Abstract
Since 1997, an emergent fungal disease named lethargic crab disease (LCD) has decimated stocks of the edible mangrove land crab Ucides cordatus (Linnaeus, 1763) (Brachyura: Ocypodidae) along the Brazilian coast, threatening the mangrove ecosystem and causing socioeconomic impacts. Evidence from a variety of sources suggests that the black yeast Exophiala cancerae (Herpotrichiellaceae, Chaetothyriales) has been responsible for such epizootic events. Based on the spatiotemporal patterns of the LCD outbreaks, the well-established surface ocean currents, and the range of ecological traits of Exophiala spp., a marine dispersal hypothesis may be proposed. Using in vitro experiments, we tested the survival and growth of E. cancerae CBS 120420 in a broad combination of salinities, temperatures, and exposure times. While variation in salinity did not significantly affect the growth of colony-forming units (CFUs) (P > 0.05), long exposure times visibly influenced an increase in CFUs growth (P < 0.05). However, higher temperature (30 °C) caused a reduction of about 1.2-fold in CFUs growth (P < 0.05). This result suggests that sea surface temperatures either above or below the optimum growth range of E. cancerae could play a key role in the apparent north–south limits in the geographical distribution of LCD outbreaks. In light of our results, we conclude that a fundamental step toward the understanding of LCD epidemiological dynamics should comprise a systematic screening of E. cancerae in estuarine and coastal waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since 1997, outbreaks in populations of the mangrove land crab Ucides cordatus Linnaeus, 1763 (Brachyura: Ocypodidae) (popularly known in Brazil as “caranguejo-uçá”), have threaten the mangrove ecosystem [1] and caused drastic socioeconomic impacts on artisanal fishing communities [2, 3]. Evidence from a variety of sources (light and electron microscopy, histopathological studies of sick crabs, behavioral tests, experimental infections, molecular phylogenetics, mathematical modeling, and epidemiological assessments) strongly suggest that a black yeast species of fungus, named Exophiala cancerae de Hoog, Vicente, Najafzadeh, Badali, Seyedmousavi & Boeger, 2011 (Herpotrichiellaceae, Chaetothyriales) (strain CBS 120420, MycoBank MB515720), is responsible for the epizootic events [1, 4,5,6,7,8,9,10,11]. Based on the clinical signs of sick crabs, this infirmity has been named lethargic crab disease (LCD) [1].
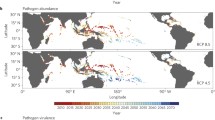
Mortalities caused by LCD have been reported from a considerable fraction of the distribution of U. cordatus, occurring on approximately 2700 km of the Brazilian coastline [1, 4,5,6,7,8,9,10,11]. Since the first record of mortalities in the year 1997, near the city of Goiana (state of Pernambuco, Brazil), LCD has spread northward (i.e., states of Paraíba, Rio Grande do Norte, Ceará, and Piauí) and southward (i.e., states of Sergipe, Bahia, and Espírito Santo) in a seemingly wave-like pattern in the same direction of the surrounding surface ocean currents, hence affecting crab populations of eight out of the total 17 Brazilian coastal states [1, 4,5,6,7,8,9,10,11] (Fig. 1). Given that U. cordatus inhabits mangroves in an extensive area in the Occidental Atlantic, from the state of Florida (USA) to the coast of the state of Santa Catarina (Brazil) [13], available evidences suggest that such dispersion of LCD outbreaks has reached an apparent north and south limit. The northernmost confirmed mortality event caused by LCD was reported from a single event in the state of Piauí in 2003, whereas the southernmost records comprise several episodes in mangroves of the state of Espírito Santo, since 2006 [7, 10].
