Abstract
Sporothrix schenckii (S. schenckii), a ubiquitous thermally dimorphic fungus, is the etiological agent of sporotrichosis, affecting immunocompromised and immunocompetent individuals. Despite current antifungal regimens, sporotrichosis results in prolonged treatment and significant mortality rates in the immunosuppressed population. The innate immune system forms the host's first and primary line of defense against S. schenckii, which has a bi-layered cell wall structure. Many components act as pathogen-associated molecular patterns (PAMPs) in pathogen-host interactions. PAMPs are recognized by pattern recognition receptors (PRRs) such as toll-like receptors, C-type lectin receptors, and complement receptors, triggering innate immune cells such as neutrophils, macrophages, and dendritic cells to phagocytize or produce mediators, contributing to S. schenckii elimination. The ultrastructure of S. schenckii and pathogen-host interactions, including PRRs and innate immune cells, are summarized in this review, promoting a better understanding of the innate immune response to S. schenckii and aiding in the development of protective and therapeutic strategies to combat sporotrichosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus of Sporothrix with worldwide distribution is a typical dimorphic fungus, leading to a chronic subcutaneous infectious fungal disease named sporotrichosis [1]. As genotyping technology develops, 53 species of Sporothrix have been identified, and only seven members were found to cause deleterious effects on humans [2]. S. schenckii, S. brasiliensis, and S. globosa are the primary pathogens, and S. schenckii is the most studied species of a clinical clade [2]. Like other dimorphic fungi, S. schenckii grows as mycelia in a saprotrophic environment or culturing at 25 °C, whereas in host tissues or culturing at 37 °C, it undergoes dimorphic transition and division into pathogenic yeast cells [3]. Furthermore, the pathogenic yeast cells induce a poor pro-inflammatory compared with mycelia, suggesting an enhanced survival for S. schenckii [4].
The first case of sporotrichosis in the world was reported by Benjamin R.Schenck in 1898 [2]. However, sporotrichosis has now been considered an emergent health problem, owing to the increasing number of reports of Sporothrix infection in immunocompromised patients [5]. In addition, sporotrichosis has been classified as one of the Neglected Tropical Diseases by The World Health Organization [6]. Sporotrichosis occurs due to traumatic inoculation of materials contaminated with Sporothrix, causing papules, nodules, plaques, ulcers, granulomas, and crusting of the face and limbs [2, 7]. Meanwhile, primary lung disease can occur through the inhalation of spores, causing pulmonary sporotrichosis [8]. Occurrences of severe clinical forms of sporotrichosis such as disseminated [9], pulmonary [8], intravascular granuloma [10], ocular [11], and osteoarticular [12] sporotrichosis were described, especially among immunocompromised individuals [13]. Moreover, increasing evidence indicates that disseminated and extracutaneous forms of sporotrichosis occur in immunocompetent individuals [14,15,16,17,18,19,20]. Therefore, oral administration of antifungal agents must be maintained until the clinical cure is reached, which usually takes several months [7]. Besides, current antifungal drugs are expensive and usually invalid due to the development of drug toxicity and fungal resistance [21]. Despite treatment with antifungal drugs, patients with disseminated and extracutaneous sporotrichosis continue to have high morbidity and mortality. Among them, the mortality rates of disseminated, osteoarticular, and pulmonary sporotrichosis are 21.9% [22], 22% [12], and 42.9% [23], respectively.
The innate immune system forms the host's first line of defense against pathogens [24]. Innate immune cells such as macrophages play a vital role and are likely the primary effector cell in killing and ultimately eliminating Sporothrix infection [25]. More adequate approaches to treating sporotrichosis may necessitate the incorporation of immunomodulatory therapies, as the compromised status of the immune system prevents the host from responding optimally to conventional therapy. A new strategy named the Trained Immunity-based Vaccine indicates that immunomodulation via PRR ligands in innate immune cells could generate broad-spectrum anti-infectious formulations [26]. Therefore, effective disease control requires the engagement of host receptors by pathogen-derived PAMPs to stimulate the immune response. In this review, the ultrastructure of yeast of S. schenckii is reviewed, contributing to recognition of PAMPs on the cell wall. It will also highlights the role of the innate cellular immune members and the arsenal of PRRs utilized by these cells to detect S. schenckii, contributing to a better understanding of the innate immune in response to S. schenckii and assisting in developing protective and therapeutic strategies against sporotrichosis.
The Cell Wall Components of Sporothrix
The fungal cell wall is the first point of contact between the host and the pathogen, which also contributes to establishing communication with the environment and the host [27]. Innate immunity is triggered by the interaction between the host cell surface PRRs and the pathogen-associated molecular patterns (PAMPs) from fungi [28]. PAMPs are conserved molecular structures on the pathogen surface, whereas PRRs are conserved transmembrane or soluble receptors on host [28]. Therefore, many cell wall components of S. schenckii were regarded as PAMPs.
The Cell Wall Structure of S. schenckii
The ultrastructural data reveal that S. schenckii has a bi-layered cell wall structure which includes an external microfibrillar layer and an inner electron-dense layer (Fig. 1) [29].
A schematic diagram for pathogenic yeast cells of Sporothrix in host tissues or culturing at 37 °C. S. schenckii has a bi-layered cell wall structure, including external microfibrillar and inner electron-dense layers. The outer layer is composed of peptidorhamnomanna containing peptide, mannose and rhamnose. Meanwhile, the chitin, β1,3-glucan, β1,4-glucan and β1,6-glucan constitute the inner layer of cell wall. Melanin granules distribute on the external cell wall, and some are released into the peripheral space separated from the cell wall. Extracellular vesicles with bi-layered biological structures could be secreted by Sporothrix yeast cells. (Created with BioRender.com)
The outer layer, i.e., fibrils, is composed of peptidorhamnomannan, a complex of molecules with a wide molecular weight range, containing 16% of peptide, 51% of mannose and 33% of rhamnose [30]. Chitin and β1,3-glucan are covered by mannan and glycoprotein on the cell wall of S. schenckii sensu stricto [31,32,33]. Further studies demonstrate that β1,3-glucan, β1,4-glucan, β1,6-glucan and chitin constitute the inner cell wall layer [29, 32, 34].
Interestingly, glycogen α-particles could be observed in the cytoplasm adjacent to the cell wall and the plasma membrane and were localized at budding poles of yeast cells, indicating that it serves as a source of glucose, whereas it vanished after 7and/or 10 days in culture [29]. Notably, α-glucan found in other pathogenic species such as Scedosporium, Pseudallescheria and Aspergillus complex was not present on the cell surfaces of Sporothrix.spp [29, 35].
Melanin is vital to the survival of fungi and can keep them from being phagocytosed [36, 37]. Melanin granules distribute on the external cell wall, and some melanin granules are released into the peripheral space, separate from the cell wall [37, 38]. The cell wall thickness of S. schenckii correlates with the presence of melanin. S. schenckii with melanin has a thicker cell wall than S. schenckii without melanin [39]. Furthermore, the yeast phases of Sporothrix shows a reduced production of melanin compared with conidia [37].
Extracellular vesicles (EVs), bi-layered biological structures that communicate between host cells and fungi cells, are secreted from Sporothrix yeast cells [40, 41]. The phagocytosis index of macrophages increased after co-culture with extracellular vesicles, suggesting that EVs play a protective role during Sporothrix infections [41].
Adhesion to extracellular matrix proteins is crucial to the invasion of S. schenckii, and cell surface glycoconjugates can bind to extracellular protein fibronectin via their carbohydrate or peptide moieties [42]. S. schenckii has 37–92 kDa of fibronectin on the surface, which contributes to the adhesion to host cells [43]. Surprisingly, research shows that S. schenckii can molt sheets of intact cell wall layers and deliver into the extracellular milieu, suggesting that it can cause antigenemia or inflammation far from the original site, which maybe the immunological basis of the disseminated type [29]. However, the mechanism of cell wall-shedding remains unknown. In general, the biofilm matrix contains polysaccharides, lipids, proteins, and nucleic acids, providing the stability of biofilms [44]. Many fungi such as Candida, Cryptococcus, and Aspergillus can produce biofilms that can decrease the effectiveness of antifungal therapies [45,46,47,48]. Like other fungi, S. schenckii, S. globosa and S. brasiliensis have the same ability to form biofilm in the filamentous phase, leading to a less susceptible to antifungal agent [49, 50]. However, it remains to explore whether yeast has the same biofilm.
