Abstract
Enzymes play an essential role in catalyzing various reactions. However, their instability upon repetitive/prolonged use, elevated temperature, acidic or alkaline pH remains an area of concern. α-Amylase, a widely used enzyme in food industries for starch hydrolysis, was covalently immobilized on the surface of two developed matrices, amino-functionalized silica-coated magnetite nanoparticles (AFSMNPs) alone and covered with chitosan. The synthesis steps and characterizations of NPs were examined by FT-IR, VSM, and SEM. Modified nanoparticles with average diameters of 20–80 nm were obtained. Enzyme immobilization efficiencies of 89 and 74 were obtained for AFSMNPs and chitosan-coated AFSMNPs, respectively. The optimum pH obtained was 6.5 and 8.0 for the enzyme immobilized on AFSMNPs and chitosan-coated AFSMNPs, respectively. Optimum temperature for the immobilized enzyme shifted toward higher temperatures. Considerable enhancements in thermal stabilities were observed for the immobilized enzyme at elevated temperatures up to 80 °C. A frequent use experiment demonstrated that the immobilized enzyme retained 74 and 85 % of its original activity even after 20 times of repeated use in AFSMNPs and chitosan-coated AFSMNPs, respectively. Storage stability demonstrated that free enzyme lost its activity completely within 30 days. But, immobilized enzyme on AFSMNPs and chitosan-coated AFSMNPs preserved 65.73 and 78.63 % of its initial activity, respectively, after 80 days of incubation. In conclusion, a substantial improvement in the performance of the immobilized enzyme with reference to the free enzyme was obtained. Furthermore, the relative activities of immobilized enzyme are superior than free enzyme over the broader pH and temperature ranges.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent times, enzyme technology has offered many approaches that facilitate their practical applications (Jiang et al. 2013; Sohrabi et al. 2014). Enzymes are macromolecular, highly selective biocatalysts which accelerate the chemical reactions of living cells and exhibit some excellent properties such as high activity, selectivity, and specificity that make them superior than chemical ones (Kumari and Kayastha 2011; Türünç et al. 2009). Biocatalysis is gradually gaining importance in green chemistry, where chemical synthesis routes are being replaced by enzymatic ones that produce chemicals, which are also safer. Recent technological progress in the field of immobilized biocatalysts resulted in their competence use in industry, waste treatment, medicine, and biosensor (Abdelmajeed et al. 2012; Mahmoud and Helmy 2009). The most important problems of the enzymes application are the recovery of enzymes from reaction solutions and separation of the enzymes from substrates and products. On the other hand, many of the enzymes are stable just under mild experimental and environmental situations and their function is limited due to their inactivation by organic solvents, extreme pH, or temperatures, and their short half-lives. These problems can be resolved by immobilization and enzyme engineering (Demir et al. 2012). Recently, nanoparticles play a key role in food sciences and industries (Ghaderi et al. 2014, 2015; Mohammadi et al. 2014; Pezeshki et al. 2014; Rasaie et al. 2014). Novel carriers with immobilized enzymes and without necessity of separation are of main focus of interest. Organic materials like polymers have a little frequent use as carriers for the immobilization of enzymes. The effectiveness of immobilized enzymes could be normally enhanced by managing the structure for enzyme leakage and decreasing the size of the carrier materials for increasing the surface area to maximize enzyme loading (Ashtari et al. 2012; Kim et al. 2006; Zhang et al. 2009b).Therefore, in the recent past, nanosized materials especially inorganic ones have been introduced for enzyme immobilization. The enzyme immobilization onto water insoluble inorganic carriers is a desired biological method to increase the applications of the enzyme on continuous procedures and industries (Homaei et al. 2013; Khan and Alzohairy 2010). Among inorganic materials, silica nanostructures for the immobilization of enzymes have gained many interests because they are in the range of nanometer, environmentally more acceptable, structurally more stable, and more resistant to microbial contamination (Ashtari et al. 2012). On the other hand, magnetic nanoparticles (MNPs) have a wide range of applications in the immobilization of cells, enzymes, and nucleic acids, bio-separation systems, immunoassays, drug delivery, and biosensors (Jiang et al. 2009; Khan et al. 2012; Mandal et al. 2005; Sohrabi et al. 2014; Xu et al. 2014). The MNPs can simply be stabilized in a fluidized bed reactor for continuous function of enzyme by complete and easy recovery of these materials from reaction systems, thus reducing the operational expenses of the processes (Ashtari et al. 2012; Atacan and Özacar 2015). Surface modification of MNPs is a key tool to minimize their toxicity and overcome the agglomeration or precipitation of MNPs in media (Arruebo et al. 2007). Chitosan is a promising support material for use in the food industry because it is a biocompatible, antibacterial, and environmentally friendly polyelectrolyte which has a great number of free reactive amino and hydroxyl groups on the surface, which provide the possibility of enzymes immobilization. The excellent capacity of chitosan for the immobilization of carbohydrate-degrading enzymes has been reported previously (Sureshkumar and Lee 2011; Tripathi et al. 2007). Alpha-amylases catalyze the hydrolysis of internal-1,4-glycosidic linkages in starch in low molecular weight products, such as glucose, maltose, and maltotriose units, which is one of the most important and widely used commercial enzymatic processes (Kumari and Kayastha 2011; Tripathi et al. 2007). The present study plans to develop a magnetically responsive enzyme carrier via simple immobilization which enables better binding of the enzyme to the magnetite substrate as well as in better reuse of the enzyme through its recovery using a magnetic field. A protective silica shell does not only serve to protect the magnetic nanoparticles against degradation, but can also be used for further functionalization with other functional groups. In the present study, α-amylase has been immobilized on our newly developed nanoparticles and its physicochemical properties are compared with respect to soluble enzyme. Also, the comprehensive kinetic and stability studies were carried out at different pH and temperatures.