Timeline of LCD outbreaks depicting a schematic distribution of the main sea surface currents, mangrove areas, and 200-m isobath. Asterisk: localities affected by LCD outbreaks; Dagger Cape São Tomé; triangle: Cape Frio, and section sign São Paulo Bight. Map modified from Magris and Barreto [12]
Although the original habitat of the strain of E. cancerae associated with the LCD outbreaks still remains to be elucidated [11], several lines of evidence have supported a waterborne origin for the disease. The genus was proposed by Carmichael [14] based on Exophiala salmonis Carmichael, 1966, responsible for a systemic infection in trout. Subsequently, two other pathogens of fish were reported, E. pisciphila [15] and E. psychrophila [16]. Recent molecular phylogenetic analyses have indicated that E. cancerae (CBS 120420) belongs to a particular clade of waterborne species that infect a range of cold-blooded animals, including the previously mentioned fishes as well as turtles, and frogs [7]. Interestingly, strains of E. cancerae isolated to date are characterized by a cosmopolitan distribution and have been regularly recovered from water sources, showing meso-/psychrophilic and halophilic tendencies [7]. Regarding specifically the strain associated with the LCD, Schmidt [17] suggested that the most likely dispersion pathway is the water, because during his studies, mangrove crabs near the lower intertidal zones had a more significant decrease in population densities.
Confronting the spatiotemporal patterns of the LCD events, the well-established surface ocean currents, and the range of ecological traits of Exophiala spp (Fig. 1), an epidemiological scenario may be proposed. In such context, if E. cancerae CBS 120420 is capable to withstand relatively long exposure to pronounced variations in environmental conditions (i.e., water surface salinity and temperature), this etiological agent may persist in the estuarine, inshore, and offshore waters and the disease could spread over relatively long distances (≈100 km) affecting naive populations of U. cordatus in adjacent estuaries. Alternatively, if fungal forms’ viability is hampered by exposure to some specific salinity and temperature regime, similar to any particular condition observed in the Brazilian estuarine and coastal environments, factors limiting the dispersion of the disease outbreaks could be elucidated.
Herein, we tested the survival and growth of E. cancerae using in vitro experiments that emulate a varied combination of environmental conditions in space and time. The understanding of the dispersal pathways of this etiologic agent is fundamental for the improvement of predictive models in epidemiological studies, risk assessment, and management of the LCD.
Materials and Methods
Experimental Strain: Source and Preparation
The strain of Exophiala cancerae CBS 120420 (Fig. 2) evaluated in this study was isolated through oil flotation technique [18] from tissues of moribund crabs collected during mortality events in the states of Bahia and Sergipe (Brazil) (beginning of 2004 and 2005) [1]. Prior to the experiments, fresh subcultures of E. cancerae were plated on Sabouraud agar (HiMedia) and incubated at 25 °C. Conidia and hyphal elements collected from the surface of fresh colonies were re-suspended in saline solution (2.5%, the same physiological salinity of U. cordatus hemolymph [19]) with Tween®20 (1%) [7, 20]. Subsequently, a test solution of about 2 × 107 fungal elements per mL was obtained with the aid of a Neubauer chamber (Optik Labor) under a phase-contrast microscope (Olympus BX51) (adapted from [18]). Manipulations were performed in a horizontal laminar airflow workstation (LabCon Co., Purifier Class II).
Experimental Design
Test solution with E. cancerae was exposed at simultaneous combinations of seawater salinities, temperatures, and exposure times estimated to accurately simulate the conditions faced by this black yeast in a putative scenario of marine dispersal among estuaries. A total of 24 treatments were delineated to quantify the growth of E. cancerae at combinations of four salinities (0, 10, 25, and 38 ppt) and two temperatures (25 °C and 30 °C), over a period of 1, 24, and 168 h (Table 1). Each experimental treatment was replicated five times. Specifically, aliquots (50 μL) of the diluted (tenfold dilution) test solution were added to 1 mL of sterilized seawater with different levels of salinity, adjusted through evaporation and dilutions (with sterile double-distilled water). Salinity of the samples was measured in a scale of parts per thousand (ppt) using a hand refractometer (PCE-0100, UK). The resulting solutions comprising 0, 10, 25, and 38 ppt were distributed into 1.5-mL Eppendorf tubes, totaling 30 replicates per salinity level. Subsequently, each of these levels was divided into two subgroups, one incubated at 25 °C and another at 30 °C. Tubes of each subgroup were kept in these temperatures for 1, 24, and 168 h. Subsequently, their contents were carefully spread onto Mycosel agar (Pronadisa, Spain) plates with the aid of a Drigalski spatula. Noteworthy is that the chosen seawater salinities and temperatures approximated the average monthly values reported in the Brazilian coastal zone (https://www.nodc.noaa.gov/) [21, 22]. Also, selected exposure times are based on average speed of sea surface currents [23, 24] and thus assessed the possibility of survival and growth of colony-forming units (CFUs) of E. cancerae when transported within and between estuaries.
