Abstract
FeVO4@TiO2 nanocomposite was fabricated via a simple and cost-effective approach. The FeVO4 nanorods were synthesized by a hydrothermal method combined with calcination route without using any template and then coated with TiO2 through an annealing process of dihydroxybis titanium. The FeVO4 nanocomposite has a significantly enhanced electrochemical performance by coating with TiO2. The FeVO4@TiO2 delivered a specific capacity of 1147 mAh g−1, the discharge capacity remaining at 596 mAh g−1 after 100 cycles (at 200 mA g−1), which is higher than that of pure FeVO4. The discharge capacity of FeVO4@TiO2 could be as high as 337 mAh g−1 (at a high load current density of 10,000 mA g−1). Compared with pure FeVO4, FeVO4@TiO2 shows a better rate performance. The amorphous TiO2 coating on a layer of FeVO4 created efficient improved stability of the structure during the charge/discharge process. The excellent rate capability and cyclic stability of the sample proved that FeVO4@TiO2 could be used as a new anode for lithium ion battery application. The synthesis method can also be applied to synthesize other related materials with typical morphologies and properties.

The FeVO4@TiO2 nanocomposite has a significantly enhanced electrochemical performance. The FeVO4@TiO2 delivers a specific capacity of 1147 mAh g−1 at 200 mA g−1. The discharge capacity remained at 596 mAh g−1 after 100 cycles, which is much higher than that of pure FeVO4 at the same condition. The amorphous TiO2 coating on the surface of FeVO4 can improve cycle stability during the charging/discharging process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In modern times, rechargeable Li-ion batteries (LIBs) have been universally used in plug-in electric vehicles and portable electronics owing to the advantages of its high-energy density and long life (Cheng and Chen 2011). As the traditional commercial anode material, graphite with the low theoretical capacity (372 mAh g−1) cannot meet the gradually increasing requirement for energy in modern times (Sun et al. 2013; Peng et al. 2005). Thus, novel anode materials with a much higher reversible capacity should be developed.
Recently, transitional metal vanadates showing the advantages of high theoretical capacity and rate performance based on the unique conversion mechanism have attracted much attention as electrode materials (Wang and Cao 2008; Huang et al. 2010; Yang et al. 2016; Lei et al. 2007; Pan et al. 2011). A wide variety of transition metal vanadates (such as FeVO4, ZnV2O4, Zn3V2O8, ZnV2O6, MoV2O8, CuV2O6, CoV2O6, and Ag2V4O11) has been fabricated and their electrochemical performance was studied (Xi and Ye 2010; Zhu et al. 2013; Sun et al. 2011; Shi et al. 2011; Li et al. 2013; Wang et al. 2014; Zhang et al. 2015; Liang et al. 2015; Wang et al. 2012; Xiao et al. 2009). For example, Yang et al. (2014) have prepared Co3V2O8 nanosheets, which showed excellent electrochemical performance. Ni et al. (2014) firstly reported synthesized Li3VO4 through hydrothermal and annealed route method. The Li3VO4 delivered a good initial discharge, 396 mAh g−1 at a rate of 0.25 C after 100 cycles. Gan and workers (2014) synthesized hexagonal Zn3V2O8 nanosheets, which displayed an excellent reversible capacity of 1103 mAh g−1. The non-spherical structures Co3V2O8·nH2O exhibited impressive electrochemical properties with superior lithium storage capability (after 255 cycles, maintaining 847 mAh g−1) (Wu et al. 2015). Yin et al. (2016) have first reported MoV2O8 nanorods were evaluated as an anode material and showed excellent performance.