Materials and methods
Materials
α-amylase from origin of Aspergillus Oryzae with an activity of 30 U/mg, soluble starch, 3,5-Dinitrosalicylic acid as reagents for investigation of enzyme activity, Glutaraldehyde (25 %) as a linker, sodium potassium tartrate, and chitosan (medium molecular weight) were purchased from Sigma-Aldrich. Bradford kit (Bio-Rad Laboratories Inc., Hercules, California, USA) was used for protein concentration assay. Fe3O4 and SiO2 were purchased from US Nano, LLC (Sarasota, FL). 3-Aminopropyl-trimethoxysilane (95 % purity) (APTES) was purchase from Acros Co. (New Jersey, US) and used as linker. Toluene was purchased from Scharlua Co., Spain. Maltose, sodium meta bisulfate, phenol, phosphoric acid, sodium hydroxide, and di-potassium hydrogen phosphate were all purchased from Merck Chemicals (Darmstadt, Germany).
Surface-modified Fe3O4–SiO2 nanoparticles
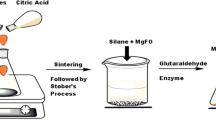
Silica and magnetic nanoparticles were both activated separately by dispersing in NaOH (1 M) solution for 24 h with vigorous stirring and then washed with deionized water until the pH of solution became natural. Then the nanoparticles were vacuum dried at room temperature for 48 h. Then the activated Silica nanoparticles (1 g) and magnetic nanoparticles (0.1 g) were dispersed in toluene with the aid of probe type sonicator for 5 min followed by addition of 1 g APTES to the mixture in order to introduce the amino groups on the surface of nanoparticle. The mixture was reacted at room temperature for 72 h under argon flow. The amine-functionalized silica-coated magnetic nanoparticles (AFSMNPs) were separated from the cooled mixture by an external magnet. These nanoparticles were washed successively with ethanol and THF. The obtained nanoparticles were dispersed in deionized water using bath sonicator for 30 min and then immediately reacted with glutaraldehyde by vigorous stirring at room temperature for 50 min. The weight ratios of AFSMNPs to glutaraldehyde were 10/1 and 5/1. The obtained nanoparticles were washed three times with de-ionized water and separated by an external magnet to remove unreacted glutaraldehyde. Finally, the glutaraldehyde-functionalized AFSMNPs were coated with chitosan. For this purpose, 0.5 g chitosan was dissolved in 50 mL acetic acid solution (2 wt%) for 24 h, and then it was filtered with a Buchner funnel. Then 10 mL of chitosan solution was added to 0.1 g glutaraldehyde-functionalized AFSMNPs and the mixture was stirred vigorously for 6 h at room temperature. Then chitosan-coated AFSMNPs were washed with deionized water and separated from unreacted chitosan solution by external magnet. The washing was continued until the pH of the solution was fixed at 6.5.