Growth of Colonies and Statistical Method
After 14 days of incubation, plated Petri dishes (90 × 15 mm) were photographed under equal light and camera parameters using a digital camera Sony MVC-CD500. These images were used to evaluate the area covered by the CFUs with the aid of the analysis tools provided by SigmaScan® Pro 5.0 software (Systat Software Inc., USA). All images were converted to gray scales; only elements with the intensity threshold higher than 60 pixels were considered [20]. The influence of the different salinities, temperature, and exposure times (predictive variables) on the growth (dependent variable) of E. cancerae (CBS 120420) was analyzed by employing the general linear model (GLM) procedure with STATISTICA 6.0 (StatSoft Inc., USA). Significance level was accepted as P < 0.05.
Results and Discussion
Our experimental results support the hypothesis of the marine dispersion of the etiologic agent of LCD between adjacent Brazilian estuaries. E. cancerae CBS 120420 grew in all experimental treatments, as indicated by the profiles of average CFUs growth per treatment (Figs. 3, 4). GLM summarized well the overall data, in which 55% (multiple coefficient of determination) of the variation observed in CFU growth was accounted for the measured variables (Table 2). Regarding a potential osmotic stress, E. cancerae showed a remarkable tolerance to a wide spectrum of salinity levels, persisting and growing for a relatively extended period of exposure (1 week) under a gradient that include both very low and very high salinities (0 or 38 ppt, respectively) (Figs. 3, 4). As such, while no significant effect was correlated with this variable (P > 0.05), the analysis confirmed that CFU growth was significantly dependent upon exposure time and temperature (Table 2; Figs. 3, 4). Regarding the time variable, the extended periods of exposure clearly influenced an increase in CFU growth (P < 0.05) (Figs. 3, 4). Regardless of the influence exerted by the other tested variables, E. cancerae could withstand the required conditions to be transported between estuaries by ocean currents. However, temperature increases (25–30 °C) have produced a significant depletion, about 1.2-fold, in such growth (P < 0.05) (Table 2; Figs. 3, 4, 5). This result corroborates the optimum growth between 24 and 27 °C reported by de Hoog et al. [6] in the species description. Accordingly, it seems plausible to suggest that sea surface temperatures either above or below this optimum growth range could play a key role in the apparent north–south limits in the geographical distribution of LCD events.
In the north, the last record of LCD outbreaks is from the state of Piauí in 2003 (Fig. 1). Since then, no evidence exists that the disease has stricken mangrove land crab populations in any neighboring estuarine area located up north. Noteworthy is that this particular estuary comprises part of the world’s largest continuous mangrove area [25] (see Fig. 1). However, under the hypothesis of marine dispersion of LCD, our data suggest that the high water temperature observed near to the Equatorial line, approximately 30 °C (https://www.nodc.noaa.gov/) [21, 22], could promote a reduction in the propagule pressure of E. cancerae conidia (non-motile asexual fungal spores), consequently affecting the contact rate between infective stages of this black yeast and susceptible mangrove crabs.
While our data did not indicate any clear evidences on the apparent southernmost limit of LCD outbreaks, direction and temperature of surface ocean currents as well as rarefaction and discontinuity of mangrove areas (see Fig. 1) may offer clues on the factors contributing to the present distribution. Near to the last reports of LCD-associated mortalities in Espírito Santo, in the vicinity of both capes, São Tomé and Frio (20°S and 23°S, respectively) (see Fig. 1) occur abrupt changes in the Brazilian coastline direction that turns sharply westward [26]. To conserve angular momentum, the Brazil Current (BC) develops a convoluted pattern of clockwise meanders that pitches off the current away from the continental shelf [27, 28]. This phenomenon is also associated with upwelling events [27], known to affect algal distribution [29] and the position of sardine spawning areas [30]. Additionally, drops in the sea surface temperature from 23 °C to 15 °C have been recorded in Cape Frio [31]. By applying a similar logic as that for the north limit hypothesis previously mentioned, we assume that these temperature declines associated with upwelling systems could also impact on fungal forms’ dispersal.
