Abstract
Background
The extracellular matrix (ECM) of skeletal muscle plays a pivotal role in tissue repair and growth, and its remodeling tightly regulated by matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), and inflammatory cytokines. This study aimed to investigate changes in the mRNA expression of MMPs (Mmp-2 and Mmp-14), TIMPs (Timp-1 and Timp-2), and inflammatory cytokines (Il-1β, Tnf-α, and Tgfβ1) in the soleus (SOL) and extensor digitorum longus (EDL) muscles of rats following acute treadmill exercise. Additionally, muscle morphology was examined using hematoxylin and eosin (H&E) staining.
Methods and results
Male rats were subjected to acute treadmill exercise at 25 m/min for 60 min with a %0 slope. The mRNA expression of ECM components and muscle morphology in the SOL and EDL were assessed in both sedentary and exercise groups at various time points (immediately (0) and 1, 3, 6, 12, and 24 h post-exercise). Our results revealed a muscle-specific response, with early upregulation of the mRNA expression of Mmp-2, Mmp-14, Timp-1, Timp-2, Il-1β, and Tnf-α observed in the SOL compared to the EDL. A decrease in Tgfβ1 mRNA expression was evident in the SOL at all post-exercise time points. Conversely, Tgfβ1 mRNA expression increased at 0 and 3 h post-exercise in the EDL. Histological analysis also revealed earlier cell infiltration in the SOL than in the EDL following acute exercise.
Conclusions
Our results highlight how acute exercise modulates ECM components and muscle structure differently in the SOL and EDL muscles, leading to distinct muscle-specific responses.
Graphical abstract
Exercise-induced skeletal muscle ECM remodeling via matrix metalloproteinases and inflammatory cytokines

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Skeletal muscle is a highly dynamic tissue that serves to maintain posture, facilitate movement and respiration, and regulate whole-body metabolism [1, 2]. Skeletal muscle can undergo remarkable changes to meet structural, functional, and metabolic demands in response to factors, such as contractile activity, periods of disuse, nutritional status, hormonal fluctuations, and environmental changes [1, 2]. In recent years, skeletal muscle research has focused on elucidating the systemic and molecular mechanisms that control muscle contraction, as well as its physiological, biochemical, and endocrine functions [1,2,3]. However, muscle fibers are embedded in a dynamic and complex structure known as the extracellular matrix (ECM), which is composed of collagenous components and various macromolecules [4, 5]. Moreover, the ECM plays a pivotal role in growth, tissue repair, and the transmission of contractile forces [3, 6].
The ECM of skeletal muscle is a highly organized structure that undergoes continuous remodeling in response to injury, repair, and mechanical loading, which are mediated by specific regulatory mechanisms responsible for ECM deposition and degradation [3]. The regulation of ECM homeostasis primarily involves two key mechanisms: matrix metalloproteinases (MMPs) and inflammatory cytokines [5, 7]. Matrix metalloproteinases are proteolytic enzymes responsible for degrading ECM macromolecules, such as collagen, elastin, and proteoglycans [8]. On the other hand, tissue inhibitors of metalloproteinases (TIMPs) are known to inhibit MMPs [6]. Notably, Mmp-2 (also known as gelatinase A) and Mmp-14 (also known as MT1-MMP) are well-known MMPs due to their significant involvement in skeletal muscle growth, tissue repair, and regeneration [9,10,11]. In addition to their role in ECM remodeling, MMPs regulate the recruitment of inflammatory cells to sites of tissue damage or injury by modulating cytokines and growth factors within the ECM [5, 7]. Moreover, MMPs produced by macrophages play a crucial role as regulators in the enzymatic cleavage of a wide range of cytokines and growth factors, including tumor necrosis factor-alpha (Tnf-α), interleukin-1beta (Il-1β), and transforming growth factor beta (Tgfβ) [7, 11]. Thus, understanding the interaction between MMPs, TIMPs, and inflammatory cytokines is essential to support healthy ECM remodeling under normal and pathological conditions.