Enzyme immobilization
8 mg of synthesized AFSMNPs and chitosan-coated AFSMNPs were well-dispersed in 2 mL of phosphate buffer (0.05 M, pH 6.5) with the aid of a sonicating water bath for 30 min. Then 160 μL glutaraldehyde was immediately added to the mixture. The cross-linking reaction was done in 50 min at the continuous shaking in order to modify the surface amino groups for covalent immobilization of α-amylase. In order to remove additional glutaraldehyde, the nanoparticles were washed with phosphate buffer using external magnet for three times. Afterward, different ratios of α-amylase were added to the dispersion at 30 rpm using an orbital shaker for 24 h at 4 °C. The amount of immobilized enzyme was calculated by subtracting the unbound enzyme from the total added. The protein concentration was determined using Bradford method. The synthesis of enzyme-immobilized chitosan-coated AFSMNPs is summarized as follows: In the present study, α-amylase was covalently immobilized on a novel enzyme nanocarrier. Firstly, super-paramagnetic nanoparticles were synthesized by co-precipitation method and then, silica-coated MNPs were prepared through the sol–gel reaction. Secondly, the silica-coated magnetite nanoparticles were treated with APTES in order to yield the amino-functionalized magnetic nanoparticles. In the third step, the amino-functionalized magnetic nanoparticles were activated with glutaraldehyde as a crosslinker to bind in the next step to the free amino group of Chitosan. Finally α-amylase was covalently immobilized on the AFSMNPs (Fig. 1) and the chitosan-oated AFSMNPs by glutaraldehyde (Fig. 2). Enzyme immobilization efficiency (EIE) and loading (EIL) values are calculated from the following equations:
Fourier transforms infrared (FTIR) spectroscopy
The chemical structures of the samples were studied by FTIR spectroscopy. All of three samples were mixed with KBr and were pressed to disk. Infrared (IR) spectra of the samples were scanned in the range from 400 to 4,000 cm−1 and recorded on a Fourier transform infrared spectrometer (Equniox 55 LS 101 Bruker, German). FTIR spectra were obtained at a resolution of 4 cm−1 with a minimum of 256 scan per spectrum. All measurements were taken at room temperature. The spectra of water, CO2, and KBr were subtracted from the sample spectrum and the procedure was done under nitrogen gas to prevent humidity interference.
The size and surface morphology
The size and surface morphology of the AFSMNPs and chitosan-coated AFSMNPs nanocomposites were assessed by a field emission scanning electron microscope-energy dispersive using X-ray (FESEM-EDX), S4160 Hitachi, Japan. The powder sample was spread on a SEM stub and sputtered with gold. Particle size was obtained by measuring the diameters of at least 300 particles shown in SEM using image analysis software (Image-Pro Plus 4.5; Media Cybernetics, Silver Spring, MD).
Magnetism test
Magnetic properties of the synthesized nanoparticles were assessed with a vibrating-sample magnetometer (VSM—4 inch, Daghigh Meghnatis Kashan Co., Iran) at room temperature (Akbarzadeh et al. 2012; Luo et al. 2011). A magnet (Φ 17.5 × 20 mm, 5,500 Oe) was utilized for the collection of magnetic particles.
Activity study of the immobilized α-amylase
Immobilized and free enzyme reactions were based on the same amount of α-amylase used for starch hydrolysis. Hydrolysis reaction was carried out in 20 mL phosphate buffer (50 mM, pH 6.5) containing 1 % starch (w/v) with constant shaking at 30 °C. Aliquots of 250 µL were taken every 10 min and added to 750 µL of DNS reagent. The resulting solution was incubated in a boiling water bath (90 °C) for 5 min. The reaction was stopped by placing on ice bath. After dilution with deionized water, the amount of hydrolysis products (reducing sugar) was measured spectrophotometrically at 540 nm with maltose as the standard. All the data points for reducing sugar concentration are an average of duplicated measurements.
Thermal and pH stability of immobilized α-amylase
The optimum pH of free and immobilized α-amylase was determined as the relative activity after incubation in phosphate buffer (50 mM, pH 2, 3.5, 5, 6.5, and 8.0) containing 1 % starch (w/v) for 10 min at 30 °C. Moreover, enzyme was incubated at various temperatures (30, 40, 50, 60, 70, and 80 °C) in 1 % starch (w/v) solution for 10 min to determine the effect of temperature on the activity of the free and immobilized α-amylase. The determination of reduced sugar was considered as α-amylase stability.
Effect of storage time and frequent use on activity of immobilized α-amylase
The storage time of free and immobilized α-amylase was determined by carrying out at different times (1–80 days) by triplicate sampling. The residual activities were calculated as percentage of the initial activity. Also the stability and frequent use of the immobilized enzyme α-amylase were measured by reusing it twenty times every 10 min. The immobilized enzyme was collected by external magnet and washed with phosphate buffer. The primary activity of the enzyme was considered 100 % and activity in later use was reported as a percentage of the initial activity. One milliliter of 1 % (w:v) starch in 50 mM phosphate buffer (pH = 6.5) was added to the immobilized enzyme and incubated for 10 min at 30 °C under constant shaking for each cycles. At the end of the reaction, immobilized enzyme was taken and washed with 3 mL phosphate buffer (50 mM pH 6.5) and then added to a substrate solution to start a new cycle. The supernatant was assayed for reducing maltose.
