Abstract
Background
Parkinson’s disease is a neurological disorder caused by the loss of dopaminergic neurons in the midbrain. Various mechanisms are involved in the incidence of the disease including oxidative stress. Several herbs and natural products may interfere with the oxidative-stress pathway due to their antioxidant effects.
Objective
Herein, we aimed to investigate the neuroprotective role of F. vaillantii extract on Parkinson’s in vitro and in vivo model owing to the presence of the bioactive agents with antioxidant properties.
Methods
In vitro experments showed that 6-hydroxydopamine could induce toxicity in PC12 cells. The impact of F. vaillantii extract on cell viability was measured by using MTT assay. Nuclear morphological changes were qualitatively evaluated employing Hoechst staining. The antioxidant activity of the extract was determined by ROS and lipid peroxidation assays. Tyrosine hydroxylase protein expression was measured by western blotting in PC12 cells. For in vivo study, movement parameters were evaluated.
Results
The results indicated that 75 µΜ of 6-OHDA induced 50% toxicity in PC12 cells for 24 h. Following post-treatment with F. vaillantii extract (0.1 mg/ml) for 72 h, we observed that the extract effectively prevented cell toxicity induced by 6-OHDA and reduced the apoptotic cell population. Furthermore, the extract attenuated the ROS level, lipid peroxidation and increased protein expression of TH after 72 h of treatment. In addition, oral administration of 300 mg/kg of F. vaillantii extract for 14 days improved locomotor activity, catalepsy, bradykinesia, motor coordination and reduced the apomorphine-caused rotation in 6-OHDA- induced Parkinson’s disease-like symptoms in male rats.
Conclusion
The present study suggests a protective role for the extract of F. vaillantii against oxidative stress-induced cell damage in the PC12 cells exposed to neurotoxin 6-OHDA which was verified in in vivo model by reducing the motor defects induced by 6-OHDA. This extract could be a promising therapeutic agent for the prevention of PD progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disease caused by the gradual death of dopaminergic neurons in the substantia nigra pars compacta (SNpc) of the midbrain [1]. The neuropathological hallmark of PD is aggregation of the α-Synuclein (α-Syn) proteins called Lewy body (LBs) fibrils in dopaminergic neurons [2]. PD is characterized by motor symptoms, including resting tremor, rigidity, bradykinesia, and postural and gait instability [3], and accompanied by non-motor symptoms such as cognitive impairment, apathy, depression/anxiety, sleep disorders, fatigue, autonomic dysfunctions, and sensory disturbances [4].
The exact etiology of PD is not fully elucidated due to several pathogenic mechanisms that are contributed to the neuronal degeneration in PD. Different factors such as oxidative stress [5], mitochondrial dysfunction [6], inflammation [7], calcium homeostasis [8], and excitotoxicity [9] trigger neurodegeneration in PD, among which oxidative stress plays a vital role in neuronal death. On the other hand, excessive reactive oxygen species (ROS) generation in the brain causes oxidative stress in PD patients [10]. Many sources and mechanisms are involved in brain ROS production, including the electron transport chain [11], auto-oxidation of dopamine [12], monoamine oxidase (MAO), NADPH oxidase (NOX) and intracellular calcium dysregulation [13]. Moreover, low levels of the reduced glutathione (GSH) [14] and high levels of iron deposition [15] in SNpc play significant roles in ROS generation. Growing evidence indicates that oxidative damage to the biomolecules consisting of lipids, proteins and DNA, is attributed to neural death in PD [16]. Indeed, oxidative stress in the brain renders cell membranes susceptible to lipid peroxidation resulting in impaired membrane integrity [17]. Furthermore, oxidative damage to proteins induces formation of protein aggregates which leads to mitochondrial dysfunction and apoptosis induction. It is worthy to mention that reactive species also cause DNA damage and then the accumulation of the damaged DNA results in the aberrant cell cycle entry and apoptosis [18].
6-Hydroxydopamine (6-OHDA) is a catecholaminergic neurotoxin that produces free radicals and causes oxidative stress. It is widely used to induce Parkinson’s model in the experimental cellular and animal studies. Although the primary 6-OHDA mechanism of action is the inhibition of mitochondrial complexes I and IV, 6-OHDA is oxidized to form semiquinone radicals participating in the ROS generation [19]. Dopamine is synthesized via sequential reactions catalyzed mainly by tyrosine hydroxylase (TH), a rate-limiting enzyme in biosynthesis of catecholamines neurotransmitters [20]. Current PD drugs are designed to lessen PD symptoms by increasing dopamine synthesis in the brain. L-DOPA, which is converted to dopamine in the brain, is the most common drug used for PD treatment [21]. Notably, L-DOPA could be another source of oxidative load and toxicity over time [22]. In this context, several studies have reported the neurotoxicity of L-DOPA, both in in vivo and in vitro, through ROS formation during L-DOPA auto-oxidation [23]. L-DOPA plays a double-edged role in intracellular ROS homeostasis; i.e., L-DOPA acts as ROS scavenger during the initial phase of treatment, wherase it can increase ROS level in long term as the auto-oxidation proceeds [24]. Therefore, natural products or the plants rich in polyphenols with antioxidant activity, would help the current treatment strategies to halt the disease progression.
Plant-based medicine in the past decades have attracted a great deal of attention worldwide. F. vaillantii is one of the medicinal plants used traditionally against various diseases. It is an annual plant in the genus Fumaria of the Fumariaceae (Papaveraceae) family and mainly grows around the Mediterranean region. The genus Fumaria comprises 60 species [25], of which seven have been reported in Flora Iranica [26]. These species are F. officinalis, F. parviflora, F. asepala, F. densiflora, F. schleicheri, F. vaillantii, and F. indica [27]. F. vaillantii grows in different parts of Iran, and its vernacular name is “Shatareh.”
As indicated in the Phytochemical investigations, F.vaillantii possesses chemical components, including alkaloids and various nonalkaloid compounds with pharmacological effects [28]. This herb has long been used in folk medicine as an antihypertensive, hepatoprotective, antiviral, and antimicrobial and also for treating skin diseases (rashes or conjunctivitis), arthritis, and gastrointestinal disease [28]. Recent data suggest that the genus Fumaria has anti-inflammatory, antinociceptive, and antioxidant properties [25, 29]. According to our literature survey, chloroformic, ethyl acetate, and n-butanol extracts of F. vaillantii have been determined to show antioxidant activity, among which the ethyl acetate extract possessed the highest antioxidant properties. This antioxidant activity may be associated with the phenolics and flavonoids present in the Fumaria species [25].
