Abstract
Ubiquitous nanomaterials have been extensively involved in multifarious biotransformation of diverse organic and synthetic techniques. This research study describes the robust immobilization of α-amylase on magnetic nanoparticles of magnesium ferrite (MgFO) functionalized with silane. The XRD patterns, FESEM and HRTEM images were analysed to study structural and morphological features. The crystallite size of MgFO is found to be 12 nm. The FTIR results confirmed the covalent attachment of the enzyme with the MgFO and VSM and Mössbauer spectrometric graphs were evaluated to study the magnetic behaviour of the prepared samples. The Kinetic parameters, reusability, storage capacity and catalytic activity of the enzyme at various reaction time, pH and temperature conditions before and after attachment with MgFO were examined to confirm the successful immobilization process. The reusability of the enzyme on modified MgFO is found to be good (> 50%) even after 12 consecutive usability cycles and also retain 50% catalytic efficiency over 30 days storage period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Biomolecules, exceptionally nano-biocatalysts are gaining striking consideration for multidisciplinary scientific studies as they are significant active and selective to catalyze various intricate actinic reactions in an antagonistic environment, nevertheless operational reaction stability, loading capacity, reusability and separability like constraints restrict their biotechnical applications [1,2,3]. Catalytic stability studies are comparatively recorded under easily established chemical studies and lead to a colossal requirement of enzyme immobilization science, whereas, after immobilization nano-bio catalyst may save the characteristics that, in inimical situations, there may be deformation of the active site and which leads to a decrease in activity rate [4]. For the development of an excellent immobilized bio-nanocatalyst assembly, the assorted dexterity of enzymology, protein science, nanosurface techniques and solution reaction intricacies are employed together [5]. The accomplishment rate of these biomolecules assisted reactions is immensely affected by the type of brace material employed for immobilization [6]. In recent years, biomolecules immobilized on magnetic brace materials due to expeditious separation of biomolecules under an external magnetic field. Various innovations in the biotechnological field have resulted in the diversification of carriers and immobilization technologies to augment the loading capacity, stability in reaction media and activity rate of natural enzymes which repress the cost of immobilization for industrial application [7]. These incorporate several methods like nanoparticles and mesoporous materials based immobilization, smart chemistry science, microwave-aided immobilization, etc. [8]. The exclusive characteristics including size, surface area, saturation magnetization value, etc. of nano powdered magnetic metal oxides attract the attention of researchers and become their leading choice in the immobilization of nano-biocatalysts [9]. The segregation and dissolution of nano-biocatalysts is a common limitation and not so easy to be overcome. This aim can be attained by employing a propitious option of magnetically separable nanoparticles which provides high accessibility with improved reusability [10]. These magnetic nanoparticles are non-porous in nature, so they do not have diffusion problems like a porous brace used for immobilization. The repeatedly employed magnetic nanoparticles are metal-iron oxides that have minimal toxicity, large loading limits with fewer diffusion possibilities in the reaction solution, sufficient oxygen enclosing surface functionalities and better biocompatibility like characteristics [11]. The most commonly employed magnetic metal-iron oxide particles generally have low dispersity and solution stability like drawbacks and several modification processes are promoted nowadays to make them more dispersible and biocompatible [12]. To subdue these drawbacks, the coalition of biocatalysts with insoluble substrates like ferrites has emerged as entrenched access and efforts were recently made to put them in the place of iron oxide nanoparticles to curtail these insufficiencies [13]. Amylase is one of the extensively used enzymes in food, clinical and medicinal industries. α-amylase is a subcategory of this enzyme which is widely studied in the hydrolysis process of starch breakdown into sugar units. There are many reported articles of amylase immobilization and encapsulation existing in the literature on various substrates like α-amylase on Titania/lignin composite [14], various silica encapsulated α-amylase [15], peanut β-amylase immobilized onto carbon nanotube [16], α-amylase on polyanilines [17] and α-amylase on Graphene oxide nanosheets [18]. By perusing this, in this research article, we have successfully immobilized α-amylase on Magnesium ferrite nanoparticles (MgFO). Efficacies of the new immobilized system are examined, and a correlated investigation of the various characteristics and process parameters of free and immobilized MgFO nanoparticles was also conducted.
