Abstract
This report discusses the preparation and microstructure of Co/Ni co-doped MgGa2O4 nanoparticles. The nanoparticles with the size of 20–55 nm were synthesized by sol-gel method. The phase and crystallinity were confirmed by X-ray powder diffraction (XRD) pattern. The particle size was estimated according to XRD data and transmission electron microscopy. The electronic structure was studied using X-ray photoelectron spectroscopy (XPS). The XPS studies showed that Ga3+ ions possess tetrahedral and octahedral sites of spinel structure and the inverse degree (two times of the fraction of tetrahedral Ga3+ ions) has increased with the increase of the doping concentration of Co2+ and Ni2+ ions. For Co/Ni co-doped MgGa2O4, two broad absorption bands of 350~500 and 550~700 nm were observed in the absorption spectra. The broad band at 350~500 nm was assigned to the combination of the absorption of octahedral Co2+ and Ni2+ ions, whereas the absorption band at 550~700 nm is mainly due to tetrahedrally coordinated Co2+ ions and octahedrally coordinated Ni2+ ions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Magnesium gallate (MgGa2O4) is an electroluminescent material and has highly been attracted for applications in vacuum fluorescent and field emission displays (Costa et al. 2009; Moon et al. 2008; Pedro et al. 2015) because of its good chemical stability, low excitation voltage, and high purity of luminescence (Liu et al. 2013; Choi et al. 2010). It belongs to AB2O4 spinel-type oxides, where A and B are divalent and trivalent metal cations, respectively. The oxygen ions in AB2O4 arrange in close-packed cubic structure, forming tetrahedral and octahedral vacancies which are occupied by A and B cations. MgGa2O4 is known to be mixed spinel structure (Jiang et al. 2012; da Silva et al. 2013). The distribution of these cations in tetrahedral and octahedral sites classifies the spinel-type oxide material (AB2O4) into three types: (I) normal spinel: A and B ions occupy the tetrahedral and octahedral sites, respectively; (II) inverse spinel: a half of B ions occupy the tetrahedral position and the other half of B and all of A ions occupy the octahedral position; and (III) mixed spinel: A and B ions occupy the two different sites randomly. The distribution of A and B cations can be controlled by different preparation methods, type of impurities, and its concentration that will affect the material color, absorption, and emission properties (Liu et al. 2015; Yu et al. 2002; Kumar et al. 2004; Tsai et al. 2004).
Recently, MgGa2O4 doped with transition metal ions has attracted much attention because of strong emission and absorption properties in the visible region (Song et al. 2014; da Silva et al. 2010; Li et al. 2009; Yu and Liu 2011; Sun et al. 2016; Lv et al. 2009; Costa et al. 2012). The introduction of transition metal ions can tune the optical properties of spinel oxide materials. For example, Co- and Ni-doped MgGa2O4 materials show emissions at 685 nm (red) and 590 nm, respectively. At present, much work has been reported about the luminescence properties of MgGa2O4 doped with single active ions such as Cr3+, Mn2+, Ni2+, Eu3+, and Pr3+ (Pedro et al. 2015; Lv et al. 2009; Costa et al. 2012; Suzuki et al. 2008; Suzuki et al. 2005; Lv et al. 2010). Though many reports on optical properties, electronic structure of MgGa2O4 nanoparticles has seldom been studied (Yu et al. 2002; Kumar et al. 2004). To the best of our knowledge, the co-doping of Co and Ni in MgGa2O4 nanoparticle has not been reported. In this work, Co/Ni co-doped MgGa2O4 nanoparticles have been synthesized by the sol-gel method and the microstructure were probed through different analytical methods such as X-ray powder diffraction, TEM, and X-ray photoelectron spectroscopy (XPS). The influence of co-doping of transition elements (Co2+ and Ni2+) on the structure and optical properties were discussed.