Kinetic parameters
The kinetic parameters of free and immobilized α-amylase were determined by measuring the initial rates of enzymes with different substrates (starch concentration 10–60 mM). The K m and V max values were calculated from Lineweaver and Burk plot.
Result and discussion
Characterization
FTIR spectroscopy
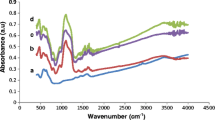
The amino group in Chitosan has a pKa value of around 6.5. Chitosan, a polysaccharide, becomes water-soluble under acidic conditions (pH < 6) and so becomes a biocompatible and often a biodegradable polymer. The cationic nature of Chitosan is mainly responsible for electrostatic interactions with negatively charged molecules. The successful step-by-step synthesize of nanocarriers and enzyme immobilization was proved by FTIR spectroscopy. The chemical structures of the Fe3O4 (Fig. 3a), SiO2 (Fig. 3b), AFSMNPs (Fig. 3c), glutaraldehyde-modified AFSMNPs (Fig. 3d), α-amylase (Fig. 3e), α-amylase immobilized on glutaraldehyde-modified AFSMNPs (Fig. 3f), chitosan (Fig. 3g), chitosan-coated AFSMNPs (Fig. 3h), and glutaraldehyde-modified chitosan-coated AFSMNPs (Fig. 3i) were studied by FTIR spectroscopy. The observation bands at 579 and 636 cm−1 in the spectrum of Fe3O4 nanoparticles are associated with the stretching vibration mode of the Fe–O bond (Fig. 3a). In Fig. 3b, the shoulder at 3,500 cm−1 could be assigned to the stretching vibrations of Si–OH groups in the structure of amorphous SiO2. The presence of the Si–OH group is proved as bonded water. The very strong and broad IR bands 1,000–1,250 cm−1, 956 cm−1, and 800 cm−1 are usually assigned to the Si–O–Si asymmetric stretching vibrations, silanol groups, and Si–O–Si symmetric stretching vibrations, respectively, whereas the IR band at 474 cm−1 is due to O–Si–O bending vibrations. In Fig. 3c, all of the peaks of Fig. 3a, b were observed proving the presence of SiO2 and Fe3O4. The C–H stretching vibration of the aliphatic section (APTES) is manifested through the strong peak at 2,890 cm−1. The peak at 3,350 cm−1 was attributed to the stretching vibrations of NH2 groups of APTES and Si–OH groups. After the modification with glutaraldehyde (Fig. 3d), the FTIR spectrum was the same as in Fig. 3c with a single peak at 1,650 cm−1 related to the imide characteristic peak. After enzyme immobilization on AFSMNPs (Fig. 3f), all the peaks of Fig. 3d appeared in addition to the peaks at 1,644 cm−1 and 1,535 cm−1 which corresponded to peptide binding (carbonyl of amide group and bending vibration of amine group, respectively). Also the stretching vibration of amine (N–H), a characteristic peak of enzyme (Fig. 3e), was observed at 3,448 cm−1. In the FTIR spectra of chitosan-coated AFSMNPs (Fig. 3h), all of the characteristic peaks of Fig. 3c were observed. Also typical bands of chitosan (Fig. 3g) appeared which were concerned with the stretching vibration bands of OH and NH at 3,446 cm−1, and the band due to the stretching vibration bands of C–O was observed at 1,089 cm−1. After attachment of glutaraldehyde (Fig. 3i), the peaks of Fig. 3h and the glutaraldehyde characteristic peak, the double peak, at 2,800 cm−1 and 1,650 were associated to COH and imide groups, respectively.
SEM study
The morphologies of the prepared silica nanoparticles (Fig. 4a), AFSMNPs (Fig. 4b), and chitosan-coated AFSMNPs (Fig. 4c) are clearly revealed by SEM. Hopefully, the sub 100-nm size of magnetic silica nanoparticles did not change after chemical modifications with APTES and chitosan. The size range of all particles was around 15–80 nm. SEM images revealed the uniformity in size and shape of the synthesized nanoparticles, as well.
