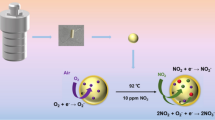
Abstract
Undoped In2O3 and 0.25–1.00 wt% M (M=Pt, Nb, and Ru)-doped/loaded In2O3 nanoparticles were successfully synthesized in a single-step flame spray pyrolysis technique using indium nitrate, platinum (II) acetylacetonate, niobium ethoxide, and ruthenium (III) acetylacetonate precursors. The undoped In2O3 and M-doped In2O3 nanoparticles were characterized by Brunauer–Emmett–Teller (BET) analysis, X-ray diffraction (XRD), and scanning and transmission electron microscopy (SEM & TEM). The BET average diameter of spherical nanoparticles was found to be in the range of 10.2–15.2 nm under 5/5 (precursor/oxygen) flame conditions. All XRD peaks were confirmed to correspond to the cubic structure of In2O3. TEM images showed that there is no Pt nanoparticle loaded on In2O3 surface, suggesting that Pt should form solid solution with the In2O3 lattice. Gas sensing studies showed that 0.5 wt% Pt doping in In2O3 nanoparticles gave a significant enhancement of NO2 sensing performances in terms of sensor response and selectivity. 0.5 wt% Pt/In2O3 exhibited a high NO2 response of ~1904 to 5 ppm NO2 at 250 °C and good NO2 selectivity against NO, H2S, H2, and C2H5OH. In contrast, Nb and Ru loading resulted in deteriorated NO2 response. Therefore, Pt is demonstrated to be an effective additive to enhance NO2 sensing performances of In2O3-based sensors.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chemical gas sensors for detections of various gases have been of great interest due to their high demand in numerous applications including environmental monitoring, safety protection, industrial process control, and quality assurance. They may be divided into many different types based on transduction principles including chemiresistive semiconducting metal oxide, chemiresistive polymer, field-effect type, acoustic wave, electrochemical, and optical gas sensors. Among these, semiconducting metal oxides such as tin oxide, tungsten oxide, and indium oxide (In2O3) have earned much attention because of their high sensitivity, fast response, low cost, and simple electronic interface. However, they suffer from low selectivity, high operating temperature, and limited long-term stability. New materials, structures, and preparation processes have extensively been explored to achieve superior gas sensing performances. Indium oxide is a relatively new wide-bandgap (3.6 eV at room temperature) semiconductor for gas sensing (Yang et al. 2004). It has good electrical conductivity and high stability and is considered as a potential gas-sensing material. It has recently been shown to be sensitive to various reducing and oxidizing gases especially environmental gases and volatile organic compounds (VOCs) (Huang et al. 2009; Chen et al. 2011). Among various target gases, NO2 is particularly important because it is a highly toxic gas widely produced from combustion and other industrial processes. Main research on NO2 sensors has been focusing on improving sensitivity, detection limit, selectivity, and operating temperature. Recently, several NO2 sensors based on metal oxide composites are reported to operate at low and room temperatures (Neri et al. 2013; Chen et al. 2013; Zhang et al. 2014; Liu et al. 2014, 2015; Su and Peng 2015). However, they still have insufficient responses at very low NO2 concentrations (<1 ppm), low selectivity, and poor stability. In addition, they are not suitable for in situ NO2 detection in harsh combustion environment at high temperatures. Thus, it is still compelling to develop novel alternative materials for NO2 sensing. Table 1 summarizes the gas response of In2O3-based materials prepared by various physical/chemical routes and recent results of the state-of-the-art NO2 sensors.
For instance, In2O3 thin films deposited by RF sputtering showed a good response of 50 toward 25 ppm NOx at a high temperature of 500 °C (Kannan et al. 2010). In addition, In2O3 thin films prepared by metal organic chemical vapor deposition (MOCVD) (Ali et al. 2008) displayed fair responses of 9 toward 10 mbar NOx and 4 toward 1 mbar O2 at 150 °C. The gas sensing performances of In2O3 sensors may be improved considerably by the formation of nanostructures. For example, flower-like In2O3 nanostructures prepared by hydrothermal and annealing treatments were reported to be highly sensitive to NO2 with a good response of 2.4 at a very low concentration of 5 ppb and a low working temperature of 125 °C (Xu et al. 2012). The incorporation of additive to semiconducting oxide is a widely used approach to enhance not only sensitivity but also selectivity toward a specific gas. Noble metals such as platinum (Pt), palladium (Pd), Niobium (Nb), or ruthenium (Ru) are particularly attractive because they can provide particularly significant gas sensing enhancement compared with other groups of materials (Neri et al. 2007; Liu et al. 2009; Tu et al. 2010). Among these, platinum (Pt) is the best understood and the most effective catalyst for sensing of reducing gas including hydrogen, carbon monoxide, and hydrocarbon. Typically, Pt additives provide higher response, higher selectivity, shorter response/recovery times, lower operating temperature, and higher stability due to the well-known chemical sensitization process via the ‘spillover’ effect. For illustration, Pt-doped In2O3 thin films prepared by in situ nanocasting method showed very high H2S response of 1850 at 150 °C (Tu et al. 2010). Additionally, Pt-loaded WO3, SnO2, and ZnO nanoparticles produced by flame spray pyrolysis (FSP) offered excellent hydrogen response and selectivity at relatively low operating temperatures (Tamaekong et al. 2009; Samerjai et al. 2014 and Liewhiran et al. 2014). Furthermore, Pt also provides gas sensing enhancement for oxidizing gases. For instance, Pt-doped In2O3 particles prepared by the impregnation and non-aqueous sol–gel methods displayed higher oxygen response at a low operating temperature 200 °C (Neri et al. 2007). Moreover, Pt-loading on WO3 nanoparticles with a low content of 0.25 wt% led to a considerable enhancement of NO2 response at 250 °C (Samerjai et al. 2014). Ru and Nb are also interesting noble metals, which have only been recently studied for gas sensing and shown to exhibit some catalytic effects toward some gases including NO2, SO2, and H2 for SnO2-, ZnO-, and WO3-based sensors (Liewhiran et al. 2009; Kruefu et al. 2011; Kruefu et al. 2014). However, they have never been applied with In2O3 and it is interesting to study their effects on gas sensing performances of In2O3.