2 Materials, preparation and characterization techniques
2.1 Materials
Magnesium nitrate, Iron nitrate, Ethylene Glycol, Ammonia, Citric acid and Ammonium hydroxide were procured from Merck Chemical Co. α-amylase (EC 3.2.1.1 from Bacillus subtilis), 3, 5-dinitrosalicylic acid (DNS), Tetra Ethoxy Silane (TEOS), Glutaraldehyde and Starch were procured from Sigma-Aldrich Chemical Co.
2.2 Synthesis of MgFO
Magnetic nanoferrite of magnesium was synthesized by a self-ignition process as reported earlier [19]. For the preparation process, a mixture of calculated ratios of metal nitrates and citric acid was made in double-distilled water and thereafter ethylene glycol and ammonia were added one by one to control agglomeration and solution pH. In the end, the mixture was allowed to convert itself into viscous gel at 80 0C which exhibit a self-ignition process to form a red-brown flocculent powder. Later, the sample was sintered for 3 h at 500 0C.
2.3 Synthesis of TEOS functionalized MgFO
An earlier reported method [10] was employed to fabricate TEOS functionalized MgFO nanoferrite (TEOS-MgFO) powder as illustrated in Fig. 1. The process is associated with the use of nanocatalyst of acidic or basic type in the hydrolysis of TEOS. The small amount (100 gm) of MgFO particles was homogeneously dispersed to a solution containing Ethanol (150 mL), ammonium hydroxide (2 mL) and distilled water (10 mL), followed by the addition of TEOS (2 mL) to the mixture. Later, the mixture formed was sonicated for two cycles of 30 min each. After one day, the MgFO particles were magnetically separated and got ethanol washed four times and later dehydrated in the oven at 60 0C. The binding agent Glutaraldehyde (2%) was transplanted on the separated particles by mixing them for 60 min at 150 rpm and later filter-separated by the reaction solution, followed by many washing and dehydration processes.
2.4 Immobilization and activity assay
Amylase dissolved in Phosphate solution was added to TEOS-MgFO particles by the process illustrated in Fig. 1, followed by 5 min incubation with N2. Finally, the mixture was kept for shaking at 100 rpm for 24 h at 37 °C for optimal immobilization of amylase onto modified MgFO. Amylase immobilized MgFO (MgFO-Amylase) was then separated by a magnet and supernatant was collected. Collected MgFO particles were washed with Phosphate buffer and were dispersed into Phosphate buffer for further use. The amount of immobilized enzyme was determined by the subtracted amount of protein determined in supernatants and washing solutions from the amount of initial protein used for immobilization. After the immobilization process, the supernatant and the washing solutions were collected and assayed for protein estimation as well as amylase activity. The Bradford method was used to determine the amount of the enzymes in solution [20], while the activities of free and immobilized amylase were assayed by the method of Bernfeld [21]. To evaluate the immobilized enzyme activity starting with the addition of modified MgFO in the 20 mM phosphate buffer with pH near 7. The solution was incubated for half an hour at 37 °C and 3, 5-dinitrosalicylic acid (DNS) was added later to it. The assay output as reduced sugar was investigated by colour change observed by UV–Vis spectrometer near 540 nm. Under standard assay conditions, for the calculation of 1 U of enzyme activity, the concentration of amylase needed to liberate 1 micromole of maltose per 60 s was calculated. A double reciprocal curve Lineweaver–Burk process was employed to evaluate the Km and Vmax results by interpreting the basic and obtained kinetic results [22]. The reusability of MgFO-Amylase was investigated up to 21 cycles for 1.5% of starch solution. After every cycle magnetically recovered MgFO-Amylase was washed three times with ethanol. The relative activity between the first cycle and the rest of the cycles is calculated to study the effect of enzyme immobilization on reusability. The commercial application of these types of immobilized catalysts is found to be dependent on the collusion between the amylase and functionalized support type which governs the storage capacity of these enzymes. The enzymatic behaviour of the prepared samples stored in phosphate buffer (20 mM, pH 7.0) at 4 °C concerning the number of storage days was investigated.
