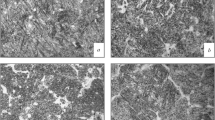
Surfaced layers formed by arc cladding of powder wires produced by the Siberian State Industrial University and used for surfacing articles operating under abrasive wear are studied. The chemical composition, the microstructure and the hardness of the deposited layers and of the nonmetallic inclusions formed in them are analyzed. Titanium addition in the wires is shown to affect positively the wear resistance of the layers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Quarry and mining equipment undergoes abrasive and impact wear in operation and is susceptible to early failure. Since purchasing of novel parts and machines takes time and money, reconditioning and repair of quarry and mining mechanisms is an important task. The repair works require development of novel surfacing materials, which prolong the service life and raise considerably the wear resistance of such mechanisms [1,2,3].
The main criterion for the development of novel surfacing materials is the choice of the alloying system [4,5,6]. This requires allowance for such factors as the cost of the surfacing material, the operating conditions of the articles, the results of tests of various materials by the standard methods and under actual operating conditions, etc. If the chemical composition for the powder wire has been selected correctly and optimally, we can obtain a clad layer with high tribological properties (the hardness and the abrasive and impact-abrasive wear resistance).
Today, reconditioning with surfacing of powder wires onto the worn surfaces of machines and mechanisms seems to be the most promising variant [7, 8]. The power wires to be surfaced on the quarry and mining equipment are manufactured using gas-cleaning dust of the aluminum production. The chemical compositions for such wires have been patented in the RF [9, 10].
The present work continues the studies devoted to development of novel compositions for powder wires to be used for surfacing of articles operating under conditions of abrasive wear in the mining industry. Specifically, we have studied the wear resistance and the hardness of layers clad from powder wires containing electric filter dust of the aluminum production, which bears carbon and fluorine, and a titanium powder component.
The aim of the present work was to study the microstructure, to determine the composition of the nonmetallic inclusions, and to perform tribological tests of a layer clad by the electric arc method with the use of powder wires of a novel developed composition.
Methods of Study
We designed and prepared blend materials for novel powder wires. The chosen blend materials were stirred, dried in an oven for 4 h, and poured into the hopper for making powder wires. The blends were composed of the following materials: aluminum production gas-cleaning dust containing carbon and fluorine (to replace amorphous carbon) [11], powders of titanium PTS (TU 14-22-57–92), iron PZhV-1 (GOST 9849–86), nickel PNK 1L5 (GOST 9722–97), chromium PKh-1S (TU 14-1-1474–75), manganese MP-0 (GOST 6008–82), and silicon KR-1 (GOST 2169–69). The housing of the powder wire was a ribbon from steel St3. The diameter of the obtained powder wire was 5 mm.
Five specimens of powder wire differing in the content of titanium powder were clad onto a substrate from steel 09G2S. The specimens were flux-cord welded using the equipment of the Research and Production Center “Welding Processes and Technologies” at the following parameters: current intensity 500 A, voltage 28 V, welding speed 15 cm_min. The flux of grade NFP had been developed earlier (TU 20.59.56.120-001-14796818–2020) and produced from the technological raw material of the West-Siberian Electrometallurgy Plant [12].
The chemical composition of the clad layers was determined using the equipment of the common access center “Materials Science” by the x-ray fluorescent technique (XRF-1900 spectrometer) and by the atomic emission technique (DFS-71 spectrometer).
The structure of the clad layers was studied using an OLYMPUS GX-51 metallographic microscope against light background with magnification from × 100 to × 1000 and the Siams Photolab 700 software. To assess the chemical composition of nonmetallic inclusions in the clad layers and the distribution of elements in the inclusions we used a Tescan MIRA 3 scanning electron microscope. The level of the contamination with nonmetallic inclusions was assessed according to GOST 1778–70. The laps with the clad layer fabricated for the analysis of the microstructure were subjected to polishing and chemical etching in a 4% solution of nitric acid. The grain size and the structural characteristics were determined according to GOST 5639–82 at a magnification × 100 and according to GOST 8233–56 and a magnification × 1000 by comparison with the standard scales.
