The process of argon arc welding of nickel onto titanium with a nonconsumable electrode with feeding of the nickel filler wire into the liquid metal pool is investigated. The influence of the welding modes on the geometrical parameters of the bead and on the susceptibility of the forming surface layer to crack formation is described. The effect of the welding modes on the chemical and phase compositions of the formed surface layer of titanium nickelide is determined. The chemical and phase compositions of the welded-on layer are shown to affect its hardness and wear resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intermetallic alloys of the Ti – Ni system promise much in various engineering applications as materials possessing shape memory, superelasticity, high mechanical properties and wear resistance [1,2,3,4]. The special tribological properties of titanium nickelide in combination with high damping capacity and corrosion and erosion resistance [5,6,7,8] make it a good candidate for parts serving in friction assemblies at elevated temperatures.
Coatings and surface layers based on nitinol are obtained by various processes, i.e., cladding (explosion, hot isostatic pressing, SHS technologies), sputtering (gas-plasma, plasma, detonation), welding-on, deposition from molten salts, deposition from organometallic compounds and from a gas phase, and various combinations of these methods [9,10,11].
Built-up layers based on intermetallics of the Ti – Ni system have been obtained on titanium by the method of double-arc cladding with titanium and nickel electrode wires. The process gives deposited layers based on titanium nickelides of different compositions [10]. However, the difficulties with control of the process of double-arc surfacing limit its application. Arc welding with a nonconsumable electrode in an argon medium with the use of nickel filling wire is an alternative method to create titanium nickelide surface layers on titanium.
This method is simple to implement and makes it possible to obtain deposited layers with a wide range of chemical and phase compositions, mechanical and operating properties, because the deposition process can be controlled.
The aim of the present work was to study to process of argon arc deposition of nickel onto titanium surface using nickel filling wires and to analyze the structure and the properties of the surface layer obtained.
Methods of Study
Sheets from titanium VT1-0 with a thickness of 10 mm were subjected to argon arc welding with a nonconsumable electrode and nickel filling wire. The surfacing was conducted in an automatic mode in a specially designed two-coordinate unit [12]. The welding current source was a Svarog 315 P AC/DC invertor apparatus; the process materials were argon (GOST 10157–2016) and filling wire NP-2 with diameter 1.2 mm. The wire was introduced and fused directly in the liquid metal pool. Titanium can be surfaced with titanium nickelide beads with different chemical and phase compositions depending on the volumes of the fused titanium in the metal pool and of the introduced nickel wire. We used the following surfacing modes: current intensity I = 270 A, deposition speed Vd = 0.18 m/min, argon flow rate 10 – 12 liters/min. The wire feeding speed (Vw ) was varied in the range of 1 – 4 m/min. Control of the feeding speed allowed us to govern the chemical composition of the surfaced alloys.
The geometrical parameters of the deposited beads were measured on transverse laps (Fig. 1) using the Universal Desktop Ruler software after specifying the scale on the photographs of the samples.
The chemical composition and the microstructure of the surfaced metal were determined by scanning electron microscopy using a LEO 1455 VP (ZEISS) microscope with an INCA Energy-300 x-ray energy spectrometer and an INCA Wave-500 x-ray wave spectrometer.
The x-ray phase analysis was made using a Bruker D8 Advance Eco x-ray diffractometer with vertical θ – θ goniometer. The alloys of the Ni – Ti system were analyzed using the Bragg–Brentano imaging scheme. The images were obtained in copper anode radiation (λ = 1.54060 Å). The tube voltage was 40 kV; the filament current was 25 mA. The Bragg–Brentano geometry was imaged using a nickel Kβ-filter. The specimens were studied for reflection; the intensity of the diffraction pattern was detected suing a linear-type position- sensitive SSD160 detector with 160 channels. The phases were identified using the Diffrac. EVA software and the Powder Diffraction File-2 data base (The International Center for Diffraction Data).
The susceptibility to formation of cracks was assessed in terms of the number of cracks and detachment of the metal on the specified surfaced area with length 100 mm.
The surface hardness HRC of the deposited beads was measured using an HBRV-187.5 stationary universal hardness tester in accordance with the GOST 9013–59 Standard. The hardness value was averaged after four measurements.