Experimental
Polycrystalline Co/Ni co-doped MgGa2O4 nanoparticles were prepared by citric acid-assisted sol-gel method. Co(CH3COO)2 .4H2O (AR, Shanghai Hengxin Chemical Reagent Factory), Ni(NO3)2 .6H2O (AR, Aladdin Chemistry Co.), Mg(NO3)2 .6H2O (AR, Tianjin Kermel Chemical Reagent Center), and Ga(NO3)3 .xH2O (AR, Aladdin Chemistry Co.) were used as starting materials. Calculated amount of these raw materials were dissolved in deionized water. Co and Ni concentrations (mol%) were varied to produce a range of Co x Ni y Mg1 − x − y Ga2O4 (0.05 ≤ x ≤ 0.3, 0.01 ≤ y ≤ 0.3) stoichiometries. Citric acid was added into the above solution as a chelating agent. The molar ratio of metal ions to citric acid was 1:2; the pH of the solution was 3.0. The final solution was stirred for 1 h using a magnetic stirrer and then heated until formation of highly viscous gels. The gels were dried in an oven at 150 °C for 8 h followed by calcination at 800 °C for 5 h. The obtained nanoparticles were used for all the characterization studies.
Powder X-ray diffraction (XRD) data of the doped MgGa2O4 nanoparticles were collected on a Rigaku D/Max-rA diffractometer using a Cu Kα radiation (λ = 0.15418 nm) and with a graphite monochromator. The samples were step scanned in steps of 0.02° (2θ) using a count time of 0.1 s/step. Transmission electron micrographs were recorded using a JEM-2100 microscope. The samples were ultrasonicated in ethanol and then, a droplet of the suspension was deposited and dried on the sample support. The X-ray photoelectron spectra (XPS) were measured using a Thermofisher ESCALAB 250 spectrometer with monochromatized Al Kα radiation under ultrahigh vacuum (< 10−7 Pa). The binding energies were calibrated using C1s peak (284.6 eV) as a reference. All the peaks were deconvoluted after background subtraction using a mixed Gaussian–Lorentzian function. Diffuse absorbance spectra were recorded on a Shimadzu UV-2550 spectrophotometer with a step of 1 nm using an integration sphere at room temperature. BaSO4 was used as reference.
Results and discussion
Synthesis of nanocrystals
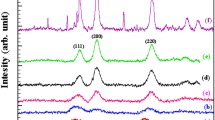
The XRD patterns of the Co x Ni y Mg1 − x − y Ga2O4 nanoparticles annealed at 800 °C are presented in Fig. 1. The peaks indexed as (220), (311), (222), (400), (422), (511), and (440) crystal planes in the XRD patterns are assigned to cubic MgGa2O4 spinel phase (JCPDS No. 10-113). The intensity of the diffraction peaks increased, and the full width at half-maximum (FWHM) decreased with the increase of Co and Ni concentrations, which indicates the improved crystallinity. It can be seen that a weak diffraction peak due to MgO impurity phase (marked as “*” in Fig. 1) appeared when x = 0.1 and y = 0.3, and the intensity of the diffraction peak of MgO impurity increased with the increase of Co and Ni concentrations.
The average particle size of the samples was calculated by means of the Scherrer formula D = 0.9λ/(βcosθ), where λ is the X-ray wavelength (0.15418 nm), β is the FWHM, and θ is the diffraction angle of the high intense peak. The size of the Co/Ni-doped MgGa2O4 nanoparticle is about 13 nm when x = 0.05 and y = 0.01, and the size increases to 50 nm when x = 0.3 and y = 0.3 (Table 1). The particle size increases dramatically when Ni content increases from 0.1 to 0.3. It may be that the doped Ni2+ ions occupy the position of Ga3+ ions, and the ionic radius of Ni2+ (69 pm) is larger than that of Ga3+ (62 pm), which makes the size of the particle increase.
The TEM images of the Co x NiyMg1 − x − y Ga2O4 nanoparticles are shown in Fig. 2. It can be seen from the figure that the particle shape is not regular. The particle size is in the range 15~55 nm and the size increases with the increase of dopant concentration. The TEM results are consistent with those of XRD (Table 1).