The hardness of the layers was measured by the Brinell and Rockwell methods in accordance with the requirements of GOST 9012–59 and GOST 9013–59. The tribological tests were performed in a 2070 SMT-1 machine in the disk-pin mode for 4 h at a load of 78.4 N and a frequency of 20 rpm.
Results and Discussion
The chemical compositions of the clad layers of the metal obtained with the use of the fabricated wire are presented in Table 1. The coefficient of withdrawal of titanium is 6.2 – 8.6%. Analysis of the mechanical and tribological characteristics (Table 2) shows that growth of the content of alloying elements in the deposited layer changes its properties substantially. For example, the increase of the titanium content in the clad layer from 0.005 to 0.075% results in a linear increase of the hardness of the ready article from 35.61 to 53.11 HRC. The wear intensity of the specimens with clad layer decreases by a factor of 3. Introduction of an addition of gas-cleaning dust of aluminum production containing carbon and fluorine into the blend results in growth of the carbon content in the clad layer.
We chose specimens T1 and T5 with minimum and maximum contents of titanium powder for further study of their surface by scanning electron microscopy. The best mechanical and tribological properties were exhibited by specimen T5.
The study of the surface of laps T1 and T5 showed the presence of inclusions of different morphologies. The size of the inclusions did not exceed 18 μm. The microscopic x-ray spectrum analysis gave us the elemental composition of an individual inclusion. The results of such an analysis are presented in Figs. 1 – 4 and Table 3. It can be seen from Table 3 that the distribution of elements in clad specimens T1 and T5 is not uniform and the elements form different compounds.
We studied three nonmetallic inclusions with diameter 7.14 μm (spectrum 1 ), 14.28 μm (spectrum 2 ) and 9.52 μm (spectrum 3 ) on the chosen area of specimen T1. Analysis of the chemical composition of the inclusions shows that their main components are oxides of aluminum, silicon, titanium and manganese; magnesium, sodium and calcium are present in inconsiderable amounts (Figs. 1 and 2, Table 3).
Energy spectra obtained from regions 1 (a), 2 (b ) and 3 (c) in the structure of specimen T1 (see Fig. 1a ).
The chemical compositions of inclusions in specimen T5 are presented in Figs. 3 and 4 and in Table 3. It can be assumed that these inclusions are mostly nitrides and sulfides of iron, manganese, titanium and aluminum. The sizes of the inclusions in this specimen vary from 0.11 to 3.76 μm.
The planes of the laps of the clad layers of specimens T1 and T5 also contain randomly distributed sulfide inclusions of different morphologies, the size of which does not exceed 2.5 μm. Sulfide inclusions are mostly located in grain boundaries (Fig. 5).
The analysis of the microstructure of the clad metal after electrolytic etching showed that specimens T1 and T5 had identical dendritic (columnar) structures typical for a cast metal and represented by martensite. Scanning electron microscopy allowed us to measure the sizes (the length) of the martensite needles (Fig. 5, Table 4). In specimen T5 (0.075% Ti), the average size of the needles was 13.05 μm.
We obtained regression models describing the effect of the content of chemical elements entering the clad layer on the wear and hardness of the clad layer. The equation for calculating the wear rate (vw , 10–4 g/rev) is as follows:
The hardness of the clad layer is describable by the equation
with an approximation error of 0.00011%.
Conclusions
We have studied inclusions in layers surfaced on steel 09G2S with the use of powder wires with different amounts of added titanium powder and gas-cleaning dust of aluminum production, which contained carbon and fluorine to replace amorphous carbon. The chemical composition of such inclusions was represented by aluminum, silicon, titanium and manganese oxides. The clad layer also contained some magnesium, sodium and calcium.
By the data of the tribological tests, elevation of the content of titanium in the powder wire blend, and hence of the content of carbon in the clad layer, increases its hardness.
Introduction of an addition containing carbon and fluorine into the powder wire blend also raises the carbon content in the clad layer. However, this is accompanied by increase in the content of nonmetallic inclusions, which may affect unfavorably the physical and mechanical properties of the layer.
The content of this addition should be optimized to improve the quality of the clad layer. Another variant is to use refining additions that should lower the contamination of the layer with nonmetallic inclusions, which will be considered in our further studies.