The Cell Wall Related to Virulence
The proportion of cell wall components can affect the recognition of host cell PRRs, thus affecting virulence of Sporothrix. S. schenckii, S. brasiliensis, and S. globosa have a similar cell wall structure [31]. What's more, the similarity of genomes between S. brasiliensis and S. schenckii is 97.5% [51], while S.brasiliens is the most virulent species, followed by S. schenckii, and S. globosa is the least virulent species [52]. Cell wall proteins, kinases and heat shock proteins, extracellular and intracellular proteinases, melanin, extracellular vesicles, lipids, and biofilm were recognized as major virulent factors of S. schenckii [53].
The components of out cell wall contribute to the virulence of Sporothrix, while the exposure of inner cell wall contribute to the protective effect in host. S. schenckii has a thinner cell wall than S. brasiliensis, with lower chitin and rhamnose contents [29]. While the latest study indicated that the increased chitin in the cell wall reduces virulence [54]. Rhamnose is a vital virulence factor for S. schenckii in the G.mellonella model of infection [32]. Furthermore, the ratio of rhamnose-to-β-glucan is proportional to the virulence, while the length of rhamnomannan is inversely proportional to the virulence [55]. The carbon or nitrogen limitation of the culture medium increases β1,3-glucan exposure at the cell surface and decreases the virulence of S. schenckii and S. brasiliensis, except for S. globosa [56].
Host Recognition of Sporothrix
PRRs, expressed on host cells, are vital components of innate immunity. In addition, PRRs can recognize and initiate an inflammatory response to invading microorganisms [57]. TLRs, CLRs, NOD-like receptors (NLRs), and RIG-I-like receptors (RLRs) are four receptor families that contribute to fungi recognition [57], especially TLRs and CLRs [58]. Fungal PAMPs contain cell wall components, such as mannan, chitin, and rhamnose. Besides, S. schenckii contain various potentially antigenic molecular components (Fig. 1).
Toll-Like Receptors
TLRs were first identified in Drosophila melanogaster [59]. TLRs are expressed in innate immune cells such as DCs and macrophages. There are two subfamilies of TLRs based on their localization, cell surface TLRs and intracellular TLRs. TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are localized on the cell surface, while TLR3, TLR7, TLR8, TLR9, TLR11, TLR12, and TLR13 are localized in the endosome [60]. TLR-2 and TLR-4 are two of the most intensively studied receptors among TLRs.
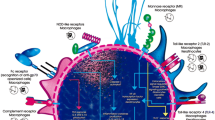
TLR-2 plays a vital role in triggering an inflammatory response to eradicate S. schenckii. Macrophages from C57BL/6 TLR-2 knock-out (KO) mice significantly reduced the percentage of macrophages with internalized yeasts and reduced the release of TNF-α, IL-1β, IL-12, NO, and IL-10 (Table 1, Fig. 2) [61, 62]. After human peripheral blood mononuclear cells (PBMCs) were pre-incubated with anti-TLR2, TNF-α, IL-1β, IL-6, and IL-10 were diminished by stimulating S. schenckii yeast cell [31]. While IL-17 liberation is independent of TLR-2, TLR-2 absence increases the release of IL-17 and TGF-β and develops Th17 response [62]. However, research reveals that an optimal fungal clearance depends on an intact Th17 response since IL-23 decrease is accompanied by fungal burden increase [63].
SolAg and LipAg can bind to TLR2, releasing TNF-α, IL-1β, IL-12, IL-6, IL-10 and NO; TLR4 recognizes lipid extraction and releases TNF-α, IL-1β, IL-10 and NO; β1,3-glucan is recognized by Dectin-1 and results in an elevated secretion of IL-10, NO, TNF-α and IL-1β; PRM binds to CR3, leading to a decreased release of IL-1β; Alkali-insoluble fraction of S. schenckii yeasts binds to NLRP3 leading to a decreased release of IL-1β, IL-18, and IL-17, while IFN-γ release was unaffected. MR and PTX3 have an impact on phagocytosis but with unknown PAMPs. UN = unknown Sporothrix ligand for the receptor. (Created with BioRender.com)
TLR-4 is also crucial for developing inflammatory responses during S. schenckii infection [64]. Cell wall rhamnose is required for S. schenckii virulence and rhamnose-based oligosaccharides are ligands that interact with TLR4 [32]. Both pro-inflammatory (NO, TNF-α) and anti-inflammatory mediators (IL-10) are reduced in TLR4-deficient peritoneal macrophages after coculturing with S. schenckii [65]. Increased release of H2O2, IL-1β, IL-6, and TGF-β was found during S. schenckii infection on macrophages from TLR-4 deficient mice, reducing the inflammatory response [64]. Interestingly, it is CD80, CD86, and CD40 but not TLR-4 that is highly expressed on S. schenckii cell wall proteins (SsCWP)-stimulated bone marrow-derived dendritic cells (BMDCs) [66]. Furthermore, studies indicate that cytokine released by Human PBMCs pre-incubated with anti-TLR4 remained unchanged after being stimulated by S. schenckii yeast cells, suggesting a modest participation of TLR4 [31], The role of other TLRs, such as TLR3, and TLR9, which localizes in the endosome, have not been elucidated. In summary, TLRs contribute to S. schenckii recognition and elimination. Due to the potentially beneficial effects of TLRs, future research may focus on developing drugs that act as TLR agonists or ligands as potential adjuvants for vaccine formulations.
C-TYPE Lectin Receptors
CLRs, including transmembrane receptors on immune cells and soluble forms in serum, can recognize carbohydrate polymers such as mannan, glucans and chitins expressed on the fungal cell wall [67]. Dectin-1, also known as a β-glucan receptor, is the primary fungal-1,3-glucan receptor on macrophages and belongs to CLRs family, which also plays a significant role in fungal elimination removal and induction of essential receptors for cytokine production during Sporothrix infection [31, 68, 69]. Peritoneal macrophages could recognize β1,3-glucan by Dectin-1, resulting in elevated secretions of IL-10, NO, TNF-α and IL-1β [70]. In vitro, the release of TNF-α, IL-1β, IL-6, and IL-10 was decreased by PBMCs, where Dectin-1 was blocked [31]. A Dectin-1 antibody-mediated blockade assay also reduced cytokine production in infected and non-infected mice [70]. There is a study reveals that the dog with sporotrichosis which is prior resistant to itraconazole results in complete elimination of the fungus with itraconazole combined with β1,3-glucan. While the masked β1,3-glucan results a weaker recognition of fungi by innate immune cells, thus aiding in the fungi to evade from innate immune clearance [71]. β1,3-glucan can act as an immunomodulator since it can be recognized by Dectin-1 and results in the activation of host protective immunity against S. schenckii infection.
MR had a minor contribution to the binding and phagocytosis of conidia of S. schenckii compared with yeast [4]. The production of pro-inflammatory cytokines released by Human PBMCs was insensitive to the blockage of MR after coculture with S. schenckii yeast cells [31]. Mannose-binding lectin (MBL) and MBL-associated serine protease-2 (MASP-2) are essential proteins in the lectin pathway of the immune system [72], and decreased levels of MBL and MASP-2 have been reported in serum samples from sporotrichosis patients compared to controls [72].
Nucleotide-Binding Oligomerization Domain (NOD)-Like Receptors
The cytoplasm contains nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), which are expressed in macrophages and dendritic cells [73]. The cytosolic NLRs are crucial regulators of inflammation and responsible for IL-1β and IL-18 maturation, whose functions depend on the caspase-1 activation that can trigger a response to microbial infection and cellular damage [74]. NLRs are present in multiprotein complexes called inflammasomes, and NOD-like receptor family pyrin domain-containing 3 (NLRP3) is the most studied [75]. NLRP3 has been implicated in a wide range of diseases, including fungal diseases [76,77,78,79], and various stimuli, including danger-associated molecular patterns (DAMPs) and PAMPs, contribute to NLRP3 inflammasome activation [80].
