Abstract
Objectives
To investigate the distribution of Candida spp., predictors of mortality, and effects of therapeutic measures on outcomes of nosocomial bloodstream infection (BSI) due to Candida spp.
Methods
This retrospective, population-based study enrolled adult patients with Candida nosocomial BSI from January 2010 to December 2014 in one tertiary care hospital. The demographics, comorbidities, species distribution, risk factors, and effects of antifungal treatment were assessed.
Results
In total, 190 episodes of Candida BSI were identified. The most prevalent species was C. albicans (38.9 %), followed by C. parapsilosis (23.2 %) and C. tropicalis (20.5 %). In vitro susceptibility testing showed that 88.9 % of Candida isolates were susceptible to fluconazole. The 30-day hospital mortality was 27.9 %, while the early mortality (within 7 days) was 16.3 %. In a multivariate regression analysis, the Acute Physiology and Chronic Health Evaluation II score [odds ratio (OR) 1.23; 95 % confidence interval (CI) 1.080–1.390; P = 0.002] and severe sepsis or septic shock (OR 15.35; 95 % CI 2.391–98.502; P = 0.004) were independently correlated with early mortality. Severe sepsis or septic shock (OR 24.75; 95 % CI 5.099–120.162; P < 0.001) was an independent risk factor for 30-day mortality, while proven catheter-related candidemia (OR 0.16; 95 % CI 0.031–0.810; P = 0.027) was a positive factor for 30-day mortality. Early central venous catheter removal and adequate antifungal treatment were closely related to decreased mortality in patients with primary candidemia.
Conclusion
The proportion of candidemia caused by C. albicans was lower than that caused by non-albicans species. The severity of illness influenced early mortality, and the origin of the central venous catheter remarkably affected 30-day mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Candida species, as a significant cause of nosocomial bloodstream infections (BSI), has risen fivefold in the past decade all over the world, and currently become the fourth cause of BSI in the USA [1]. Many risk factors contribute to the incidence of candidemia, including immunosuppressive therapies (e.g., chemotherapy, corticosteroids), neutropenia, exposure to broad-spectrum antibacterial agents, intensive care unit (ICU) admission, complicated surgery, prolonged use of central venous catheters (CVCs), and administration of total parenteral nutrition (TPN) [2].
Candida albicans has long been the most prevalent species isolated in patients with candidemia. However, recent researches have found a shift in this trend, and the proportion of non-albicans species is now even higher than that of C. albicans [3, 4]. The epidemiology, species distribution, and antifungal drug susceptibility of candidemia vary geographically. The SENTRY antimicrobial surveillance program showed that the prevalence of C. glabrata was higher in the United States than in other regions. Candida tropicalis and C. parapsilosis are more prevalent in Latin America than in the USA. In Canada and Europe, the prevalence of C. albicans is higher than that in other regions [5–7]. One study showed that in China, C. albicans was the most prevalent species causing fungal infection, while the most common non-albicans species was C. glabrata [8].
Despite the increasing use of echinocandins for the treatment of Candida infections, the mortality rate remains high ranging from 35 to 53 % [2, 9, 10]. Although one study showed that the clearance rate of candidemia was lower for mixed candidemia/bacteremia during the early stage of antifungal treatment, the survival rate did not differ regardless of concurrent bacteremia [11]. Many research data have showed that delayed CVC removal and/or inadequate antifungal treatment (e.g., inappropriate dosage, resistant isolate) could increase the mortality among patients with candidemia [4, 12–14]. Therefore, these two interventions have been recommended as the standard care in patients with candidemia.
The objectives of the present study were to describe the distribution of Candida spp. and the clinical characteristics of candidemia, identify the predictors of mortality in patients with candidemia, and determine the effect of clinical interventions on outcomes at our tertiary care hospital in Beijing, China, from 2010 to 2014. Antifungal susceptibility and antifungal therapy were also analyzed.
Methods
Hospital Setting and Study Design
This retrospective study was undertaken in a 2200-bed tertiary care hospital in Beijing, China, from January 2010 to December 2014. This center is a comprehensive hospital with medical, health, teaching, and scientific research accreditation that serves all national army personnel and nonmilitary personnel from across the country. The center has six ICUs containing more than 100 beds. All consecutive hospitalized patients aged ≥18 years with an episode of Candida bloodstream infection that was monitored by the Department of Infection Management and Disease Control and identified through the microbiological laboratory during the hospital stay were qualified for this study. We recorded the following data upon confirmation of candidemia: demographics, underlying diseases, risk factors for candidemia, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, occurrence of severe sepsis or septic shock, Candida spp. distribution, time from admission to onset, antifungal susceptibility testing results, antifungal therapy, and early (≤7-day) and overall (≤30-day) mortality. The impact of multiple comorbidities was evaluated by the Charlson comorbidity index.