Magnetism test
The magnetic properties of the nanoparticles were analyzed by vibrating sample magnetometry at room temperature. Figure 5 shows the hysteresis loops of the samples. The saturation magnetization was found to be 43 emu/g and 27 emu/g for AFSMNPs and chitosan-coated AFSMNPs, respectively, less than for the pure Fe3O4 nanoparticles (80 emu/g). This difference suggests that a large amount of Fe3O4 nanoparticles encapsulated into the nanocarriers. Regarding the observed large saturation magnetization, the Fe3O4 magnetic nanoparticles modified with APTES and chitosan could be rapidly and easily separated from the reaction medium in a magnetic field (Fig. 6). In addition, there was no hysteresis in the magnetization, with both remanence and coercivity being zero, suggesting that these magnetic nanoparticles are super paramagnetic. When the external magnetic field was removed, the magnetic nanoparticles could be well dispersed by gentle shaking. These magnetic properties are critical for application in the biomedical and bioengineering field.
α-Amylase immobilization parameters
Enzyme immobilization can be achieved by two different ways: chemical (by covalent bonds) and physical (by week interactions) methods. Covalent binding is very effective in retaining the enzyme activity, providing a maximum rigidity, preventing the diffusion of products in reaction medium, and also avoiding enzyme unfolding upon heating or in the presence of a denaturant. This also decreases the loss of enzymes and offers the chance to re-use the enzyme for many reaction cycles and thus lowering the total production cost and time of enzyme-mediated reactions.
Effect of nanocarrier/enzyme weight ratio on enzyme loading and activity
The weight ratios of different enzymes to the glutaraldehyde-modified AFSMNPs (1:1, 2:1 and 4:1) were studied to achieve the optimum enzyme immobilization condition. Results of the statistical analysis showed that although increase in enzyme amount led to an increase in enzyme immobilization on the carrier but concurrently the amount of un-immobilized enzyme increased dramatically (Table 1). Therefore, enzyme to the substrate ratio of 1:1 was chosen for enzyme immobilization on chitosan-coated AFSMNPs. The high observed enzyme loading percentage for AFSMNPs and chitosan-coated AFSMNPs in comparison to the results of previously reported studies (Bayramoğlu et al. 2008; Demir et al. 2012; Pascoal et al. 2011; Sohrabi et al. 2014; Talbert and Goddard 2013) revealed the superiority of the developed nanocarriers. The high surface to volume ratio caused by very small particle size of carrier and its increased porosity due to the application of silica particles in the structure of carriers might be responsible for the obtained results (Ashtari et al. 2012). In the next step, the immobilized enzyme activity was measured. Results of the statistical analysis revealed that different ratios of enzyme to the substrate had no significant differences in terms of activity (p > 0.05). The results of enzyme activity were well-matched with the findings of enzyme loading. After separation of unloaded enzyme, almost the same amount of enzyme remained causing the same enzyme activity. Therefore, carrier type did not affect the enzyme activity. The increased viscosity around the enzyme by the presence of chitosan might be responsible for the observed low enzyme activity, although the difference is not statistically significant.
Effect of pH on enzyme activity
pH of the solution is considered as an important factor on the enzyme activity through the ionization of amino acids of the active site. Results of the statistical analysis showed that the effect of pH on enzyme activity was significant (Table 2). In the case of free enzyme, the highest enzyme activity was observed in pH 6.5 and the lowest activity was demonstrated in pH 2 and 8. Enzyme deactivations in the low pH values (5 and 3.5) are higher for immobilized enzyme on the both nanocarriers than free enzyme. This might be attributed to the protonation of imide groups of AFSMNPs and their possible reaction with carboxylic groups of enzyme. The activity of protonated amine groups of AFSMNPs coated with chitosan polymer is less than AFSMNPs alone for interaction with immobilized enzyme due to the presence of chitosan polymer. The alkaline environment caused a dramatic decrease in the activity of free enzyme and one immobilized on AFSMNPs. The presence of amino groups of chitosan buffers the enzyme environment by adsorption of negative charges of media. The optimum pH for the immobilized enzyme is higher than the free enzyme which could be due to the basic nature of the non-modified free amine groups present on the surface of silica nanoparticles. Similar results have been reported in the literature that the optimal pH of the enzyme stabilization process has moved to alkaline pH (Erginer et al. 2000). In another study, the optimum pH values for the enzyme activity of free enzyme, and enzyme immobilized on amberlite and on chitosan obtained were 5.5, 7, and 8, respectively (Kumari and Kayastha 2011).
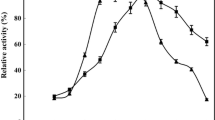
Effect of temperature on the enzyme activity
The effect of temperature on the activity of free and immobilized enzyme was examined at room temperature, 40, 50, 60, 70, and 80 °C (Table 3). The statistical analysis results showed that the effect of temperature on enzyme activity was significant (p < 0.05). The reason for low activity of free enzyme at high temperature could be due to the change in secondary structure and/or enzyme degradation that affects the active site of the enzyme which consequently caused a decrease in enzyme activity. But due to the covalent immobilization of enzymes, it is expected that the enzyme structure is largely preserved by temperature changes (Dong et al. 2012; Gupta et al. 2014). For example, in the case of lipase immobilization on organobentonite at temperatures higher than 60 °C, drastic reductions in free lipase activity was observed; but on immobilized lipase, reduction was very low which was due to the greater stability of the stabilized enzyme against thermal denaturation (Dong et al. 2012). Decrease in the viscosity of enzyme environment which had been elevated by incorporation of chitosan into the structure of carrier might be responsible for observed increased activity of chitosan-coated AFSMNPs at higher temperatures.