2.5 Characterization
The crystalline phase and size of the MgFO sample were characterized by X-Ray Diffraction and the successful binding of amylase on MgFO was investigated by FTIR results obtained by employing Pan analytical instrument in the angle range from 20 to 70° θ and Perkin Elmer spectrometer in the range 400–4000 cm−1 respectively. The particle size and morphology of MgFO were investigated by HRTEM and FESEM results obtained by TEM, 2000 FP 5022/22 FEI, USA and FESEM, Nova Nano SEM-450, JFEI (USA), respectively. The magnetic characteristics of the MgFO and modified MgFO were analysed by VSM graphs and Mossbauer spectra obtained by employing VSM, Microsense, EV7 and Mössbauer spectrometer of FAST Com Tec 070,906, respectively. All the characterization studies were performed at 300 K. The protein estimation results were calculated by dye-binding method, used to calculate the amylase concentration in the supernatant through spectrometer named tech UV 2100.
3 Results
3.1 Structural and phase characteristics
3.1.1 FTIR graph
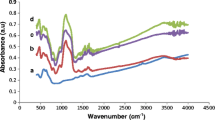
The modification of MgFO surface with various functional moieties was elucidated from recorded FTIR spectra of MgFO, TEOS-MgFO and MgFO-Amylase as shown in Fig. 2, respectively. As depicted in the figure the elemental bands in the range of 400–800 cm−1 exemplify the successful structure formation of the ferrite sample consisting of the octahedral and tetrahedral site bonds [10]. A predominant peak near 600 cm−1 proved the presence of an iron-oxygen bond in MgFO samples. In the rest two samples of TEOS-MgFO and MgFO-Amylase, the presence of silane-oxygen-silane bonding was observed by peaks at 1475 cm−1 [23]. The peaks noticed near 1010 and 1370 cm−1 are attributed to Carbon–Oxygen and carbon-hydrogen bonding [24]. The absorption peak found near 1032, 1392, 1548 and 1620 cm−1 confirms the fruitful changes in the MgFO sample done by enzyme loading on its surface [25]. A broad absorption peak near 3300 cm−1 confirmed O–H stretching [26] and a band near 2960 cm−1 symbolized the stretching vibration corresponding to aliphatic C–H bonding in the samples [26].
3.1.2 Phase and structural analysis
The crystallinity of the structure of MgFO nanoparticles was checked by XRD graphs as presented in Fig. 3. The obtained results confirmed the presence of all the required peaks of a spinel cubic structure in accordance with JCPDS file 71-1232 and indexed as (220), (311), (222), (400), (331), (422), (511), etc. [27]. The Fullproof Rietveld refinement method was employed for peak indexing. The calculated crystallite size by using Debye and Scherrer’s equation is approximately 12 nm. The synthesized sample has unit cell values as that of a cubic space group fd-3 m with a side = 8.3673 nm and crystallographic angle = 90.0.
3.1.3 Microstructural studies
The figurative FESEM images of the MgFO and modified MgFO samples are collectively shown in Fig. 4a,b. The FESEM images proved the spherical shape and homogeneity of the prepared and modified samples. The nano range particle size of MgFO contributed to the serene immobilization of amylase. Later modification leads to agglomeration and an increase in size too by keeping the shape of the particles the same. The detected size of the MgFO conforms to the XRD calculations. As presented in Fig. 4c,d, the scrutiny of the crystalline nature of the MgFO and TEOS-MgFO samples was exhibited by HRTEM results. From the obtained results the particle size of MgFO is found to be approximately 15 nm and 20 nm in diameter before and after the modification process. After TEOS modification the polycrystalline nature of the MgFO samples remains the same.
3.2 Magnetic characterization
3.2.1 Vibrating sample magnetometer results
From the VSM curve as depicted in Fig. 5, the magnetic saturation value Ms of the as-prepared and modified samples (TEOS-MgFO and MgFO-Amylase) was evaluated as 23.7 emu/gm, 11.1emu/gm and 9.1 emu/gm, respectively. This difference in the magnetic saturation values confirmed the successful immobilization and modification of the samples. The large Ms value of the nanoparticles also makes sure that the immobilized system can be expeditious separate from the reaction solution in the action of applied magnetic force. In addition, coercivity and retentivity values near zero conclude the near superparamagnetic behaviour of the samples. The calculated result values of magnetic parameters are enlisted in Table 1. The change in magnetic parameters after and before immobilization is accrediting to the large functional area of nanoparticles which permit lucrative binding of amylase with prepared nanoparticles [28]. The comprehensive magnetization of the sample is stated by Ms = V*ms Where, V and ms are the volume fragment and magnetic moment values of the sample. After modification of the prepared composite rely upon the magnetic and non-magnetic parts present in the sample. As clear from Fig. 5, a small hysteresis curve is evident proof of its nearly superparamagnetic nature.