Among transition metal vanadates, FeVO4 (iron vanadate), as a promising host for anode materials, has prominent advantages due to its layered structure (short intercalated Li+ ion distance) (Ma et al. 2011). Some reports have reported high specific capacities of the vanadates (1300 mAh g−1 for FeVO4). Yan et al. (2016) reported the synthesis of FeVO4 via a facile hydrothermal-sintering method. The FeVO4 nanoparticles show initial capacities of 527 mAh g−1, maintaining 430 mAh g−1 (after 100 cycles). A number of reports on two-dimensional (2D) nanostructure materials used to develop high performance are attributed to a large contact area and prompt Li+ diffusion paths. Sim et al. (2012) reported amorphous FeVO4 nanosheet arrays by a CVD method. The FeVO4 nanosheet arrays presented high specific capacities. Liu and coworkers (2017) reported FeVO4/graphene nanocomposites were synthesized via a hydrothermal and heat-treatment method. The FeVO4/graphene nanocomposites delivered a good specific capacity, 1046 mAh g−1 after 100 cycles. An abundance of anode materials with graphene oxide nanocomposite and carbon coating composite with outstanding electrochemical performance has been reported. Titanium dioxide (TiO2) with a protective layer is ideal for modified material, which is widely used to improve cyclic stability for the cathode material. Compared with anode materials, a few works have been conducted on anode materials coated with TiO2. Chen et al. (2015) synthesized porous cubic Mn2O3@TiO2 through precipitation-calcination route. The porous cubic Mn2O3@TiO2 delivered a superior specific capacity of 936 mAh g−1 at a rate of 200 mA g−1 after 100 cycles. The highly stable TiO2 provides protection for Mn2O3 from structural destruction due to the volume change during charge/discharge processes, and a new scheme is provided to solve the problem on capacity loss for transition metal oxides.
In this work, we reported the synthesis of nanostructures of FeVO4 nanorods without any additives and template under hydrothermal conditions followed by calcination, using ammonium lactate titanium (IV) as modified material. The synthesis method is simple and cost effective. Compared with pure FeVO4, FeVO4@TiO2 shows better electrochemical performance. The amorphous TiO2 coating layer on FeVO4 created an improvement in the stability of the structure, rate capability, and cyclic stability through the charge/discharge process. FeVO4@TiO2 synthesized by a suitable method could be used as a promising anode material for lithium-ion battery application.
Experimental
Synthesis and characterization of the samples
In the experiment, all of the chemicals were of analytical grade. One millimole of FeCl3·6H2O and 1 mmol NH4VO3 were added into 10 ml of deionized water at room temperature and stirred for 20 min, respectively. In the stirring, the NH4VO3 solution was added dropwise into the FeCl3 solution, and after 0.5 h, the mixed solution into a 50-ml autoclave and heated at 180 °C for 2 h. The expected samples were cleaned with H2O and ethanol, dried at 80 °C for 8 h, and calcined at 500 °C for 2 h. The FeVO4 nanorods were obtained.
0.2 g of the FeVO4 nanorods was added into 10 ml of NaOH solution (0.1 mmol) under stirring for 90 min, then 100 μl of ammonium lactate titanium (IV) was added under stirring for 180 min. The followed steps for synthesis of FeVO4 were the same as those described above. The FeVO4@TiO2 nanostructures were obtained.
X-ray diffraction (XRD) was characterized using Cu Kα radiation by Bruker AXS (D8 diffractometer). The sizes of the samples were detected by SEM (JEOLJSM-7400F, Japan), X-ray (EDX) detector (Oxford Instruments, INCA), and TEM/HRTEM (Tecnai G2 F30, FEI company). X-ray photoelectronic spectrometer (XPS, VGESCA-LABMK II) was used to determine the valence states of the elements in the metal oxide.
Electrochemical measurements
The electrodes were prepared by pressing a mixture of polytetrafluoroethylene (PTFE) (10%), acetylene black (20%), and active material (70%) onto a nickel net. The electrodes were dried at 110 °C in a vacuum drying oven for 12 h. Electrochemical experiments were performed using CR2032-type cells with Li foil as the counter electrode and composite electrodes of expected compounds. The typical mass of active material was about 1.0–1.2 mg cm−2. The electrolyte was a solution of l M LiPF6 in diethyl carbonate (DEC) and ethylene carbonate (EC) (1:1 by volume). The battery was assembled in a glove box filled with argon gas. The voltage range of 0.01 to 3.00 V and different current densities were controlled during battery tests. The cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) experiments were conducted by a CHI 660A electrochemical workstation.