Electronic structure
The composition and the electronic structure of as-prepared Co/Ni co-doped MgGa2O4 nanoparticles were determined through XPS analysis. Figure 3 shows the XPS survey spectra of the Co x Ni y Mg1 − x − y Ga2O4 nanoparticles. The XPS core level spectra of Ga 2p, Mg 2p, Co 2p, and Ni 2p were collected as shown in Figs. 4 and 5. The XPS survey spectra revealed that no other elements were detected except contaminated carbon. The C 1s peak at 284.6 eV were used for calibration.
Figure 4a shows the Ga 2p3/2 spectra of MgGa2O4 nanoparticles with different concentrations of Co and Ni dopants. It can be found that all the spectra are asymmetric, indicating more than one chemical state of Ga3+ ions. After deconvolution, Ga 2p3/2 spectra are divided into two peaks, indicating the Ga3+ ions occupy two different coordination sites. The compositional details and peak parameters of Ga3+ are listed in Table 2. The peaks located at 1118.8 eV and 1116.8 eV are respectively assigned to octahedral and tetrahedral Ga3+ ions. When x = 0.05 and y = 0.01, the inversion degree (two times of the fraction of tetrahedral Ga3+ ion) is 0.5, and the value increases with the increase of the doping concentration. When both x and y are equal to 0.3, the inversion parameter is increased to 0.70.
The Mg 2p XPS spectra (Fig. 4b) are also not symmetric and consist of two peaks. The peak at 50.6 eV can be assigned to Mg2+ ion in octahedral site, and the peak at 49.7 eV can be ascribed to Mg2+ ion in tetrahedral site. These values are consistent with the literature reports (Ono et al. 2001; Zakaznova-Herzog et al. 2006). The fraction of octahedral Mg2+ ion is 0.48 when x = 0.05 and y = 0.01, and this value increases to 0.67 when x = 0.3 and y = 0.3. The compositional details and peak parameters of Mg2+ are listed in Table 3.
Figure 5a shows the Co 2p XPS spectra of Co x Ni y Mg1 − x − y Ga2O4 nanoparticles with different concentrations of Co and Ni dopants. The peak around 781 eV is ascribed to high-spin nature of divalent cobalt, and this value is consistent with Co2+ in other materials (Stelmachowski et al. 2014; Sharma et al. 2008). The FWHM is relatively wide probably because of the Co2+ ions occupy two different sites in the nanoparticles. The Ni 2p XPS spectra are displayed in Fig. 5b. The peak at 855.4 eV associated with the satellite peak at 861.6 eV is assigned to Ni 2p3/2, which is consistent with the literatures (Zhang et al. 2009; Bennet et al. 2016; Mittal et al. 2006). The compositional details and peak parameters of Co 2p3/2 and Ni 2p3/2 are listed in Table 4.
Optical properties
To study the relationship between the structure and optical properties of Co x NiyMg1 − x − y Ga2O4 nanoparticles, the absorption spectra were recorded as presented in Fig. 6. The Ni-doped MgGa2O4 shows two absorption peaks at 370 and 650 nm; this can be assigned to the transition of 3A2g (3F) → 3T1g (3P) and 3A2g (3F) → 3T1g (3F) of Ni2+ in the octahedral sites, respectively (Suzuki et al. 2008; Suzuki et al. 2010). Similarly, for Co-doped sample, two broad bands at around 400~500 and 550~700 nm were observed. The absorption band at 400~500 nm is assigned to Co2+ ions in octahedral sites, and the absorption peaks at 550, 600, and 650 nm are assigned to the spin-allowed of 4A2 (4F) → 2 T2 (2G), 4A2 (4F) → 2 T1 (2G), and 4A2 (4F) → 4 T1 (4P) transition of Co2+ in tetrahedral sites, respectively (Chen et al. 2002; Gaudon et al. 2009). For Co/Ni co-doped MgGa2O4, the broad band at 350~500 nm is the combination of the absorption of octahedral Co2+ and Ni2+ ions, while the band at 550~700 nm is mainly due to tetrahedral Co2+ and octahedral Ni2+ ions. The absorption intensity of the peaks increases with increasing concentration of Co2+ and Ni2+ ions.