Effect of storage time on the stability and enzyme activity
Preserving the enzyme activity for a long time is the most important goal of the stabilization process (Zhang et al. 2009a). In this study, to assess the stability of enzyme activity over time, free and immobilized enzymes were kept in 1 mL phosphate buffer (50 mM, pH 6.5) for 80 days. The results revealed that free enzyme retained 1.2 % of its initial activity after 40 days while on the other hand the immobilized enzymes on AFSMNPs and chitosan-coated AFSMNPs retained 82.9 and 84.3 % of their initial activity, respectively. But after 80 days of incubation, free enzyme lose its activity completely but immobilized enzymes on AFSMNPs and chitosan-coated AFSMNPs preserved 65.73 and 78.63 % of their initial activity, respectively (Fig. 7). The result of this study indicated the worth of the presence of chitosan in the structure of nanocarriers for preserving enzyme activity for a longer period. These results are very similar to the result of the effect of storage time on the activity of glucose oxidase stabilized on magnetic silica-coated nanoparticles: 98 % of the glucose oxidase activity was maintained after 45 days (Ashtari et al. 2012). Demir and co-workers (Demir et al. 2012) stated that the biocatalysts with very high sensitivity to environmental conditions easily lost their activity during time and, therefore, their immobilization would be very helpful to maintaining their activity. They found only 25–30 % decrease in immobilized α-amylase activity after several days that were similar to the results achieved in this study. In another study, Sohrabi et al. showed the increase in immobilized α-amylase activity in comparison to free enzyme (Sohrabi et al. 2014).
Effect of frequent use on the stability of immobilized enzyme activity
One of the main advantages of enzyme immobilization is the possibility of frequent usage of enzyme. The results of frequent use on the stability of immobilized enzyme activity showed that for AFSMNPs and chitosan-coated AFSMNPs 82 and 91 % of their initial activity, respectively, remained after 10 repeated uses (Fig. 8). About 74 and 85 % of their initial activity was preserved after repeated use of 20 times in AFSMNPs and chitosan-coated AFSMNPs, respectively. Also the addition of chitosan to AFSMNPs led to the increase of enzyme stability in frequent use. These results indicated the superiority of both nanocarriers compared to the previous studies (Pascoal et al. 2011). In another study conducted with Ashley et al. (2011) in the stabilization of the enzyme with substrate polyaniline, 90 % of enzyme activity after 4 times and 70 % after 10 times was preserved.
The results of the kinetic properties of enzyme
To determine the kinetic parameters of free and immobilized enzyme on AFSMNPs and chitosan-coated AFSMNPs, the enzyme activity was observed in the presence of different concentrations of starch (0.2–3 %) as substrate. Kinetic parameters of V max and K m were obtained from Michaelis–Menten Graph and Lineweaver–Burk diagram (Fig. 9). Obtained values of kinetic parameters are shown in Table 4. The V max amount depends on the amount of active enzyme and K m represents the tendency of the enzyme to the substrate. K m is much smaller, indicating a greater tendency of the enzyme to the substrate. The results show that the K m of the immobilized enzyme in both AFSMNPs and chitosan-coated AFSMNPs was more than the free enzyme. Though these changes are not very impressive, it can be concluded that the proper complex between the enzyme and the substrate has been created. There have not been drastic changes in the three-dimensional structure of the enzyme, and the catalytic properties of the enzyme were largely maintained. These results showed the same tendency of the enzyme to the substrate and the formation of appropriate complex between the enzyme and substrate and confirmed that the stabilization of enzyme on the substrate did not reduce the tendency of the enzyme to the substrate. The k max for free and immobilized enzyme on AFSMNPs and chitosan-coated AFSMNPs were 0.39, 0.86, and 1.36, respectively. Little differences between k m are probably due to the changes in the tertiary structure of amylase, how the enzyme is immobilized on the substrate, and fewer availability of substrate for the active site that was affected by the immobilization process. Therefore, a slight decrease in the catalytic efficiency of enzymes and increase in k m have been observed (Kumari and Kayastha 2011). The V max for free and immobilized enzyme on AFSMNPs and chitosan-coated AFSMNPs obtained were 1.92, 1.157, and 1.255 µmol/mg min, respectively. The decrease in V max value that is dependent on the enzyme activity represents that the action of the enzyme immobilization reduced the enzyme activity. This reduction probably happened due to the covalent stabilization of enzyme from multiple points or might be due to the damaging of the structure of the active site of the enzyme when hydrolysis process was done. In a study, the same k m values were achieved for the immobilized glucose oxidase on the ultra-filtration membranes and free enzyme (Godjevargova et al. 2000).