Results and discussion
Both FeVO4 nanorods and FeVO4@TiO2 nanoparticles were characterized by XRD analysis, as presented in Fig. 1. The XRD indicated that the two samples had the same single-phase structure, and the diffraction peaks of two samples are in agreement with the ordered triclinic FeVO4 structure (JCPDS No. 071-1592), without any impurity phase. There are no TiO2 diffraction peaks in the FeVO4@TiO2 sample, which might be due to the low content and amorphous state of TiO2. However, compared with porous FeVO4 nanorods, the main diffraction peaks of FeVO4@TiO2 were weak in Fig. 1b.
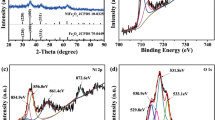
As shown in Fig. 2, the X-ray photoelectron spectroscopy measurements were used to confirm the oxidation states of Fe, V, O, and Ti in FeVO4@TiO2. The peaks located at 711.3 and 724.5 eV identified with the binding energy of Fe 2p3/2 and 2p1/2. The peaks appearing at 516.8, 524.6, and 529.8 eV are attributable to the binding energy of V2p3/2, V2p1/2, and O1s. The weaker peaks at 458.6 and 464.2 eV correspond to the binding energy of Ti 2p3/2 and 2p1/2 (Fig. 2d), meaning the presence of Ti4+ in TiO2. The combined results demonstrated that the FeVO4@TiO2 composite was obtained.
The particle morphologies of the as-prepared FeVO4 and FeVO4@TiO2 were examined by SEM and TEM. As shown in Fig. 3a and b, uniform FeVO4 nanorods were successfully obtained. Figure 3c and d show that the FeVO4@TiO2 composites retain the nanorods’ structure, which would not be destroyed by TiO2 encapsulation and thermal treatment. The EDX analyses show that Fe, V, and O are presented in the FeVO4 samples, as shown in Fig. 3g; the uniform elemental Ti was distributed on the surface of FeVO4@TiO2 which is shown in Fig. 3f and Ti atom content in the composite was about 5%. The TEM images of the as-prepared FeVO4 and FeVO4@TiO2 are shown in Fig. 4; these nanorods have widths of 100 nm and lengths of 0.3–2.0 μm, respectively. From Fig. 4b, it clearly shows that FeVO4 nanorods have a hollow cavity structure (diameters of 5–25 nm). Upon TiO2 encapsulation and calcination at 450 °C, the surfaces of the FeVO4 nanorods (Fig. 4c) become relatively smooth and the HRTEM image in Fig. 2f showed apparent lattice fringes. The HRTEM image taken from the edge of the FeVO4@TiO2 samples showed the lattice fringe spacings of 0.62 and 0.51 nm (conforming to the interplanar spacings of the (100) and (011) lattice planes) of triclinic FeVO4, respectively. A gray amorphous border is observed on the wall, which refers to TiO2 layers (~5.6 nm).
Figure 5 presents the CV curves of the FeVO4@TiO2 samples. The CV curves were collected in a potential range of 0.01 to 3.0 V at a sweep rate of 0.3 mV s−1. In the first curve, two reduction peaks at around 2.6–1.8 and 0.63 V, and the two obvious cathodic peaks observed at ~0.19 and ~0.46 V, can be assigned to the transformation of FeVO4 into LixV2O5 and the reduction of Fe3+ to Fe0, respectively. In this process, the electrochemical reactions can be described as: xLi++ xe− + FeVO4 → Fe + LixV2O5 (Sim et al. 2012; Liu et al. 2017). From the following cycle, the peak of ~0.19 V disappeared, which implied an irreversible reaction occurring in this potential. The observation of phase transition of FeVO4 in cycling is similar to that reported in literature (Ma et al. 2015; Ni et al. 2015). Due to the generated SEI layer and dissolution of the electrolyte solvent between the electrolyte and electrode, the electrode materials presented irreversible performance in the first cycle. The reduction peak at 0.46 V shifted to a positive direction at 0.54 V. The two obvious oxidation peaks at 1.3 and 2.04 V, a weak oxidation peak at ~2.5 V, are not changed from the initial cycle. Additionally, peaks at 1.7 V, which are attributed to the reduction peaks of anatase TiO2 (Chen et al. 2015), suggest that the TiO2 coating is active for lithium-ion intercalation. Except for the first cycle, the followed curves are nearly overlapped, indicating good reversibility for lithium ions to be intercalated and deintercalated in the FeVO4@TiO2 samples.