3.2.2 Mössbauer spectra
Figure 6 depicts the Mössbauer spectra of the prepared and modified samples at room temperature. The spinel structure of ferrite has tetrahedral (A) and octahedral (B) sites which correspond to magnetic sub-lattices in the structure. The electrostatic interplay among the nucleus and inner-shell electrons in an atom engendered shifting in the nuclear energy states of absorber and source both. Also due to nonuniform cubic arrangements and different bond separation at both sites, they have different specific site magnetic values and isomer shift results. The calculated Mössbauer parameters isomer shift (IS), Quadrupole splitting (QS), Line width (LW) from the recorded spectra are presented in Table 1. The recorded results confirmed the presence of two combined existed wide sextets with large line width and single doublet, representing two sites of the spinel structure and also the existence of superparamagnetic relaxation with ferromagnetic ordering in the samples [25].
3.3 Effect of different process parameters on amylase immobilization
3.3.1 Determination of amylase activity at different reaction conditions
The catalytic activity of an enzyme predominantly depends on the change in starch percentage, reaction time, reaction pH and temperature. The optimum concentration of amylase immobilized on MgFO was investigated for the different starch solutions and the calculated results are presented in Fig. 7a. Hence, 36 (U/mg) is the best-optimized amylase captivation in the 1.5% starch solution. The obtained data made it clear that the catalytic rate of the MgFO-Amylase sample increased with the amylase concentration and confirms that active groups on MgFO coated with the TEOS sample are not changing and the conformational changes in the amylase structure are negligible [24].
The variation of reaction time and reaction pH and the corresponding change in the catalytic rate of amylase are given in Fig. 7b, c. The obtained results for variation of reaction pH at different values in the range of 6–9, revealed that the catalytic rate of MgFO-Amylase is maximum at pH 7. Also, the obtained results confirm that the catalytic rate of amylase and MgFO-Amylase samples were investigated for 10, 20, 30 and 40 min and is found to be highest at 20 min incubation. After 20 min, activity decline again this is attributed to a possible decrease in the availability of the surface functional group as steric hindrance rises [10].
The variation in reaction temperature strongly regulates the amylase catalytic activity as temperature variations decidedly influence the active sites. The catalytic behaviour of amylase and MgFO-Amylase with the variation in reaction temperature is illustrated in Fig. 7d. The results revealed that the optimum reaction temperature for amylase is 37 °C and for MgFO-Amylase is in-between the range of 37–45 °C. The obtained results proved that the immobilization process does not alter the amylase conformation and also increased the catalytic behaviour to the range of 37°45 °C effectively with similar activity. This increase in stability at a higher temperature range is attributed to a rise in thermo-kinetic energy of MgFO which results in more uncovering of catalytic sites of amylase to the chosen substrate and also improved than the few already obtained immobilization results [29]. This increase in temperature stability also advances the chances of the amylase binding to MgFO and hence, enhances the potency of the used process.
3.3.2 Kinetic parameters
The Kinetic behaviour of Amylase and MgFO-Amylase was determined by investigating the elementary reaction behaviour of the immobilized enzyme in various substrate concentrations. After evaluating, the double reciprocal curve, the Km values are calculated as 41.1 and 39.3 and Vmax values are 0.81 and 0.49 for Amylase and MgFO-Amylase, respectively. The increase in Michaelis constant results shows that after immobilization on MgFO Amylase affinity for substrate in the reaction mixture rises. The activity behaviour of the enzyme for the substrate is depicted in Fig. 8 as the Lineweaver–Burk plot. The obtained values confirmed the lower binding affinity of amylase in comparison to MgFO-Amylase samples. The obtained results show that the Km value of Amylase-MgFO was low than the free amylase sample and this is credited to the surrounding changes of amylase enzyme after immobilization on MgFO particles. The prepared MgFO particles have a small size, which helps us to conclude that the amylase enzyme may undergo expansion on MgFO particles and provide high affinity towards the substrate and increase active sites availability too [30].