The ECM of skeletal muscle is responsive to various stimuli, including contraction and exercise, leading to the upregulation of MMPs and cytokines as part of an adaptive remodeling process [10, 11]. Previous studies in rodents have shown that both mRNA and protein expression of Mmp-2 and Mmp-14 increase during the initial stages following electrical stimulation, thereby promoting the breakdown of ECM proteins [12, 13]. This transient elevation persists for several hours and days, indicating its significant role in ongoing remodeling processes [12, 13]. A study using mechanically overloaded rodent muscle to mimic muscle hypertrophy found that Mmp-14 promoted the degradation of collagen, which may be crucial for ECM remodeling to facilitate hypertrophy [14]. Despite the well-known roles of MMPs and inflammatory cytokines in skeletal muscle, there is limited literature addressing the muscle-specific response to acute exercise over a 24 h period.
In this study, our aim was to investigate the effect of acute treadmill exercise on gene expression related to ECM remodeling in two distinct muscle types: SOL, predominantly composed of slow-oxidative muscle fibers (~ 96% type I fibers, ~ 3.9% type IIa fibers), and EDL, predominantly composed of fast-glycolytic muscle fibers (~ 5.5% type I fibers, ~ 18.8% type IIa fibers, ~ 75.7% type IIb fibers) [15]. Specifically, we sought to determine the time-course changes in mRNA expression of MMPs (Mmp-2 and Mmp-14), TIMPs (Timp-1 and Timp-2), and inflammatory cytokines (Il-1β, Tnf-α, and Tgfβ1) in both the SOL and EDL muscles.
Materials and methods
Animals
Forty-two male Sprague Dawley rats (9–12 weeks old, weighing 280–300 g) were purchased from Kobay A.S. (Ankara, Turkey). All animals were housed in groups of two per cage at a temperature of 20–24 °C on a 12 h light–dark cycle (lights on: 20:00–08:00, lights off: 08:00–20:00) with free access to a standard chow diet (Bilyem, Ankara) and water at the Animal Facility Laboratory, Faculty of Sport Sciences, Hacettepe University.
Experimental groups
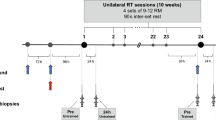
After one week of acclimatization, the animals were randomly assigned to sedentary (SED, n = 6) or exercise (n = 36) group, which underwent acute treadmill exercise on a motorized rodent treadmill. Following completion of the acute treadmill exercise protocol, the animals were euthanized at six time points: immediately (0 h, n = 6), 1 h (n = 6), 3 h (n = 6), 6 h (n = 6), 12 h (n = 6), and 24 h (n = 6) post-exercise (Fig. 1). Animals in the SED group were housed under standard cage conditions.
Acute treadmill exercise protocol
Animals in the exercise groups underwent a 6 day acclimatization period on a motorized rodent treadmill with a gradual increase in running speed (from 10 to 20 m/min) and duration (from 10 to 30 min) each day. Following the acclimatization period, the animals rested for 2 days and then underwent a single session of acute treadmill exercise lasting 60 min at a running speed of 25 m/min with a 0% slope. This exercise intensity has been demonstrated to correspond to moderate-to-high intensity treadmill exercise [16, 17]. During the exercise session, the tails of the animals were stimulated with a brush to encourage them to maintain a constant speed. As rats are nocturnal animals, both treadmill acclimatization and acute exercise sessions were carried out during the dark cycle, which is the active phase for rats (at approximately between 10 a.m. and 3:00 pm) [18]. This approach aimed to minimize stress on the animals, which could otherwise affect their physiological and molecular responses [18]. Food was removed 1 h before the start of each exercise session. Following the completion of the exercise sessions, the animals were returned to their cages with free access to the food and water.
Tissue extraction
After completing the experimental procedure, the animals were euthanized under anesthesia using ketamine (90 mg/kg, ip) and xylazine (10 mg/kg, ip). Subsequently, the SOL and EDL were removed, immediately frozen in liquid nitrogen, and stored at − 80 °C for quantitative real-time PCR (qPCR) and histological analysis.
Total RNA isolation and cDNA synthesis
Approximately 50 mg of muscle sample was homogenized using a homogenizer (Ultraturrax T25, IKA, Germany). Total RNA extraction was performed using the Total RNA Mini Kit (catalog no: W72070, Wizbiosolutions, Seongnam, Korea) according to the manufacturer’s instructions. The concentration of total RNA was quantified using a microplate reader (Thermo-Multiskan GO, USA). Subsequently, cDNA was synthesized from 100 ng of total RNA using a cDNA synthesis kit (catalog no: W2211, Wizbiosolutions, Seongnam, Korea) and cDNA was stored at − 20 °C until further use.
qPCR analysis
Gene expression analysis of target genes (Mmp-2, Mmp-14, Timp-1, Timp-2, Il-1β, Tnf-α, and Tgfβ1) and a housekeeping gene (β-actin) was performed using a PikoReal™ Real-Time PCR System (Thermo Scientific, USA) with RealQ Plus 2 × Master Mix Green (catalog no: A323402, Ampliqon, Denmark). Relative gene expression levels were calculated by normalizing each gene to β-actin using the 2−ΔΔCT method [19]. The primer sequences used for the qPCR analysis are listed in Table 1.