Conclusion
In this work, α-amylase was successfully immobilized on glutaraldehyde-activated amino-functionalized silica-coated magnetite nanoparticles alone (AFSMNPs) and covered with chitosan using the covalent binding method. FTIR spectrum proved that the chemical modification and immobilization were carried out successfully. The α-amylase-bounded glutaraldehyde-activated AFSMNPs and chitosan-coated AFSMNPs nanoparticles have a mean size of around 20–80 nm. Immobilized α-amylase was found to be stable against various types of physical and chemical denaturants. Enzyme assays demonstrated substantial improvement of thermal, pH, storage stability, and frequent use of the α-amylase due to immobilization. The kinetic studies verified the Michaelis–Menten behavior and proposed an overall improvement in the performance of the immobilized enzyme in comparison to the free enzyme. The results presented here revealed the potential applicability of the developed nanoparticles for biomedical and biotechnological applications, which could be suggested to be used for the efficient conversion of starch in continuous reactors at the industrial level.
References
Abdelmajeed NA, Khelil OA, Danial EN (2012) Immobilization technology for enhancing bio-products industry. Afr J Biotechnol 11:13528–13539
Akbarzadeh A et al (2012) Synthesis, characterization, and in vitro evaluation of novel polymer-coated magnetic nanoparticles for controlled delivery of doxorubicin. Nanotechnol Sci Appl 5:13
Arruebo M, Fernández-Pacheco R, Ibarra MR, Santamaría J (2007) Magnetic nanoparticles for drug delivery. Nano today 2:22–32
Ashly P, Joseph M, Mohanan P (2011) Activity of diastase α-amylase immobilized on polyanilines (PANIs). Food Chem 127:1808–1813
Ashtari K, Khajeh K, Fasihi J, Ashtari P, Ramazani A, Vali H (2012) Silica-encapsulated magnetic nanoparticles: enzyme immobilization and cytotoxic study. Int J Biol Macromol 50:1063–1069
Atacan K, Özacar M (2015) Characterization and immobilization of trypsin on tannic acid modified Fe3O4 nanoparticles. Colloids Surf B Biointerfaces 128:227–236
Bayramoğlu G, Kiralp S, Yilmaz M, Toppare L, Arıca MY (2008) Covalent immobilization of chloroperoxidase onto magnetic beads: catalytic properties and stability. Biochem Eng J 38:180–188
Demir S, Gök SB, Kahraman MV (2012) α-Amylase immobilization on functionalized nano CaCO3 by covalent attachment. Starch-Stärke 64:3–9
Dong H, Li J, Li Y, Hu L, Luo D (2012) Improvement of catalytic activity and stability of lipase by immobilization on organobentonite. Chem Eng J 181:590–596
Erginer R, Toppare L, Alkan S, Bakir U (2000) Immobilization of invertase in functionalized copolymer matrices. React Funct Polym 45:227–233
Ghaderi S, Ghanbarzadeh S, Mohammadhassani Z, Hamishehkar H (2014) Formulation of gammaoryzanol-loaded nanoparticles for potential application in fortifying food products Adv. Pharm Bull 4:549–554
Ghaderi S, Ghanbarzadeh S, Hamishehkar H (2015) Evaluation of different methods for preparing nanoparticle containing gammaoryzanol for potential use in food fortification. Pharm Sci 20:130–134
Godjevargova T, Konsulov V, Dimov A, Vasileva N (2000) Behavior of glucose oxidase immobilized on ultrafiltration membranes obtained by copolymerizing acrylonitrile and N-vinylimidazol. J Membr Sci 172:279–285
Gupta A, Kumar V, Dubey A, Verma A (2014) Kinetic Characterization and Effect of Immobilized Thermostable β-Glucosidase in Alginate Gel Beads on Sugarcane Juice. ISRN Biochemistry 2014:8
Homaei AA, Sariri R, Vianello F, Stevanato R (2013) Enzyme immobilization: an update. J Chem Biol 6:185–205
Jiang Y, Guo C, Xia H, Mahmood I, Liu C, Liu H (2009) Magnetic nanoparticles supported ionic liquids for lipase immobilization: enzyme activity in catalyzing esterification. J Mol Catal B Enzym 58:103–109