The electrochemical performances of FeVO4 and FeVO4@TiO2 have been evaluated utilizing the coin-type cell. The cycling performance of the FeVO4 and FeVO4@TiO2 electrodes for 100 cycles at 200 mA g−1 in the range from 0.01 to 3.0 V (vs. Li/Li+) is shown in Fig. 6. The first discharge capacities of FeVO4 and FeVO4@TiO2 were 1032 and 1147 mAh g−1, respectively. The initial charge specific capacities of the FeVO4 and FeVO4@TiO2 electrodes were 714 and 829 mAh g−1; the first coulombic efficiencies were 69.2 and 72.3%, respectively. The cycle performance in Fig. 6c shows that FeVO4@TiO2 retains a capacity of 597 mA g−1 after 100 cycles. In contrast, the discharge capacity of FeVO4 decreased to 470 mA g−1 at the same cycles. It is apparent that FeVO4@TiO2 has a better cyclic stability than FeVO4. The highly stable amorphous TiO2 provides protection for FeVO4 from structural destruction through the charge/discharge processes.
In addition, the rate performance of the FeVO4 and FeVO4@TiO2 is shown in Fig. 6d, in which the current density varied from 200 to 10,000 mA g−1. The discharge capacities were obtained as 819, 536, 439, 436, 387, and 337 mAh g−1, respectively, at discharge current densities of 200, 500, 1000, 2000, 5000, and 10,000 mA g−1. The capacity was increased to 562 mAh g−1 when the current density was reverted to 100 mA g−1, these results further implied that the FeVO4@TiO2 electrode behaved at a better cycle and rate performance than the bare FeVO4 electrode (especially, 285 and 176 mAh g−1 at high current densities of 2000 and 5000 mA g−1). The better rate capability of the FeVO4@TiO2 electrode can be ascribed to the coating of TiO2 on the surface of FeVO4.
To reveal the superior electrochemical performance of FeVO4@TiO2 compared with FeVO4 for lithium energy storage, the charge-transfer resistance was tested by EIS over the frequency domain from 0.01 Hz to 100 kHz (Fig. 7). The result showed that the resistance of the FeVO4@TiO2 electrode was 97 Ω, which is lower than that of pure FeVO4 (236 Ω). So, FeVO4@TiO2 shows better conductivity than FeVO4, that is, the FeVO4@TiO2 electrode has accommodated the high current density during the cycles. It is why FeVO4@TiO2 has a better cycling stability than FeVO4.
Conclusions
In summary, a facile route to prepare porous FeVO4 nanorods without any additives and template under hydrothermal conditions followed by calcinations was reported. To improve structural stability and cycle life, TiO2 is used to coat the porous FeVO4 nanorods. The FeVO4@TiO2 delivered a specific capacity of 1147 mAh g−1 at 200 mA g−1. The discharge capacity remained at 596 mAh g−1 after 100 cycles, higher than that of pure FeVO4. Compared with pure FeVO4, FeVO4@TiO2 showed better electrochemical performances. The amorphous TiO2 coating layer on FeVO4 efficiently enhanced stability during the charging and discharging process, and this interesting synthesis method can also be applied to synthesize other materials with typical morphologies and properties.