Conclusion
Co x Ni y Mg1 − x − y Ga2O4 nanoparticles with the size of 15~55 nm were synthesized by citric acid-assisted sol-gel method. The powder XRD pattern confirmed the synthesized MgGa2O4 phase and particles size were increased with increase of dopant concentrations. The XPS spectra indicate that Ga3+ and Mg2+ ions occupy both the tetrahedral and octahedral sites. The fraction of tetrahedral Ga3+ ions increases with increasing doping concentration, which is consistent with the change of the proportion of octahedral Mg2+ ions. The inverse parameter increases with the increase of the doping concentrations. Two broad absorption bands at 350~500 and 550~700 nm were observed for Co/Ni co-doped MgGa2O4. The absorption is attributed to the combination of octahedral or tetrahedral Co2+ and octahedral Ni2+ ions. The intensity of the absorption bands increases with the increase of dopant concentrations of Co and Ni.
References
Bennet J, Tholkappiyan R, Vishista K, Victor Jaya N, Hamed F (2016) Attestation in self-propagating combustion approach of spinel AFe2O4 (A = Co, Mg and Mn) complexes bearing mixed oxidation states: magnetostructural properties. Appl Surf Sci 383:113–125
Chen ZZ, Shi EW, Li WJ, Zheng YQ, Zhong WZ (2002) Hydrothermal synthesis and optical property of nano-sized CoAl2O4 pigment. Mater Lett 55:281–284
Choi S, Kim K, Moon YM, Park BY, Jung HK (2010) Rapid synthesis of spherical-shaped green-emitting MgGa2O4: Mn2+ phosphor via spray pyrolysis. Mater Res Bull 45:979–981
Costa GKB, Pedro SS, Carvalho ICS, Sosman LP (2009) Preparation, structure analysis and photoluminescence properties of MgGa2O4: Mn2+. Opt Mater 31:1620–1627
Costa GKB, Sosman LP, Lopez A, Cella N, Barthem RB (2012) Optical and structural properties of Ni2+-doped magnesium gallate polycrystalline samples. J Alloys Compd 534:110–114
da Silva MAFM, da Silva Pedro S, Sosman LP (2010) Cr3+ impurity concentration and excitation dependences of magnesium gallate spinel photoluminescent data. J Alloys Compd 492:282–285
da Silva MAFM, Carvalho ICS, Cella N, Bordallo HN, Sosman LP (2013) Evidence of broad emission band in the system MgGa2O4–Ga2O3 doped with Cr3+ ions. Opt Mater 35:543–546
Gaudon M, Apheceixborde A, Menetrier M, Le Nestour A, Demourgues A (2009) Synthesis temperature effect on the structural features and optical absorption of Zn1-xCoxAl2O4 oxides. Inorg Chem 48:9085–9091
Jiang C, Sickafus KE, Stanek CR, Rudin SP, Uberuaga BP (2012) Cation disorder in MgX2O4 (X = Al, Ga, In) spinels from first principles. Phys Rev B 86:024203
Kumar VR, Narasimhulu KV, Gopal NO, Jung HK, Chakradhar RPS, Rao JL (2004) EPR, luminescence and IR studies of Mn activated ZnGa2O4 phosphor. J Phys Chem Solids 65:1367–1372
Li YX, Niu PJ, Hu L, Xu XW, Tang CC (2009) Monochromatic blue-green and red emission of rare-earth ions in MgGa2O4 spinel. J Lumin 129:1204–1206
Liu H, Yu LX, Li FH (2013) Photoluminescent properties of Eu3+ and Dy3+ ions doped MgGa2O4 phosphors. J Phys Chem Solids 74:196–199
Liu J, Duan XL, Li NN, Jiang HD (2015) Effects of synthesis method on cation distribution and optical properties of Co/Cr co-doped MgGa2O4 nanoparticles. J Alloys Compd 640:169–174
Lv HL, Du JY, Tang ZQ, Liu CN (2009) Nanocrystals of Co2+-doped MgGa2O4: preparation by a low-temperature combustion method and optical properties. J Chin Chem Soc 56:323–326