Blood was cultured using a BacT/AlerT 3D automatic blood and sterile body fluid detection system (Becton–Dickinson, Sparks, MD, USA), and positive cultures were automatically inoculated with an automated agar plate inoculation system (PREVI Isola; bioMérieux, Marcy l’Etoile, France). Identification of Candida spp. was confirmed with the VITEK-2 system (bioMérieux). The minimum inhibitory concentration clinical breakpoints were defined based on recommendations from the Clinical and Laboratory Standards Institute [15, 16].
Definitions
The definition of an episode of candidemia was detection of at least one positive peripheral blood culture for Candida spp. with relevant signs or symptoms. The onset of candidemia was once drawing a positive blood culture. We only recorded the first episode of candidemia in each case. Patients whose cultures grew more than one species of Candida were not included in the study. Mixed candidemia/bacteremia was confirmed by the bacterial species isolated from a single blood culture or different cultures within 2 days [11].
Primary candidemia was defined as an infection that had no obvious sources or was catheter-related [17]. Catheter-related candidemia was defined as the detection of the same Candida spp. in both peripheral blood and semiquantitative catheter tip culture (>15 cfu) [18]. Secondary candidemia was considered when the same Candida spp. was identified in both blood culture and other potential infection sources. All potential risk factors for candidemia within 30 days before the first positive blood culture were noted. Neutropenia was defined as <500 cells/mm3 absolute neutrophil count. Systemic corticosteroid therapy was defined as >1 week of treatment with prednisone at >1 mg/kg/day or equivalent before diagnosis of Candida BSI. We defined “broad-spectrum antibiotics” as fourth-generation cephalosporins and carbapenems. Exposure to broad-spectrum antibiotics was defined as the use of these drugs for at least 5 days during the 30 days prior to the blood cultures being drawn.
Appropriate antifungal treatment was defined as an adequate dosage and initiation within 5 days from the blood cultures draw based on the Infectious Diseases Society of America guidelines, including fluconazole, initial dose of 12 mg/kg/day, followed by 6 mg/kg/day (adjusted according to renal function) for all Candida spp. except for C. glabrata (≥12 mg/kg once daily); voriconazole, initial dose of 6 mg/kg twice daily for two doses followed by 3 mg/kg twice daily; amphotericin B deoxycholate, ≥0.5 mg/kg/day; liposomal amphotericin B, ≥3 mg/kg/day; caspofungin, initial dose of 70 mg followed by 50 mg/day; and micafungin, 100 mg/day [13]. It was considered inappropriate to treat C. krusei candidemia with fluconazole. The definition of early CVC removal was catheter removal within the first 48 h of sampling the first blood culture that was positive for Candida spp.
Statistical Analysis
The baseline and subgroup characteristics were routinely described and analyzed in the results. Continuous variables are represented as median and interquartile range (IQR). The differences in categorical variables were analyzed by the chi-squared test or Fisher’s exact test. We predicted and analyzed the risk factors for mortality by generating a multivariate logistic regression model. Variables statistically related (P < 0.10) to early and 30-day mortality in the univariate analyses were used to build the multivariate model. We used Kaplan–Meier curves to estimate the survival rates, and differences were evaluated using the log-rank test. A two-tailed P value of <0.05 indicated statistical significance. All statistical analyses were performed using SPSS 19.0 (IBM Corp., Armonk, NY, USA).
Results
Demographic and Clinical Characteristics of the Patients
In total, 204 episodes of candidemia occurred among 197 patients and 7 patients were excluded because they were infected with two species. The overall incidence was 0.29 cases/1000 admissions and decreased from 0.43 to 0.25 episodes/1000 admissions from 2010 to 2014 (0.43 in 2010, 0.36 in 2011, 0.30 in 2012, 0.15 in 2013, and 0.25 in 2014). The median patient age in this study was 68 years, and 64.7 % of the patients were male. Table 1 shows the demographics and clinical characteristics of the patients with Candida BSI. The median length of prior hospital stay before Candida BSI was 21 days (IQR, 11.5–35.0 days). A total of 52.6 % of patients were residents in the ICU at the time of candidemia diagnosis. In terms of risk factors for Candida BSI, 150 patients (78.9 %) had a CVC for at least 24 h at the time of candidemia diagnosis, 139 (73.2 %) were exposed to broad-spectrum antibiotics, and 115 (60.5 %) were given TPN before Candida isolation. Overall, 64.2 % of Candida isolates (122 of 190) were found among the primary infections, among which 42 cases proved to be catheter-related.