Histological analysis
The skeletal muscles from each animal were fixed with 4% formaldehyde (catalog no: 722841, Adekim Kimya, Turkey) and processed using a tissue processor device (Sakura, Tissue TEK, Japan). Subsequently, 4-micron-thick sections were cut from a paraffin block using a microtome (Leica RM2255, Germany) and mounted on adhesive slides. These sections were then stained with H&E using a slide stainer (Sakura, Tissue TEK, Japan) and the respective solutions of hematoxylin (catalog no: 05-06004/L, Bio Optica, Italy) and eosin (catalog no: 05 10007/L, Bio Optica, Italy). The stained sections were observed under a light microscope (Nikon Eclipse 80i, Japan), and images were captured at a magnification of × 200 using NIS Element 3.0 software (Japan). Inflammatory cells infiltrating the muscles were identified in five randomly selected fields within each section.
Statistical analysis
All values were analyzed using GraphPad Prism version 8.0 (GraphPad Software, USA). One-way analysis of variance (ANOVA) with Fisher’s least significant difference (LSD) post hoc test was used to assess differences among multiple groups. All values are presented as the mean ± standard error of the mean (SEM), and statistical significance was set at p < 0.05.
Results
Acute treadmill exercise increases the mRNA expression of MMPs and TIMPs
To investigate the impact of acute treadmill exercise on the gene expression of MMPs and TIMPs, we measured the mRNA expression levels of Mmp-2, Mmp-14, Timp-1, and Timp-2 in both the SOL and EDL.
Compared to the SED, we observed an increase in Mmp-2 mRNA expression at 0 and 1 h post-exercise in the SOL (Fig. 2A, Table 2). In the EDL, Mmp-2 mRNA expression increased at 3 h post-exercise and remained elevated at 6, 12, and 24 h post-exercise compared to the SED (Fig. 2B, Table 2). Furthermore, mRNA expression of Mmp-14 increased at 0, 6, and 24 h post-exercise in the SOL (Fig. 2C, Table 2), while in the EDL, mRNA expression of Mmp-14 increased at 6 and 24 h post-exercise compared to the SED (Fig. 2D, Table 2).
mRNA expression of Mmp-2 (A, B), Mmp-14 (C, D), Timp-1 (E, F), and Timp-2 (G, H) following acute treadmill exercise at different post-exercise time points (0, 1, 3, 6, 12, and 24 h) compared to the SED in the SOL (A, C, E, G) and EDL (B, D, F, H). Data are presented as mean ± SEM. Statistical analysis was performed using one-way ANOVA with the LSD post hoc test. n = 6 for all time points. #Significantly different from the SED (p < 0.05)
The fold changes in mRNA expression of Timp-1 and Timp-2 are illustrated in Fig. 2 and Table 2. Compared to the SED, Timp-1 mRNA expression increased at 0 and 6 h post-exercise in the SOL (Fig. 2E, Table 2) and at 6 and 24 h post-exercise in the EDL (Fig. 2F, Table 2). Similarly, Timp-2 mRNA expression increased at 0 h in the SOL (Fig. 2G, Table 2) and 3, 6, and 24 h post-exercise in the EDL (Fig. 2H, Table 2).
