Abstract
Mucormycosis is an aggressive and high-mortality opportunistic fungal infection, especially in immunocompromised patients. Conventional antifungals or surgery showed a limited effect on this disease. The antimicrobial photodynamic therapy (aPDT) has been proven to be a promising therapeutic choice against multiple pathogenic fungi. We evaluated the effect of aPDT by using methylene blue (MB) combined with a light emitting diode (LED) on the viability of Rhizopus oryzae, as well as the antifungal susceptibility after aPDT treatment in vitro. A total of six strains were included in this study; MB (8, 16, and 32 μg/ml) was chosen for the photosensitizer, and a light source of LED (635 ± 10 nm, 12 J/cm2) device was used to active it. aPDT with MB (32 μg/ml) and LED was highly effective in cell growth inhibition and exhibited colony-forming unit reductions of up to 4.3log10. The minimal inhibitory concentration ranges of itraconazole, posaconazole, and amphotericin B decreased from > 32 μg/ml to 4–8 μg/ml, 8–16 μg/ml to 0.5–2 μg/ml, and 2–4 μg/ml to 0.25–0.5 μg/ml, respectively, after pre-treatment with MB (8 μg/ml) and LED. In conclusion, aPDT with MB and LED was a promising therapeutic option against R. oryzae infections alone or combined with antifungal agents. However, further investigation is needed to determine the potential for clinic therapy and to elucidate the underlying mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mucormycosis is an aggressive and frequently fatal opportunistic fungal infection that has emerged in patients with severe underlying immunosuppression, especially those with hematological malignancies or recipients of allogeneic hematopoietic stem cell transplantation [1]. Rhizopus oryzae is by far the most common cause of mucormycosis, which accounts for approximately 60% of all disease manifestation and 90% of all rhinocerebral cases [2]. Mucorales are resistant to most triazoles, except for posaconazole [3, 4]. Treatment strategies are based on high doses of amphotericin B (occasionally used in combination with echinocandins) and posaconazole, associated with surgical resections when possible. However, even with aggressive antifungal treatment, the estimated attributable mortality rate for mucormycosis exceeds 50% [2]. In the absence of surgical debridement of the infected tissue, antifungal agents alone are rarely curative, resulting in 100% mortality rate for patients with disseminated disease [5]. Therefore, there is an urgent need for alternative new treatment strategies.
In recent years, antimicrobial photodynamic therapy (aPDT) alone or in combination with antifungals has arisen as a promising approach for mycoses [6, 7]. Previous studies have demonstrated the efficacy of aPDT against a variety of pathogenic fungi in vitro, including Candida spp., Fusarium spp., Fonsecaea spp., Exophiala dermatitidis, Sporothrix schenckii, Trichophyton rubrum, Scedosporium, and Lomentospora spp., ect [8,9,10,11,12,13]. In addition, aPDT has also been successfully applied in vivo to treat chromoblastomycosis and onychomycosis [14,15,16]. However, little is known about the effects of aPDT on the viability and antifungal susceptibility of R. oryzae. In the present study, the growth inhibition effects of aPDT mediated by methylene blue and light emitting diode (LED) on R. oryzae and the impacts of pre-treatment of aPDT on antifungal susceptibilities were evaluated.
Materials and Methods
Samples
A total of six strains of R. oryzae were studied, including the sequencing strain RA99880 (purchased from FGSC), ATCC56536, ATCC44170, and three clinical strains isolated from rhinocerebral mucormycosis patients. All isolates were identified by standard morphological criteria and confirmed by molecular sequencing of the internal transcribed spacer (ITS) ribosomal DNA (rDNA) as required. The quality control strain for the in vitro antifungal susceptibility test is ATCC22019.
Inoculum Preparation and Photodynamic Treatment
Conidia were freshly harvested from cultures grown for 2 days on Sabouraud dextrose agar (SDA) by sterile distilled water and further diluted to a concentration of 1–3 × 106 CFU/ml. The methylene blue was applied as photosensitive agents and tested at concentrations of 8 μg/ml (T1), 16 μg/ml (T2), and 32 μg/ml (T3), with 100 μl of each concentration mixed with 100 μl of the prepared inocula prealiquoted into sterile 96-well microtiter plates. After incubation in dark at 37 °C for 2 h, the plates were then irradiated with LED (Type: Carnation-86C, Lifotronic Technology Co., Ltd., Shenzhen, China) at a wavelength of 635 ± 10 nm and at a distance of 1 cm for 120 s (irradiance: 100 mW/cm2, 12 J/cm2). The control groups included conidial suspension in saline without LED irradiation (C1), conidial suspension with 32 ug/mL of methylene blue and without LED irradiation (C2), and conidial suspension in saline and irradiated (C3). After aPDT treatment, 10 μL aliquots of each well was diluted with sterile saline to a total volume of 1L, subsequently 100 μL of the mixture were inoculated on SDA and incubated at 37 °C in 24 h before counting colony-forming unit (CFU).