Based on the reported results, FSP-made metal oxide gas sensors showed relatively high gas sensing performances compared with those prepared by several other methods. FSP is thus a very promising technique for synthesis of gas-sensing materials. FSP method uses high-temperature flame to drive chemical reactions of sprayed precursors resulting in atomic clusters which further grow to nanoparticles by surface growth, coagulation, and coalescence at high temperatures (Tamaekong et al. 2009; Samerjai et al. 2014; Liewhiran et al. 2014). It has previously been employed to synthesize various metal oxide particles with well-controlled size and high surface area in one step (M’adler et al. 2002 ). Recently, it has been used to produce In2O3 and indium tin oxide nanoparticles for formaldehyde sensing (Kemmler et al. 2012 ). However, there is no report of its application for synthesis of In2O3 doped or loaded with Pt, Ru, and Nb. In this research, In2O3 nanoparticles doped or loaded with 0.25–1.00 wt% of three noble metals including Pt, Nb, and Ru are synthesized by the FSP technique for the first time and characterized for gas sensing toward NO2 in the working temperature ranging from 200 to 350 °C.
Methods
Flame synthesis of nanoparticles
Indium (III) nitrate (In(NO3)3, Aldrich, 99.9%), platinum (II) acetylacetonate (C10H14O4Pt, Aldrich, 97%), niobium (V) ethoxide (C10H25NbO5, Aldrich, 97%), and ruthenium (III) acetylacetonate [(C5H7O2)3Ru, Aldrich, 97% were used as precursors and dissolved in ethanol (Carlo Erba, 98.5%) to obtain a 0.5 mol/L precursor solution. The details of FSP synthesis have been previously reported in the literature (Liewhira et al. 2014). In a typical synthesis procedure, the precursors were fed into a FSP reactor using a syringe pump at a rate of 5 mL/min while 5 L/min of O2 was being dispersed (5/5 flame condition). The gas flow rates of methane and O2 supporting flamelets were 1.19 and 2.46 L/min, respectively. The pressure drop at the capillary tip was kept constant at 1.5 bars by adjusting the orifice gap area at the nozzle. The reactor’s water-cooling system was used to avoid any evaporation of precursors within the liquid feed lines or overheating of the nozzle. In order to assure enough oxidant for complete conversion of reactants, an additional outer oxygen flow (5 L/min) was supplied. The liquid precursor mixture was rapidly dispersed by a gas stream and ignited by a premixed methane/oxygen flame. After evaporation and combustion of precursor droplets, particles were formed by nucleation, condensation, coagulation, and coalescence. Finally, nanoparticles were collected on glass microfiber filters (GF/D Whatman, 25.7 cm in diameter) with an aid of a vacuum pump. During the experiment, the filter was placed 40.0 cm above the nozzle in a water-cooled holder to keep the off-gas temperature below 200 °C.
Powder characterizations
The undoped In2O3 and 0.25–1.00 wt% M(M=Pt, Nb, and Ru)-doped In2O3 nanoparticles were characterized by X-ray diffraction (XRD) using a Cu Kα radiation source (JEOL JDX-3530), scanning electron microscopy (FE-SEM, JEOL JSM-6335F operated at 20 kV), and transmission electron microscopy (TEM, JEOL JEM-2010 operated at 200 kV). The Brunauer–Emmett–Teller (BET) surface area (SSA BET) of the nanoparticles was measured by nitrogen adsorption at 77 K (Micromeritics Tristar) after degassing for 1 h at 150 °C in nitrogen.
Sensing film fabrication
Sensing films were prepared from undoped In2O3 and 0.25–1.00 wt% M (M=Pt, Nb, and Ru)-doped/loaded In2O3 by mixing 60 mg of nanoparticles into an organic paste composed of ethylcellulose (Fluka, 30–70 mPa s) and terpineol (Aldrich, 90%) 0.25 mL, which acted as a vehicle binder and solvent, respectively. The mixtures were spin-coated on alumina substrates equipped with interdigitated gold electrodes on the front side. Lastly, the sensing films then were annealed at 450 °C for 2 h at a heating rate of 2 °C/min for binder removal.
Gas sensing measurement
For gas sensing measurements, undoped In2O3 and M (M=Pt, Nb, and Ru)-doped/loaded In2O3 sensors were heated by the external NiCr heater at the operating temperatures of 200, 250, 300, and 350 °C in dry air before exposure to target gases into a stainless steel chamber [the detailed setup is reported in our previous work (Kruefu et al. 2010)]. The gas sensing response (S) for an oxidizing gas including (NO2: 0.125–5 ppm) is given by S = R g/R a, where R a and R g are the steady-state resistances of the sensor measured in the presence of pure dry air and test gas, respectively. The definition is reversed (R a/R g) for a reducing gas including hydrogen sulfide (H2S: 0.5–10 ppm), hydrogen (H2: 2500–30,000 ppm), ethanol (C2H5OH: 200–2000 ppm), and nitric oxide (NO: 25–1000 ppm). The response time (t res) is the time required to reach 90 % of the steady-state response signal upon gas exposure while the recovery time (trec) is the time needed to recover 90 % of the baseline signal.