Recently, a study revealed that NLRP3 inflammasome linked the innate recognition of S. schenckii to the adaptive immune response and triggered a protective response to the host during S. schenckii infection. On the other hand, KO mice (NLRP3−/−, ASC−/−, and caspase-1−/−) were more susceptible to infection than the wild-type (WT). Furthermore, NLRP3 inflammasome could promote the release of IL-1β, IL-18, and IL-17 by macrophage-splenocyte coculture in vitro, leading to the elimination of fungi, while eradicating IFN-γ was unaffected [81]. Most recently, it has been reported that neodymium-doped yttrium aluminum garnet (Nd:YAG) 1,064-nm is effective in treating sporotrichosis by inducing apoptosis and pyroptosis via NLRP3/caspase-1 pathway [82].
Complement and Other Soluble Mediators
Preimmune human serum opsonization plays a critical role in optimal phagocytosis of S. schenckii. Internalization of yeast cells in macrophages significantly decreased in heat-inactivated serum, suggesting the role of complement components in yeast uptake [4]. The peptidorhamnomannan(PRM) is a new PAMP that is the component of cell walls of S. schenckii and S. brasiliensis, and it showed a direct interaction with the complement receptor-3(CR3). IL-1β secretion by human monocyte-derived macrophages (hMDMs) decreased when CR3 was blocked [83]. Pentraxin 3 (PTX3) is a soluble receptor that can enhance pathogen clearance by promoting C1q deposition. However, the mechanism remains to be further elucidated (Table 1, Fig. 2) [83, 84].
Effector Functions of Innate Immune Cells
Macrophages
Macrophages are the primary host protection cells, which can regulate inflammatory responses by eliminating invading fungal pathogens through phagocytosis [64]. Macrophages can adopt various phenotypes and are divided into "classic" and "alternatively" activated populations, known as M1 and M2 macrophages, respectively. In general, M1 can promote to tissue injury and result in pathogen eradication, while M2 cells contribute to tissue mimicry and repair, leading to infection persistence [85].
Macrophages undergo a phenotypic switch during the infection of S. schenckii. IFN-γ and IL-12 were increased in the murine model of disseminated sporotrichosis in the first two weeks, while the predominant IL-4 was presented after the fifth week [86], suggesting a classical and alternative response of macrophage activation in the early and late phase of infection, respectively. After being challenged with cell wall peptide-polysaccharide, the peritoneal exudate cells showed a predominance of M1 macrophage population with an increased NO and IL-12 production during the second week of infection, while a predominance of M2 macrophage population with an increased release of IL-10, TGF-β, and Arg-1 were present during the sixth and eighth weeks after infection [87]. S. schenckii infection increased the expression of disabled homolog 2 (DAB2) through JNK/c-JUN pathway and revealed a mixed M1/M2-like type of gene expression in bone marrow-derived macrophages (BMDMs), accompanied by increased of TNF-α, IL-10, and Mgl-1 and reduced IL-1β, IL-6, and Arg-1 [88].
Cryptococcus gattii and C.neoformans may bias the immune response toward Th2 response, helping its escape from the phagosome and resulting in disease progression [47]. Similarly, M2 macrophage populations may contribute to immune evasion, thus promoting S. schenckii infection [87]. Ingested conidia could survive and transform into the yeast cell in the macrophage with a complete structure [89,90,91]. S. schenckii can reverse ergosterol peroxide to ergosterol and dampen the effects on reactive oxygen species (ROS) during phagocytosis [92, 93]. This is advantageous to S. schenckii as an immune evasion strategy, which may be the reason for recurrent and disseminative sporotrichosis.
NO production by macrophages is a double-edged sword. It not only contributed to pathogen killing but also inhibited TNF-α release, lymphoproliferation, and MHC-2 expression, with immunosuppression consequences [86]. In addition, research proved that NO overproduction could suppress Th1 responses against S. schenckii and cause infection susceptibility [86, 94].
Cell wall components can suppress or promote macrophage phagocytosis. Melanin, a well-recognized virulence factor of S. schenckii complex, can inhibit the innate immune functions of macrophages, such as phagocytosis and killing. MHC class II, CD86 CIITA, and PIV expressions in macrophages were inhibited when infected with a black S. globosa strain (MEL +) [37]. TLR2 and TLR4 receptors and the release of TNF-α and IL-6 in THP-1 macrophages were suppressed after incubation with melanin [36]. The lipid compound from the cell wall was found to inhibit the phagocytic process of macrophages while promoting the release of NO and TNF-α in macrophage culture [95].
Chitin-rich heteroglycan extracted from S. schenckii sensu stricto promoted fungus phagocytosis by macrophages and upregulated TNF-α expression at 24 h and IL-12 expression at 72 h after incubation [90]. Extracellular vesicles (EVs) play a crucial role in the biological process. EVs increase the phagocytic activity of macrophages and result in decreased colony-forming units [41]. In contrast, more immunoreactive components exist in EVs from S. schenckii compared with S. brasiliensis [40]. EVs have shed new light on their great potential as a therapeutic tool in modulating the immune response [40].
Dendritic Cells
DCs, known as antigen-presenting cells (APC), play an essential sentinel function by taking up antigen or infectious agents and transporting them to the lymph node for T cell recognition and the priming of immune responses [96]. In addition, DCs can sense fungi in a morphotype-specific manner and activate protective and non-protective Th cells as well as regulatory T cells, thus affecting the outcome of the infections [97].
BMDCs can phagocytize the S. schenckii. The expressions of CD40, CD80, and CD86 on the surface of S. schenckii-pulsed mouse bone marrow-derived DC increased indicating that BMDCs undergo the maturation program after stimulation with S. schenckii. Then, the secretion of IL-12 increased, with subsequent activation of Th1-prone immune responses [98]. S. schenckii of cutaneous origin is much more potent in activating DCs and induces Th1-prone immune responses, while S. schenckii from visceral are only weak activators for DCs with minimal induction of IFN-γ and positively induce Th2-prone immune responses [99]. The life of S. schenckii and its exoantigen activated BMDCs and made them capable of triggering T cell responses, and, surprisingly, the exoantigen induces an inflammatory Th17 response rather than a Th1 response [100]. SsCWP-stimulated BMDCs can induce a Th1-prone cytokine such as IFN-γ and IL-2 when cocultured with splenocytes [66].
Neutrophils
Neutrophils are the most abundant innate immunity cells in the blood and can rapidly migrate to the site of infection [101, 102]. Neutrophils represent the primary inflammatory cells associated with sporotrichosis lesions [103]. An in vitro study showed that human polymorphonuclear leukocytes (PMNLs) could phagocyte and kill yeast-phase cells of S. schenckii in the presence of 10% unheated serum [104], while other in vitro studies revealed that human PMNs could kill S. schenckii hyphae, and yeasts are resistant to be killed by neutrophils [105]. PMNs show a high capacity to bind or ingest S. schenckii cells, release intracellular content, and establish a pro-inflammatory environment. Meanwhile, the interaction of human PMNs with S. schenckii cells cannot affect fungal viability and S. schenckii cells can undergo dimorphic switching within PMNs [106]. Exogenous local hyperthermia at 41 °C could serve as an effective therapy for fixed cutaneous sporotrichosis, while this ability does not involve the formation of neutrophil extracellular traps (NETs) [107]. Administration of potassium iodide to regular volunteers does not increase the killing of S. schenckii by their neutrophils or monocytes [108]. Therefore, the role of neutrophils during the protective immune responses against S. schenckii is complex.
Natural Killer Cells
Natural killer (NK) cells are lymphocytes of the innate immune system, playing a critical role in the initial defense against various pathogens, including fungi [109, 110]. NK cells expand in the spleen and mature more after S. schenckii infection, and CD62L and KLRG1 are upregulated on NK cells. Furthermore, the fungal load in the spleens increased more than eightfold in NK cell-depleted infected mice, accompanied by an augmented systemic production of inflammatory cytokines of TNF-α, IFN-γ, and IL-6 [111], suggesting an indispensable role of NK cells against S. schenckii. Recently, researchers have used expanded NK cells as therapy for invasive Aspergillosis resulting in a significantly reduced fungal burden in the mice model [112], providing a reference for treating sporotrichosis.
Mast Cells
Mast cells (MCs) are well recognized for their complex role in fungal infections and their critical role in allergic diseases [113]. Five PRRs have been documented in MCs: TLRs, CLRs, nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), a retinoic acid-inducible gene I (RIG-I) like receptors (RLRs), and absent-in-melanoma (AIM)-like receptors (ALRs) [114], while TLRs and CLRs are the most reported PRRs in antifungal host defense [113]. Positive or negative immunoregulatory cells can function depending on the situation [115].