Considering the oxidative stress as an essential pathway in the neurodegeneration of PD and the antioxidative potential of F. vaillantii, in the present study, we examined the protective effects of post-treatment with F. vaillantii extract in in vitro and in vivo 6-OHDA model of PD.
Materials and methods
Drugs and reagents
RPMI 1640, fetal bovine serum (FBS), phosphate-buffered saline (PBS), and penicillin/ streptomycin were purchased from Gibco. 6-hydroxydopamine hydrochloride (6-OHDA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), Hoechst 33342, propidium iodide (PI), and 2′-7′- dichlorofluorescein diacetate were provided by Sigma-Aldrich. DMSO was obtained from Takara. Thiobarbituric acid (TBARS) was purchased from Merck.
Plant material and extraction procedure
F.vaillantii plant was collected from north of Iran in August 2014. A voucher specimen (No. 6563 TEH) was deposited at the Herbarium of the Faculty of Pharmacy at Tehran University of Medical Sciences and authenticated by Dr. Gholamreza Amin. The plant was collected following the national guidelines and regulations. The permission to collect plant was provided by the Faculty of Pharmacy at Tehran University of Medical Sciences. After the aerial parts of the plant were separated, they were dried in the dark for three days. 320 g of the dried powder were thoroughly mixed with ethanol: water (80:20) to make the total extract via maceration procedure three times at room temperature for 72 h. The extracts were evaporated to dryness and then kept at 4 °C [30]. The Research and Ethics Committee of Tehran University of Medical Sciences, School of Advanced Technologies in Medicine, approved the experimental protocol. All methods complied with relevant institutional, national and international guidelines and legislation.
Cell culture
PC12 cell line was purchased from Pasture Institute of Iran. Cells were grown in RPMI 1640 containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin. Cells were then maintained in a humidified atmosphere of 5% CO2 at 37 °C. We further waited for the cells to reach 80% confluence and split the cells in a ratio of 1:3 [31].
Cell viability assay
The effect of F. vaillantii on the viability of PC12 cells treated with 6-OHDA was determined using MTT assay. In the MTT assay, mitochondrial succinate dehydrogenase of living cells reduces the yellow reagent 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT) to purple MTT-formazan which is water-insoluble crystals dissolved in DMSO [32]. PC12 cells were seeded in a 96-well plate at 5000 cells/well density. They were then incubated for 24 h to allow cell attachment. Afterwards, the medium was replaced by a fresh one containing 6-OHDA (25 µM, 50 µM, and 75 µM). After 24 h of incubation, the medium was collected and replaced by the medium containing F. vaillantii extract (0.01, 0.1, 0.25 mg/ml) and then further incubated for 24 h, 48 h, and 72 h. Each treatment replicated 5 times. Finally, the medium was removed and 200 µL of MTT solution (5 mg/mL) was added to each well. The plates were incubated for another 4 h to allow the formation of formazan crystal. DMSO was added to dissolve the formed formazan crystal in each well. The absorbance was read at 570 nm using a microplate reader (BioTek, ELx800, USA), with background correction performed at 690 nm [33]. The percentage of viability was calculated by this formula:
% of Cell viability = 100*(ODsample/ODcontrol).
Hoechst staining
Hoechst staining was carried out to assess the impact of F. vaillantii extract on the apoptosis induced by 6-OHDA in PC12 cells. Indeed, Hoechst 33342 is used to observe nuclear alteration upon apoptosis induction. It binds to DNA and emits an intense blue fluorescence that is visualized under a fluorescent microscope [34]. To do it, cells were seeded in 24-well plates at 30,000 cells/well density in 500 µL media and then incubated for 24 h. The medium was removed and replaced by the fresh medium containing 6-OHDA (75 µM). After 24 h, 0.1 mg /ml of F. vaillantii extract was treated as above-mentioned. Cells were incubated for 72 h, rinsed with PBS, and 1 ml of paraformaldehyde 4% was added to each well and kept at 4 °C for half an hour to be fixed. Having washed thoroughly three times with PBS, the cells were stained with 3 mg/mL of Hoechst 33342, and further incubated for 30 min in the dark at room temperature. After washing with PBS, they were observed using a fluorescence microscope (inverted fluorescence microscope model: IM-3FL4) at 357 nm excitation and 447 nm emission wavelengths [35].
ROS assay
ROS assay is a fluorescence-based quantitative experiment. The effect of F. vaillantii on ROS generation induced by 6-OHDA was measured by using 2′,7′- dichlorofluorescein diacetate (DCFH-DA). DCFH-DA is a cell-permeable dye that freely enters the cell and is oxidized to DCF by ROS inside the cell which produces a green fluorescence signal detected by a flow cytometer [36]. PC12 cells were seeded and treated as previously mentioned, and then 1 ml of DCF solution was added to each well. The plate was incubated for 30 min at 37 °C in the dark. After washing with PBS to remove an unincorporated dye, the plate was read using a Cytation 3 imaging reader (BioTek instruments) set to 37 °C. Filter pair of a 485 excitation and a 528 emission with a photomultiplier tubes (PMT) sensitivity setting of 55 were used. The percentage of MFI (mean fluorescence intensity) values was calculated relative to the untreated control cells [35]. The cells were also photographed using a fluorescence microscope (Nikon Eclipse TS100). For this purpose, PC12 cells were pretreated with the indicated concentration of 6-OHDA for 24 h and then incubated with F. vaillantii extract as mentioned before. After washing the cells with PBS, DCFH-DA (10 µM) was added to the cells and further incubated for 30 h min at 37 h°C in the dark.
Lipid peroxidation assay
The number of lipid peroxides within a cell was measured via thiobarbituric acid assay (TBA test) [37]. PC12 cells were seeded in 12-well plates and treated with 75 µM of 6-OHDA (24 h) alone or in combination with F. vaillantii extract for further 24 h (post-treatment). Cells were then detached by a cell scraper, centrifuged and washed with 1× PBS. Following resuspending the cells in 120 µL of 1×PBS,100 µL of 1% SDS was added to the cell suspension and mixed with 4 mL of color reagent (prepared by mixing 320 mg of TBARS dissolved in 30 mL of 0.1 NaOH and 30 mL of 3.5 M diluted acetic acid). After boiling the mixture for 1 h at 100 °C in the dark, fluorescence intensity was measured at 520 nm excitation and 550 nm emission wavelengths using a BioTek microplate reader (ELx800, USA) [35].