3.3.3 Reusability and storage stability of MgFO-amylase
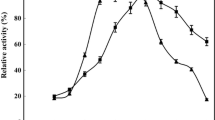
To increase the possible technical uses of amylase the enzyme reusability and storage stability factors were investigated. The obtained data for these factors is illustrated in Fig. 9a, b, respectively, and the results revealed that the enzymatic catalytic rate of amylase remain 50% even after 12 recycles. The observed decrease in the enzymatic catalytic rate concerning the number of recycles could be explained by the inactivation of active sites which are inhibited by accumulated by the reaction left product after a few cycles. The observed results confirmed that the immobilization of Amylase on MgFO provides a sheltered covering to amylase that bestows its enlarged catalytic behaviour and could raise its practical stability on functionalized magnetic support matrix [31].
The catalytic behaviour of amylase and MgFO- Amylase concerning the number of days was investigated over the period of 56 days. The storage stability results as shown in Fig. 9b revealed that after immobilization of Amylase the catalytic activity remains up to 50% even after 35 days. The probable reason behind the observed results is could be the interaction of Amylase with MgFO precluding the denaturation of MgFO-Amylase. The obtained results are in accordance with those earlier reported results that the magnetic nanoparticles could enhance enzyme stability and preserve its activity in adverse conditions. When Navya Antony et al. immobilized Amylase on Zinc Oxide, the obtained optimum pH was 6 and temperature was 60 °C, but the enzyme activity after six cycles of use and 30 days of storage remained at approximately 20% and 50% respectively [32]. Similarly, F. Eslamipour et al. analysed Fe3O4 nanoparticles for Amylase immobilization and found that the enzyme activity get reduced 40% and 50% after six cycles of use and 12 days of storage [33]. In the present study, the immobilized sample could be reused for seven cycles and 21 days with enzyme activity remaining at > 75% of its initial activity. These observed results are explained by the increased stability and rigidity in the immobilized enzyme structures after immobilization [34]. Hence the obtained results of the immobilized enzyme on the surface of prepared nanoferrite are found to be better than these earlier results and confirmed more suitability of the prepared sample in the repetitive use for bioindustrial applications of immobilized enzyme.
4 Conclusion
This article provides a lucrative immobilization strategy to immobilize amylase enzyme on magnetic MgFO particles to achieve easy dissolution of the enzyme by reaction mixture with enhanced catalytic activity. The crystallite size was calculated by the X-Ray Diffractometer result and found to be 12 nm. The FESEM and HRTEM studies concluded that the particle size increased after modification but the crystallinity and spherical shape remain the same. The investigation of FTIR results confirmed the effective surface modification and attachment of the enzyme with the MgFO particles. The MgFO-Amylase samples were found to have more reusability, enhanced reaction pH and thermal stability than free amylase samples. The analysis of obtained mössbauer spectra confirmed the presence of a doublet and two sextets pattern. The observed pattern of mössbauer spectra confirmed that the local magnetic field does not get altered by the modification process. The calculated kinetic parameters confirmed that the used progressive immobilization strategy is a noble and acceptable way for effective industrial applications of amylase enzyme.
References
V. Atiroğlu, A. Atiroğlu, M. Özacar, Immobilization of α-amylase enzyme on a protein @metal–organic framework nanocomposite: a new strategy to develop the reusability and stability of the enzyme. Food Chem. 349(September), 2021 (2020). https://doi.org/10.1016/j.foodchem.2021.129127
A. Basso, S. Serban. Industrial applications of immobilized enzymes—a review. Mol. Catal. 479, 110607 (2019). https://doi.org/10.1016/j.mcat.2019.110607
U. Hanefeld, L. Gardossi, E. Magner, Understanding enzyme immobilisation. Chem. Soc. Rev. 38(2), 453–468 (2009). https://doi.org/10.1039/b711564b
S. Santos, J. Puna, J. Gomes. A review on bio-based catalysts (immobilized enzymes) used for biodiesel production. Energies 13(11). https://doi.org/10.3390/en13113013
R. A. Wahab, N. Elias, F. Abdullah, S. K. Ghoshal. On the taught new tricks of enzymes immobilization: an all-inclusive overview. React. Funct. Polym. 152, 104613 (2020). https://doi.org/10.1016/j.reactfunctpolym.2020.104613.