MCs can improve immunity by triggering degranulation and the release of cytokines, while it seems that MCs act as negative immunoregulators in S. schenckii infection [113]. MC-deficient mice developed fewer skin lesions than WT mice after infection with S. schenckii, significantly decreasing the fungal burden [116]. The severity of cutaneous lesion of sporotrichosis was significantly reduced after depleting peritoneal mast cells [117]. Furthermore, the severity of S. schenckii infection in humans correlates with IL-6 and TNF levels. It has been demonstrated that MCs exacerbate mouse and human skin infection by releasing the pro-inflammatory cytokines TNF and IL-6 rather than degranulation [116, 117]. Furthermore, when the mast cells were activated by the yeast cells of S. schenckii, TNF-α and IL-6 could be induced by the activation of the extracellular signal-regulated kinase(ERK) signaling pathway [118].
Epithelial Cells
The epithelial lining of the skin is a protective barrier against infection [119]. The fungus's adhesion to host tissue has been identified as a critical step in colonization and invasion, including S. schenckii [120]. S. schenckii yeast cells can adhere to epithelial cells via fungal surface glycoprotein with glucose residue and mannose [121]. Antimicrobial peptides (AMPs) are released by epithelial cells and play a vital role in the innate immune system. AMPs contribute to pathogen elimination, including fungi such as Cryptococcus neoformans [59, 122]. Recently, AMP ToAP2D has been revealed ability to inhibit the growth of S. globosa and trigger apoptosis, suggesting a potential drug for treatment [123]. However, more AMPs with therapeutic effects require further research.
Epidermal keratinocytes can participate in the cutaneous inflammatory response to invading pathogens by producing pro-inflammatory cytokines and chemokines that recruit and activate neutrophils and macrophages to the infection site. Upon S. schenckii cells were implanted into the epidermis and dermis, keratinocytes could release IL-6 and IL-8 via TLR-2, TLR-4, and NF-κB signaling pathways [124]. Recently, it has been demonstrated that MR, CR3, TRL2, and TLR6 on keretinocytes contribute to S.schencki recognition, except TLR4. A pro-inflammatory environment including cytokines, chemokines, and growth factors was created to recruit other immune cells to the infection site. Besides, keratinocytes infected with S.schencki change the actin cytoskeleton to facilitate S.schencki internalization (Fig. 3) [125].
Innate immune cells involved in Sporothrix-host interactions. Neutrophils are the first cells to migrate to the site of infection and can phagocytose S. schenckii as well as chemotactic for other immune cells. Macrophages play a central role in regulating the disease outcome, adopting to M1 phenotype, which promotes the clearance of S. schenckii and M2 phenotype, contributing to tissue remodel and repair. Dendritic cells not only phagocytose S. schenckii but also act as a bridge between innate and adaptive immunity. Additionally, natural killer cells, epidermal keratinocytes and epithelial cells contribute to S. schenckii clearance. Meanwhile, as in other diseases, MCs act as negative immunoregulators in S. schenckii infection. (Created with BioRender.com)
Summary and Future Prospects
Emerging data on the ultrastructure of S. schenckii contributes to a better understanding of sporotrichosis. Many components of the cell wall of S. schenckii could act as PAMPS and play a vital role in the interaction between pathogen and host. PRRs like TLRs, CLRs, NLRs, and complements are vital for recognizing PAMPs and trigger a cytokine response and phagocyte recruitment to the clearance of Sporothrix. As PRRs have been characterized as a protective role against sporotrichosis, further exploration of the interactions and signaling pathways is required.
The interactions between Sporothrix and innate immune cells play a critical role in disease progression in the host. Neutrophils are the first cells to migrate to the site of infection and can phagocytose S. schenckii and chemotactic for other immune cells. Macrophages play a central role in regulating the disease outcome, adopting the M1 phenotype, which promotes the clearance of S. schenckii and M2 phenotype, contributing to tissue remodel and repair. Dendritic cells not only phagocytose S. schenckii but also act as a bridge between innate and adaptive immunity. Additionally, natural killer cells, epidermal keratinocytes, and epithelial cells contribute to the clearance of S. schenckii. While as in other diseases, MCs act as negative immunoregulators in S. schenckii infection [113]. However, the role of basophils cannot be excluded and warrants further investigation in sporotrichosis [116]. As the biological agents develop, monoclonal antibodies such as anti-TNF-α [126], anti-IL-17A [127], and anti-IL4/13 [128] have been increasingly used in dermatosis. Patients being treated with or prescribed biologics should be alerted since biological agents could suppress innate immunity.
This convincing evidence has emerged from studies suggesting a strong relationship between innate immune and S. schenckii. However, further fundamental mechanisms underlying innate immunity against S. schenckii remain to be elucidated. Despite adaptive immune systems, cells of the innate immune system appear to be able to gain memory characteristics after transient stimulation, resulting in an enhanced responsiveness to subsequent triggers and this phenomenon is called trained immunity [129]. The cell wall component of pathogen including LPS and β-glucan can induce trained immunity, resulting an activation of the innate immune system [130]. Therefore, we propose innovative therapeutic approaches targeting innate cells to combat sporotrichosis, especially for creating of future vaccines [21].
References
Chakrabarti A, Bonifaz A, Gutierrez-Galhardo MC, Mochizuki T, Li S. Global epidemiology of sporotrichosis. Med Mycol. 2015;53:3–14. https://doi.org/10.1093/mmy/myu062.
Rodrigues AM, Della Terra PP, Gremião ID, Pereira SA, Orofino-Costa R, de Camargo ZP. The threat of emerging and re-emerging pathogenic Sporothrix species. Mycopathologia. 2020;185:813–42. https://doi.org/10.1007/s11046-020-00425-0.
He D, Zhang X, Gao S, You H, Zhao Y, Wang L. Transcriptome analysis of dimorphic fungus Sporothrix schenckii exposed to temperature stress. Int Microbiol. 2021;24:25–35. https://doi.org/10.1007/s10123-020-00136-y.
Guzman-Beltran S, Perez-Torres A, Coronel-Cruz C, Torres-Guerrero H. Phagocytic receptors on macrophages distinguish between different Sporothrix schenckii morphotypes. Microbes Infect. 2012;14:1093–101. https://doi.org/10.1016/j.micinf.2012.06.001.
López-Romero E, Reyes-Montes MDR, Pérez-Torres A, Ruiz-Baca E, Villagómez-Castro JC, Mora-Montes HM, et al. Sporothrix schenckii complex and sporotrichosis, an emerging health problem. Future Microbiol. 2011;6:85–102. https://doi.org/10.2217/fmb.10.157.
Hay R, Denning DW, Bonifaz A, Queiroz-Telles F, Beer K, Bustamante B, et al. The diagnosis of fungal Neglected Tropical Diseases (Fungal NTDs) and the role of investigation and laboratory tests: an expert consensus report. Trop Med Infect Dis. 2019;4:122. https://doi.org/10.3390/tropicalmed4040122.
Orofino-Costa R, Macedo PMD, Rodrigues AM, Bernardes-Engemann AR. Sporotrichosis: an update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An Bras Dermatol. 2017;92:606–20. https://doi.org/10.1590/abd1806-4841.2017279.
Rojas FD, Fernández MS, Lucchelli JM, Lombardi D, Malet J, Vetrisano ME, et al. Cavitary pulmonary sporotrichosis: case report and literature review. Mycopathologia. 2017;182:1119–23. https://doi.org/10.1007/s11046-017-0197-6.
Bonifaz A, Tirado-Sánchez A, Paredes-Solís V, Cepeda-Valdés R, González GM, Treviño-Rangel RJ, et al. Cutaneous disseminated sporotrichosis: clinical experience of 24 cases. J Eur Acad Dermatol. 2018;32:e77–9. https://doi.org/10.1111/jdv.14533.
Nomoto Y, Higashi Y, Uchida Y, Fujii K, Ooka T, Kanekura T. Disseminated cutaneous sporotrichosis with intravascular granuloma. J Dermatol. 2022;49:e301–2. https://doi.org/10.1111/1346-8138.16429.