Species Distribution
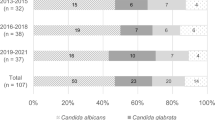
Overall, 74 (38.9 %) of the infections were due to C. albicans, followed by C. parapsilosis (44 cases, 23.2 %), C. tropicalis (39 cases, 20.5 %), C. glabrata (25 cases, 13.2 %), C. krusei (4 cases, 2.1 %), and other Candida spp. (4 cases, 2.1 %). Non-albicans Candida species comprised more than half of the isolates. Most candidemia episodes (n = 99) occurred in the ICU, followed by the internal medicine department. The proportions of C. albicans and non-albicans Candida strains were differently distributed among different departments. In the surgery ward, C. albicans was detected in 51.4 % of the patients and C. tropicalis in 8.6 %, while no C. krusei was isolated; in the surgical ICU, C. tropicalis accounted for 27.3 % of the cases (Fig. 1a).
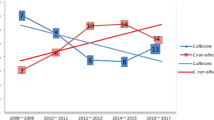
The numbers of cases of non-albicans Candida were higher than the number of cases of C. albicans from 2010 to 2014 (25 vs. 20, 32 vs. 13, 23 vs. 19 and 27 vs. 10, respectively), except 2013 (9 vs. 12, respectively) (Fig. 1b).
Drug Susceptibility and Antifungal Therapy
In vitro susceptibility testing showed that 88.9 % of Candida isolates (112 of 126) were susceptible to fluconazole. Specifically, no C. albicans isolates showed fluconazole resistance, and 25.0 % of C. glabrata, 18.8 % of C. parapsilosis, and 11.1 % of C. tropicalis isolates were intermediately resistant or fully resistant to fluconazole. Because echinocandin in vitro susceptibility testing has not been carried out in our hospital, the relevant clinical data could not be obtained.
The patients’ antifungal therapies are shown in Table 1. In total, 63.7 % of patients (121 of 190) were given azoles as the initial antifungal agents, 51.1 % (97 of 190) were given fluconazole, 14.2 % (27 of 190) were given echinocandin, and 2.6 % (5 of 190) were given a combination of an azole and echinocandin; 36 patients never received targeted antifungals. The median time from positive blood culture to initial antifungal therapy was 2 days (IQR, 0–4 days). Thirty-three patients (17.4 %) were undergoing an antifungal treatment at the onset of candidemia.
Outcome and Predictors of Mortality
The 30-day cumulative mortality rate was 27.9 % (53 of 190), and more than half of those patients died within 7 days (31 of 53). The median time of death was 15 days (IQR, 5–46 days) after blood sample collection. Candida albicans and non-albicans Candida had similar hospital mortality rates; the highest mortality rate was observed for C. glabrata (60 %, 15 of 25 patients).
Univariate predictors of poor outcomes of candidemia are shown in Table 2. In the univariate analysis, patients who were treated with TPN, urinary catheters, and mechanical ventilation had a higher early (≤7-day) mortality rate. Patients residing in the ICU when Candida BSI was diagnosed were more likely to have early death [odds ratio (OR) 2.538; 95 % confidence interval (CI) 1.101–5.854; P = 0.025]. As shown in Table 2, predictors of 30-day mortality by univariate analysis among patients with candidemia were the presence of a CVC (OR 2.586; 95 % CI 1.016–6.580), the presence of a urinary catheter (OR 2.045; 95 % CI 1.017–4.112), and treatment with renal replacement therapy (OR 2.571; 95 % CI 1.101–6.005).
On multivariate analysis, the APACHE II score (OR 1.23; 95 % CI 1.080–1.390; P = 0.002) and severe sepsis or septic shock (OR 15.35; 95 % CI 2.391–98.502; P = 0.004) were independently associated with early mortality. Severe sepsis or septic shock (OR 24.75; 95 % CI 5.099–120.162; P < 0.001) was an independent risk factor for 30-day mortality, while proven catheter-related candidemia was a protective factor (OR 0.16; 95 % CI 0.031–0.810; P = 0.027) (Table 3).