The samples were tested for abrasive wear by friction against fixed abrasive particles. The relative wear resistance was calculated by the equation
where ls is the linear wear of the standard sample and lm is the linear wear of the material tested. The material of the standard was titanium VT1-0.
Results and Discussion
It has been shown that the tested modes of surfacing of titanium with Ti – Ni alloys produce beads with steady geometrical parameters (Fig. 2).
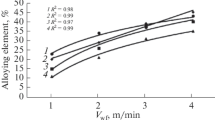
The depth of penetration varies within 2.7 – 3.5 mm. The width of the beads amounts to 13.5 – 15.0 mm, and the reinforcement is 0.2 – 2.3 mm. Increase of the speed of feeding of the filling wire raises the height of the reinforcement and the width of the beads. The penetration depth decreases with increase of the speed of feeding of the filling wire (Fig. 3).
The chemical composition of surfaced beads is determined mostly by the speed of feeding of the filling wire. The nickel content varies from 15 to 70% (Fig. 3b ) upon variation of Vw from 1.0 to 3.5 m/min.
The deposited layer had the following phase composition: Ti-based α-phase, Ti2Ni, TiNi and TiNi3. When the filling wire was fed at a speed of 1 m/min, the deposited layer consisted of a matrix based on an α-phase supersaturated with nickel to 11.5% and an α (Ti) + Ti2Ni eutectic (Fig. 5a – c) according to the data of the x-ray phase analysis and of the Ti – Ni phase diagram (Fig. 4) [11]. The supersaturation of the α-Ti with nickel and the higher concentration of nickel in the eutectic are explainable by the high rate of cooling of the fused metal under the surfacing [13].
Titanium – nickel phase diagram [11].
At Vw = 1.5 – 2.0 m/min, the structure was represented by a primary Ti2Ni phase with about 36% Ni and an α(Ti) + Ti2Ni eutectic (Fig. 5d – f ). The content of nickel in the alloys deposited at Vw = 2.5 m/min was 46 – 48%, and the structure consisted of two phases, i.e., Ti2Ni and TiNi (Fig. 5g – i ).
At Vw = 3.0 m/min, the average content of nickel in the layer was about 55%. It had a single-phase structure represented by TiNi nitinol according to the chemical composition and to the data of the x-ray phase analysis (Fig. 5j and k ). Increase of the feeding speed to Vw = 3.5 m/min caused appearance of phase TiNi3 in the deposited layer.
Assessment of the susceptibility of the deposited layer to formation of cracks in terms of their number in the controlled region showed that all the beads except for the nitinol-based ones deposited at the sped Vw = 3.0 m/min had transverse cracks (see Table 1).
The number of cracks was the highest in the alloys based on the Ti2Ni phase deposited with the feeding speed of 2.0 m/min (see Table 1). These beads had high brittleness, and the base metal flaked off during preparation of the laps and of the samples (Fig. 6).
The hardness of the beads was chiefly determined by their chemical and phase compositions and varied within 41 – 55 HRC (Fig. 7a ).
The growth in the hardness with the increase of Vw from 1.0 to 2.0 m/min was connected with elevation of the content of the hard Ti2Ni phase in the structure of the deposited metal. At Vw = 2.0 – 2.5 m/min and nickel content of 35 – 45%, the hardness of the deposited layer was at a level of 52 – 55 HRC. The dominant phase in the structure of layer was Ti2Ni.
At Vw = 2.0 m/min, the structure of the layer acquired a TiNi phase, which lowered the hardness of the deposited metal. At Vw = 3.0 m/min, the deposited metal had a virtually single-phase (TiNi) structure with hardness 45 – 46 HRC, which matches the data on the hardness of nitinol. Further increase of the speed of feeding of the filling wire caused appearance of phase TiNi3 in the deposited layer and raised its hardness.
