In addition, despite the fact that the ventilated enclosure contained particles released from this task, the low face velocity (25.5 cm/s) operated in this enclosure should be checked against manufacturer’s specifications. Most enclosures require an inlet face velocity of at least 30–41 cm/s (60–80 ft/min) and generally on the higher side (41 cm/s) to ensure good containment.
Conclusions and recommendations
This field survey was conducted to monitor particle emissions from processes using CNTs for product research and development. The primary objective of this observational study was to identify potential exposures to CNTs in a laboratory setting during the handling of small amounts of nanomaterials. The conduct of experimental studies to obtain multiple measurements from every activity/task for statistical analysis is often not possible in field surveys. In this field study, every process (including weighing, sonication, and coating) was repeated multiple times during the preparation of the two different suspensions. For example, the 20-min probe sonication process was performed two times (40 min) for suspension A and three times (60 min) for suspension B. We collected a sound sample dataset by continuously monitoring the processes with real-time measurements at a 1-s logging rate. Therefore, the data reported in this manuscript were considered valid for the purpose of identifying the emission sources.
Particle emissions were found during the probe sonication process but limited from other processes. These limited emissions were due to the small production scale and limited quantity of CNTs being handled. Some EC concentrations were slightly above the NIOSH REL or near the level seen in ambient air pollution (0.60 ± 0.72 μg/m3). However, most of the EC concentrations measured from the collected samples were estimated values because of the short sampling time (less than 8 h) and low concentrations below the limit of quantitation (0.67 μg/sample in this case). For the probe sonication process with no engineering controls, the EC concentrations in the PBZ were highly variable, while those at the source were more consistently above the room background (except for the test done for suspension A shown in Table 2).
TEM results confirmed that CNT release from the open beaker during probe sonication was possible, though only one CNT was found on the 7402 filter TEM sample. However, CNT clusters and fibers were consistently found during probe sonication on the other samples collected using the grid-attached diffusion method and the ESPnano. The inlet tube of the ESPnano was visually contaminated with CNTs, thus demonstrating the potential for surface contamination in areas near the process if no control is employed.
The following suggestions are provided to improve particle containment during CNT weigh-out and preparation of CNT suspension with probe sonication. Handling nanomaterials in dry powder form potentially releases nanoparticles to the workplace (Tsai et al. 2009; Evans et al. 2010; Dahm et al. 2012). Because only a few micrograms of CNTs were handled in this case, no particle releases were identified by direct-reading instruments during CNT weigh-out without control measures. This result should not be interpreted that it is safe to handle small quantities of nanomaterials in an open environment. To prevent accidental releases of nanoparticles, it is strongly recommended that fume hoods, ventilated enclosures equipped with high efficiency particulate air (HEPA) filters, or gloveboxes be used for nanomaterial weighing and transferring processes (NIOSH 2012, 2013). Moreover, ducted control measures that exhaust to the outside are preferred to disperse HEPA-filtered air into the work or indoor atmosphere. As confirmed by TEM analysis on the filter samples, probe sonication for the CNT suspension in an open beaker poses a risk of nanomaterial emissions and work surface contamination. If a closed lid for probe sonication of the CNT suspension is not feasible, the task should be contained properly as suggested to limit exposure to nanomaterials.