Building on knowledge derived from our unpublished results, a mathematical model on epidemiological aspects of LCD has corroborated our hypothesis of marine dispersion. In an attempt to understand LCD transmission between mangrove areas, Avila et al. [10] formulated a model incorporating the dispersion of E. cancerae in the ocean. Noteworthy is that this assumption was based on a personal communication of the preliminary (unpublished) results of the present study that pointed out the tolerance of this etiologic agent to the range of salinities found in the ocean. The model indicated the existence of traveling wave solutions connecting the disease-free and affected populations of the mangrove land crab Ucides cordatus. Interestingly, the numerical simulations suggested that the direction of the outbreaks could be influenced by the ocean currents [10]. In a previous mathematical modeling study, Ferreira et al. [5] successfully recovered the cyclic pattern observed in nature of the mortality events within a mangrove area. Although both models predicted that the observed oscillations may be a consequence of the relationship between demographic and epidemiological parameters, thereby rejecting the influence of an external forcing parameter (e.g., stress of U. cordatus during mating season) [5], the seasonality present in the records of LCD outbreaks still remains to be clarified. Most epizootic events have occurred in the summer, also known as the rainy season. Given that, we believe that the formulation of future models under the scenario of water dispersion of E. cancerae should incorporate seasonal environmental drivers related to rainfall in order to elucidate the timing for exportation out of estuary of fungal infective stages. These variables could comprise tidal amplitude, freshwater riverine discharge rates, as well as density and distribution of populations of mangrove land crab within mangrove intertidal zones.
In conclusion, route of infection within a single host individual as well as transmission between mangrove land crabs within a population is a significant knowledge gap that needs closure. Despite the epidemiological concerns, these data could also provide basis to understand whether the LCD-associated strain of E. cancerae is a true pathogen only found in its host or this particular lineage represents an opportunistic strain colonizing a new host via a resource tracking process [32,33,34,35,36]. To date, no study had assessed the presence of E. cancerae in either estuarine or coastal (inshore and offshore) waters. Guerra et al. [11] screened for E. cancerae in plant and soil material derived from mangrove areas affected by LCD. No strain of the etiological agent of LCD was isolated from these environmental compartments. This observation aligns with the marine dispersal hypothesis and stresses the need for environmental screenings that focus efforts on recovering isolates from exclusively this particular source.
References
Boeger WA, Pie MR, Ostrensky A, Patella L. Lethargic crab disease: multidisciplinary evidence supports a mycotic etiology. Mem Inst Oswaldo Cruz. 2005;100:161–7.
Nóbrega RR, Nishida AK. Aspectos socioeconômicos e percepção ambiental dos catadores de caranguejo-uçá, Ucides cordatus (L. 1763) (Decapoda, Brachyura) do estuário do rio Mamanguape, Nordeste do Brasil (in Portuguese). Interciencia. 2003;28:36–43.
Alves RR, Nishida AK, Hernández MI. Environmental perception of gatherers of the crab “caranguejo-uçá” (Ucides cordatus, Decapoda, Brachyura) affecting their collection attitudes. J Ethnobiol Ethnomed. 2005;. doi:10.1186/1746-4269-1-10.
Boeger WA, Pie MR, Vicente VA, Ostrensky A, Hungria D, Castilho GG. Histopathology of the mangrove land crab Ucides cordatus (Ocypodidae) affected by lethargic crab disease. Dis Aquat Organ. 2007;78:73–81.
Ferreira CP, Pie MR, Esteva L, Mancera PFA, Boeger WA, Ostrensky A. Modelling the lethargic crab disease. J Biol Dyn. 2009;3(6):620–34.
De Hoog GS, Vicente VA, Najafzadeh MJ, Harrak MJ, Badali H, Seyedmousavi S. Waterborne Exophiala species causing disease in cold-blooded animals. Persoonia. 2011;. doi:10.3767/003158511X614258.