C. Bernal, K. Rodríguez, R. Martínez, Integrating enzyme immobilization and protein engineering: An alternative path for the development of novel and improved industrial biocatalysts. Biotechnol. Adv. 36(5), 1470–1480 (2018). https://doi.org/10.1016/j.biotechadv.2018.06.002
H.M. Salvi, G.D. Yadav, Process intensification using immobilized enzymes for the development of white biotechnology. Catal. Sci. Technol. 11(6), 1994–2020 (2021). https://doi.org/10.1039/d1cy00020a
M. Bilal, Y. Zhao, S. Noreen, S.Z.H. Shah, R.N. Bharagava, H.M.N. Iqbal, Modifying bio-catalytic properties of enzymes for efficient biocatalysis: A review from immobilization strategies viewpoint. Biocatal. Biotransf. 37(3), 159–182 (2019). https://doi.org/10.1080/10242422.2018.1564744
M. Razzaghi et al. Industrial applications of immobilized nano-biocatalysts. Bioprocess Biosyst. Eng. 0123456789 (2021). https://doi.org/10.1007/s00449-021-02647-y.
A. Sharma, A. Kumar, K.R. Meena, S. Rana, M. Singh, S.S. Kanwar, Fabrication and functionalization of magnesium nanoparticle for lipase immobilization in n-propyl gallate synthesis. J. King Saud Univ. Sci. 29(4), 536–546 (2017). https://doi.org/10.1016/j.jksus.2017.08.005
M. Bilal, Y. Zhao, T. Rasheed, H.M.N. Iqbal, Magnetic nanoparticles as versatile carriers for enzymes immobilization: a review. Int. J. Biol. Macromol. 120, 2530–2544 (2018). https://doi.org/10.1016/j.ijbiomac.2018.09.025
B.I. Kharisov, H.V.R. Dias, O.V. Kharissova, Mini-review: ferrite nanoparticles in the catalysis. Arab. J. Chem. 12(7), 1234–1246 (2019). https://doi.org/10.1016/j.arabjc.2014.10.049
M. Amiri, K. Eskandari, M. Salavati-Niasari, Magnetically retrievable ferrite nanoparticles in the catalysis application. Adv. Colloid Interface Sci. 271, 101982 (2019). https://doi.org/10.1016/j.cis.2019.07.003
Ł Klapiszewski, J. Zdarta, T. Jesionowski, Titania/lignin hybrid materials as a novel support for α-amylase immobilization: a comprehensive study. Colloids Surf. B Biointerfaces 162, 90–97 (2018). https://doi.org/10.1016/j.colsurfb.2017.11.045
K. Hisamatsu et al., α-Amylase immobilization capacities of mesoporous silicas with different morphologies and surface properties. J. Porous Mater. 19(1), 95–102 (2012). https://doi.org/10.1007/s10934-011-9452-2
R. Das, M. Talat, O.N. Srivastava, A.M. Kayastha, Covalent immobilization of peanut β-amylase for producing industrial nano-biocatalysts: a comparative study of kinetics, stability and reusability of the immobilized enzyme. Food Chem. 245, 488–499 (2018). https://doi.org/10.1016/j.foodchem.2017.10.092
P.C. Ashly, M.J. Joseph, P.V. Mohanan, Activity of diastase α-amylase immobilized on polyanilines (PANIs). Food Chem. 127(4), 1808–1813 (2011). https://doi.org/10.1016/j.foodchem.2011.02.068
K. Singh, G. Srivastava, M. Talat, O.N. Srivastava, A.M. Kayastha, α-Amylase immobilization onto functionalized graphene nanosheets as scaffolds: its characterization, kinetics and potential applications in starch based industries. Biochem. Biophys. Rep. 3, 18–25 (2015). https://doi.org/10.1016/j.bbrep.2015.07.002
M. Dhiman, S. Rana, M. Singh, J.K. Sharma, Magnetic studies of mixed Mg–Mn ferrite suitable for biomedical applications. Integr. Ferroelectr. 202(1), 29–38 (2019). https://doi.org/10.1080/10584587.2019.1674821
Z. Emami Bistgani, S. A. Siadat, A. Bakhshandeh, A. Ghasemi Pirbalouti, M. Hashemi, Interactive effects of drought stress and chitosan application on physiological characteristics and essential oil yield of Thymus daenensis Celak. Crop J. 5(5), 407–415 (2017). https://doi.org/10.1016/j.cj.2017.04.003.