Ramírez-Soto MC, Tirado-Sánchez A, Bonifaz A. Ocular sporotrichosis. J Fungi. 2021;7:951. https://doi.org/10.3390/jof7110951.
Ramos V, Astacio GS, Do Valle ACF, de Macedo PM, Lyra MR, Almeida-Paes R, et al. Bone sporotrichosis: 41 cases from a reference hospital in Rio de Janeiro. Brazil Plos Neglect Trop D. 2021;15:e9250. https://doi.org/10.1371/journal.pntd.0009250.
Queiroz-Telles F, Buccheri R, Benard G. Sporotrichosis in immunocompromised hosts. J Fungi. 2019;5:8. https://doi.org/10.3390/jof5010008.
Queiroz-Telles F, Cognialli RC, Salvador GL, Moreira GA, Herkert PF, Hagen F. Cutaneous disseminated sporotrichosis in immunocompetent patient: case report and literature review. Med Mycol Case Rep. 2022;36:31–4. https://doi.org/10.1016/j.mmcr.2022.05.003.
Garcia BM, Bond AR, Barry AK, Steen AJ, LeBoit PE, Ashbaugh C, et al. Disseminated-cutaneous sporotrichosis in an immunocompetent adult. JAAD Case Rep. 2021;11:102–4. https://doi.org/10.1016/j.jdcr.2021.03.003.
Medeiros KB, Landeiro LG, Diniz LM, Falqueto A. Disseminated cutaneous sporotrichosis associated with ocular lesion in an immunocompetent patient. An Bras Dermatol. 2016;91:537–9. https://doi.org/10.1590/abd1806-4841.20164859.
Martínez-Herrera E, Arenas R, Hernández-Castro R, Frías-De-León MG, Rodríguez-Cerdeira C. Uncommon clinical presentations of sporotrichosis: a two-case report. Pathogens. 2021;10:1249. https://doi.org/10.3390/pathogens10101249.
Yap FB. Disseminated cutaneous sporotrichosis in an immunocompetent individual. Int J Infect Dis. 2011;15:e727–9. https://doi.org/10.1016/j.ijid.2011.05.005.
Sendrasoa FA, Ranaivo IM, Sata M, Razanakoto NH, Andrianarison M, Ratovonjanahary V, et al. Osteoarticular sporotrichosis in an immunocompetent patient. Med Mycol Case Rep. 2021;32:50–2. https://doi.org/10.1016/j.mmcr.2021.03.007.
Do Monte Alves M, Pipolo Milan E, Da Silva-Rocha WP, De Sena S, Da Costa A, Araújo Maciel B, Cavalcante Vale PH, et al. Fatal pulmonary sporotrichosis caused by Sporothrix brasiliensis in Northeast Brazil. Plos Neglect Trop D. 2020;14:8141. https://doi.org/10.1371/journal.pntd.0008141.
Téllez-Martínez D, Batista-Duharte A, Portuondo DL, Carlos IZ. Prophylactic and therapeutic vaccines against sporotrichosis. feasibility and prospects. Microbes Infect. 2019;21:432–40. https://doi.org/10.1016/j.micinf.2019.05.003.
Fichman V, Freitas DFS, Do Valle ACF, de Souza RV, Curi ALL, Valete-Rosalino CM, et al. Severe sporotrichosis treated with Amphotericin B: a 20-year cohort study in an endemic area of zoonotic transmission. J Fungi. 2022;8:469. https://doi.org/10.3390/jof8050469.
Fichman V, Mota-Damasceno CG, Procópio-Azevedo AC, Almeida-Silva F, de Macedo PM, Medeiros DM, et al. Pulmonary sporotrichosis caused by Sporothrix brasiliensis: a 22-year, single-center, retrospective cohort study. J Fungi. 2022;8:536. https://doi.org/10.3390/jof8050536.
Kirkland TN, Fierer J. Innate immune receptors and defense against primary pathogenic fungi. Vaccines. 2020;8:303. https://doi.org/10.3390/vaccines8020303.
Ruiz-Baca E, Pérez-Torres A, Romo-Lozano Y, Cervantes-García D, Alba-Fierro CA, Ventura-Juárez J, et al. The role of macrophages in the host’s defense against Sporothrix schenckii. Pathogens. 2021;10:905. https://doi.org/10.3390/pathogens10070905.
Sánchez-Ramón S, Conejero L, Netea MG, Sancho D, Palomares Ó, Subiza JL. Trained immunity-based vaccines: a new paradigm for the development of broad-spectrum anti-infectious formulations. Front Immunol. 2018;9:2936. https://doi.org/10.3389/fimmu.2018.02936.
Gow NAR, Latge J, Munro CA. The fungal cell wall: structure, biosynthesis, and function. Microbiol Spectr. 2017;5. https://doi.org/10.1128/microbiolspec.FUNK-0035-2016.
Hatinguais R, Willment JA, Brown GD. PAMPs of the fungal cell wall and mammalian PRRs. Curr Top Microbiol. 2020;425:187–223. https://doi.org/10.1007/82_2020_201.
Lopes-Bezerra LM, Walker LA, Niño-Vega G, Mora-Montes HM, Neves GWP, Villalobos-Duno H, et al. Cell walls of the dimorphic fungal pathogens Sporothrix schenckii and Sporothrix brasiliensis exhibit bilaminate structures and sloughing of extensive and intact layers. Plos Neglect Trop D. 2018;12: e6169. https://doi.org/10.1371/journal.pntd.0006169.
Lloyd KO, Bitoon MA. Isolation and purification of a peptido-rhamnomannan from the yeast form of Sporothrix schenckii. structural and immunochemical studies. J Immunol. 1971;107:663–71.
Martínez-Álvarez JA, Pérez-García LA, Mellado-Mojica E, López MG, Martínez-Duncker I, Lópes-Bezerra LM, et al. Sporothrix schenckii sensu stricto and Sporothrix brasiliensis are differentially recognized by human peripheral blood mononuclear cells. Front Microbiol. 2017;8:843. https://doi.org/10.3389/fmicb.2017.00843.
Tamez-Castrellón AK, van der Beek SL, López-Ramírez LA, Martínez-Duncker I, Lozoya-Pérez NE, van Sorge NM, et al. Disruption of protein rhamnosylation affects the Sporothrix schenckii-host interaction. Cell Surf. 2021;7:100058. https://doi.org/10.1016/j.tcsw.2021.100058.
Erwig LP, Gow NAR. Interactions of fungal pathogens with phagocytes. Nat Rev Microbiol. 2016;14:163–76. https://doi.org/10.1038/nrmicro.2015.21.
Lozoya-Pérez NE, Casas-Flores S, de Almeida JF, Martínez-Álvarez JA, López-Ramírez LA, Pereira Jannuzzi G, et al. Silencing of OCH1 unveils the role of Sporothrix schenckii N -linked glycans during the host-fungus interaction. Infect Drug Resist. 2019;12:67–85. https://doi.org/10.2147/IDR.S185037.
Höft MA, Hoving JC, Brown GD. Signaling C-type lectin receptors in antifungal immunity. Curr Top Microbiol. 2020;429:63–101. https://doi.org/10.1007/82_2020_224.
Guan M, Yao L, Zhen Y, Song Y, Cui Y, Li S. Melanin of Sporothrix globosa affects the function of THP-1 macrophages and modulates the expression of TLR2 and TLR4. Microb Pathogenesis. 2021;159: 105158. https://doi.org/10.1016/j.micpath.2021.105158.
Song Y, Yao L, Zhen Y, Cui Y, Zhong S, Liu Y, et al. Sporothrix globosa melanin inhibits antigen presentation by macrophages and enhances deep organ dissemination. Braz J Microbiol. 2021;52:19–31. https://doi.org/10.1007/s42770-020-00345-7.
Morris-Jones R, Youngchim S, Gomez BL, Aisen P, Hay RJ, Nosanchuk JD, et al. Synthesis of melanin-like pigments by Sporothrix schenckii in vitro and during mammalian infection. Infect Immun. 2003;71:4026–33. https://doi.org/10.1128/IAI.71.7.4026-4033.2003.
Madrid IM, Mattei AS, Soares MP, de Oliveira NM, Meireles MC. Ultrastructural study of the mycelial phase of clinical isolates of Sporothrix schenckii obtained from feline, canine and human cases of sporotrichosis. Braz J Microbiol. 2011;42:1147–50. https://doi.org/10.1590/S1517-838220110003000037.