Lv HL, Du JY, Ma Y, Ma WL, Liu JQ (2010) Synthesis and characterization of ZnxMg1−xGa2O4: Co2+ fabricated by low-temperature burning method. Powder Technol 203:428–431
Mittal VK, Chandramohan P, Bera S, Srinivasan MP, Velmurugan S, Narasimhan SV (2006) Cation distribution in NixMg1−xFe2O4 studied by XPS and Mossbauer spectroscopy. Solid State Commun 137:6–10
Moon YM, Choi S, Jung HK, Lim SH (2008) Sensitized photoluminescent properties of manganese-activated magnesium gallate phosphor. J Lumin 128:1491–1495
Ono S, Asami K, Masuko N (2001) Mechanism of chemical conversion coating film growth on magnesium and magnesium alloys. Mater Trans 42:1225–1231
Pedro SS, Dasilva MAFM, Lopez A, Sosman LP (2015) Structural and photoluminescent properties of the MgGa2O4: Co2+ ceramic compound revisited after two decades. J Adv Ceram 4:267–271
Sharma Y, Sharma N, Rao GVS, Chowdari BVR (2008) Studies on spinel cobaltites, FeCo2O4 and MgCo2O4 as anodes for Li-ion batteries. Solid State Ionics 179:587–597
Song EH, Wang JL, Yu DC, Ye S, Zhang QY (2014) Anomalous tunable visible to near infrared emission in the Mn2+-doped spinel MgGa2O4 and room-temperature upconversion in the Mn2+ and Yb3+ co-doped spinel. J Mater Chem C 2:8811–8816
Stelmachowski P, Maniak G, Kaczmarczyk J, Zasada F, Piskorz W, Kotarba A, Sojka Z (2014) Mg and Al substituted cobalt spinels as catalysts for low temperature deN2O—evidence for octahedral cobalt active sites. Appl Catal B Environ 146:105–111
Sun JJ, Yu LX, Li FH, Wei SL, Li SC (2016) Up-conversion and near infrared luminescence in Er3+/Yb3+ co-doped glass-ceramic containing MgGa2O4 nanocrystals. J Lumin 170:444–450
Suzuki T, Murugan GS, Ohishi Y (2005) Spectroscopic properties of a novel near-infrared tunable laser material Ni: MgGa2O4. J Lumin 113:265–270
Suzuki T, Arai Y, Ohishi Y (2008) Quantum efficiencies of near-infrared emission from Ni2+-doped glass-ceramics. J Lumin 128:603–609
Suzuki T, Hughes M, Ohishi Y (2010) Optical properties of Ni-doped MgGa2O4 single crystals grown by floating zone method. J Lumin 130:121–126
Tsai BS, Chang YH, Chen YC (2004) Nanostructured red-emitting MgGa2O4: Eu3+ phosphors. J Mater Res 19:1504–1508
Yu LX, Liu H (2011) Photoluminescent properties of Dy3+ in MgO-Ga2O3-SiO2 nano-glass-ceramic prepared by sol–gel method. Phys B 406:3101–3103
Yu M, Lin J, Zhou YH, Wang SB (2002) Citrate–gel synthesis and luminescent properties of ZnGa2O4 doped with Mn2+ and Eu3+. Mater Lett 56:1007–1013
Zakaznova-Herzog VP, Nesbitt HW, Bancroft GM, Tse JS (2006) High resolution core and valence band XPS spectra of non-conductor pyroxenes. Surf Sci 600:3175–3186
Zhang L, Li F, Xiang X, Wei M, Evans DG (2009) Ni-based supported catalysts from layered double hydroxides: tunable microstructure and controlled property for the synthesis of carbon nanotubes. Chem Eng J 155:474–482
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Nos. 51172130 and 51672160).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, N., Liu, J., Duan, X. et al. Synthesis and microstructure of Co/Ni: MgGa2O4 nanoparticles. J Nanopart Res 19, 285 (2017). https://doi.org/10.1007/s11051-017-3985-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-017-3985-5