Orélis-Ribeiro R, Boeger WA, Vicente VA, Chammas M, Ostrensky A. Fulfilling Koch’s postulates confirms the mycotic origin of lethargic crab disease. Antonie Van Leeuwenhoek. 2011;99:601–8.
Pie MR, Boeger WA, Patella L, Vicente VA, Orélis-Ribeiro R, Ostrensky A. Specific primers for the detection of the black-yeast fungus associated with the lethargic crab disease. Dis Aquat Organ. 2011;94:73–5.
Vicente VA, Orélis-Ribeiro R, Najafzadeh MJ, Sun J, Guerra RS, Miesch S, Ostrensky A, Meis JF, Klaassen CH, de Hoog GS, Boeger WA. Black yeast-like fungi associated with lethargic crab disease (LCD) in the mangrove-land crab, Ucides cordatus (Ocypodidae). Vet Microbiol. 2012;158:109–22.
Avila RP, Mancera PFA, Esteva L, Pie MR, Ferreira CP. Traveling waves in the lethargic crab disease. Appl Math Comput. 2012;218(19):9898–910.
Guerra RS, do Nascimento MMF, Miesch S, Najafzadeh MJ, Orélis-Ribeiro R, Ostrensky A, de Hoog GS, Vicente VA, Boeger WA. Black yeast biota in the mangrove, in search of the origin of the lethargic crab disease (LCD. Mycopathologia. 2013;175(5–6):421–30.
Magris RA, Barreto R. Mapping and assessment of protection of mangrove habitats in Brazil. Pan Am J Aquat Sci. 2010;5:546–56.
Melo GAS. Manual de identificação dos Brachyura (caranguejos e siris) do litoral brasileiro. São Paulo: Plêiade; 1996.
Carmichael JW. Cerebral mycetoma of trout due to a Phialophora-like fungus. Sabouraudia. 1966;5:120–3.
Mcginnis MR, Ajello LA. A new species of Exophiala isolated from channel catfish. Mycologia. 1974;66:518–20.
Pedersen OA, Langvad F. Exophiala psychrophila sp. nov., a pathogenic species of the black yeasts isolated from farmed Atlantic salmon. Mycol Res. 1989;92:153–6.
Schmidt AJ. Estudo da dinâmica populacional do caranguejo-uçá, Ucides cordatus cordatus e dos efeitos de uma mortalidade em massa desta espécie em manguezais do Sul Bahia (in Portuguese). Dissertation, IOUSP, São Paulo; 2006.
Iwatsu T, Miyajii M, Okmoto S. Isolation of Phialophora verrucosa and Fonsecaea pedrosoi from nature in Japan. Mycopathologia. 1981;75:149–58.
Harris RR. Santos MCF. Sodium uptake and transport (Na + K+) ATPase changes following Na + depletion and low salinity acclimation in the mangrove crab Ucides cordatus (L.). Comp Biochem Physiol. 1993;105:35–42.
Orélis-Ribeiro R, Chammas MA, Ostrensky A, Boeger WA. Viability of the etiologic agent of the Lethargic Crab Disease, Exophiala cancerae, during cooking of the mangrove-land crab: Does this traditional dish represent a risk to humans? Food Control. 2012;25:591–3.
Ffield A. North Brazil current rings viewed by TRMM Microwave Imager SST and the influence of the Amazon Plume. Deep Sea Res Part I Oceanogr Res Pap. 2005;52(1):137–60.
Miloslavich P, Klein E, Díaz JM, Hernández CE, Bigatti G, Campos L, Artigas F, Castillo J, Penchaszadeh PE, Neill PE, Carranza A, Retana MV, de Astarloa JMD, Lewis M, Yorio P, Piriz ML, Rodríguez D, Yoneshigue-Valentin Y, Gamboa L, Martín A. Marine biodiversity in the Atlantic and Pacific Coasts of South America: knowledge and gaps. PLoS ONE. 2011;. doi:10.1371/journal.pone.001463.