Ikeda MAK, Ferreira KS. Extracellular vesicles from Sporothrix yeast cells. Curr Top Microbiol. 2021;432:35–44. https://doi.org/10.1007/978-3-030-83391-6_4.
Campos RMS, Jannuzzi GP, Ikeda MAK, de Almeida SR, Ferreira KS. Extracellular vesicles from Sporothrix brasiliensis yeast cells increases fungicidal activity in macrophages. Mycopathologia. 2021;186:807–18. https://doi.org/10.1007/s11046-021-00585-7.
Lima OC, Figueiredo CC, Previato JO, Mendonç Previato L, Morandi V, Lopes Bezerra LM. Involvement of fungal cell wall components in adhesion of Sporothrix schenckii to human fibronectin. Infect Immun. 2001;69:6874–80. https://doi.org/10.1128/IAI.69.11.6874-6880.2001.
Teixeira PAC, de Castro RA, Nascimento RC, Tronchin G, Pérez Torres A, Lazéra M, et al. Cell surface expression of adhesins for fibronectin correlates with virulence in Sporothrix schenckii. Microbiology. 2009;155:3730–8. https://doi.org/10.1099/mic.0.029439-0.
Flemming H, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623–33. https://doi.org/10.1038/nrmicro2415.
Mitchell KF, Zarnowski R, Andes DR. The extracellular matrix of fungal biofilms. Adv Exp Med Biol. 2016;931:21–35. https://doi.org/10.1007/5584_2016_6.
Tournu H, Van Dijck P. Candida biofilms and the host: models and new concepts for eradication. Int J Microbiol. 2012;2012:1–16. https://doi.org/10.1155/2012/845352.
Campuzano A, Wormley F. Innate Immunity against Cryptococcus: from recognition to elimination. J Fungi. 2018;4:33. https://doi.org/10.3390/jof4010033.
Kaur S, Singh S. Biofilm formation by Aspergillus fumigatus. Med Mycol. 2013;52:1–8. https://doi.org/10.3109/13693786.2013.819592.
Brilhante RSN, de Aguiar FRM, Da Silva MLQ, de Oliveira JS, de Camargo ZP, Rodrigues AM, et al. Antifungal susceptibility of Sporothrix schenckii complex biofilms. Med Mycol. 2018;56:297–306. https://doi.org/10.1093/mmy/myx043.
Sánchez-Herrera R, Flores-Villavicencio LL, Pichardo-Molina JL, Castruita-Domínguez JP, Aparicio-Fernández X, Sabanero López M, et al. Analysis of biofilm formation by Sporothrix schenckii. Med Mycol. 2021;59:31–40. https://doi.org/10.1093/mmy/myaa027.
Teixeira MM, de Almeida LG, Kubitschek-Barreira P, Alves FL, Kioshima ÉS, Abadio AK, et al. Comparative genomics of the major fungal agents of human and animal sporotrichosis: Sporothrix schenckii and Sporothrix brasiliensis. BMC Genomics. 2014;15:943. https://doi.org/10.1186/1471-2164-15-943.
Arrillaga-Moncrieff I, Capilla J, Mayayo E, Marimon R, Marine M, Genis J, et al. Different virulence levels of the species of Sporothrix in a murine model. Clin Microbiol Infec. 2009;15:651–5. https://doi.org/10.1111/j.1469-0691.2009.02824.x.
García-Carnero LC, Martínez-Álvarez JA. Virulence factors of Sporothrix schenckii. J Fungi. 2022;8:318. https://doi.org/10.3390/jof8030318.
Huang L, Zhang J, Song T, Yuan L, Zhou J, Yin H, et al. Antifungal curcumin promotes chitin accumulation associated with decreased virulence of Sporothrix schenckii. Int Immunopharmacol. 2016;34:263–70. https://doi.org/10.1016/j.intimp.2016.03.010.
Villalobos-Duno HL, Barreto LA, Alvarez-Aular Á, Mora-Montes HM, Lozoya-Pérez NE, Franco B, et al. Comparison of cell wall polysaccharide composition and structure between strains of Sporothrix schenckii and Sporothrix brasiliensis. Front Microbiol. 2021;12:726958. https://doi.org/10.3389/fmicb.2021.726958.
Lozoya-Pérez NE, Clavijo-Giraldo DM, Martínez-Duncker I, García-Carnero LC, López-Ramírez LA, Niño-Vega GA, et al. Influences of the culturing media in the virulence and cell wall of Sporothrix schenckii, Sporothrix brasiliensis, and Sporothrix globosa. J Fungi. 2020;6:323. https://doi.org/10.3390/jof6040323.
Plato A, Hardison SE, Brown GD. Pattern recognition receptors in antifungal immunity. Semin Immunopathol. 2015;37:97–106. https://doi.org/10.1007/s00281-014-0462-4.
Hernández-Chávez M, Pérez-García L, Niño-Vega G, Mora-Montes H. Fungal strategies to evade the host immune recognition. J Fungi. 2017;3:51. https://doi.org/10.3390/jof3040051.
Brown GD. Innate Antifungal immunity: the key role of phagocytes. Annu Rev Immunol. 2011;29:1–21. https://doi.org/10.1146/annurev-immunol-030409-101229.
Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461. https://doi.org/10.3389/fimmu.2014.00461.
Negrini TDC, Ferreira LS, Alegranci P, Arthur RA, Sundfeld PP, Maia DCG, et al. Role of TLR-2 and fungal surface antigens on innate immune response against Sporothrix schenckii. Immunol Invest. 2012;42:36–48. https://doi.org/10.3109/08820139.2012.719982.
Negrini TDC, Ferreira LS, Arthur RA, Alegranci P, Placeres MCP, Spolidorio LC, et al. Influence of TLR-2 in the immune response in the infection induced by fungus Sporothrix schenckii. Immunol Invest. 2013;43:370–90. https://doi.org/10.3109/08820139.2013.879174.
Ferreira LS, Gonçalves AC, Portuondo DL, Maia DCG, Placeres MCP, Batista-Duharte A, et al. Optimal clearance of Sporothrix schenckii requires an intact Th17 response in a mouse model of systemic infection. Immunobiology. 2015;220:985–92. https://doi.org/10.1016/j.imbio.2015.02.009.
Sassá MF, Ferreira LS, de Abreu Ribeiro LC, Carlos IZ. Immune response against Sporothrix schenckii in TLR-4-deficient Mice. Mycopathologia. 2012;174:21–30. https://doi.org/10.1007/s11046-012-9523-1.
Sassá MF, Saturi AET, Souza LF, de Abreu Ribeiro LC, Da Graça Sgarbi DB, Carlos IZ. Response of macrophage Toll-like receptor 4 to a Sporothrix schenckii lipid extract during experimental sporotrichosis. Immunology. 2009;128:301–9. https://doi.org/10.1111/j.1365-2567.2009.03118.x.
Quinello C, Souza Ferreira L, Picolli I, Loesch M, Portuondo D, Batista-Duharte A, et al. Sporothrix schenckii cell wall proteins-stimulated BMDCs are able to induce a Th1-prone cytokine profile in vitro. J Fungi. 2018;4:106. https://doi.org/10.3390/jof4030106.
Goyal S, Castrillón-Betancur JC, Klaile E, Slevogt H. The interaction of human pathogenic fungi with C-type lectin receptors. Front Immunol. 2018;9:1261. https://doi.org/10.3389/fimmu.2018.01261.
Kalia N, Singh J, Kaur M. The role of Dectin-1 in health and disease. Immunobiology. 2021;226:152071. https://doi.org/10.1016/j.imbio.2021.152071.
Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, et al. Dectin-1 is a major β-Glucan receptor on macrophages. J Exp Med. 2002;196:407–12. https://doi.org/10.1084/jem.20020470.
Jellmayer JA, Ferreira LS, Manente FA, Gonçalves AC, Polesi MC, Batista-Duharte A, et al. Dectin-1 expression by macrophages and related antifungal mechanisms in a murine model of Sporothrix schenckii sensu stricto systemic infection. Microb Pathog. 2017;110:78–84. https://doi.org/10.1016/j.micpath.2017.06.025.