Acute treadmill exercise differentially affects the mRNA expression of Il-1β, Tnf-α, and Tgfβ1
The time-course analyses of changes in mRNA expression of Il-1β, Tnf-α, and Tgfβ1 in response to acute exercise in both the SOL and EDL are presented in Fig. 3 and Table 2. Compared to the SED, the mRNA expression of Il-1β increased at 0 h post-exercise in the SOL (Fig. 3A, Table 2). In the EDL, the mRNA expression of Il-1β decreased at 1 h, increased at 3 h, and then decreased again at 12 h post-exercise compared to the SED (Fig. 3B, Table 2).
mRNA expression of Il-1β (A, B), Tnf-α (C, D), and Tgfβ1 (E, F) following acute treadmill exercise at different post-exercise time points (0, 1, 3, 6, 12, and 24 h) compared to the SED in the SOL (A, C, E) and EDL (B, D, F). Data are presented as mean ± SEM. Statistical analysis was performed using one-way ANOVA with the LSD post hoc test. n = 6 for all time points. #Significantly different from the SED (p < 0.05)
Compared to the SED, the mRNA expression of Tnf-α increased at 0, 1, and 6 h post-exercise in the SOL (Fig. 3C, Table 2). In contrast, the mRNA expression of Tnf-α in the EDL increased at 24 h post-exercise compared to the SED (Fig. 3D, Table 2).
In the SOL, the mRNA expression of Tgfβ1 decreased at 0, 1, 3, 6, 12, and 24 h post-exercise compared to the SED (Fig. 3E, Table 2). In contrast, the mRNA expression of Tgfβ1 in the EDL increased at 0 and 3 h but decreased at 12 h post-exercise compared to the SED (Fig. 3F, Table 2).
Acute treadmill exercise initiates inflammatory cell infiltration in the SOL and EDL
To assess the effects of acute exercise on muscle structure, we performed H&E staining to examine the morphology of both the SOL and EDL. Our results revealed that acute exercise did not lead to inflammatory cell infiltration into the interstitial space in the SED and immediately (0 h) post-exercise in the SOL (Fig. 4). However, in the SOL, we observed minimal inflammatory cell infiltration at 1, 3, 6, and 24 h post-exercise, with moderate inflammatory cell infiltration noted at 12 h post-exercise (Fig. 4).
Inflammatory cell infiltration in the SOL and EDL following acute treadmill exercise was evaluated at different post-exercise time points (0, 1, 3, 6, 12, and 24 h) and in the SED. Representative images of H&E staining are shown for each group at a magnification of × 200. n = 6 for all time points. Black arrows (→) indicate inflammatory cell infiltration. Scale bar = 100 μm
In the EDL, acute exercise did not result in inflammatory cell infiltration into the interstitial space at 0, 1, 3, and 6 h post-exercise (Fig. 4). However, minimal inflammatory cell infiltration was observed at 12 and 24 h post-exercise in the EDL (Fig. 4).
Discussion
Overview of the main findings
The skeletal muscle ECM displays remarkable plasticity through remodeling of its structure and function to meet the demands imposed by muscle contraction and exercise in skeletal muscle. This plasticity of the ECM is regulated by the activation and/or repression of molecular pathways, which provide the basis for exercise adaptations. Therefore, understanding the underlying mechanisms will provide important details on the time-course and muscle-specific analysis of these molecular pathways in response to acute exercise and exercise adaptation [1,2,3].
To our knowledge, these are the first experiments to elucidate the time-course and muscle-specific expression of MMPs, TIMPs, and inflammatory cytokines in response to acute treadmill exercise for up to 24 h, providing important findings regarding the effects of exercise on ECM components and structure in skeletal muscle. Our results showed that acute treadmill exercise increased the mRNA levels of Mmp-2, Mmp-14, Timp-1, and Timp-2 in the SOL and EDL. However, the mRNA expression of MMPs and TIMPs elevated immediately and during the early stages of the post-exercise time points in the SOL compared to the EDL. A similar trend was also observed for the mRNA expression of Il-1β and Tnf-α in the SOL compared to the EDL. In contrast, the mRNA expression of Tgfβ1 decreased in the SOL at all post-exercise time points, while Tgfβ1 mRNA expression initially increased at 0 and 3 h post-exercise and then decreased at 12 h post-exercise in the EDL.
Acute treadmill exercise differentially induces the mRNA expression of MMPs and TIMPs in the SOL and EDL at various post-exercise time points
The maintenance of the ECM is regulated by specialized enzymes responsible for the degradation of ECM components [4, 11, 20]. Several studies have shown that Mmp-2 and Mmp-14 are upregulated in skeletal muscle [21,22,23], possibly due to ECM remodeling initiated by muscle contraction, mechanical loading, injury, and regeneration of muscle fibers [9, 24].