Results and discussion
The undoped In2O3 and 0.25–1.00 wt% M (M=Pt, Nb, and Ru)-doped/loaded In2O3 nanoparticle powders have successfully been synthesized by FSP process under 5/5 (precursor/oxygen) flame conditions. Characterization results will be mainly reported for the Pt additive because it is found to be the most useful additive compared with the other two noble metals. Figure 1 demonstrates the BET specific surface area (SSA BET) and corresponding BET particle diameter of In2O3 nanoparticles as a function of Pt content. The error bars represent the variation of BET data from three distinct samples of each material. It is seen that the surface area initially increases from 54.9 to 81.6 m2/g and the corresponding particle diameter decreases considerably from 15.2 to 10.2 nm after the small Pt addition of 0.25 wt% but the surface area becomes decreasing and particle size tends to increase with slight fluctuation as the Pt content increases further from 0.25 to 1.0 wt%. The results may be explained based on the mechanisms of particle sintering during FSP process. The presence of Pt may affect the sintering rate of In2O3 and reduce particle growth by coagulation and sintering mechanisms, leading to the increase of SSA. As the Pt precursor content increases, the flame temperature will, however, increase due to increasing combustion rate by the organic component of Pt precursor, leading to the crystal/particle growth by sintering and the decrease of SSA.
Figure 2 shows the XRD patterns of undoped In2O3 and Pt-doped In2O3 nanoparticles. It is seen that all peaks correspond with the cubic structure of In2O3 (JCPDS No. 03–065–3170) and diffraction peaks of Pt cannot be observed. The absence of Pt phases may be due to the fact that the Pt concentrations are below the detection limit of XRD instrument. The other plausible reason is that Pt may form solid solution with In2O3 host because Pt4+ ions has very similar ionic radius (0.76 Å) to that of In3+ (0.765 Å) so that Pt4+ can easily occupy In3+ sites in In2O3 lattice (Lu and Yin 2011). The lattice constant of cubic In2O3 for undoped and Pt-doped In2O3 nanoparticles from the XRD data is found to be nearly the same at 1.011–1.012 nm, demonstrating that Pt doping causes insignificant lattice distortion. The result is expected since the ionic radius of Pt4+ is very close to that of In3+ (0.65 % difference) so that the change of lattice constant due to Pt doping will be too small to be observed at the resolution of a standard XRD instrument. Thus, Pt atoms should be doped into rather than loaded onto In2O3 nanoparticles. Moreover, it can be noticed that In2O3 peaks tend to be narrower as the Pt content increases, indicating the possible change of crystallite size. The average crystallite sizes of Pt-doped In2O3 nanoparticles with different Pt contents are determined using Scherrer's equation from three dominant planes including (100), (220), and (400) as shown in the inset of Fig. 2. The error bars again correspond to the variation of BET data from three distinct samples of each material. It is seen that the average crystallite size initially decreases after Pt doping with the smallest Pt content of 0.25 wt% but then monotonically increases as the Pt concentration increases further to 1 wt%. The trend is quite similar to that of BET particle diameter (Fig. 1). Thus, XRD results confirm the role of Pt precursor content on the crystal and particle growth as previously explained for BET data.
TEM-bright field images of undoped In2O3 and 1.0 wt% Pt-doped In2O3 nanoparticles presented in Fig. 3a,c reveal spherical and cubic morphologies with the particle size in the range of 10–20 nm. The corresponding electron diffraction patterns (Insets of Fig. 3a, c) display dotted-ring features of a semi-polycrystalline structure that can be indexed as the In2O3 cubic phase (JCPDS No. 03–065–3170) presenting (222), (400), (420), (431), (440), (620), and (721) planes. The respective HRTEM images shown in Fig. 3b, d) clearly demonstrate lattice fringes on various nanoparticles whose d-spacings can be matched well with various planes of the In2O3 phase. Since Pt nanoparticles cannot be seen even with near atomic-resolution TEM images, it could be primarily concluded that In2O3 lattice is doped with Pt4+ ions and not loaded with Pt nanoparticles. When comparing the sizes of In2O3 nanoparticles synthesized by FSP with those of other methods, the size of FSP-made particles is comparable with that of cubic In2O3 nanocrystals prepared by annealing of precursor particles (10–20 nm) (Chen et al. 2011). In addition, the FSP-made nanoparticles are relatively small compared with undoped In2O3, Eu3+/In2O3, Gd3+/In2O3, and Ho3+/In2O3 nanopowders synthesized by a sol-gel method having the mean particle sizes of 20, 19, 25, and 23 nm, respectively (Liewhiran et al. 2014). Moreover, the FSP-made particles are considerably smaller than In2O3 nanoparticles produced by a hydrothermal method showing the average particle size of ~40 nm (Cai et al. 2012).