P. Bernfeld, Amylases, alpha and beta. Methods Enzymol. I I(540), 149–158 (1955)
H. Lineweaver, D. Burk, The determination of enzyme dissociation constants. J. Am. Chem. Soc. 56(3), 658–666 (1934). https://doi.org/10.1021/ja01318a036
M. Soleimani, A. Khani, K. Najafzadeh, α-Amylase immobilization on the silica nanoparticles for cleaning performance towards starch soils in laundry detergents. J. Mol. Catal. B Enzym. 74(1–2), 1–5 (2012). https://doi.org/10.1016/j.molcatb.2011.07.011
M. Defaei, A. Taheri-Kafrani, M. Miroliaei, P. Yaghmaei, Improvement of stability and reusability of α-amylase immobilized on naringin functionalized magnetic nanoparticles: A robust nanobiocatalyst. Int. J. Biol. Macromol. 113(2017), 354–360 (2018). https://doi.org/10.1016/j.ijbiomac.2018.02.147
S. Rana, A. Sharma, A. Kumar, S.S. Kanwar, M. Singh, Utility of silane-modified magnesium-based magnetic nanoparticles for efficient immobilization of bacillus thermoamylovorans lipase. Appl. Biochem. Biotechnol. 192(3), 1029–1043 (2020). https://doi.org/10.1007/s12010-020-03379-7
A. N. Ananth, A. N. Ananth, S. P. Jose, S. Umapathy, T. Mathavan. Influence of α-amylase template concentration on systematic entrapment of highly stable and monodispersed colloidal gold nanoparticles. AIP Adv. 6(1) (2016). https://doi.org/10.1063/1.4939849.
M. V. Nikolic, M. D. Lukovic. Influence of SnO2 content on the humidity dependent impedance of the MgFe2O4-Fe2O3-SnO2 compound. Chemosensors 8(2). https://doi.org/10.3390/CHEMOSENSORS8020039.
M. Defaei, A. Taheri-Kafrani, M. Miroliaei, P. Yaghmaei, Alpha-amylase immobilized on polycaprolactone-grafted magnetic nanoparticles: improving stability and reusability. J. Chem. Technol. Biotechnol. 95(8), 2243–2250 (2020). https://doi.org/10.1002/jctb.6412
Z.M. Milani, R. Jalal, E.K. Goharshadi, Carbodiimide for covalent α-amylase immobilization onto magnetic nanoparticles. Int. J. Nanosci. 16(5–6), 1–8 (2017). https://doi.org/10.1142/S0219581X17500156
M. Talebi, S. Vaezifar, F. Jafary, M. Fazilati, S. Motamedi, Stability improvement of immobilized α-amylase using nano pore zeolite. Iran. J. Biotechnol. 14(1), 33–38 (2016). https://doi.org/10.15171/ijb.1261
A. Sharma, T. Sharma, K.R. Meena, A. Kumar, S.S. Kanwar, High throughput synthesis of ethyl pyruvate by employing superparamagnetic iron nanoparticles-bound esterase. Process Biochem. 71(April), 109–117 (2018). https://doi.org/10.1016/j.procbio.2018.05.004
N. Antony, S. Balachandran, P.V. Mohanan, Immobilization of diastase α-amylase on nano zinc oxide. Food Chem. 211, 624–630 (2016). https://doi.org/10.1016/j.foodchem.2016.05.049
F. Eslamipour, P. Hejazi, Evaluating effective factors on the activity and loading of immobilized α-amylase onto magnetic nanoparticles using a response surface-desirability approach. RSC Adv. 6(24), 20187–20197 (2016). https://doi.org/10.1039/c5ra26140f
K. Salem et al., Enzyme storage and recycling: Nanoassemblies of α-amylase and xylanase immobilized on biomimetic magnetic nanoparticles. ACS Sustain. Chem. Eng. 9(11), 4054–4063 (2021). https://doi.org/10.1021/acssuschemeng.0c08300
Funding
No funding was provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rana, S., Sharma, A., Batoo, K.M. et al. Fabrication and characteristic studies of doped metal oxide-silane magnetic nanocomposite for enhancement of stability of α-amylase. Appl. Phys. A 128, 729 (2022). https://doi.org/10.1007/s00339-022-05882-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-05882-6