References
Chen XQ, Lin HB, Zheng XW, Cai X, Xia P, Zhu YM, Li XP, Li WS (2015) Fabrication of core–shell porous nanocubic Mn2O3@TiO2 as a high-performance anode for lithium ion batteries. J Mater Chem A 3:18198–18206. doi:10.1039/C5TA04238K
Cheng FY, Chen J (2011) Transition metal vanadium oxides and vanadate materials for lithium batteries. J Mater Chem 21:9841–9848. doi:10.1039/C0JM04239K
Gan LH, Deng DR, Zhang YJ, Li G, Wang XY, Jiang L, Wang CR (2014) Zn3V2O8 hexagon nanosheets: a high-performance anode material for lithium-ion batteries. J Mater Chem A 2:2461–2466. doi:10.1039/C3TA14242F
Huang WD, Gao SK, Ding XK (2010) Crystalline MnV2O6 nanobelts: synthesis and electrochemical properties. J Alloys Compd 495:185–188. doi:10.1016/j.jallcom.2010.01.116
Lei SJ, Tang KB, Jin Y, Chen CH (2007) Preparation of aligned MnV2O6 nanorods and their anodic performance for lithium secondary battery use. Nanotechnology 18:175605. doi:10.1088/0957-4484/18/17/175605
Li D, Duan XC, Qin Q (2013) Facile synthesis of novel α-Ag3VO4 nanostructures with enhanced photocatalytic activity. CrystEngComm 15:8933–8936. doi:10.1039/C3CE41365A
Liang LY, Xu Y, Wang X, Wang CL, Zhou M, Fu Q, Wu MH, Lei Y (2015) Intertwined Cu3V2O7(OH)2·2H2O nanowires/carbon fibers composite: a new anode with high rate capability for sodium-ion batteries. J Power Sources 294:193–200. doi:10.1016/j.jpowsour.2015.06.076
Liu XL, Cao YC, Zheng H, Chen X, Feng CQ (2017) Synthesis and modification of FeVO4 as novel anode for lithium-ion batteries. Appl Surf Sci 394:183–189. doi:10.1016/j.apsusc.2016.09.133
Ma H, Yang XJ, Tao ZL, Liang J, Chen J (2011) Controllable synthesis and characterization of porous FeVO4 nanorods and nanoparticles. CrystEngCommun 13:897–901. doi:10.1039/C0CE00273A
Ma JJ, Ni SB, Zhang JC, Yang XL, Zhang LL (2015) The charge/discharge mechanism and electrochemical performance of CuV2O6 as a new anode material for Li-ion batteries. Phys Chem Chem Phys 17:21442–21447. doi:10.1039/C5CP03435C
Ni SB, Lv XH, Ma JJ, Yang XL, Zhang LL (2014) Electrochemical characteristics of lithium vanadate, Li3VO4 as a new sort of anode material for Li-ion batteries. J Power Sources 248:122–129. doi:10.1016/j.jpowsour.2013.09.050
Ni SB, Ma JJ, Zhang JC, Yang XL, Zhang LL (2015) Electrochemical performance of cobalt vanadium oxide/natural graphite as anode for lithium ion batteries. J Power Sources 282:65–69. doi:10.1016/j.jpowsour.2015.01.187
Pan AQ, Zhang JG, Cao GZ, Liang SQ, Liu J (2011) Nanosheet-structured LiV3O8 with high capacity and excellent stability for high energy lithium batteries. J Mater Chem 21:10077–10084. doi:10.1039/C1JM10976F
Peng B, Fan ZC, Qiu XM (2005) A novel transparent vanadate glass for use in fiber optics. Adv Mater 17:857–859. doi:10.1002/adma.200401271
Shi R, Wang YJ, Zhou F, Zhu YF (2011) Zn3V2O7(OH)2(H2O)2 and Zn3V2O8 nanostructures: controlled fabrication and photocatalytic performance. J Mater Chem 21:6313–6320. doi:10.1039/C0JM04451B
Sim DH, Rui XH, Chen J, Tan HT, Lim TM, Yazami R, Hng HH, Yan QY (2012) Direct growth of FeVO4 nanosheet arrays on stainless steel foil as high-performance binder-free Li ion battery anode. RSC Adv 2:3630–3633. doi:10.1039/C2RA20058A