The morphologies of undoped In2O3 and Pt-doped In2O3 nanoparticles were examined by FE-SEM. Figure 4a, b illustrates cross-sectional SEM micrographs of undoped and 0.5 wt% Pt-doped In2O3 sensing films on Al2O3 substrates, respectively. It is seen that both In2O3-based sensing films comprise highly dense and very fine nanoparticles uniformly arranged on a solid alumina substrate. The high magnification images (insets) show that most nanoparticles still retain individual boundaries while some of them are fused into larger particles or rod-like structures. Despite their similar morphologies, the Pt-doped In2O3 film is apparently thinner with the thickness of ~12 µm, which is about 60 % compared with that of undoped ones (~20 µm thick). Similar morphologies and thickness of sensing film with different Pt contents are observed and their images are thus omitted for presentation conciseness. The notable difference of film thickness between undoped and Pt-doped In2O3 films may be due to the effect of Pt dopants on wettability of In2O3 nanoparticles in the paste solution. It has been observed that the contact angle of Pt-doped In2O3 paste on an alumina substrate is smaller than that of undoped one under the same preparation condition with a constant sample volume. Thus, Pt-doped In2O3 materials could exhibit higher paste mobility and lower film thickness after spin coating. Nevertheless, both sensing films show regularity and uniformity in film thickness, demonstrating that the binder/powder mixing and spin coating method can be used to fabricate gas sensing films (Samerjai et al. 2014 and Tamaekong et al. 2009).
Figure 5 shows the change in resistance of In2O3 sensing films with different Pt doping concentrations under exposure to various NO2 concentrations ranging from 0.125 to 5 ppm at an optimal operating temperature of 250 C. It can be seen that the baseline resistance in air slightly increases as the Pt content increases from 0.0 to 0.25 wt% but become decreasing by roughly one order of magnitude (from 2 × 103 to 2 × 102 Ω) as the Pt doping concentration increases further to 1.0 wt%. The error bars in the figure demonstrate the resistance variation of three different sensors fabricated under the same condition. It is seen that the variation of sensor resistances of the same material is less than 40 % and the resistance tends to decrease with increasing Pt doping level. Thus, FSP/spin coating method can produce gas sensors with good reproducibility. The low baseline resistance of In2O3 sensing films is in the similar range as those of other reported In2O3 sensors, which have the baseline resistance in the range of 103–104 Ω (Ali et al. 2008; Kannan et al. 2010; Xu et al. 2012). The trend of baseline resistance change with Pt doping may be explained based on particle size n-type doping effects as modeled in Fig. 6.
For undoped In2O3, In2O3 lattice naturally contains large amount of defects, particularly oxygen vacancy, resulting in n-type conductivity. When In2O3 is exposed to air, oxygen will be chemisorbed on its surface, capturing electrons from the conduction band to form oxygen ions, including O2−, O−, and O2 −. At the operating temperature in the range of 200–350 °C, O− and O−2 are predominant (Dong et al. 2013). As a result, depletion regions are formed on the surface of nanoparticles as displayed in Fig. 6a. With Pt addition, Pt4+ ions would substitute In3+ sites, leading to the creation of additional free electrons and the reduction of depletion region as illustrated in Fig. 6b, causing a decrease of electrical resistance, which is clearly observed at moderate and high Pt doping levels (0.5–1.0 wt%). However, the resistance for the sensor with the lowest Pt doping level of 0.25 wt% unexpectedly increases compared with that of undoped one. The unexpected result may be explained by the effect of particle size reduction (according to BET and XRD data). Due to the increased surface area, the amount of chemisorbed oxygen species on nanoparticle surface increases. The oxygen ions will effectively take away additional electrons donated by Pt, resulting in wider depletion regions on the nanoparticle surfaces. In addition, there could be additional resistive contribution from increased amount of crystallite boundaries. Thus, the particle size reduction effect can override the doping effect by Pt atoms at a low Pt concentration, leading to an increase in resistance. As Pt doping level increases further, particle and crystallite sizes increase again, leading to less resistive contributions of crystallite boundaries and depletion regions by chemisorbed oxygen species while the electron concentration increases substantially due to additional Pt dopants. Thus, the doping effect dominates, resulting in a significant reduction of depletion region and resistance.
The roles of Pt doping level on NO2 response may also be explained based on particle size and n-type doping effects, which are correspondingly demonstrated in Fig. 6. Upon exposure to NO2, the resistances of all sensors increase at all gas concentrations, confirming typical behaviors of n-type semiconductor toward an oxidizing gas. It is generally accepted that NO2 molecules will chemically adsorb on a metal oxide surface according to the following oxidizing reaction:
The reaction involves large electrostatic states of NO2 − ions that capture more electrons from the conduction band of the base material leading to the resistance increase of n-type In2O3 films (Fig. 6a). At the low Pt doping concentration (0.25 wt%), NO2 molecules will be less adsorbed on the surface of Pt-doped nanoparticles than that of undoped ones because the Pt-doped nanoparticles are relatively small but have thick depletion region so that there are much less electrons available for the NO2 adsorption reaction (Eq. 1), leading to a lower NO2 response. As Pt content increases moderately to 0.5 wt%, the particle size increases and Pt dopants give considerable amount of electrons to the In2O3 conduction band leading to less oxygen pre-adsorption and the contraction of depletion region. Consequently, the NO2 adsorption rate increases considerably as more electrons can be extracted through thinner depletion regions for the reaction (Eq. 1), resulting in deeper extension of depletion regions into nanoparticles and higher NO2 response upon NO2 exposure as depicted in Fig. 6b. When the Pt content increases further (0.75–1.0 wt%), the NO2 adsorption rate decreases possibly due to excessive surface disorder due to natural defects caused by Pt dopants located near the surface, which could result in semi-amorphous structure that is less active for NO2 chemisorption, leading to an inferior NO2 response.