Western blotting analysis of TH protein
PC12 cells were seeded in a 6-well plate at 50,000 cells/well density and were treated with 6-OHDA (75 µM) for 24 h and post-treated with F. vaillantii extract (0.1 mg/ml) for 72 h. Total protein was extracted using RIPA buffer (radioimmunoprecipitation assay buffer). The BCA Protein quantification kit was used to determine protein concentration. 40 µg of protein was loaded onto the 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to a 0.2 μm immune-Blot™ polyvinylidene difluoride (PVDF) membrane (Cat No: 162-017777; Bio-Rad Laboratories, CA, USA). The membranes were then blocked with 5% BSA (Cat No: A-7888; Sigma Aldrich, MO, USA) and incubated with Anti-Tyrosine Hydroxylase (cat No: ab137869, Abcam), and Anti-β actin-loading control Antibodies (Cat No: ab8227, Abcam). After washing, the membranes were incubated with Goat Anti-Rabbit IgG H&L (HRP) (Cat No: ab6721; Abcam) secondary antibody. The blots were visualized after incubating membranes with enhanced chemiluminescence (ECL) for 1–2 min. Protein expression was normalized to β-actin and was determined using Image J software [38].
Animals
Adult male albino Wistar rats, weighing 220–250 g, were purchased from Royan Institute of Iran and were kept in standard laboratory conditions (room temperature: 23 + 2 °C; illumination: 12 h light / dark cycle). The procedures of animal research and the experimental protocol were performed according to the International Guideline for the Care and Use of Laboratory Animals which was approved by the Research and Ethics Committee of Tehran University of Medical Sciences, School of Advanced Technologies in Medicine.
Group1: sham-operated: surgery without any treatment.
Group 2: 6-OHDA model, injected with 6-OHDA.
Group 3: injected with 6-OHDA and F. vaillantii (300 mg/kg. P.O, 14 days).
Surgery and drug treatment
The rats were anesthetized with an intraperitoneal injection of 80 mg/kg of ketamine and 10 mg/kg of xylazine and were fixed in a stereotaxic apparatus. 6-OHDA (12 µg 6-OHDA in 4 µL DMSO) was injected unilaterally into the right striatum with the following coordinates at two different points: 1st point (AP, + 0.5; ML, + 2.5; DV, − 5.0) and 2nd point (AP, − 0.9; ML, + 3.7; DV, − 6.5) via a microsyringe (Hamilton Bonaduz AG, Bonaduz, Switzerland) during 5 min to assure the solution diffusion (Fig. 1). 24 h after 6-OHDA microinjection, F. vaillantii (300 mg/kg) dissolved in saline and administered orally via gavage once daily for 14 consecutive days. The dose of F. vaillantii was selected based on previous studies [39] Various types of movement assessments were performed to observe the effect of plant extract on motor functions of 6-OHDA-induced PD in rats. All tests were conducted on the 14th day, 5 h after the last F. vaillantii gavage administration (Fig. 2).
Apomorphine-induced rotation
Apomorphine-induced rotation test reflects the hypersensitivity of the lesioned striatum which confirms the effects of selective neurotoxin (6-OHDA). Apomorphine (1 mg/kg in 0.5% ascorbic acid-saline) was injected intraperitoneally and five minutes after, the animals were monitored for the number of contralateral rotations induced by apomorphine. The results were expressed as rotation per 30 min and 5–7 rotations/min were considered the Parkinsonian model [40].
Rotarod test
To assess fore- and hind-limb motor coordination and balance in a rat model, the rotarod test was performed. The animal was trained for 3 consecutive days by placing on a cylindrical rotarod bar (80 mm) which rotated along its long axis with a constant speed of 5 rpm for 300s. On the test day, rats were placed on the rotarod bar with accelerating speeds, range 5– 44 rpm over a period of 600s. The time spent on the rotarod and the travel distance were recorded. The experimental protocol consisted of administrating the test procedure three times for each rat. The time to complete the task was recorded for each of three replicates. Subsequently, the mean response time was calculated across the triplicate trials for each rat.
Pole test
The motor coordination in rats was determined by pole test. Rat was placed facing upward on the top of a pole (1 cm diameter and 70 cm height). The time the rat took to turn head down and climb down to the bottom of the pole was recorded. The test was replicated three times for each rat, and the average time for three tests was calculated.
Bar test
Bar test was performed to measure Parkinson’s disease–induced catalepsy-like immobility in rats. In this test, the forepaws of rat were placed onto a bar (0.9 cm diameter and 10 cm above the platform) and the time took the rat removed one or both paws from the bar was recorded. The experiment was conducted three times for each rat. The mean, or average, time value was then calculated across the three trials.
Beam walking
Beam Walking was examined to evaluate akinesia and bradykinesia symptoms in rats. The animal was placed in one corner of the narrow beam (105 cm length, 4 cm width) and allowed to walk across the narrow beam from one end to the other, while recording the latency time to start walking within 1 min and the total time it took to arrive to the other end of the beam within 2 min. The results are expressed as the average total time (s) it took for the animals from each experimental treatment group to run the test.
Open field
Locomotor activity was evaluated by the open field test. After adaptation, the rats were placed in the center of the glass and were allowed to explore the center area for 15 min. The number of squares the animal crossed and total distance were recorded.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Significant differences were determined for multiple groups using one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison post-hoc test. In this study, statistical significance was set at P < 0.05.
Results
The effect of F. Vaillantii extract on cell viability
PC12 cells were exposed to different concentrations of 6-OHDA (25, 50, and 75 µM) dissolved in 0.05% DMSO for 24 h. Our data revealed that 6-OHDA at a concentration of 75 µM significantly decreased PC12 cell viability (Fig. 3a), which was selected for subsequent in vitro experiments.
Post-treatment of various concentrations (0.01, 0.1, 0.25 mg/ml) of F. vaillantii for 24 h, 48 h, and 72 h could significantly reverse 6-OHDA-induced cell toxicity and enhance cell viability compared with the 6-OHDA group. 0.1 mg/ml of F. vaillantii extract was the most effective concentration that could increase cell viability at 24 h and 48 h, although three concentrations acted similarly to revese the 6-OHDA –induce PC12 cells cytotoxicity after 72 h of treatment. Therefore, we selected 0.1 mg/ml of the extract as the most effective concentration for subsequent in vitro experiments at 72 h (Fig. 3b, c, d).