References
ACGIH (2016) Industrial ventilation: a manual of recommended practice for design (29th edition), 29th edn. American Conference of Governmental Industrial Hygienists, Cincinnati
AIHA (2003) ANSI/AIHA Z9.5-2003 American national standard for laboratory ventilation. American Industrial Hygiene Association, Falls Church
Bayer MaterialScience (2010) Occupational exposure limit (OEL) for Baytubes defined by Bayer MaterialScience. Bayer MaterialScience, Leverkusen
BSI (2007) Nanotechnologies—part 2: guide to safe handling and disposal of manufactured nanomaterials. vol PD 6699–2:2007. British Standards Institute, London,
Carrero-Sanchez JC, Elias AL, Mancilla R, Arrellin G, Terrones H, Laclette JP, Terrones M (2006) Biocompatibility and toxicological studies of carbon nanotubes doped with nitrogen. Nano Lett 6(8):1609–1616
Dahm MM, Evans DE, Schubauer-Berigan MK, Birch ME, Fernback JE (2012) Occupational exposure assessment in carbon nanotube and nanofiber primary and secondary manufacturers. Ann Occup Hyg 56(5):542–556. doi:10.1093/annhyg/mer110
Evans D, Ku BK, Birch ME, Dunn KH (2010) Aerosol monitoring during carbon nanofiber production: mobile direct-reading sampling. Ann Occup Hyg 54(5):514–531
Grosse Y, Loomis D, Guyton KZ, Lauby-Secretan B, Ghissassi FE, Bouvard V, Benbrahim-Tallaa L, Guha N, Scoccianti C, Mattock H, Straif K (2014) Carcinogenicity of fluoro-edenite, silicon carbide fibres and whiskers, and carbon nanotubes. Lancet Oncol 15(13):1427–1428
Heitbrink WA, Collingwood S (2005) Aerosol generation by blower motors as a bias in assessing aerosol penetration into cabin filtration systems. JOEH 2(1):45–53
Heitbrink WA, Lo LM (2015) Effect of carbon nanotubes upon emissions from cutting and sanding carbon fiber-eposy composites. J Nanopart Res 17(8):355
Heitbrink WA, Lo LM, Dunn KH (2015) Exposure controls for nanomaterials at three manufacturing sites. J Occup Environ Hyg 12:16–18
International Agency for Research on Cancer (IARC) (2017) Agents classified by the IARC monographs. vols 1–119, Last updated on June 28, 2017
Johnson DR, Methner MM, Kennedy AJ, Steevens JA (2010) Potential for occupational exposure to engineered carbon-based nanomaterials in environmental laboratory studieo. Environ Health Perspect 118:49–54
Kostarelos K (2008) The long and short of carbon nanotube toxicity. Nat Biotechnol 26(7):774–776
Kuempel ED, Jaurand MC, Møller P, Morimoto Y, Kobayashi N, Pinkerton KE, Sargent LM, Vermeulen RC, Fubini B, Kane AB (2016) Evaluating the mechanistic evidence and key data gaps in assessing the potential carcinogenicity of carbon nanotubes and nanofibers in humans. Crit Rev Toxicol 18:1–58
Methner M, Hodson L, Dames A, Geraci C (2010) Nanoparticle emission assessment technique (NEAT) for the identification and measurement of potential inhalation exposure to engineered nanomaterials—part B: results from 12 field studies. JOEH 7(3):163–176
Muller J, Huaux F, Moreau N, Misson P, Heilier JF, Delos M, Arras M, Fonseca A, Nagy JB, Lison D (2005) Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol 207(3):221–231. doi:10.1016/j.taap.2005.01.008
NIOSH (1994) NIOSH manual of analytical methods (NMAM) Method 7402: asbestos by TEM. National Institute for Occupational Safety and Health, Cincinnati
NIOSH (2003) NIOSH manual of analytical methods (NMAM) Method 5040: elemental carbon (diesel particulate). National Institute for Occupational Safety and Health, Cincinnati
NIOSH (2012) General safe practices for working with engineered nanomaterials in research laboratories, DHHS (NIOSH) Publication No. 2012–147. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati
NIOSH (2013) Current Intelligence Bulletin 65: occupational exposure to carbon nanotubes and nanofibers, DHHS (NIOSH) Publication No. 2013–145. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Cincinnati