Figure 7 shows the sensor response and response time of In2O3 sensing films calculated from the change in resistance (Fig. 5). It is seen that the responses of all sensors increase monotonically with increasing NO2 concentration and are found to follow the power law with the exponent values close to one (~0.8–1.3) while the response times decrease monotonically with increasing NO2 concentration. The response and response time of undoped In2O3 sensor toward 5 ppm NO2 are ~643 and ~255 s, respectively. Upon Pt doping with the lowest content of 0.25 wt%, the response sharply decreases to 98 while the response time increases to ~375 s. In contrast, the 0.5 wt% Pt-doped In2O3 sensor gives the highest response but relatively long response time of ~1904 and ~330 s, respectively. At the higher Pt doping levels (0.75–1.0 wt%), responses and response times deteriorate (S = 278–417 and t res = ~412–422 s). Similar trends have been observed at various NO2 concentrations and operating temperatures. The corresponding recovery times (not shown) are relatively long (7–14 min) and exhibit similar dependencies on Pt and NO2 concentrations to those of response time. Moreover, the variations of responses for the same materials demonstrated by the corresponding error bars are less than 30 % for all conditions, confirming good reproducibility of the FSP and spincoating process.
The effect of operating temperature ranging from 200 to 350 °C on NO2 response of In2O3 with different Pt doping concentrations is demonstrated in Fig. 8. It is seen that the responses of all sensing films increase substantially as the operating temperature increases from 200 to 250 °C and then decrease considerably as the temperature increases further to 350 °C. Consequently, the 0.5 wt% Pt-doped In2O3 sensor exhibits the highest response of ~1904 to 5 ppm NO2 at the optimal working temperature of 250 °C. The dependence of NO2 response on operating temperature may be explained as follows. The NO2 adsorption reaction generally requires activation energy to overcome adsorption energy barrier and needs available adsorption sites on a metal oxide surface. When the operating temperature initially increases, NO2 adsorption rate and NO2 response will increase due to increasing thermal activation energy. As the temperature increases further, NO2 adsorption rate and NO2 response will decrease despite the increasing activation energy because there will be less available adsorption sites, which have been taken away by the pre-adsorbed oxygen species (O−and O−2) at the elevated operating temperature in air. For In2O3, O−, and O−2, adsorptions will become dominant at the temperatures above 250 °C. Thus, NO2 adsorption rate and corresponding response will reduce as the operating temperature goes above 250 °C.
Figures 9, 10 show the NO2 response of Nb/In2O3 and Ru/In2O3 sensing films with different loading concentrations as a function of temperature. It is evident that NO2 response reduces considerably with increasing Nb and Ru concentrations at all operating temperatures, and the reduction magnitude is larger in the case of Ru addition. Among these, undoped In2O3 sensor exhibits relatively high response of ~643.1 to 5 ppm NO2 at an optimal temperature of 250 °C. Thus, Nb and Ru are not beneficial catalysts for NO2 sensing on In2O3 support. A possible explanation for the degraded response due to Nb and Ru additions is that Nb and Ru form nanoparticles loaded on In2O3 surface, resulting in the expansion of depletion regions at the interfaces and the decrease of electron concentration in In2O3 conduction band, which in turn leads to reduced NO2 adsorption. Therefore, Pt is confirmed to be the most effective additive for NO2 oxidation on In2O3 support.
The attained NO2 response (~1904 to 5 ppm NO2 at the optimal operating temperature of 250 °C) of the optimal Pt-doped In2O3 sensor is considerably better than several other responses reported In2O3-based sensors as listed in Table 1, which offer optimal NO2 responses of less than 200 at NO2 concentrations of 5 ppm or higher (Ali et al. 2008; Huang et al. 2009; Kannan et al. 2010; Chen et al. 2011). However, it is slightly inferior compared with flower-like In2O3 nanostructures prepared by hydrothermal method, which exhibits a high response of 198 at a low NO2 concentration of 0.5 ppm (Xu et al. 2012). Regarding the response time, the Pt-loaded In2O3 sensors have comparable dynamic performances as those other In2O3 sensors, which exhibit response time in the range of 100-450 s depending on NO2 concentration and working temperature (Ali et al. 2008; Kannan et al. 2010; Xu et al. 2012).
When comparing with the results of other semiconducting metal oxides (also shown in Table 1), 0.5wt% Pt-doped In2O3 exhibits substantially higher NO2 response at similar concentrations. For instance, Pt-loaded WO3 thin films prepared by reactive radio-frequency sputtering had low responses of 3.45 to 10 ppm NO2 at 150 °C (Penza et al. 1998) and 11.73 to 10 ppm NO2 at 260 °C (Stankova et al. 2006). Similarly, Pt-loaded WO3 films prepared by screen printing gave an inferior response of ~4 to 10 ppm NO2 at 450 °C (Srivastava and Jain 2008). In contrast, NO2 sensors made of other FSP-prepared materials show high response but the values are still lower than that of the optimal Pt-doped In2O3 sensor. For example, the FSP-made 0.5 mol% Nb/ZnO thick film displayed a high response of ~1640 to 5 ppm NO2 at 300 °C (Kruefu et al. 2011). In addition, the flame-made 0.25 wt% Pt-loaded WO3 sensor showed responses of 954 and 637 at 150 °C toward 20 and 10 ppm NO2, respectively (Samerjai et al. 2014). Recently, the FSP-made 0.25 mol% MnOx-loaded ZnO sensor was reported to have a high NO2 response of ∼2700 to 50 ppm NO2 at 350 °C (Tamaekong et al. 2014). In comparison with state-of-the-art NO2 sensors based on graphene-metal oxide composite (also see Table 1), the Pt-loaded In2O3 sensors also exhibit relatively high response at lower gas concentration but these sensors can operate at lower operating temperatures ranging from 25 to 150 °C (Neri et al. 2013; Chen et al. 2013; Zhang et al. 2014; Liu et al. 2014, 2015; Su and Peng 2015).