Clinical Management of Candidemia
Kaplan–Meier 30-day survival curves for adequate antifungal therapy and early CVC removal showed that these patients had lower mortality, although no statistically significant differences were noted (P = 0.115 and 0.220, respectively, by log-rank test) (Fig. 2).
We also explored the effect of early interventions on mortality in patients with primary candidemia (Fig. 3).
Discussion
The current study included 190 patients with candidemia who were investigated with respect to species distribution, clinical features, and outcomes of Candida BSI.
The incidence of candidemia is reportedly 0.08–1.73 episodes per 1000 admissions worldwide [19–21], and several studies have revealed a substantial tread toward an increase in this incidence [19, 20]. However, we found that the incidence of candidemia steadily decreased steadily in our hospital over time (0.43–0.25 episodes per 1000 admissions from 2010 to 2014), which may reflect the improvements in the diagnosis and treatment of candidemia and differences in blood culture or antifungal resistance patterns.
The distribution of Candida spp. varies geographically. An international surveillance of bloodstream infections attributable to Candida species, the SENTRY antimicrobial surveillance program, showed that the prevalence of C. glabrata was higher in the United States than in other regions. In Latin America, C. tropicalis and C. parapsilosis were reported to be more prevalent than in the USA [5]. Candida albicans was also reportedly more prevalent in Canada and Europe than in other regions [6]. Additionally, because of the complexity of fungal culture and the longer time required, physicians usually used empiric therapy. Thus, clinicians should pay more attention to local distribution. A retrospective analysis from Shanghai showed that the episodes of non-albicans infections were more frequent than those of C. albicans [19], although C. albicans remained the most common species causing candidemia (37.2 %). This is similar to our observation (38.9 % for C. albicans vs. 61.1 % for non-albicans Candida); however, no C. guilliermondii or C. sake was found in our hospital. The reasons for the different distribution among regions are not fully understood, although prior exposure to antifungal drugs [22] and different antifungal tactics may partly explain the difference [13, 23].
In the present study, the proportion of C. albicans and non-albicans Candida species varied across different departments. Almost 52.1 % of episodes were found in the ICU, and C. glabrata was observed with a lower percentage (9.1 %) in the surgical ICU than in the internal medicine ICU (16.4 %), while Candida albicans (51.4 %) was more frequent in surgery wards.
We found that 88.9 % of Candida isolates (112 of 126) were susceptible to fluconazole, and no C. albicans isolates showed fluconazole resistance; however, 25.0 % of C. glabrata, 18.8 % of C. parapsilosis, and 11.1 % of C. tropicalis isolates were intermediately resistant or fully resistant to fluconazole. Susceptibility testing for five types of antifungal drugs for Candida spp. began only in November 2013 as a part of routine practice in our institution; susceptibility testing only for fluconazole had been performed before this time. With the exception of itraconazole (91.7 % susceptibility), these antifungal drugs exhibited 100 % susceptibility for C. albicans. Additionally, 5-flucytosine and amphotericin B showed 100 % susceptibility against non-albicans Candida strains. The ratios of susceptibility to voriconazole for C. parapsilosis and C. tropicalis were higher than those of fluconazole and itraconazole, and C. glabrata showed 100 % susceptibility (data not shown).
In our study, 18.9 % of patients received no targeted antifungal agent; this is similar to other Asian publications [10, 19]. We drew the following major conclusions after a review of our cases. First, some clinicians judged the blood culture result as false-positive, including pollution caused by non-standard blood sample collection. Second, a small portion of patients with Candida BSI had been discharged, been transferred, or died before the final blood culture results were obtained. Finally, some patients and their families refused to use antifungal drugs because of economic factors or other considerations. Most physicians selected an azole as the initial empiric antifungal therapy, especially fluconazole. Although echinocandin has great efficacy in the treatment of candidemia and has been recommended for use as first-line therapy [23], only 14.2 % of patients received echinocandin, while 2.6 % received a combination of an azole and echinocandin as first-line antifungal therapy. No unified treatment guideline exists, and the higher price of echinocandin may partly explain the differing empiric antifungal therapy plans.
Our study showed a lower overall 30-day mortality rate (27.9 %) than that in other reports [2, 9, 10]. Additionally, most of our patients died within 7 days (58.5 %, 31 of 53). To further explore the reason for the high early mortality of candidemia and to precisely analyze the impact of the therapeutic strategy at different time points, we further investigated the predictors of early (≤7-day) and overall 30-day mortality among our patients.