Our results showed that acute treadmill exercise was sufficient to increase Mmp-2 and Mmp-14, as well as their inhibitors Timp-1 and Timp-2, in both the SOL and EDL. These findings align with previous studies indicating that acute and short-term exercise can increase the expression of Mmp-2, Mmp-14, Timp-1, and Timp-2 in skeletal muscles [23, 25, 26]. However, an early response of Mmp-2 and Mmp-14 was observed in the SOL compared to the EDL, with the induction observed at 0, 3, and 6 h post-exercise. Additionally, the mRNA expression of Timp-1 and Timp-2 increased at 0 and 6 h post-exercise, while a delayed mRNA response (3–24 h post-exercise) was observed in the EDL compared to the SOL, suggesting a trend similar to that of Mmp-2 and Mmp-14.
The transient upregulation of MMPs is typically considered the first step in proper repair following muscle contraction, as it promotes the stimulation of regenerative capacity and activation of satellite cells [10]. Our data support previous findings regarding the induction of MMPs and provide important details on the time-course and muscle-specific response to exercise [10, 23]. One of the hallmarks of adaptation to endurance exercise is that type I muscle fibers are recruited more than type II muscle fibers during an endurance exercise session, making them more susceptible to skeletal muscle damage [2, 27]. Thus, the initiation of ECM remodeling may occur earlier in the SOL (predominantly composed of type I fibers) than in the EDL (predominantly composed of type II fibers). These effects may also be somewhat specific to collagen abundance, as slow-oxidative muscle fibers have significantly higher concentrations of collagen than fast-glycolytic muscle fibers [28, 29]. Takala et al. emphasized the significance of the fiber type-specific response of ECM remodeling to endurance training [30]. The authors also noted that collagen turnover in the quadriceps muscle of mice is more pronounced during the initial phase of exercise training in red muscles compared to white muscles [30]. This observation may explain the early-phase induction of MMPs observed in the SOL compared to the EDL, possibly due to the muscle-specific response to endurance exercise. Another possible explanation is that muscle-specific differences may also contribute to more pronounced exercise-induced ECM remodeling through the induction of MMPs and their inhibitors, TIMPs, in the SOL compared to the EDL. Immunohistochemical staining of Mmp-2 revealed that basal intracellular levels of Mmp-2 are much more prominent in type II muscle fibers in the gastrocnemius muscle of mice [31], suggesting that the SOL could be more sensitive to exercise-induced induction Mmp-2 expression than the EDL due to lower basal Mmp-2.
Similar to Mmp-2, Mmp-14 mRNA expression has also been reported in skeletal muscle [9]; however, there is no information on whether its expression differs in type I or type II muscle fibers. In our study, the exercise-induced increase in Mmp-14 mRNA expression was more pronounced in the SOL compared to the EDL. An explanation for the increase in Mmp-14 could be that Mmp-14 is required for proteolytic cleavage of proMmp2 [6]. A single bout of exercise did not increase the mRNA expression of Mmp-2 or Mmp-14 in the vastus lateralis muscle, which is predominantly composed of type II muscle fibers, immediately after and 120 min following exercise [26]. However, the mRNA expression of Mmp-2 and Mmp-14 increased after 10 days of exercise [25]. Part of this discrepancy in the mRNA expression of Mmp-2 and Mmp-14 related to acute exercise might be due to the skeletal muscle fiber composition since the vastus lateralis muscle is composed of type II fibers [32]. We observed delayed mRNA expression of Mmp-2 and Mmp-14 in response to acute treadmill exercise in the EDL, suggesting that muscle-specific mRNA expression of Mmp-2 was dependent on the timing of muscle isolation. Thus, further studies are needed to fully elucidate the exercise-induced changes in the mRNA expression of MMPs at multiple post-exercise time points.
Time-course and muscle-specific changes in the mRNA expression of Il-1β, Tnf-α, and Tgfβ1 in response to acute treadmill exercise
The inflammatory response is a complex and vital mechanism that enables the elimination of pathogens and the maintenance of tissue homeostasis. Adaptive inflammation, in which stress conditions are resolved, involves the activation of immune cells that infiltrate the injury site to remove cellular debris and promote tissue repair, ultimately restoring tissue homeostasis. However, if stress cannot be resolved and persists, maladaptive inflammation contributes to the sustained infiltration of immune cells orchestrated by cytokines and chemokines, resulting in impaired tissue function [33]. Thus, proper functioning of the inflammatory response is necessary to maintain tissue homeostasis under both physiological and pathophysiological conditions.