The gas sensing selectivity of Pt-doped In2O3 gas sensors was characterized toward NO2, NO, H2S, H2, and C2H5OH at their critical concentrations and operating temperature of 250 °C as shown in Fig. 11. It is seen that the undoped In2O3 exhibits high response to NO2, moderate responses to NO and C2H5OH, and low responses to H2S and H2, displaying good selectivity to NO2. At the lowest Pt doping level of 0.25 wt%, the response to H2S slightly increases while those to all other gases especially NO2 decrease, indicating lower NO2 selectivity against other gases. With the optimal Pt doping concentration of 0.5 wt%, the response to NO2 substantially increases while those to other gases increase at lower rate or slightly change, suggesting the higher NO2 selectivity against these gases. At higher Pt doping levels (0.75–1.0 wt%), responses to all gases reduce monotonically, displaying minor changes of NO2 selectivity. Therefore, the optimal Pt doping level can substantially improve NO2 selectivity against NO, H2S, H2, and C2H2OH. The observed high NO2 selectivity suggests specifically high reaction rate between NO2 and Pt-doped In2O3 nanoparticles, which may be attributed to the n-type doping effect. It is interesting that the response to H2 and C2H2OH is very low for all Pt-doped In2O3 sensors. The results are in contrast to other Pt-incorporated metal oxide gas sensors, which normally exhibit very high responses to H2 and other hydrocarbon-based gases. However, they are in agreement with Pt-doped In2O3 sensors prepared by the nanocasting method (Tu et al. 2010). The main reason for the disparity is that Pt is doped in In2O3 lattice instead of being loaded on the surface resulting in the absence of the spillover mechanism and Pt does not provide the response enhancement toward H2 and other related gases.
Conclusion
In summary, undoped In2O3 and 0.25–1.00 wt% M(M=Pt, Nb, and Ru)-doped In2O3 nanoparticles were successfully synthesized by a single-step FSP technique of under 5/5 (precursor/oxygen) flame conditions. Gas sensing studies revealed that Pt doping at the optimal content of 0.5 wt% provided an enhancement in NO2 sensing performances in terms of response and selectivity but lower or higher doping levels led to degraded NO2 response. The roles of Pt doping level on NO2 sensing response were explained based on n-type doping and particle size effects. The optimal Pt-doped In2O3 sensor exhibited a notably high NO2 response of 1904 toward 5 ppm NO2 at 250 °C. In addition, it displayed good NO2 selectivity against NO, H2S, C2H5OH, and H2. Moreover, Pt provided significant NO2 response enhancement for In2O3 nanoparticles in contrast to Nb and Ru that led to inferior NO2 sensing performances.
References
Ali M, Wang CY, Röhlig CC, Cimalla V, Stauden T, Ambacher O (2008) NOx sensing properties of In2O3 thin films grown by MOCVD. Sens Actuators, B 129:467–472. doi:10.1016/j.snb.2007.08.011
Cai P, Bai W, Zhang L, Song H, Su Y, Lv Y (2012) Hierarchical hollow microspheres and flower-like indium oxide: controllable synthesis and application as H2S cataluminescence sensing materials. Mater Res Bull 47:2212–2218. doi:10.1016/j.materresbull.2012.06.002
Chen C, Wei Y, Chen D, Jiao X (2011) Indium oxide nanocrystals: capping-agent-free synthesis, size-control mechanism, and high-gas sensing performance. Mater Chem Phys 125:299–304. doi:10.1016/j.matchemphys.2010.09.042
Chen N, Li X, Wang X, Yu J, Wang J, Tang Z, Akbar SA (2013) Enhanced room temperature sensing of Co3O4-intercalated reduced graphene oxide based gas sensors. Sens Actuators, B 188:902–908. doi:10.1016/j.snb.2013.08.004
Choi Y, Hwang I, Park J, Choi K, Park J, Lee J (2008) Novel fabrication of an SnO2 nanowire gas sensor with high sensitivity. Nanotechnology 19:095508. doi:10.1088/0957-4484/19/9/095508
Dong H, Liu Y, Li G, Wang X, Xu D, Chen Z, Zhang T, Wang J, Zhang L (2013) Hierarchically rosette-like In2O3 microspheres for volatile organic compounds gas sensors. Sens Actuators B Chem 178:302–309. doi:10.1016/j.snb.2012.12.103
Guo P, Pan H (2006) Selectivity of Ti-loaded In2O3 ceramics as an ammonia sensor. Sens Actuators, B 114:762–767. doi:10.1016/j.snb.2005.07.040
Huang BR, Lin JC (2012) Core-shell structure of zinc oxide/indium oxide nanorod based hydrogen sensors. Sens Actuators, B 174:389–393. doi:10.1016/j.snb.2012.08.065