(a) Concentration-dependent cytotoxicity of 6-OHDA on cell viability after 24 h. (b) Effect of F. vaillantii extract at different concentrations (0.01, 0.1, 0.25 mg/ml) on PC12 cell viability for 24 h (b), 48 h (c), (d) 72 h (d) after exposure to 6-OHDA. Values are shown as means ± SEM of three independent experiments performed in 4 replicates. *p < 0.05 versus the untreated control cells; #p < 0.05, ##p < 0.01 and ###p < 0.001 versus 6-OHDA treated cells
Effects of F. Vaillantii extract on the 6-OHDA-Induced changes in nuclear morphology
Using Hoechst 33342 staining, changes in nuclear morphology were evaluated in the cells exposed to 6-OHDA. As shown in Fig. 4, nucleus of the cells receiving 6-OHDA are more condensed and also brighter under fluorescence, compared with the control group in which the nucleus staining is homogeneous and regular.Treating by F. vaillantii extract at 0.1 mg/ml for 72 h, could significantly prevent the nuclear morphology changes induced by 6-OHDA.
The effect of F. Vaillantii on 6-OHDA-induced intracellular ROS production
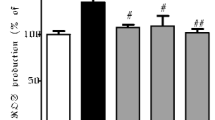
A quantitative and qualitative evaluation of oxidative stress in the PC12 cells was carried out by using the conversion of DCF-DA to fluorescent 2,7-dichlorodihydrofluorescein (DCFH). In the cells exposed to 75 µM of 6-OHDA, ROS production significantly increased. When the cells were treated with F. vaillantii extract at 0.1 mg/ml, ROS level reduced as much as to the control group (Fig. 5a), suggesting that F. vaillantii can prevent ROS production induced by 6-OHDA. Besides it, the cells were visualized by a fluorescence microscope. As shown in Fig. 5b, the green-fluorescent cells in the 6-OHDA treated group increased compared to the vehicle control, wherase the live stained cells after treatment with F. vaillantii were more than the 6-OHDA treated cells.
Effects of F.vaillantii extract (0.1 mg/ml) on 6-OHDA induced-ROS level in PC12 cells. (a) Spectrophotometric fluorescence intensity measurement. Values shown are as means ± SEM of three independent experiments performed in 4 replicates. ***p < 0.001 versus the untreated control cells, ###p < 0.001 versus the 6-OHDA treated cells. (b) Fluorescence microscopic image of treated cells
Effect of F. Vaillantii on 6-OHDA-induced lipid peroxidation by TBARS assay
6-OHDA (75 μM) induced lipid peroxidation in the PC12 cells. Post-treatment of the cells with 0.1 mg/ml of F. vaillantii extract for 72 h, significantly decreased lipid peroxidation induced by 6-OHDA (Fig. 6).
Effects of F. vaillantii extract (0.1 mg/ml), 6-OHDA, and 6-OHDA in the presence of F. vaillantii on lipid peroxidation in the PC12 cells. Values shown are means ± SEM of three independent experiments performed in 4 replicates. **p < 0.01 versus the untreated control cells; #p < 0.05 versus the 6-OHDA treated cells
Effect of F. Vaillantii on protein expression of tyrosine hydroxylase
TH protein expression was measured using western blotting. As it is shown in Fig. 7, exposing cells to 75 µM of 6-OHDA, reduced protein expression of TH to half compared with control cells (P < 0.01). However, the expression of TH significantly increased when cells were post-treated with F. vaillantii extract (0.1 mg/ml) for 72 h.
Representative Western blotting analysis of TH proteins expression. 6-OHDA decreased TH/β-actin ratio expression however F. vaillantii extract (0.1 mg/ml) reversed TH expression in the PC12 cells. Values shown are means ± SEM of three independent experiments performed in 2 replicates. **p < 0.01 versus the untreated control cells; #p < 0.05 versus the 6-OHDA treated cells
In vivo assay
Effect of F. Vaillantii on apomorphine-induced rotation in 6-OHDA-lesioned rats
At the end of the second week, the number of rotations during 30 min after intraperitoneal administration of apomorphine (1 mg/kg) in the lesion group was significantly higher compared to the sham group. As shown in Fig. 8a, treatment with 300 mg/kg of F.vaillantii significantly decreased apomorphine-induced rotations (F [2, 21] = 26.89, p < 0.001).
Effect of F. Vaillantii on rotarod test in 6-OHDA-lesioned rats
The rotarod test was performed two weeks after the injection of 6-OHDA. Our results showed that the time spent on the rotarod at a speed of 5–44 rpm in 600 s in rats treated with 6-OHDA was significantly decreased compared to the sham group. Gavage administration of 300 mg/kg F. vaillantii for 14 consecutive days after exposure to 6-OHDA, significantly increased time spent on the rotary (F [2, 21] = 11.81, p < 0.001). In addition, the distance traveled at 5–44 rpm in 600 s was significantly reduced in rats treated with 6-OHDA compared to the sham group. However, travel distance in F. vaillantii post-treated group was significantly more than the lesion group (F [2, 21] = 4.803, P <0.05) (Fig. 8c, b).
Effect of F. Vaillantii on pole test in 6-OHDA-lesioned rats
To further assess the neuroprotective effects of F.vaillantii extract in 6-OHDA-induced neurotoxicity model in rats, pole test was used to assess the motor coordination. 6-OHDA-treated rats exhibited apparent deficits in motor coordination. The motor function impairment was severe for the rat to turn at the top of the pole (F [2, 21] = 9.225, p <0.01) and climb down (F [2, 21] = 6.913, P <0.01) compared to the control group. Treatment with 300 mg/kg of F. vaillantii significantly exerted a recovery impact on the movement impairment (Fig. 8d, e).
Effect of F. Vaillantii on catalepsy test in 6-OHDA-lesioned rats
The 6-OHDA-lesion group showed a significant increase in the time on the bar compared with the sham group, implying muscle rigidity or catalepsy in 6-OHDA-treated rats. Treatment with 300 mg/kg of F. vaillantii counteracted such an increase. (F [2, 21] = 16.06, p < 0.001) and could effectively mitigate 6-OHDA-induced catalepsy (Fig. 8f).
Effect of F. Vaillantii on beam test in 6‑OHDA‑lesioned rats
In the beam balance test, the number of foot faults was increased in 6-OHDA treated rats compared to the control groups in 20 cm (F [2, 21] = 19.87, p < 0.001) and total (F [2, 21] = 5.578, p< 0.05) beam walking. Treatment with 300 mg/kg of F. vaillantii significantly decreased the number of foot faults and improved motor impairment (Fig. 8g, h).