In vitro Antifungal Susceptibility Testing
The effects of aPDT on antifungal susceptibility were evaluated via determination of the minimal inhibitory concentrations (MICs) of itraconazole (ITC), posaconazole (POS), and amphotericin B (AMB) (all purchased from Sigma Chemical Co., St. Louis, MO, USA) against photodynamic treated (T1) and untreated planktonic cells(C1). The working concentrations of all tested agents were 0.06–32 μg/ml. According to the broth microdilution method M38-A2 of the Clinical and Laboratory Standards Institute [17], the 96-well plate was inoculated with 100 μL of conidial suspensions of T1 or C1 and 100 μL of the serially diluted drugs. After 24 h of incubation at 35 °C, the MICs were determined visually as the lowest drug concentrations resulting in complete growth inhibition [17]. All experiments were conducted in triplicate.
Results
Effects of Photodynamic Treatment on R. oryzae
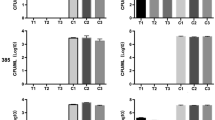
As shown in Table 1, all aPDT treatment groups resulted in reduction in CFU counts. Notably, aPDT with 32 μg/ml methylene blue in T3 group inhibited all cell growth and exhibited CFU reductions of up to 4.3 log10, revealing biologically relevant antimicrobial activity (Fig. 1). As for T1 and T2 group, the average CFU reductions were 1.1 log10 and 2.2 log10, respectively. For each particular treatment group, there were no significance differences in response to aPDT treatment among individual tested strains.
Photodynamic Effects on Antifungal Susceptibilities
The MIC results presented in Table 2 revealed that both ITC (> 32 μg/ml) and POS (8–16 μg/ml) were inactive against all strains, while AMB exhibited moderate activity with a MIC range of 2–4 μg/ml. However, with aPDT pre-treatment, the MIC ranges of ITC, POS, and AMB decreased to 4–8 μg/ml, 0.5–2 μg/ml, and 0.25–0.5 μg/ml, respectively, resulting a fourfold to eightfold reduction in MICs.
Statistical Analysis
All the data presented in the Tables are expressed as mean value of three independent experiments. Analysis of variance (ANOVA) and Bonferroni post hoc test were carried out by SPSS V.13 to assess values obtained between different groups. All tests were two-tailed, and P ≤ 0.05 was considered statistically significant.
Discussions
Rhizopus oryzae is the primary cause of mucormycosis, especially the most frequently reported rhinocerebral form [18]. The infection always starts in the nasal tissues and spreads into the paranasal sinuses and deep organs. The characteristic rapid angioinvasive growth nature leads to thrombosis and tissue ischemia. Timely control of the infections prevents dissemination of the infection. However, surgical debridement may result in disfiguration and available antifungals showed limited activity against Mucorales. In contrast, aPDT with methylene blue and LED, which allows maximal tissue transmission with low invasive character, is very convenient and inexpensive. The antimicrobial effects of PDT are based on the combination of a non-toxic photosensitizer (PS) and a specific wavelength of visible light, which can promote a phototoxic reaction and produce reactive oxygen species (ROS) at the presence of oxygen. ROS were highly cytotoxic to microorganisms through inducing oxidation of cellular structures, modifying the plasma membrane structures or the DNA, and inhibiting enzymatic systems. However, the effect of PDT is selective since ROS were only produced in microorganisms or diseased tissue accumulated with PS. And PDT is a noninvasive procedure which could be repeatedly applied without causing cumulative toxicity or damage of normal tissue [19, 20]. From the present study, the results have proved the efficacy of aPDT in both inhibiting the cell growth of R. oryzae and significantly enhancing the effects of azoles and AMB against R. oryzae. These results suggest aPDT can be a therapeutic method against mucormycosis caused by R. oryzae, or aPDT can combine with antifungal agents, which may help to overcome the antifungal drug resistance, and has the potential to decrease drug dosages, side effects, and improving patients’ compliance. Nevertheless, further investigations including in vivo experimental and clinical studies are warranted to determine the safe and reliable application in clinical practice.
Conclusion
In summary, our results expand the knowledge regarding the photodynamic inactivation of R. oryzae. The aPDT mediated by methylene blue and LED revealed antimicrobial activity against R. oryzae and rendered R. oryzae more sensitive to azoles and AMB. The findings encourage further studies to evaluate the effectiveness of aPDT in mucormycosis.
References
Bitar D, Van Cauteren D, Lanternier F, Dannaoui E, Che D, Dromer F, Desenclos JC, Lortholary O. Increasing incidence of zygomycosis (mucormycosis), France, 1997–2006. Emerg Infect Dis. 2009;15(9):1395–401.
Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–53.