Bischof B, Mariano AJ, Ryan EH: “The North Brazil Current”. Ocean Surf Curr. http://oceancurrents.rsmas.miami.edu/atlantic/north-brazil.html.
Bischof B, Rowe E, Mariano AJ, Ryan EH: “The Brazil Current.” Ocean Surf Curr. http://oceancurrents.rsmas.miami.edu/atlantic/brazil.html.
Kjerfve B, Perillo GME, Gardner LR, Rine JM, Dias GTM, Mochel FR. Morphodynamics of muddy environments along the Atlantic coasts of North and South America. In: Healy TR, Wang Y, Healy J-A, editors. Muddy coasts of the world: processes, deposits and functions. Amsterdam: Elsevier; 2002. p. 479–532.
Oliveira LR, Piola AR, Mata MM, Soares ID. Brazil current surface circulation and energetics observed from drifting buoys. J Geophys Res. 2009;. doi:10.1029/2008JC004900.
Campos EJD, Velhote D, Silveira ICA. Shelf break upwelling driven by Brazil current cyclonic meanders. Geophys Res Lett. 2000;. doi:10.1029/1999GL010502.
Calado L, Gangopadhyay A, Silveira ICA. A parametric model for the Brazil current meanders and eddies off Southeastern Brazil. Geophys Res Lett. 2006;. doi:10.1029/2006GL026092.
Guimaraens MA, Coutinho R. Temporal and spatial variation of Ulva spp. and water properties in the Cabo Frio upwelling region of Brazil. Aquat Bot. 2000;66:101–14.
Matsuura Y. Brazilian sardine (Sardinella brasiliensis) spawning in the Southeast Brazilian bight over the period 1976–1993. Rev Bras Oceanogr. 1998;46(1):33–43.
Campos PC, Moller OO Jr, Piola AR, Palma ED. Seasonal variability and coastal upwelling near Cape Santa Marta (Brazil). J Geophys Res Oceans. 2013;. doi:10.1002/2012JC008492.
Sav H, Ozakkas F, Altınbas R, Kiraz N, Tümgör A, Gümral R, Döğen A, Ilkit M, de Hoog GS. Virulence markers of opportunistic black yeast in Exophiala. Mycoses. 2016;59:343–50.
Agosta SJ, Klemens JA. Ecological fitting by phenotypically flexible genotypes: implications for species associations, community assembly and evolution. Ecol Lett. 2008;. doi:10.1111/j.1461-0248.2008.01237.x.
Agosta SJ, Janz N, Brooks DR. How specialists can be generalists: resolving the “parasite paradox” and implications for emerging infectious disease. Zoologia (Curitiba). 2010;27:151–62.
Brooks DR, Agosta SJ. Children of time: the extended synthesis and major metaphors of evolution. Zoologia (Curitiba). 2012;29:497–514.
Araujo SBL, Braga MP, Brooks DR, Agosta SJ, Hoberg EP, von Hartenthal FW, Boeger WA. Understanding host-switching by ecological fitting. PLoS ONE. 2015;. doi:10.1371/journal.pone.0139225.
Acknowledgements
We thank Robert W. Pilchowski (GIA, UFPR) for supplying the seawater used in the in vitro experiments, and Marcio R. Pie (Department of Zoology, UFPR) for advice in the experimental design and statistical analysis. This study was supported by the Companhia de Desenvolvimento Industrial e de Recursos Minerais de Sergipe (CODISE) (Sergipe, Brazil), the Secretaria de Estado da Ciência, Tecnologia e Ensino Superior do Estado do Paraná (SETI) (Paraná, Brazil), and also by a grant to WAB and a Postdoctoral Fellowship to ROR under the project entitled “Exploring a new paradigm for the evolution of host-parasites associations,” number 404344/2013-5 of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brazil) (http://www.cnpq.br).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Orélis-Ribeiro, R., Vicente, V.A., Ostrensky, A. et al. Is Marine Dispersion of the Lethargic Crab Disease Possible? Assessing the Tolerance of Exophiala cancerae to a Broad Combination of Salinities, Temperatures, and Exposure Times. Mycopathologia 182, 997–1004 (2017). https://doi.org/10.1007/s11046-017-0169-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-017-0169-x