Jiang YZ, Shi YQ, Tian LZ, Xu HY (2013) Synthesis and Dye Degradation Properties of Cu2 + Complexes with Benzimidazole Derivatives. Adv Mater Res 726:2449–2452
Khan AA, Alzohairy MA (2010) Recent advances and applications of immobilized enzyme technologies: a review. Res J Biol Sci 5:565–575
Khan MJ, Husain Q, Azam A (2012) Immobilization of porcine pancreatic α-amylase on magnetic Fe2O3 nanoparticles: applications to the hydrolysis of starch. Biotechnol Bioproc E 17:377–384
Kim J, Grate JW, Wang P (2006) Nanostructures for enzyme stabilization Chem Eng Sci 61:1017–1026
Kumari A, Kayastha AM (2011) Immobilization of soybean (Glycine max) α-amylase onto Chitosan and Amberlite MB-150 beads: optimization and characterization. J Mol Catal B Enzym 69:8–14
Luo Y-B, Shi Z-G, Gao Q, Feng Y-Q (2011) Magnetic retrieval of graphene: extraction of sulfonamide antibiotics from environmental water samples. J Chromatogr 1218:1353–1358
Mahmoud DA, Helmy WA (2009) Potential Application of Immobilization Technology in Enzyme and Biomass Production. J Appl Sci Res 5:2466–2476
Mandal M, Kundu S, Ghosh SK, Panigrahi S, Sau TK, Yusuf S, Pal T (2005) Magnetite nanoparticles with tunable gold or silver shell. J Colloid Interface Sci 286:187–194
Mohammadi M, Ghanbarzadeh B, Hamishehkar H (2014) Formulation of nanoliposomal vitamin D3 for potential application in beverage fortification. Adv Pharm Bull 4:569–575
Pascoal AM, Mitidieri S, Fernandes KF (2011) Immobilisation of α-amylase from Aspergillus niger onto polyaniline. Food Bioprod Process 89:300–306
Pezeshki A, Ghanbarzadeh B, Mohammadi M, Fathollahi I, Hamishehkar H (2014) Encapsulation of vitamin A palmitate in nanostructured lipid carrier (NLC)-effect of surfactant concentration on the formulation properties. Adv Pharm Bull 4:563–568
Rasaie S, Ghanbarzadeh S, Mohammadi M, Hamishehkar H (2014) Nano Phytosomes of Quercetin: a Promising Formulation for Fortification of Food Products with Antioxidants. Pharm Sci 20:96
Sohrabi N, Rasouli N, Torkzadeh M (2014) Enhanced stability and catalytic activity of immobilized α-amylase on modified Fe3O4 nanoparticles. Chem Eng J 240:426–433
Sureshkumar M, Lee C-K (2011) Polydopamine coated magnetic-chitin (MCT) particles as a new matrix for enzyme immobilization. Carbohydr Polym 84:775–780
Talbert JN, Goddard JM (2013) Characterization of lactase-conjugated magnetic nanoparticles. Process Biochem 48:656–662
Tripathi P, Kumari A, Rath P, Kayastha AM (2007) Immobilization of α-amylase from mung beans (Vigna radiata) on Amberlite MB 150 and chitosan beads: a comparative study. J Mol Catal B Enzym 49:69–74
Türünç O, Kahraman MV, Akdemir ZS, Kayaman-Apohan N, Güngör A (2009) Immobilization of α-amylase onto cyclic carbonate bearing hybrid material. Food Chem 112:992–997
Xu J, Sun J, Wang Y, Sheng J, Wang F, Sun M (2014) Application of iron magnetic nanoparticles in protein immobilization. Molecules 19:11465–11486
Zhang L, Jiang Y, Jiang Z, Sun X, Shi J, Cheng W, Sun Q (2009a) Immobilized transglucosidase in biomimetic polymer–inorganic hybrid capsules for efficient conversion of maltose to isomaltooligosaccharides. Biochem Eng J 46:186–192
Zhang Y, Zhang Y, Wang H, Yan B, Shen G, Yu R (2009b) An enzyme immobilization platform for biosensor designs of direct electrochemistry using flower-like ZnO crystals and nano-sized gold particles. J Electroanal Chem 627:9–14
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hosseinipour, S.L., Khiabani, M.S., Hamishehkar, H. et al. Enhanced stability and catalytic activity of immobilized α-amylase on modified Fe3O4 nanoparticles for potential application in food industries. J Nanopart Res 17, 382 (2015). https://doi.org/10.1007/s11051-015-3174-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-015-3174-3