Huang SZ, Lin W, Chen WZ (2009) Gas sensitivity of indium oxide. Trans NonferrMet Soc China 19:s80–s82. doi:10.1016/S1003-6326(10)60249-X
Kannan S, Rieth L, Solzbacher F (2010) NOx sensitivity of In2O3 thin film layers with and without promoter layers at high temperatures. Sens Actuators, B 149:8–19. doi:10.1016/j.snb.2010.06.042
Kemmler JA, Pokhrel S, Birkenstock J, Schowalter M, Rosenauer A, Barsan N, Weimar U, Madler L (2012) Quenched, nanocrystalline In4Sn3O12 high temperature phase for gas sensing applications. Sens Actuators, B 161:740–747. doi:10.1016/j.snb.2011.11.026
Kruefu V, Peterson E, Chantha C, Siriwong C, Phanichphant S, Carroll DL (2010) Flame –made niobium doped zinc oxide nanoparticles in bulk heterojunction solar cells. Appl Phys Lett 97:053302. doi:10.1063/1.3465866
Kruefu V, Liewhiran C, Wisitsoraat A, Phanichphant S (2011) Selectivity of flame-spray-made Nb/ZnO thick films towards NO2 gas. Sensors and Actuators B Chem 156:360–367. doi:10.1016/j.snb.2011.04.046
Kruefu V, Wisitsoraat A, Phanichphant S (2014) Effects of niobium-loading on sulfur dioxide gas-sensing characteristics of hydrothermally prepared tungsten oxide thick film. J Nanomater 51:7318. doi:10.1016/j.snb.2011.04.046
Liewhiran C, Tamaekong N, Wisitsoraat A, Phanichphant S (2009) H2 sensing response of flame-spray-made Ru/SnO2 thick films fabricated from spin-coated nanoparticles. Sensors 9:8996–9010. doi:10.3390/s91108996
Liewhiran C, Tamaekong N, Tuantranont A, Wisitsoraat A, Phanichphant S (2014) The effect of Pt nanoparticles loading on H2 sensing properties of flame-spray-made SnO2 sensing films. Mater Chem Phys 147:661–672. doi:10.1016/j.matchemphys.2014.06.005
Lim SK, Hwang SH, Chang D, Kim S (2010) Preparation of mesoporous In2O3 nanofibers by electrospinning and their application as a CO gas sensor. Sens Actuators, B 149:28–33. doi:10.1016/j.snb.2010.06.039
Liu L, Zhang T, Li S, Wang L, Tian Y (2009) Preparation, characterization, and gas-sensing properties of Pd-loaded In2O3 nanofibers. Mater Lett 63:1975–1977. doi:10.1016/j.matlet.2009.05.060
Liu X, Wang R, Zhang T, He Y, Tu J, Li X (2010) Synthesis and characterization of mesoporous indium oxide for humidity-sensing applications. Sens Actuators, B 150:441–448. doi:10.1016/j.snb.2010.05.033
Liu S, Yu B, Zhang H, Fei T, Zhang T (2014) Enhancing NO2 gas sensing performances at room temperature based on reduced graphene oxide-ZnO nanoparticles hybrids. Sens Actuators, B 202:272–278. doi:10.1016/j.snb.2014.05.086
Liu S, Wang Z, Zhang Y, Zhang C, Zhang T (2015) High performance room temperature NO2 sensors based on reduced graphene oxide-multiwalled carbon nanotubes-tin oxide nanoparticles hybrids. Sens Actuators, B 211:318–324. doi:10.1016/j.snb.2015.01.127
Lu X, Yin L (2011) Porous indium oxide nanorods: synthesis, characterization and gas sensing properties. J Mater Sci Technol 27(8):680–684. doi:10.1016/S1005-0302(11)60125-4
Luyo C, Ionescu R, Reyes LF, Topalian Z, Estrada W, Llober E, Graqvist CG, Heszler P (2009) Gas sensing response of NiO nanoparticle films made by reactive gas deposition. Sens Actuators B Chem 138:14–20. doi:10.1016/j.snb.2008.11.057
M’adler L, Kammler HK, Mueller R, Pratsinis SE (2002) Controlled synthesis of nanostructured particles by flame spray pyrolysis. Aerosol Sci 33:369–389. doi:10.1016/S0021-8502(01),00159-8
Min Y, Tuller HL, Palzer S, llenstein JW, Bo H (2003) Gas response of reactively sputtered ZnO films on Si-based micro-array. Sens Actuators B Chem 93:435–441. doi:10.1016/S0925-4005(03)00170-9
Neri G, Bonavita A, Micali G, Rizzo G, Pinna N, Niederberger M (2007) In2O3 and Pt-In2O3 nanopowders for low temperature oxygen sensors. Sens Actuators, B 127:455–462. doi:10.1016/j.snb.2007.04.046
Neri G, Leonardi SG, Latino M, Donato N, Baek S, Conte DE, Russo PA, Pinna N (2013) Sensing behavior of SnO2/reduced graphene oxide nanocomposites toward NO2. Sens Actuators, B 179:61–68. doi:10.1016/j.snb.2012.10.031
Niu X, Zhong H, Wang X, Jiang K (2006) Sensing properties of rare earth oxide loaded In2O3 by a sol–gel method. Sens Actuators, B 115:434–438. doi:10.1016/j.snb.2005.10.004
Penza M, Martucci C, Cassano G (1998) NOx gas sensing characteristics of WO3 thin films activated by noble metals (Pd, Pt, Au) layers. Sens Actuators, B 50:52–59. doi:10.1016/S0925-4005(98),00156-7