The relative wear resistance of the layer varied within 0.78 – 2.2. The minimum values of the relative wear resistance were detected in the deposited layer based on a brittle intermetallic Ti2Ni phase at Vw ~ 2.0 m/min [9] (Fig. 7b ). The relative wear resistance was the highest at Vw = 3.0 m/min (Fig. 7b ), when the deposited layer had a single-phase nitinol-based structure (TiNi). The relative wear resistance of the beads deposited a Vw > 3 m/min decreased due to formation of a brittle TiNi3 phase in their structure.
Conclusions
-
1.
Arc surfacing with a nonconsumable electrode in an argon medium with nickel filling wire makes it possible to form satisfactory-quality beads with steady geometric parameters on titanium. The phase composition of the deposited intermetallic layer involves α(Ti), Ti2Ni, TiNi, and TiNi3 phases depending on the surfacing mode.
-
2.
The deposited layer based on the TiNi intermetallic phase has the lowest susceptibility to formation of cracks and the highest relative wear resistance as compared to the properties of the layers based on phases Ti2Ni and TiNi3. The hardness of the layer based on phase TiNi amounts to 45 – 46 HRC.
References
V. E. Gunter, V. N. Khodorenko, Yu. F. Yasenchuk, et al., Titanium Nickelide. New-Generation Medical Material [in Russian], Izd. “MITS,” Tomsk (2006), 296 p.
I. V. Protokovilov, I. A. Skiba, and D. A. Petrov, “Technological aspects of magnetically controlled electroslag melting and thermomechanical treatment of titanium nickelide,” Sovr. Elektrometall., No. 2, 17 – 20 (2012).
M. A. Khusainov, “Phase transitions in shape memory titanium nickelide alloys,” Vestn. Novgorod. Gos. Univ., No. 3(86), Pt. 2, 81 – 84 (2015).
S. Yu. Kondrat’ev, O. G. Zotov, G. Ya. Yaroslavskii, et al., “Investigation of interrelationship between dumping capacity and mechanical properties as well as morphology of martensite in alloys with reversible martensite transformation,” Probl. Prochn., 14B(3), 79 – 82 (1983).
S. Yu. Tarasov, “A study of tribotechnical properties of titanium nickelide,” Perspect. Mater., No. 5, 24 – 30 (1998).
D. Y. Li and D. Y. Liu, “The mechanism responsible for high wear resistance of pseudo-elastic TiNi alloy — a novel tribo-material,” Wear, 225 – 229(2), 777 – 783 (1999).
D. Y. Li, “Development of novel tribo composites with TiNi shape memory alloy matrix,” Wear, 255(1 – 6), 617 – 628 (2003).
S. Yu. Kondrat’ev, G. Yu. Yaroslavskii, and B. S. Chaikovskii, “Classification of high-damping metallic materials,” Strength Mater., 18(10), 1325 – 1329 (1986).
Zh. M. Blednova, N. A. Makhutov, and M. I. Chaevskii, Surface Modification of Shape Memory Material [in Russian], Izd. Dom-Yug, Krasnodar (2009), 354 p.
A. I. Kovtunov, D. I. Pakhotnyi, T. V. Semistenova, et al., “A study of the structure and properties of welded-on coatings based on titanium nickelide,” Perpekt. Mater., No. 4, 81 – 88, (2021).
Zh. M. Blednova, N. A. Makhutov, and P. O. Rusinov, “Prospects of application of shape memory materials for formation of multifunctional coatings on machine-building articles,” Zavod. Lab., Diag. Mater., 79(11), 49 – 56 (2013).
A. I. Kovtunov and A. G. Bochkarev, “A study of composition, structure and properties of welded-on alloys of the chromium-alloyed titanium-aluminum system,” Uproch. Tekhnol. Pokr., 17[10(202)], 470475 (2021).
N. P. Lyakishev (ed.), Phase Diagrams of Binary Metallic Systems, A Reference Book, Vol. 3, Book 1 [in Russian], Mashinostroenie, Moscow (2001), 872 p.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 8, pp. 43 – 47, August, 2023
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kovtunov, A.I., Khokhlov, Y.Y., El’tsov, V.V. et al. Investigation of the Processes of Formation and Properties of Titanium Nickelides Welded on Titanium. Met Sci Heat Treat 65, 501–505 (2023). https://doi.org/10.1007/s11041-023-00961-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-023-00961-8