According to our univariate analysis, C. parapsilosis may be a positive factor for 30-day mortality (OR 0.414; 95 % CI 0.171–0.992; P = 0.043). Other studies also showed that this species was associated with a better outcome [24, 25]. Candida glabrata had the highest mortality rate (60 %) compared with other Candida spp., but there was no statistically significant relationship between C. glabrata and death. Candida glabrata usually had a lower susceptibility rate to commonly used antifungal drugs, including amphotericin B, and C. glabrata infections were more prevalent in older than younger cohorts [7, 26].
In our multivariate model, the independent risk factor for early mortality was the severity of illness as indicated by the APACHE II score or the presence of severe sepsis or septic shock. Because of the limitations of retrospective studies, we researched all-cause rather than candidemia infection-related mortality. The APACHE II score reflected the patients’ comprehensive clinical situation and was influenced by many factors. This may also explain the lack of an effective impact on reducing the mortality of patients who received appropriate antifungal treatment. Thus, further studies on mortality attributable to Candida are still needed.
Severe sepsis or septic shock was also independently associated with overall 30-day mortality. Meanwhile, the data of 30-day mortality highlighted the protective effect of CVC-related candidemia. The comorbid status at baseline, risk factors for the onset of candidemia, and Candida spp. distribution were not independent influences on early or 30-day mortality in the multivariate model.
Controversy remains about whether catheter removal can truly reduce the mortality rate. A systematic review of 14 studies demonstrated that catheter removal did not reduce complications or death in patients with candidemia [27]. In recent guidelines for candidiasis, removal of all CVCs was recommended at the initial treatment of candidemia even in neutropenic patients [13, 28]. A study by Garnacho-Montero et al. [4] showed that delayed catheter withdrawal increased the mortality of patients with candidemia, although catheter management did not improve the prognosis of secondary candidemia, and adequate antifungal therapy (<48 h) reduced the in-hospital death rate in patients with secondary candidemia. In the present study, the Kaplan–Meier survival curve showed that delayed CVC removal had higher 30-day mortality than the initial 48-h CVC removal, although the difference was not statistically significant.
The present study also assessed the influence on mortality of another intervention (appropriate antifungal therapy). In our study, 87 patients (45.8 %) received initial appropriate antifungal treatment (i.e., an appropriate sensitive drug was given at an adequate dosage within 5 days from the first blood culture draw). The Kaplan–Meier survival curve did not indicate statistical significance. We also explored the role of source control in primary candidemia. As shown in Table 2, primary candidemia infections had lower mortality and better protection for 30-day mortality, although there was no statistically significant difference. Figure 3 shows that early CVC removal and adequate antifungal therapy were related to decreased mortality in patients with primary candidemia; this is in agreement with the findings of Garnacho-Montero et al. [4].
Several limitations of this study should be taken into consideration. First, this was a retrospective study performed at a single center over a 5-year period, which could lead to selection bias. Second, the small sample size of this study might have affected the outcome of the multivariate analyses. Third, susceptibility testing for five sorts of antifungal drugs for Candida spp. began only in November 2013 as part of the routine practice in our institution; susceptibility testing only for fluconazole was performed before this time.
In conclusion, this study comprised a large cohort of patients diagnosed with nosocomial Candida BSI. Non-albicans Candida spp. ranked at the top (61.1 %), and the distribution of Candida spp. differed according to department. The APACHE II score and severe sepsis or septic shock were independently associated with early mortality. The origin of CVCs was a protective factor for 30-day mortality. Kaplan–Meier 30-day survival curves for adequate antifungal treatment and early CVC removal showed lower mortality, although there was no statistically significant difference. However, among the patients with primary candidemia, adequate antifungal therapy and early CVC removal did demonstrate a lower 30-day mortality rate.
References
Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39(3):309–17.
Horn DL, Neofytos D, Anaissie EJ, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis. 2009;48(12):1695–703.
Al TAH, Farahat FM, Al HMI, Al AAF, Perfect JR. Predictors and outcomes of Candida bloodstream infection: eight-year surveillance, western Saudi Arabia. Int J Infect Dis. 2014;21:5–9.
Garnacho-Montero J, Diaz-Martin A, Garcia-Cabrera E, de Pipaon MRP, Hernandez-Caballero C, Lepe-Jimenez JA. Impact on hospital mortality of catheter removal and adequate antifungal therapy in Candida spp. bloodstream infections. J Antimicrob Chemother. 2013;68(1):206–13.
Pfaller MA, Diekema DJ, Jones RN, et al. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J Clin Microbiol. 2001;39(9):3254–9.