Effect of F. Vaillantii on open‑field test in 6‑OHDA‑lesioned rats
Rats were placed in a 60 × 60 open field box and were observed for 10 min. There was a significant reduction in the total distance (F [2,21]=7.24; p<0.01) and number of crossings after 6-OHDA lesion (F [2,21]=4.555; p<0.05)which was then compensated by F. vaillantii post-treatment (Fig. 8j, i).
Effects of oral administration of F. vaillantii extract (300 mg/kg) for 14 consecutive days on 6-OHDA-induced motor impairment. Apomorphine-induced rotational behavior (a) distance and time spent on rotarod test (b, c), motor coordination, and balance in the pole test: both inversion and total time (d, e), time in bar test (f), beam walking test (g, h) and the open-field behavior (i, j). The values are means ± SEM for 8 rats in each group. *p < 0.05, **p < 0.01, ***p < 0.001, versus the untreated sham groups; #p < 0.05, ##p < 0.01###,p < 0.001 versus the 6-OHDA treated groups
Discussion
The present study was designed to examine whether F.vaillantii exctract provides neuroprotective properties against oxidative stress. The findings of this study demonstrated that the F. vaillantii plant extract possessed the capability to protect PC12 cells from the deleterious effects induced by 6-OHDA exposure. Furthemore, the in vivo animal investigation revealed that administration of the plant extract was able to remarekably ameliorate the motor impairment that was caused by 6-OHDA in male rat model.
F. vaillantii has been investigated in treating various diseases; however, no study has been yet described its neuroprotective role, particularly in PD. To our knowledge, this is the first study to reveal the protective role of F. vaillantii extract against the neurotoxicity induced by 6-OHDA in both in vitro and in vivo models of PD.
6-OHDA, a hydroxylated analog of dopamine, is a neurotoxin used in the experimental PD cells and animal models [41]. It generates cytotoxic free radicals through oxidation, which results in cell death [42]. In this context, our data exhibited that 6-OHDA induced 50% toxicity in PC12 cells, when the cells were exposed to 75 µΜ of 6-OHDA for 24 h. This finding is in agreement with a previous study reporting that exposing the PC12 cells to 75 µM of 6-OHDA for 24 h, declines cell viability to 54.21% [43]. Noteworthy, 6-OHDA induces cell death via increasing ROS level and inhibiting the mitochondrial respiratory chain complexes I and IV [19].
To asses post-treatment effect of F.vaillantii extract on inhibiting the 6-OHDA-induced toxicity, we treated cells with the increasing concentrations of plant exctract for 24, 48 and 72 h, following exposure to 6-OHDA. Our results revealed that 0.1 mg/ml of F.vaillantii extract effectively prevented cell toxicity induced by 6-OHDA at 72 h. Hence, we selected this concentration for our further analysis at 72 h. We then verified our MTT results using Hoechst 33342 staining. In this experiment, we observed that numbers of the apoptotic cells remarkably increased in the PC12 cells treated with 6-OHDA for 24 h compared with the control cells; however, F. vaillantii extract at 0.1 mg/ml could significantly decrease the apoptotic cells population after 72 h. Interestingly, we found a study in which the antiapoptotic effect of F. parviflora had been explored in the testicular tissue of male rats through enhancing Bcl-2 and decreasing Bax mRNA and protein expressions, inhibiting the mitochondrial depolarization, cytochrome c release, caspase activities along with suppressing the DNA fragmentation [39, 44]. Given these data and knowing that extract of other Fumaria species caused no cytotoxicity in the cells [45, 46], we suggest that F. vaillantii extract might be a suitable neuroprotective candidate. To prove this assumption, we performed additional experiments.
One of the mechanisms that reverses apoptosis in the neuronal cells is suppressing the oxidative-stress pathway [47]. To further corroborate the protective role of F. vaillantii against apoptosis under oxidative stress condition in PC12 cells, we quantitatively and qualitatively evaluated the intracellular ROS level upon post-treatment of 6-OHDA-treated PC12 cells with F. vaillantii extract. Our results showed that the extract attenuated the ROS level generating by 6-OHDA after 72 h of treatment. However, the green cells in the fluorescence imaging slightly elevated after incubating with extract, which may be due to the increased numbers of live cells after treatment with F. vaillantii extract compared to the 6-OHDA treated cells, alone. Consistently, Jaberian et al. reported the antioxidant characteristics of F. vaillantii extracts determined by the DPPH method [48]. Furthermore, Moghaddam et al. found that ethanolic extract of the aerial parts of F. vaillantii displays antioxidant activity and suggested F. vaillantii extract as a potential source of natural antioxidants [49]. In the current work, the antioxidant activity of F. vaillantii extract was also evaluated through the cell-based lipid peroxidation using TBARS, a potent oxidizing agent. In the TBARS test, the level of malondialdehyde (MDA) as the end product of lipid peroxidation is determined, which reflects the amount of lipid peroxidation in the cells [50]. We, herein, demonstrated that 6-OHDA increased MDA content, while F. vaillantii extract at 0.1 mg/ml could significantly decrease the amount of lipid peroxidation induced by 6-OHDA. In this regard, it was shown that F. vaillantii extract reduced MDA level that had been induced by acetaminophen and also increased glutathione content in mice liver tissue [51].
Evaluating TH expression is a hallmark to study dopaminergic neurons in different cell cultures [52]. For this reason, here, we revealed that 6-OHDA significantly reduces protein expression of TH compared with the control cells. Preveious studies have consistently shown that the expression of mRNAs and protein levels for TH in PC12 cells decreases 24 h after the 6-OHDA treatment [53]. It is also well-established that a number of TH-immunoreactive cells decreases after 6-OHDA injection in dopaminergic neurons in SNc in the rat brain [54, 55]. Furthermore, reports indicate that the mRNA expression of TH in the brain tissue of PD rats is reduced [56]. Additionally, the intraventricular administration of 6-OHDA has been found to decrease the total TH activity in the striatum [57, 58]. Postmortem samples from PD patients have also shown the loss of TH + fibres within the striatum [59,60,61]. Peventing the depletion of TH has been obsereved to induce neuroprotective effects in both in vitro and in vivo model of PD [62]. Interestingly, in our study, F.vaillantii treatment for 72 h, after exposure to 6-OHDA, increased TH protein expression to the level of the control cells. In this regard, it has been reported that ethanol extract of Gynostemma pentaphyllum, a phenolic acid [63], ameliorates the reduction of TH-immunopositive neurons induced by 6-OHDA in the rat brain and exerts neuroprotective effects [64]. Kaempferol, a flavonoid phytoestrogen, can also prevent the loss of TH-positive neurons induced by MPTP and shows anti-parkinsonian properties in the mouse model of PD [65]. Besides it, extract of safflower containing flavonoids, are found to be effective in models of neurodegenerative disease and prevents the loss of TH in a rotenone-induced rat model of PD [66].