References
D. B. Slinko, R. Yu. Sobolev, and A. B. Kaveshnik, “Experience in the use of plasmapowder surfacing in the reduction of the semi-axes stages subway escalators,” Trudy GOSNITI, Moscow, 212, 243 – 249 (2015).
V. G. Durakov, S. F. Gnyusov, B. V. Dampilon, and S. Z. Dekhonova, “Effect of technological parameters of electron-beam cladding on the structure of copper-chromium composites,” Izv. TPU, 320(2), 80 – 86 (2012).
A. I. Gusev, N. V. Kibko, N. A. Kozyrev, et al., “A study on the properties of the deposited metal by flux cord wires 40GMFR and 40H3G2MF,” IOP Conf. Ser., Mater. Sci. Eng., 150, 1 – 7 (012033) (2016).
Z. M. Khaltarov, A. S. Milonov, N. N. Smirnyagina, and V. M. Khaltanova, “Special features of phase composition and structure of layers based on TiB2 diboride formed in surface layers of carbon steels under intense electron beams in vacuum,” Vest. Buryat. Gos. Univ., 203 – 209 (2011).
N. N. Milushin, V. L. Osetkovskii, and I. V. Osetkovskii, “Surfacing with low-temperature heating of parts of metallurgical equipment with heat-resistant steels,” Zagot. Proizvod. Mahinostr., No. 10, 6 – 10 (2014).
V. F. Gromov, E. V. Kapralov, S. V. Raikov, et al., “Structure and properties of wear-resistant coatings clad by the electric arc method onto steel with powder wires,” Usp. Fiz. Met., 15, 211 – 232 (2014).
E. V. Kapralov, E. A. Budovskikh, V. E. Gromov, et al., “Formation of nanostructure-phase conditions and properties of wear-resistant cladding on steel,” Nanoinzheneriya, No. 4(46), 14 – 23 (2015).
A. I. Gusev, N. A. Kozyrev, N. V. Kibko, et al., “Effect of introduction of tungsten and chromium on the properties of the metal clad with powder wire of the Fe – C – Si – Mn – Mo – Ni – V – Co system,” Zagot. Proizvod. Mashinostr. (Lit. Svar. Proizvod.), 17(2), 56 – 60 (2019).
N. A. Kozyrev, A. I. Gusev, G. V. Galevskii, et al., “Powder wire. Patent RF No. 2641590, MPK8 B23 K35/36 B23 K35/36, FGBOU VO “Sibir. Gos. Industr. Univ.,’ No. 201612508/02 (03992),” appl. 22.06.2016.
A. A. Umanskii, N. A. Kozyrev, A. R. Mikhno, et al., “Powder wire. Patent RF No. 2726230, MPK8 B23 35/368, FGBOU VO ‘Sibir. Gos. Industr. Univ.,’ No. 2020100811,” Byull. Izobr. Polezn. Modeli, No. 19 (2020), appl. 09.01.2020, publ. 10.07.2020.
E. V. Kapralov, E. A. Budovskikh, V. E. Gromov, and Yu. F. Ivanov, “Nanostructure states and properties of a cladding formed on steel using powder wire,” Izv. Vysh. Uchebn. Zaved., Fiz., 58(4), 39 – 45 (2015).
N. A, Kozyrev, D. A. Titov, S. N. Starovatskaya, et al., “Effect of introduction of carbon-fluorine-containing addition and nickel into the blend for manufacturing powder wire of the C – Si – Mn – Cr – V – Mo system,” Izv. Vysh. Uchebn. Zaved., Chern. Metall., No. 4, 34 – 37 (2014).
The work has been performed within a state assignment (topic identifier 0809-2021-0013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallovedenie i Termicheskaya Obrabotka Metallov, No. 12, pp. 27 – 33, December2022
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shlyarov, V.V., Komarov, A.A., Kozyrev, N.A. et al. Microstructure and Tribological Properties of Metal Layer Deposited by Arc Cladding of Powder Wire Containing Titanium Powder. Met Sci Heat Treat 64, 705–711 (2023). https://doi.org/10.1007/s11041-023-00876-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11041-023-00876-4