Bassetti M, Merelli M, Righi E, et al. Epidemiology, species distribution, antifungal susceptibility, and outcome of candidemia across five sites in Italy and Spain. J Clin Microbiol. 2013;51(12):4167–72.
Milazzo L, Peri AM, Mazzali C, et al. Candidaemia observed at a university hospital in Milan (northern Italy) and review of published studies from 2010 to 2014. Mycopathologia. 2014;178(3–4):227–41.
Wu SX, Guo NR, Li XF, et al. Human pathogenic fungi in China—emerging trends from ongoing national survey for 1986, 1996, and 2006. Mycopathologia. 2011;171(6):387–93.
Wisplinghoff H, Ebbers J, Geurtz L, et al. Nosocomial bloodstream infections due to Candida spp. in the USA: species distribution, clinical features and antifungal susceptibilities. Int J Antimicrob Agents. 2014;43(1):78–81.
Kim SH, Yoon YK, Kim MJ, Sohn JW. Clinical impact of time to positivity for Candida species on mortality in patients with candidaemia. J Antimicrob Chemother. 2013;68(12):2890–7.
Kim SH, Yoon YK, Kim MJ, Sohn JW. Risk factors for and clinical implications of mixed Candida/bacterial bloodstream infections. Clin Microbiol Infect. 2013;19(1):62–8.
Garey KW, Rege M, Pai MP, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006;43(1):25–31.
Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(5):503–35.
Parkins MD, Sabuda DM, Elsayed S, Laupland KB. Adequacy of empirical antifungal therapy and effect on outcome among patients with invasive Candida species infections. J Antimicrob Chemother. 2007;60(3):613–8.
Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts: third informational supplement M27-S3.CLSI, Wayne, PA, USA, 2008.
Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts: 4th informational supplement M27-S4.CLSI, Wayne, PA, USA, 2012.
Rodriguez D, Park BJ, Almirante B, et al. Impact of early central venous catheter removal on outcome in patients with candidaemia. Clin Microbiol Infect. 2007;13(8):788–93.
Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1–45.
Yang ZT, Wu L, Liu XY, et al. Epidemiology, species distribution and outcome of nosocomial Candida spp. bloodstream infection in Shanghai. BMC Infect Dis. 2014;14:241.
Bassetti M, Taramasso L, Nicco E, et al. Epidemiology, species distribution, antifungal susceptibility and outcome of nosocomial candidemia in a tertiary care hospital in Italy. PLoS One. 2011;6(9):e24198.
Arendrup MC, Dzajic E, Jensen RH, et al. Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: data from a nationwide fungaemia surveillance programme. Clin Microbiol Infect. 2013;19(8):E343–53.
Garnacho-Montero J, Diaz-Martin A, Garcia-Cabrera E, et al. Risk factors for fluconazole-resistant candidemia. Antimicrob Agents Chemother. 2010;54(8):3149–54.
Cornely OA, Bassetti M, Calandra T, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18(Suppl 7):19–37.
Almirante B, Rodriguez D, Cuenca-Estrella M, et al. Epidemiology, risk factors, and prognosis of Candida parapsilosis bloodstream infections: case-control population-based surveillance study of patients in Barcelona, Spain, from 2002 to 2003. J Clin Microbiol. 2006;44(5):1681–5.
Malani A, Hmoud J, Chiu L, Carver PL, Bielaczyc A, Kauffman CA. Candida glabrata fungemia: experience in a tertiary care center. Clin Infect Dis. 2005;41(7):975–81.
Malani AN, Psarros G, Malani PN, Kauffman CA. Is age a risk factor for Candida glabrata colonisation. Mycoses. 2011;54(6):531–7.
Nucci M, Anaissie E. Should vascular catheters be removed from all patients with candidemia? An evidence-based review. Clin Infect Dis. 2002;34(5):591–9.
Limper AH, Knox KS, Sarosi GA, et al. An official American Thoracic Society statement: treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med. 2011;183(1):96–128.
Acknowledgments
The collection, analysis, and interpretation of data in this work were supported by Research Support Foundation, Military health and disease prevention and control research (13BJYZ32).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no declared conflict of interests.
Ethical Approval
This study was approved by the local institutional review board.
Additional information
Ying Li and Mingmei Du have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, Y., Du, M., Chen, La. et al. Nosocomial Bloodstream Infection Due to Candida spp. in China: Species Distribution, Clinical Features, and Outcomes. Mycopathologia 181, 485–495 (2016). https://doi.org/10.1007/s11046-016-9997-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-016-9997-3