Wang Y, Zou Y, Chen X, Li H, Yin Z, Zhang B, et al. Innate immune responses against the fungal pathogen Candida auris. Nat Commun. 2022;13:3553. https://doi.org/10.1038/s41467-022-31201-x.
Bao F, Fu X, Yu G, Wang Z, Liu H, Zhang F. Mannose-binding lectin and mannose-binding lectin-associated serine protease-2 genotypes and serum levels in patients with sporotrichosis. Am J Trop Med Hyg. 2019;101:1322–4. https://doi.org/10.4269/ajtmh.19-0470.
Kanneganti T, Lamkanfi M, Núñez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–59. https://doi.org/10.1016/j.immuni.2007.10.002.
Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 Inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328. https://doi.org/10.3390/ijms20133328.
Gonçalves AC, Maia DCG, Ferreira LS, Monnazzi LGS, Alegranci P, Placeres MCP, et al. Involvement of major components from Sporothrix schenckii cell wall in the Caspase-1 activation, nitric oxide and cytokines production during experimental sporotrichosis. Mycopathologia. 2015;179:21–30. https://doi.org/10.1007/s11046-014-9810-0.
Ma H, Chan JFW, Tan YP, Kui L, Tsang C, Pei SLC, et al. NLRP3 inflammasome contributes to host defense against talaromyces marneffei infection. Front Immunol. 2021;12:760095. https://doi.org/10.3389/fimmu.2021.760095.
Rogiers O, Frising UC, Kucharíková S, Jabra-Rizk MA, van Loo G, Van Dijck P, et al. Candidalysin crucially contributes to Nlrp3 Inflammasome activation by Candida albicans hyphae. Mbio. 2019;10:e02221–18. https://doi.org/10.1128/mBio.02221-18.
Zhong J, Liu L, Lu Y, Gu Y, Zhao J, Chen B, et al. NLRP3, NLRC4 and NLRC5 gene polymorphisms associate with susceptibility of pulmonary aspergillosis in non-neutropenic patients. J Clin Med. 2022;11:1870. https://doi.org/10.3390/jcm11071870.
Chen M, Xing Y, Lu A, Fang W, Sun B, Chen C, et al. Internalized Cryptococcus neoformans activates the canonical caspase-1 and the noncanonical caspase-8 inflammasomes. J Immunol. 2015;195:4962–72. https://doi.org/10.4049/jimmunol.1500865.
Yang Y, Wang H, Kouadir M, Song H, Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10:128. https://doi.org/10.1038/s41419-019-1413-8.
Gonçalves AC, Ferreira LS, Manente FA, de Faria CMQG, Polesi MC, de Andrade CR, et al. The NLRP3 inflammasome contributes to host protection duringSporothrix schenckii infection. Immunology. 2017;151:154–66. https://doi.org/10.1111/imm.12719.
Yan T, Li F, Li J, Chen F. Antifungal activity of a neodymium-doped yttrium aluminum garnet 1,064-nanometer laser against Sporothrix globosa by inducing apoptosis and pyroptosis via the NLRP3/Caspase-1 signaling pathway: in vitro and in vivo study. Microbiol Spectr. 2021;9:e136421. https://doi.org/10.1128/Spectrum.01364-21.
Neves GWP, Wong SSW, Aimanianda V, Simenel C, Guijarro JI, Walls C, et al. Complement-mediated differential immune response of human macrophages to Sporothrix species through interaction with their cell wall peptidorhamnomannans. Front Immunol. 2021;12:749074. https://doi.org/10.3389/fimmu.2021.749074.
Moalli F, Jaillon S, Inforzato A, Sironi M, Bottazzi B, Mantovani A, et al. Pathogen recognition by the long pentraxin PTX3. J Biomed Biotechnol. 2011;2011:1–15. https://doi.org/10.1155/2011/830421.
Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541–66. https://doi.org/10.1146/annurev-physiol-022516-034339.
Maia DCG, Sassá MF, Placeres MCP, Carlos IZ. Influence of Th1/Th2 cytokines and nitric oxide in murine systemic infection induced by Sporothrix schenckii. Mycopathologia. 2006;161:11–9. https://doi.org/10.1007/s11046-005-0142-y.
Alegranci P, de Abreu Ribeiro LC, Ferreira LS, Negrini TDC, Maia DCG, Tansini A, et al. The predominance of alternatively activated macrophages following challenge with cell wall peptide-polysaccharide after prior infection with Sporothrix schenckii. Mycopathologia. 2013;176:57–65. https://doi.org/10.1007/s11046-013-9663-y.
Zhao S, Qi RQ, Gao XH, Chen HD. Sporothrix schenckii regulates macrophage inflammatory responses via the c-JUN-induced Dab2 transcription. Exp Dermatol. 2022;31:1330–40. https://doi.org/10.1111/exd.14580.
Hiruma M, Kawada A, Noda T, Yamazaki M, Ishibashi A. Tissue response in sporotrichosis: light and electron microscopy studies. Mycoses. 1992;35:35–41. https://doi.org/10.1111/j.1439-0507.1992.tb00816.x.
Huang L, Zhang J, Du W, Liang Z, Li M, Wu R, et al. Chitin-rich heteroglycan from Sporothrix schenckii sensu stricto potentiates fungal clearance in a mouse model of sporotrichosis and promotes macrophages phagocytosis. Bmc Microbiol. 2021;21:190. https://doi.org/10.1186/s12866-021-02243-w.
Boyce KJ, Andrianopoulos A. Fungal dimorphism: the switch from hyphae to yeast is a specialized morphogenetic adaptation allowing colonization of a host. Fems Microbiol Rev. 2015;39:797–811. https://doi.org/10.1093/femsre/fuv035.
Alba-Fierro CA, Pérez-Torres A, Toriello C, Romo-Lozano Y, López-Romero E, Ruiz-Baca E. Molecular components of the Sporothrix schenckii complex that induce immune response. Curr Microbiol. 2016;73:292–300. https://doi.org/10.1007/s00284-016-1045-5.
Sgarbi DB, Da SA, Carlos IZ, Silva CL, Angluster J, Alviano CS. Isolation of ergosterol peroxide and its reversion to ergosterol in the pathogenic fungus Sporothrix schenckii. Mycopathologia. 1997;139:9–14. https://doi.org/10.1023/a:1006803832164.
van der Veen RC. Nitric oxide and T helper cell immunity. Int Immunopharmacol. 2001;1:1491–500. https://doi.org/10.1016/S1567-5769(01)00093-5.
Carlos IZ, Sgarbi DBG, Santos GC, Placeres MCP. Sporothrix schenckii lipid inhibits macrophage phagocytosis: involvement of nitric oxide and tumour necrosis factor-alpha. Scand J Immunol. 2003;57:214–20. https://doi.org/10.1046/j.1365-3083.2003.01175.x.
Ronchese F, Hilligan KL, Mayer JU. Dendritic cells and the skin environment. Curr Opin Immunol. 2020;64:56–62. https://doi.org/10.1016/j.coi.2020.03.006.
Bozza S, Montagnoli C, Gaziano R, Rossi G, Nkwanyuo G, Bellocchio S, et al. Dendritic cell-based vaccination against opportunistic fungi. Vaccine. 2004;22:857–64. https://doi.org/10.1016/j.vaccine.2003.11.031.
Kusuhara M, Qian H, Li X, Tsuruta D, Tsuchisaka A, Ishii N, et al. Mouse bone marrow-derived dendritic cells can phagocytize theSporothrix schenckii, and mature and activate the immune response by secreting interleukin-12 and presenting antigens to T lymphocytes. J Dermatol. 2014;41:386–92. https://doi.org/10.1111/1346-8138.12472.
Uenotsuchi T, Takeuchi S, Matsuda T, Urabe K, Koga T, Uchi H, et al. Differential induction of Th1-prone immunity by human dendritic cells activated with Sporothrix schenckii of cutaneous and visceral origins to determine their different virulence. Int Immunol. 2006;18:1637–46. https://doi.org/10.1093/intimm/dxl097.
Verdan FF, Faleiros JC, Ferreira LS, Monnazzi LGS, Maia DCG, Tansine A, et al. Dendritic cell are able to differentially recognize Sporothrix schenckii antigens and promote Th1/Th17 response in vitro. Immunobiology. 2012;217:788–94. https://doi.org/10.1016/j.imbio.2012.04.006.
de Oliveira S, Rosowski EE, Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol. 2016;16:378–91. https://doi.org/10.1038/nri.2016.49.