Piccinno F, Gottschalk F, Seeger S, Nowack B (2012) Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J Nanopart Res 14:1–11
Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WA, Seaton A, Stone V, Brown S, MacNee W, Donaldson K (2008) Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol 3(7):423–428. doi:10.1038/nnano.2008.111
Porter DW, Hubbs AF, Mercer RR, Wu N, Wolfarth MG, Sriram K, Leonard S, Battelli L, Schwegler-Berry D, Friend S, Andrew M, Chen BT, Tsuruoka S, Endo M, Castranova V (2010) Mouse pulmonary dose- and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology 269:136–147
Porter DW, Hubbs AF, Chen BT, McKinney W, Mercer RR, Wolfarth MG, Battelli L, Wu N, Sriram K, Leonard S, Andrew M, Willard P, Tsuruoka S, Endo M, Tsukada T, Munekane F, Frazer DG, Castranova V (2013) Acute pulmonary dose–responses to inhaled multi-walled carbon nanotubes. Nanotoxicology 7(7):1179–1194
Schulte PA, Kuempel ED, Zumwalde RD, Geraci CL, Schubauer-Berigan MK, Castranova V, Hodson L, Murashov V, Dahm MM, Ellenbecker M (2012) Focused actions to protect carbon nanotube workers. Am J Ind Med 55(5):395–411
SEFA (2006) Recommended practices for laboratory fume hoods. Scientific Equipment and Furniture Association, Garden City
Shinde A, Tsai CSJ (2016) Toxicity mechanism in fetal lung fibroblast cells for multi-walled carbon nanotubes defined by chemical impurities and dispersibility. Toxicol Res 5:248–258. doi:10.1039/C5TX00211G
Siegrist KJ, Reynolds SH, Kashon ML, Lowry DT, Dong C, Hubbs AF, Young S-H, Salisbury JL, Porter DW, Benkovic SA, McCawley M, Keane MJ, Mastovich JT, Bunker KL, Cena LG, Sparrow MC, Sturgeon JL, Dinu CZ, Sargent LM (2014) Genotoxicity of multi-walled carbon nanotubes at occupationally relevant doses. Part Fibre Tocicol 11:6
Tian FR, Cui DX, Schwarz H, Estrada GG, Kobayashi H (2006) Cytotoxicity of single-wall carbon nanotubes on human fibroblasts. Toxicol in Vitro 20(7):1202–1212. doi:10.1016/j.tiv.2006.03.008
Toyokuni S (2013) Genotoxicity and carcinogenicity risk of carbon nanotubes. Adv Drug Deliv Rev 65:2098–2110
Tsai S-J, Ada E, Isaacs JA, Ellenbecker MJ (2009) Airborne nanoparticle exposures associated with the manual handling of nanoalumina and nanosilver in fume hoods. J Nanopart Res 11:147–161
Wang L, Luanpitpong S, Castranova V, Tse W, Lu Y, Pongrakhananon V, Rojanasakul Y (2011) Carbon nanotubes induce malignant transformation and tumorigenesis of human lung epithelial cells. Nano Lett 11:2796–2803
Wick P, Manser P, Limbach LK, Dettlaff-Weglikowska U, Krumeich F, Roth S, Stark WJ, Bruinink A (2007) The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol Lett 168:121–131
Yu S, Dennis RL, Bhave PV, Eder BK (2004) Primary and secondary organic aerosols over the United States: estimates on the basis of observed organic carbon (OC) and elemental carbon (EC), and air quality modeled primary OC/EC ratios. Atmos Environ 38:5257–5268
Acknowledgements
The authors are also grateful to Charles Geraci, Laura Hodson, and Appavoo Rengasamy for their insightful comments and suggestions on the early version of the manuscript. This research was funded by the National Institute for Occupational Safety and Health under Nanotechnology Research Center project 927ZJLR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Disclaimer
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health (NIOSH). Mention of any company or product does not constitute endorsement by NIOSH.
Rights and permissions
About this article
Cite this article
Lo, LM., Tsai, C.SJ., Heitbrink, W.A. et al. Particle emissions from laboratory activities involving carbon nanotubes. J Nanopart Res 19, 293 (2017). https://doi.org/10.1007/s11051-017-3990-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-017-3990-8