Qurashi A, Maghraby EME, Yamazaki T, Kikuta T (2010) Catalyst supported growth of In2O3 nanostructures and their hydrogen gas sensing properties. Sens Actuators, B 147:48–54. doi:10.1016/j.snb.2010.03.024
Samerjai T, Liewhiran C, Wisitsora-at A, Tamaekong N, Phanichphant S (2014) NO2 Gas sensing of flame-made Pt-loaded WO3 thick films. J Solid State Chem 214:47–52. doi:10.1016/j.jssc.2013.10.041
Seetha M, Meena P, Mangalaraj D, Masuda Y, Senthil K (2012) Synthesis of indium oxide cubic crystals by modified hydrothermal route for application in room temperature flexible ethanol sensors. Mater Chem Phys 133:47–54. doi:10.1016/j.matchemphys.2011.12.034
Singh N, Ponzoni A, Comini E, Lee PS (2012) Chemical sensing investigations on Zn-In2O3 nanowires. Sens Actuators, B 171–172:244–248. doi:10.1016/j.snb.2012.03.054
Srivastava V, Jain K (2008) Highly sensitive NH3 sensor using Pt catalyzed silica coating over WO3 thick films. Sens Actuators, B 133:46–52. doi:10.1016/j.snb.2008.01.066
Stankova M, Vilanova X, Calderer J, Llobet E, Brezmes J, Gràcia I, Cané C, Correig X (2006) Sensitivity and selectivity improvement of rf-sputtered WO3 micro hotplate gas sensors. Sens Actuators, B 113:241–248. doi:10.1016/j.snb.2005.02.056
Su PG, Peng SL (2015) Fabrication and NO2 gas-sensing properties of reduced graphene oxide/WO3 nanocomposite films. Talanta 132:398–405. doi:10.1016/j.talanta.2014.09.034
Suchea M, Katsarakis N, Christoulakis S, Nikolopoulou S, Kiriakidis G (2006) Low temperature indium oxide gas sensors. Sens Actuators, B 118:135–141. doi:10.1016/j.snb.2006.04.020
Tamaekong N, Liewhiran C, Wisitsoraat A, Tuantranont A, Phanichphant S (2009) Sensing characteristics of flame-spray-made Pt/ZnO thick films as H2 gas sensor. Sensors 9:6652–6669. doi:10.3390/s90906652
Tamaekong N, Liewhiran C, Wisitsoraat A, Tuantranont A, Phanichphant S (2014) NO2 sensing properties of flame-made MnOx-loaded ZnO-nanoparticle thick film. Sens Actuators B Chem 204:239–249. doi:10.1016/j.snb.2014.07.089
Tu J, Li N, Lai X, Chi Y, Zhang Y, Wang W, Xi X (2010) H2S-sensing properties of Pt-loaded mesoporous indium oxide. Appl Surf Sci 256:5051–5055. doi:10.1016/j.apsusc.2010.03.055
Wagner T, Sauerwald T, Kohl CD, Waitz T, Weidmann C, Tiemann M (2009) Gas sensor based on ordered mesoporous In2O3. Thin Solid Films 517:6170–6175. doi:10.1016/j.tsf.2009.04.013
Xu J, Chen Y, Shen J (2008) Ethanol sensor based on hexagonal indium oxide nanorods prepared by solvothermal methods. Mater Lett 62:1363–1365. doi:10.1016/j.matlet.2007.08.054
Xu L, Dong B, Wang Y, Bai X, Liu Q, Song H (2010) Electrospinning preparation and room temperature gas sensing properties of porous In2O3 nanotubes and nanowires. Sens Actuators, B 147:531–538. doi:10.1016/j.snb.2010.04.003
Xu X, Wang D, Liu J, Sun P, Guan Y, Zhang H, Sun Y, Liu F, Liang X, Gao Y, Lu G (2012) Template-free synthesis of novel In2O3 nanostructures and their application to gas sensors. Sens Actuators, B 185:32–38. doi:10.1016/j.snb.2013.04.078
Yang H, Tang A, Zhang X, Yang W, Qiu G (2004) In2O3 nanoparticles synthesized by mechanochemical processing. Scr Mater 50:413–415. doi:10.1016/j.scriptamat.2003.11.011
Zhang H, Feng J, Fei T, Liu S, Zhang T (2014) SnO2 nanoparticles-reduced graphene oxide nanocomposites for NO2 sensing at low operating temperature. Sens Actuators, B 190:472–478. doi:10.1016/j.snb.2013.08.067
Zheng W, Lu X, Wang W, Li Z, Zhang H, Wang Y, Wang Z, Wang C (2009) A highly sensitive and fast-responding sensor based on electrospun In2O3 nanofibers. Sens Actuators, B 142:61–65. doi:10.1016/j.snb.2009.07.031
Acknowledgments
The authors would like to gratefully acknowledge the financial support from the National Research University Project under the Office of the Higher Education Commission, Ministry of Education, Thailand. The Graduate School, Chiang Mai University, Thailand and Graduate Institute of Science and Technology (TGIST–01–56–026), National Science and Technology Development Agency, Thailand are also greatly appreciated for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inyawilert, K., Channei, D., Tamaekong, N. et al. Pt-doped In2O3 nanoparticles prepared by flame spray pyrolysis for NO2 sensing. J Nanopart Res 18, 40 (2016). https://doi.org/10.1007/s11051-016-3341-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-016-3341-1