Marty FM, Cosimi LA, Baden LR. Breakthrough zygomycosis after voriconazole treatment in recipients of hematopoietic stem-cell transplants. N Engl J Med. 2004;350(9):950–2.
Lewis RE, Kontoyiannis DP. Epidemiology and treatment of mucormycosis. Future Microbiol. 2013;8(9):1163–75.
Husain S, Alexander BD, Munoz P, Avery RK, Houston S, Pruett T, Jacobs R, Dominguez EA, Tollemar JG, Baumgarten K, Yu CM, Wagener MM, Linden P, Kusne S, Singh N. Opportunistic mycelial fungal infections in organ transplant recipients: emerging importance of non-Aspergillus mycelial fungi. Clin Infect Dis. 2003;37(2):221–9.
Qiao J, Li R, Ding Y, Fang H. Photodynamic therapy in the treatment of superficial mycoses: an evidence-based evaluation. Mycopathologia. 2010;170(5):339–43.
Calzavara-Pinton P, Rossi MT, Sala R, Venturini M. Photodynamic antifungal chemotherapy. Photochem Photobiol. 2012;88(3):512–22.
Lu Q, Sun Y, Tian D, Xiang S, Gao L. Effects of Photodynamic Therapy on the Growth and Antifungal Susceptibility of Scedosporium and Lomentospora spp. Mycopathologia. 2017;182(11–12):1037–43.
Gao L, Jiang S, Sun Y, Deng M, Wu Q, Li M, Zeng T. Evaluation of the Effects of Photodynamic Therapy Alone and Combined with Standard Antifungal Therapy on Planktonic Cells and Biofilms of Fusarium spp. and Exophiala spp. Front Microbiol. 2016; 7: 617.
Carmello JC, Alves F, Ribeiro AP, Basso FG, de Souza Costa CA, Tedesco AC, Primo FL, Mima EG, Pavarina AC. In vivo photodynamic inactivation of Candida albicans using chloro-aluminum phthalocyanine. Oral Dis. 2016;22(5):415–22.
Hu Y, Huang X, Lu S, Hamblin MR, Mylonakis E, Zhang J, Xi L. Photodynamic therapy combined with terbinafine against chromoblastomycosis and the effect of PDT on Fonsecaea monophora in vitro. Mycopathologia. 2015;179(1–2):103–9.
Nunes Mario DA, Denardi LB, Brayer Pereira DI, Santurio JM, Alves SH. In vitro photodynamic inactivation of Sporothrix schenckii complex species. Med Mycol. 2014;52(7):770–3.
Morton CO, Chau M, Stack C. In vitro combination therapy using low dose clotrimazole and photodynamic therapy leads to enhanced killing of the dermatophyte Trichophyton rubrum. BMC Microbiol. 2014;14:261.
Gilaberte Y, Aspiroz C, Martes MP, Alcalde V, Espinel-Ingroff A, Rezusta A. Treatment of refractory fingernail onychomycosis caused by nondermatophyte molds with methylaminolevulinate photodynamic therapy. J Am Acad Dermatol. 2011;65(3):669–71.
Lyon JP, Pedroso e Silva Azevedo Cde M, Moreira LM, de Lima CJ, de Resende MA. Photodynamic antifungal therapy against chromoblastomycosis. Mycopathologia. 2011;172(4):293–7.
Souza LW, Souza SV, Botelho AC. Distal and lateral toenail onychomycosis caused by Trichophyton rubrum: treatment with photodynamic therapy based on methylene blue dye. An Bras Dermatol. 2014;89(1):184–6.
Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard-2nd ed. CLSI document M38-A2. CLSI, Wayne, PA. 2008.
Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236–301.
Ribeiro AP, Andrade MC, da Silva Jde F, Jorge JH, Primo FL, Tedesco AC, Pavarina AC. Photodynamic inactivation of planktonic cultures and biofilms of Candida albicans mediated by aluminum–chloride–phthalocyanine entrapped in nanoemulsions. Photochem Photobiol. 2013;89(1):111–9.
Carrera ET, Dias HB, Corbi SCT, Marcantonio RAC, Bernardi ACA, Bagnato VS, Hamblin MR, Rastelli ANS. The application of antimicrobial photodynamic therapy (aPDT) in dentistry: a critical review. Laser Phys. 2016;26(12).
Acknowledgements
This work was supported by National Natural Science Foundation of China (31400131 to Lujuan Gao and 81401677 to Yi Sun), and Hubei Province Health and Family Planning Scientific Research Project (WJ2018H178 to Yi Sun).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Handling Editor: Cunwei Cao.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Z., Tang, J., Sun, Y. et al. Effects of Photodynamic Inactivation on the Growth and Antifungal Susceptibility of Rhizopus oryzae. Mycopathologia 184, 315–319 (2019). https://doi.org/10.1007/s11046-019-00321-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-019-00321-2