Unilateral microinjection of 6-OHDA is widely used as a model of PD, resulting in motor deficits that closely mimic the symptomatic manifestations characteristic of PD in the animal [67]. As demonstrated by our results, post-treatment administration of F. vaillantii for 14 days, improved motor coordination and balance, catalepsy, bradykinesia and locomotor behavior in the 6-OHDA induced PD model in rats. It also considerably diminished the apomorphine-induced rotation, suggesting its neuroprotective role. To the extent of our knowledge, there is no previous study supporting the effect of this extract in an animal PD model. However, further experiments are needed to elucidate the exact mechanism underlying the neuroprotective effect of F. vaillantii.
Overall, our findings indicated that F. vaillantii helps to fight against oxidative stress in PC12 cells induced by 6-OHDA through decreasing ROS level, lipid peroxidation and preventing TH depletion. The antioxidant activity of F. vaillantii may be attributed to its phenolics and flavonoid contents [68]. According to our literature survey, the phenolic compounds are neuroprotective in the 6-OHDA model of PD through antioxidant activity [69]. Besides, polyphenols can inhibit nitric oxide (NO) and ROS level in the 6-OHDA rat model [70]. It has been well-known that flavonoids containing hydroxyl functional groups, are highly effective as free radical scavengers and antioxidant agents [48].
There are some limitations in our study that should be addressed in future research. First, we focused on evaluating the cell viability, antioxidant and antiapoptotic effect of F. vaillantii extract in the in vitro model of PD. Yet, further studies are necessary to confirm the results described. Hence, determing the expression level of the apoptosis-related proteins as well as the anti-oxidant enzymes would be of value to underly the mechanism of action of F. vaillantii as a neuroprotective agent.
Conclusion
In summary, the present study suggests a protective role for the extract of F. vaillantii against oxidative stress-induced cell damage in the PC12 cells exposed to neurotoxin 6-OHDA. Furthermore, oral administration of the plant extract was able to improve 6-OHDA-induced movement deficit in male rats. This extract could be a promising therapeutic agent for the prevention of PD progression. However, further experiments are higly required to elucidate the underlying mechanisms and explore the clinical applications of this natural extract for PD management.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- MDA:
-
Malondialdehyde
- 6-OHDA:
-
6-Hydroxydopamine
- PD:
-
Parkinson’s disease
- ROS:
-
Reactive Oxygen Species
- SNpc:
-
Substantia Nigra Pars Compacta
- TH:
-
Tyrosine Hydroxylase
Refrences
Md F (2011) Etiology and pathophysiology of Parkinson’s disease. BoD–Books on Demand
Wakabayashi K, Tanji K, Mori F, Takahashi H (2007) The Lewy body in Parkinson’s disease: molecules implicated in the formation and degradation of alpha-synuclein aggregates. Neuropathology 27(5):494–506
Lang AE (1998) Parkinson’s disease. N Engl J Med 339:1130–1153
Sauerbier A, Jenner P, Todorova A, Chaudhuri KR (2016) Non motor subtypes and Parkinson’s disease. Parkinsonism Relat Disord 22(Suppl 1):S41–S46
Jenner P (2003) Oxidative stress in Parkinson’s disease. Ann Neurol 53(Suppl 3):S26–36 discussion S-8
Beal MF (2005) Mitochondria take center stage in aging and neurodegeneration. Ann Neurol 58(4):495–505
Tansey MG, Wallings RL, Houser MC, Herrick MK, Keating CE, Joers V (2022) Inflammation and immune dysfunction in Parkinson disease. Nat Rev Immunol 22(11):657–673
Zaichick SV, McGrath KM, Caraveo G (2017) The role of ca(2+) signaling in Parkinson’s disease. Dis Model Mech 10(5):519–535
Iovino L, Tremblay ME, Civiero L (2020) Glutamate-induced excitotoxicity in Parkinson’s disease: the role of glial cells. J Pharmacol Sci 144(3):151–164
Dias V, Junn E, Mouradian MM (2013) The role of oxidative stress in Parkinson’s disease. J Parkinsons Dis 3(4):461–491
Yan MH, Wang X, Zhu X (2013) Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic Biol Med 62:90–101
Munoz P, Huenchuguala S, Paris I, Segura-Aguilar J (2012) Dopamine oxidation and autophagy. Parkinsons Dis 2012:920953
Mosharov EV, Larsen KE, Kanter E, Phillips KA, Wilson K, Schmitz Y et al (2009) Interplay between cytosolic dopamine, calcium, and α-synuclein causes selective death of substantia nigra neurons. Neuron 62(2):218–229
Chinta SJ, Kumar MJ, Hsu M, Rajagopalan S, Kaur D, Rane A et al (2007) Inducible alterations of glutathione levels in adult dopaminergic midbrain neurons result in nigrostriatal degeneration. J Neurosci 27(51):13997–14006
Nunez MT, Urrutia P, Mena N, Aguirre P, Tapia V, Salazar J (2012) Iron toxicity in neurodegeneration. Biometals 25(4):761–776
Dorszewska J, Kowalska M, Prendecki M, Piekut T, Kozlowska J, Kozubski W (2021) Oxidative stress factors in Parkinson’s disease. Neural Regen Res 16(7):1383–1391
Montine KS, Quinn JF, Zhang J, Fessel JP, Roberts LJ 2nd, Morrow JD, Montine TJ (2004) Isoprostanes and related products of lipid peroxidation in neurodegenerative diseases. Chem Phys Lipids 128(1–2):117–124
Thanan R, Oikawa S, Hiraku Y, Ohnishi S, Ma N, Pinlaor S et al (2014) Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int J Mol Sci 16(1):193–217
Glinka Y, Gassen M, Youdim MB (1997) Mechanism of 6-hydroxydopamine neurotoxicity. J Neural Transm Suppl 50:55–66
Nagatsu T, Nakashima A, Ichinose H, Kobayashi K (2019) Human tyrosine hydroxylase in Parkinson’s disease and in related disorders. J Neural Transm (Vienna) 126(4):397–409