Sun XJ, Wang JW, Xing Y, Zhao Y, Liu XC, Liu B, Hou SY (2011) Surfactant-assisted hydrothermal synthesis and electrochemical properties of nanoplate-assembled 3D flower-like Cu3V2O7(OH)2·2H2O microstructures. CrystEngCommun 13:367–370. doi:10.1039/C0CE00083C
Sun Y, Hu X, Luo W, Xia FF, Huang YH (2013) Reconstruction of conformal nanoscale MnO on graphene as a high-capacity and long-life anode material for lithium ion batteries. Adv Funct Mater 23:2436–2444. doi:10.1002/adfm.201202623
Wang Y, Cao GZ (2008) Developments in nanostructured cathode materials for high-performance lithium-ion batteries. Adv Mater 20:2251–2269. doi:10.1002/adma.200702242
Wang M, Shi YJ, Jiang GQ (2012) 3D hierarchical Zn3 (OH)2V2O7·2H2O and Zn3 (VO4)2 microspheres: synthesis, characterization and photoluminescence. Mater Res Bull 47:18–23. doi:10.1016/j.materresbull.2011.10.020
Wang JX, Yang X, Chen J (2014) Photocatalytic activity of novel Ag4V2O7 photocatalyst under visible light irradiation. J Am Ceram Soc 97:267–274. doi:10.1111/jace.12639
Wu FF, Xiong SL, Qian YT, Yu SH (2015) Hydrothermal synthesis of unique hollow hexagonal prismatic pencils of Co3V2O8·n H2O: a new anode material for lithium-ion batteries. Angew Chem Int Ed 54:10787–10791. doi:10.1002/anie.201503487
Xi GC, Ye JH (2010) Synthesis of bismuth vanadate nanoplates with exposed {001} facets and enhanced visible-light photocatalytic properties. Chem Commun 46:1893–1895. doi:10.1039/B923435G
Xiao LF, Zhao YQ, Yin J (2009) Clewlike ZnV2O4 hollow spheres: nonaqueous sol–gel synthesis, formation mechanism, and lithium storage properties. Chem Eur J 15:9442–9450. doi:10.1002/chem.200901328
Yan N, Xu Y, Li HJ, Chen W (2016) The preparation of FeVO4 as a new sort of anode material for lithium ion batteries. Mater Lett 165:223–226. doi:10.1016/j.matlet.2015.11.061
Yang GZ, Cui H, Yang GW, Wang CX (2014) Self-assembly of Co3V2O8 multilayered nanosheets: controllable synthesis, excellent li-storage properties, and investigation of electrochemical mechanism. ACS Nano 8(5):4474–4487. doi:10.1021/nn406449u
Yang GZ, Wu MM, Wang CX (2016) Ultrathin Zn2 (OH)3VO3 nanosheets: first synthesis, excellent lithium-storage properties, and investigation of electrochemical mechanism. ACS Appl Mater Interfaces 8(36):23746–23754. doi:10.1021/acsami.6b08048
Yin ZG, Xiao Y, Wang X, Wang W, Zhao D, Cao MH (2016) MoV2O8 nanostructures: controlled synthesis and lithium storage mechanism. Nano 8:508–516. doi:10.1039/C5NR05602K
Zhang SY, An W, Wu G (2015) Cu5 (VO4)2(OH)4·H2O nanobelts as anode materials for lithium-ion batteries. Chem Phys Lett 621:1–4. doi:10.1016/j.cplett.2014.12.047
Zhu Q, Wang WS, Lin L (2013) Facile synthesis of the novel Ag3VO4/AgBr/Ag plasmonic photocatalyst with enhanced photocatalytic activity and stability. J Physical Chemistry C 117:5894–5900. doi:10.1021/jp400842r
Acknowledgments
This work was financially supported by the Natural Science Foundation of China (No. 21476063) and is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zheng, H., Yang, Y., Liu, X. et al. Controllable synthesis of FeVO4@TiO2 nanostructures as anode for lithium ion battery. J Nanopart Res 19, 243 (2017). https://doi.org/10.1007/s11051-017-3940-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-017-3940-5