Gazendam RP, van de Geer A, Roos D, van den Berg TK, Kuijpers TW. How neutrophils kill fungi. Immunol Rev. 2016;273:299–311. https://doi.org/10.1111/imr.12454.
Zhang Y, Xu X, Zhang M, Jiang P, Zhou X, Li Z, et al. Sporotrichosis: clinical and histopathological manifestations. Am J Dermatopathol. 2011;33:296–302. https://doi.org/10.1097/DAD.0b013e3181f5b622.
Cunningham KM, Bulmer GS, Rhoades ER. Phagocytosis and intracellular fate of Sporothrix schenckii. J Infect Dis. 1979;140:815–7. https://doi.org/10.1093/infdis/140.5.815.
Schaffner A, Davis CE, Schaffner T, Markert M, Douglas H, Braude AI. In vitro susceptibility of fungi to killing by neutrophil granulocytes discriminates between primary pathogenicity and opportunism. J Clin Investig. 1986;78:511–24. https://doi.org/10.1172/JCI112603.
Curtiellas-Piñol V, Ventura-Juárez J, Ruiz-Baca E, Romo-Lozano Y. Morphological changes and phagocytic activity during the interaction of human neutrophils with Sporothrix schenckii: an in vitro model. Microb Pathogenesis. 2019;129:56–63. https://doi.org/10.1016/j.micpath.2019.01.041.
Wang YL, Qi RQ, Lan J, Li ZX, Gao XH. Exogenous local hyperthermia at 41 is effective to eliminate mouse model of sporotrichosis, independent of neutrophil extracellular traps formation. Ann Dermatol. 2021;33:37–45. https://doi.org/10.5021/ad.2021.33.1.37.
Rex JH, Bennett JE. Administration of potassium iodide to normal volunteers does not increase killing of Sporothrix schenckii by their neutrophils or monocytes. J Med Vet Mycol. 1990;28:185–9. https://doi.org/10.1080/02681219080000241.
Abel AM, Yang C, Thakar MS, Malarkannan S. Natural killer cells: development, maturation, and clinical utilization. Front Immunol. 2018;9:1869. https://doi.org/10.3389/fimmu.2018.01869.
Schmidt S, Tramsen L, Lehrnbecher T. Natural killer cells in antifungal immunity. Front Immunol. 2017;8:1623. https://doi.org/10.3389/fimmu.2017.01623.
Ferreira LS, Portuondo DL, Polesi MC, Carlos IZ. Natural killer cells are pivotal for in vivo protection following systemic infection by Sporothrix schenckii. Immunology. 2018;155:467–76. https://doi.org/10.1111/imm.12986.
Soe WM, Lim JHJ, Williams DL, Goh JG, Tan Z, Sam QH, et al. Using expanded natural killer cells as therapy for invasive aspergillosis. J Fungi. 2020;6:231. https://doi.org/10.3390/jof6040231.
Yu M, Song X, Liu B, Luan T, Liao S, Zhao Z. The emerging role of mast cells in response to fungal infection. Front Immunol. 2021;12:688659. https://doi.org/10.3389/fimmu.2021.688659.
Agier J, Pastwińska J, Brzezińska-Błaszczyk E. An overview of mast cell pattern recognition receptors. Inflamm Res. 2018;67:737–46. https://doi.org/10.1007/s00011-018-1164-5.
Jiao Q, Luo Y, Scheffel J, Zhao Z, Maurer M. The complex role of mast cells in fungal infections. Exp Dermatol. 2019;28:749–55. https://doi.org/10.1111/exd.13907.
Jiao Q, Luo Y, Scheffel J, Geng P, Wang Y, Frischbutter S, et al. Skin mast cells contribute to Sporothrix schenckii Infection. Front Immunol. 2020;11:469. https://doi.org/10.3389/fimmu.2020.00469.
Romo-Lozano Y, Hernández-Hernández F, Salinas E. Mast cell activation by conidia of Sporothrix schenckii: role in the severity of infection. Scand J Immunol. 2012;76:11–20. https://doi.org/10.1111/j.1365-3083.2012.02706.x.
Romo-Lozano Y, Hernández-Hernández F, Salinas E. Sporothrix schenckii yeasts induce ERK pathway activation and secretion of IL-6 and TNF-α in rat mast cells, but no degranulation. Med Mycol. 2014;52:862–8. https://doi.org/10.1093/mmy/myu055.
Roan F, Obata-Ninomiya K, Ziegler SF. Epithelial cell–derived cytokines: more than just signaling the alarm. J Clin Invest. 2019;129:1441–51. https://doi.org/10.1172/JCI124606.
Sabanero López M, Flores Villavicencio LL, Soto Arredondo K, Barbosa Sabanero G, Villagómez-Castro JC, Cruz Jiménez G, et al. Proteases of Sporothrix schenckii: cytopathological effects on a host-cell model. Rev Iberoam Micol. 2018;35:32–8. https://doi.org/10.1016/j.riam.2017.05.003.
Sandoval-Bernal G, Barbosa-Sabanero G, Shibayama M, Perez-Torres A, Tsutsumi V, Sabanero M. Cell wall glycoproteins participate in the adhesion of Sporothrix schenckii to epithelial cells. Mycopathologia. 2011;171:251–9. https://doi.org/10.1007/s11046-010-9372-8.
Schop J. Protective immunity against cryptococcus neoformans infection. McGill J Med. 2007;10:35–43.
Yan T, Li F, Li J, Chen F. Antifungal activity of ToAP2D peptide against Sporothrix globosa. Front Bioeng Biotechnol. 2021;9:761518. https://doi.org/10.3389/fbioe.2021.761518.
Li M, Chen Q, Sun J, Shen Y, Liu W. Inflammatory response of human keratinocytes triggered by Sporothrix schenckii via toll-like receptor 2 and 4. J Dermatol Sci. 2012;66:80–2. https://doi.org/10.1016/j.jdermsci.2012.01.003.
Paredes-Rojas A, Palma-Ramos A, Castrillón-Rivera LE, Mendoza-Pérez F, Navarro-González MDC, Arenas-Guzmán R, et al. Keratinocyte response to infection with Sporothrix schenckii. J Fungi. 2022;8:437. https://doi.org/10.3390/jof8050437.
Gisondi P, Geat D, Conti A, Dapavo P, Piaserico S, De Simone C, et al. TNF-α inhibitors biosimilars as first line systemic treatment for moderate-to-severe chronic plaque psoriasis. Expert Rev Clin Immu. 2020;16:591–8. https://doi.org/10.1080/1744666X.2020.1771182.
Berg SH, Balogh EA, Ghamrawi RI, Feldman SR. A review of secukinumab in psoriasis treatment. Immunotherapy-Uk. 2021;13:201–16. https://doi.org/10.2217/imt-2020-0195.
Blauvelt A, Teixeira HD, Simpson EL, Costanzo A, De Bruin-Weller M, Barbarot S, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis. Jama Dermatol. 2021;157:1047. https://doi.org/10.1001/jamadermatol.2021.3023.
Bekkering S, Domínguez-Andrés J, Joosten LAB, Riksen NP, Netea MG. Trained immunity: reprogramming innate immunity in health and disease. Annu Rev Immunol. 2021;39:667–93. https://doi.org/10.1146/annurev-immunol-102119-073855.
Netea MG, Domínguez-Andrés J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:375–88. https://doi.org/10.1038/s41577-020-0285-6.
Acknowledgements
This research was funded by Tianjin Municipal Health Commission-Research Projects in Key Areas of Traditional Chinese Medicine (Grant Number 2019002), Tianjin Scientific Research Project of Integration of Traditional Chinese Medicine and Western Medicine (Grant Number 2021001) and Tianjin Municipal Health Commission Health Science and Technology Project (Grant Number ZC20129). These agencies had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
The first draft of the manuscript was written by Peng Lin and Jianfeng Zhang, and all authors critically revised the work. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Rights
This review does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: Min Chen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, P., Zhang, J., Xie, G. et al. Innate Immune Responses to Sporothrix schenckii: Recognition and Elimination. Mycopathologia 188, 71–86 (2023). https://doi.org/10.1007/s11046-022-00683-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-022-00683-0