Gandhi KR, Saadabadi A (2022) Levodopa (l-dopa). StatPearls Publishing, StatPearls [Internet]
Shulman LM (2000) Levodopa toxicity in Parkinson disease: reality or myth? Reality—practice patterns should change. Arch Neurol 57(3):406–408
Kostrzewa RM, Kostrzewa JP, Brus R (2002) Neuroprotective and neurotoxic roles of levodopa (L-DOPA) in neurodegenerative disorders relating to Parkinson’s disease. Amino Acids 23(1–3):57–63
Hormann P, Delcambre S, Hanke J, Geffers R, Leist M, Hiller K (2021) Impairment of neuronal mitochondrial function by L-DOPA in the absence of oxygen-dependent auto-oxidation and oxidative cell damage. Cell Death Discov 7(1):151
Ivanov I, Vrancheva R, Marchev A, Petkova N, Aneva I, Denev P et al (2014) Antioxidant activities and phenolic compounds in Bulgarian Fumaria species. Int J Curr Microbiol Appl Sci 3(2):296–306
Keshavarzi M, Ebrahimzadeh Araii F, Ghadam P, Habibi Tirtash F, Sheidaii M (2012) BIOSYSTEMATIC STUDY OF FUMARIA L.(PAPAVERACEAE) SPECIES IN IRAN. Iran J Bot 18(2):294–301
Tirtash FH, Keshavarzi M, Fazeli F (2011) Antioxidant components of Fumaria species (Papaveraceae). Int J Biomedical Biol Eng 5(2):57–60
Zhang R, Guo Q, Kennelly EJ, Long C, Chai X (2020) Diverse alkaloids and biological activities of Fumaria (Papaveraceae): an ethnomedicinal group. Fitoterapia 146:104697
Bribi N, Algieri F, Rodriguez-Nogales A, Garrido-Mesa J, Vezza T, Maiza F et al (2015) Antinociceptive and Anti-inflammatory effects of Total Alkaloid Extract from Fumaria capreolata. Evid Based Complement Alternat Med 2015:736895
Davoodi-Roodbordeii F, Afshar M, Haji Abas Tabrizi F, Choopani S, Torkaman G, Moayer F, Salimi M (2019) Topical hydrogel containing Fumaria vaillantii Loisel. Extract enhances wound healing in rats. BMC Complement Altern Med 19(1):254
Manouchehrabadi M, Farhadi M, Azizi Z, Torkaman-Boutorabi A (2020) Carvacrol protects against 6-Hydroxydopamine-Induced neurotoxicity in in Vivo and in Vitro models of Parkinson’s Disease. Neurotox Res 37(1):156–170
Farmani F, Moein M, Amanzadeh A, Kandelous HM, Ehsanpour Z, Salimi M (2016) Antiproliferative evaluation and apoptosis induction in MCF- 7 cells by Ziziphus Spina Christi Leaf extracts. Asian Pac J Cancer Prev 17(1):315–321
Tabrizi FH, Irian S, Amanzadeh A, Heidarnejad F, Gudarzi H, Salimi M (2016) Anti-proliferative activity of Fumaria vaillantii extracts on different cancer cell lines. Res Pharm Sci 11(2):152–159
Chazotte B (2011) Labeling nuclear DNA with hoechst 33342. Cold Spring Harb Protoc 2011(1):pdb prot5557
Shah M, Rajagopalan S, Xu L, Voshavar C, Shurubor Y, Beal F et al (2014) The high-affinity D2/D3 agonist D512 protects PC 12 cells from 6‐OHDA‐induced apoptotic cell death and rescues dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. J Neurochem 131(1):74–85
Afsharirad T, Tahmasvand R, Amini M, Daraei B, Salimi M (2020) Two novel anticancer compounds with minimum cardiotoxic property. BMC Pharmacol Toxicol 21(1):79
Draper H, Hadley M (1990) [43] Malondialdehyde determination as index of lipid peroxidation. Methods in enzymology, vol 186. Elsevier, pp 421–431
Babaei H, Alibabrdel M, Asadian S, Siavashi V, Jabarpour M, Nassiri SM (2018) Increased circulation mobilization of endothelial progenitor cells in preterm infants with retinopathy of prematurity. J Cell Biochem 119(8):6575–6583
Dolatkhah MA, Shokoohi M, Charvandeh S, Tvrda E, Shoorei H, Moghimian M, Alihemmati A (2020) Fumaria parviflora regulates oxidative stress and apoptosis gene expression in the rat model of varicocele induction. Andrologia 52(11):e13826
Bigham M, Mohammadipour A, Hosseini M, Malvandi AM, Ebrahimzadeh-Bideskan A (2021) Neuroprotective effects of garlic extract on dopaminergic neurons of substantia nigra in a rat model of Parkinson’s disease: motor and non-motor outcomes. Metab Brain Dis 36:927–937
Hernandez-Baltazar D, Zavala-Flores LM, Villanueva-Olivo A (2017) The 6-hydroxydopamine model and parkinsonian pathophysiology: novel findings in an older model. Neurologia 32(8):533–539
Hou JG, Cohen G, Mytilineou C (1997) Basic fibroblast growth factor stimulation of glial cells protects dopamine neurons from 6-hydroxydopamine toxicity: involvement of the glutathione system. J Neurochem 69(1):76–83
Zhang Z, Wang T, Cao X, Sun S, Wang L (2009) 6-OHDA induces cycle reentry and apoptosis of PC12 cells through activation of ERK1/2 signaling pathway. J Huazhong Univ Sci Technol [Medical Sciences] 29:97–100
Tripathi M, Singh BK, Raisuddin S, Kakkar P (2011) Abrogation of nimesulide induced oxidative stress and mitochondria mediated apoptosis by Fumaria parviflora Lam. Extract. J Ethnopharmacol 136(1):94–102
Tripathi M, Singh BK, Mishra C, Raisuddin S, Kakkar P (2010) Involvement of mitochondria mediated pathways in hepatoprotection conferred by Fumaria parviflora Lam. Extract against nimesulide induced apoptosis in vitro. Toxicol Vitro 24(2):495–508
Brice Landry K, Tariq S, Malik A, Sufyan M, Ashfaq UA, Ijaz B, Shahid AA (2022) Berberis lyceum and Fumaria indica: in vitro cytotoxicity, antioxidant activity, and in silico screening of their selected phytochemicals as novel hepatitis C virus nonstructural protein 5A inhibitors. J Biomol Struct Dynamics 40(17):7829–7851
Kahl R, Kampkotter A, Watjen W, Chovolou Y (2004) Antioxidant enzymes and apoptosis. Drug Metab Rev 36(3–4):747–762
Jaberian H, Piri K, Nazari J (2013) Phytochemical composition and in vitro antimicrobial and antioxidant activities of some medicinal plants. Food Chem 136(1):237–244
Moghaddam M, Khaleghi Miran SN, Mehdizadeh L (2018) Total phenolic content and antioxidant activity of Fumaria vaillantii extract at three phenological stages assayed by various methods. Int J Hortic Sci Technol 5(1):93–102
Garcia YJ, Rodriguez-Malaver AJ, Penaloza N (2005) Lipid peroxidation measurement by thiobarbituric acid assay in rat cerebellar slices. J Neurosci Methods 144(1):127–135
Zamani-Moghaddam E, Azami K, Minaei-Zangi B, Mousavi SZ, Sabzevari O (2012) Protective activity of Fumaria vaillantii extract and monomethyl fumarate on acetaminophen induced hepatotoxicity in mice. Int J Pharmacol 8(3):177–184
Rausch WD, Wang F, Radad K (2022) From the tyrosine hydroxylase hypothesis of Parkinson’s disease to modern strategies: a short historical overview. J Neural Transm (Vienna) 129(5–6):487–495
Xu YQ, Long L, Yan JQ, Wei L, Pan MQ, Gao HM et al (2013) Simvastatin induces Neuroprotection in 6-OHDA‐Lesioned PC 12 via the PI 3K/AKT/Caspase 3 pathway and anti‐inflammatory responses. CNS Neurosci Ther 19(3):170–177
Stanic D, Finkelstein DI, Bourke DW, Drago J, Horne MK (2003) Timecourse of striatal re-innervation following lesions of dopaminergic SNpc neurons of the rat. Eur J Neurosci 18(5):1175–1188
Sauer H, Oertel WH (1994) Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience 59(2):401–415
Chen Y, Lian Y, Ma Y, Wu C, Zheng Y, Xie N (2017) The expression and significance of tyrosine hydroxylase in the brain tissue of Parkinson’s disease rats. Experimental Therapeutic Med 14(5):4813–4816
Lapchak PA, Miller PJ, Collins F, Jiao S (1997) Glial cell line-derived neurotrophic factor attenuates behavioural deficits and regulates nigrostriatal dopaminergic and peptidergic markers in 6-hydroxydopamine-lesioned adult rats: comparison of intraventricular and intranigral delivery. Neuroscience 78(1):61–72
Breese GR, Traylor TD (1971) Depletion of brain noradrenaline and dopamine by 6-hydroxydopamine. Br J Pharmacol 42(1):88–99
Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH et al (2013) Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 136(Pt 8):2419–2431
McGeer PL (1971) Tyrosine hydroxylase and parkinsonism. Lancet 2(7716):165
Mogi M, Harada M, Kiuchi K, Kojima K, Kondo T, Narabayashi H et al (1988) Homospecific activity (activity per enzyme protein) of tyrosine hydroxylase increases in parkinsonian brain. J Neural Transm 72(1):77–82
Feng Y, Ma J, Yuan L (2020) beta-methylphenylalanine exerts neuroprotective effects in a Parkinson’s disease model by protecting against tyrosine hydroxylase depletion. J Cell Mol Med 24(17):9871–9880
Šamec D, Valek-Žulj L, Martinez S, Grúz J, Piljac A, Piljac-Žegarac J (2016) Phenolic acids significantly contribute to antioxidant potency of Gynostemma pentaphyllum aqueous and methanol extracts. Ind Crops Prod 84:104–107
Choi HS, Park MS, Kim SH, Hwang BY, Lee CK, Lee MK (2010) Neuroprotective effects of herbal ethanol extracts from Gynostemma pentaphyllum in the 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Molecules 15(4):2814–2824
Li S, Pu XP (2011) Neuroprotective effect of kaempferol against a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson’s disease. Biol Pharm Bull 34(8):1291–1296
Ablat N, Lv D, Ren R, Xiaokaiti Y, Ma X, Zhao X et al (2016) Neuroprotective effects of a standardized flavonoid extract from safflower against a rotenone-induced rat model of Parkinson’s disease. Molecules 21(9):1107
Guimarães RP, Ribeiro DL, Dos Santos KB, Godoy LD, Corrêa MR, Padovan-Neto FE (2021) The 6-hydroxydopamine rat model of Parkinson’s disease. JoVE (Journal Visualized Experiments). (176):e62923
Srivastava S, Choudhary G (2014) Pharmacognostic and pharmacological study of Fumaria vaillantii Loisel: a review. J Pharmacognosy Phytochemistry 3(1)
Zbarsky V, Datla KP, Parkar S, Rai DK, Aruoma OI, Dexter DT (2005) Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic Res 39(10):1119–1125
Guo S, Yan J, Yang T, Yang X, Bezard E, Zhao B (2007) Protective effects of green tea polyphenols in the 6-OHDA rat model of Parkinson’s disease through inhibition of ROS-NO pathway. Biol Psychiatry 62(12):1353–1362
Acknowledgements
Declared none.
Funding
None.
Author information
Authors and Affiliations
Contributions
HJ and EHS and R.R. conducted the experiments and analyzing the data. H.J. analyzing, interpreting the data and writing the manuscript. M.S. and M.F. contributed in analyzing and interpreting the data. A.T.B. developed the theory, designed the experiments, contributed in analyzing and interpreting the data, writing and editing the manuscript. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Research and Ethics Committee of Tehran University of Medical Sciences, School of Advanced Technologies in Medicine, approved the experimental protocol. All methods complied with relevant institutional, national, and international guidelines and legislation. The plant was collected in accordance with national guidelines and regulations. It was kindly identified by Dr. Gholamreza Amin, former professor of pharmacognosy, Tehran University of Medical Sciences. A voucher specimen (No. 6563 TEH) gwas deposited at the Herbarium of Faculty of Pharmacy at Tehran University of Medical Sciences and authenticated by Dr. Gholamreza Amin.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Javid, H., Rahimian, R., Salimi, M. et al. Fumaria vaillantii extract protects PC12 cells against neurotoxicity induced by 6-OHDA. Mol Biol Rep 51, 768 (2024). https://doi.org/10.1007/s11033-024-09673-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09673-5