Acute exercise may represent a major challenge to whole-body homeostasis, caused by the contractile activity of skeletal muscles, resulting in the activation of an inflammatory response through cytokines and chemokines [1]. Here, we demonstrated that the early response of Il-1β and Tnf-α mRNA expression to acute treadmill exercise in the SOL compared to the EDL appears to be muscle-specific. Both Il-1β and Tnf-α are classic proinflammatory cytokines that are released in response to cellular damage [33]. This transient increase in the muscle-specific inflammatory response leads to the activation of several signaling pathways that contribute to the myogenesis, repair, and remodeling of the skeletal muscle [34]. Despite their important role in skeletal muscle, little is known about exercise-induced changes in inflammatory cytokine expression. A single bout of acute eccentric exercise, known to cause structural damage to skeletal muscle fibers, is a potent stimulus for the increase in Il-1β and Tnf-α in skeletal muscle [35, 36]. Importantly, muscle- and fiber-specific mRNA and protein expression of Il-1β and Tnf-α differ, as basal Il-1β does not change between the SOL and EDL in rats, whereas type II fibers express more Tnf-α than type I fibers in both rats and humans [37, 38]. These results make it difficult to provide a simple explanation for muscle type-specific mRNA expression of Il-1β and Tnf-α in response to acute treadmill exercise. However, a possible explanation is that endurance exercise causes more damage to type I fibers and results in greater recruitment of type I fibers [2, 27]. Additionally, the recruitment of type I fibers depends on the exercise intensity [2]. Consequently, different inflammatory responses can be observed owing to muscle damage and the recruitment of fiber types associated with the type and intensity of exercise.
A critical feature of Il-1β and Tnf-α is that they are highly interconnected and can influence each other at the transcriptional and protein levels. The gene expression of Il-1β and Tnf-α is bidirectional, with Tnf-α being upregulated by Il-1, while Il-1β stimulates the expression of Tnf-α via nuclear factor kappa B, a central switch of the inflammatory response [39]. Our time-course and muscle-specific analyses revealed that acute treadmill exercise resulted in an increase in the mRNA expression of Il-1β and Tnf-α in the SOL compared to the EDL in the early stage of post-exercise. This observation also suggests that the early response of these cytokines to acute exercise might exert their effects in a paracrine and endocrine-dependent manner on the target tissues, thereby promoting the delayed expression of Il-1β and Tnf-α in the EDL. Additionally, the exercise-induced inflammatory response via macrophages that express Il-1β and Tnf-α is associated with early stage regeneration to clear damaged fibers [40]. The underlying mechanism is not entirely clear, but we speculate that the increased gene expression of Il-1β and Tnf-α may trigger tissue regeneration. However, further studies are needed to elucidate the cellular and molecular mechanisms underlying exercise-induced inflammation.
In contrast to the early phase increase in the expression of Il-1β and Tnf-α, a decrease in Tgfβ1 expression was observed at all post-exercise time points in the SOL. However, the mRNA expression of Tgfβ1 dramatically increased at 0 and 3 h post-exercise in the EDL. These results are in line with previous findings that acute exercise increases the expression of Tgfβ1. For example, Heinemeier et al. reported an increase in muscle Tgfβ1 after 1 h of one-leg kicking exercise [41]. Similar results were also reported for the gene expression of Tgfβ1 in the gastrocnemius muscle of rats immediately after acute exercise [42] and in the EDL muscle of rats after 30 day of exercise [43]. Based on previous findings, Tgfβ1 expression can be induced by contractile activity. The literature lacks comprehensive data on the mRNA and protein expression of Tgfβ1 in response to exercise, especially concerning muscle-specific variations. Tgfβ1 signaling has been identified as a key regulator of skeletal muscle regeneration, ECM remodeling, and fibrosis [44]. In the acute phase of skeletal muscle damage caused by injury or exercise, Tgfβ1 has the potential to stimulate the production of ECM components, such as collagen and fibronectin [44, 45]. Furthermore, there is a bidirectional relationship between Tgfβ and MMPs, suggesting that MMPs proteolytically activate Tgfβ, while Tgfβ suppresses MMP upregulation [46, 47]. Our results are similar to those of previous studies, and it can be speculated that reduced Tgfβ1 expression is essential for facilitating post-exercise skeletal muscle remodeling by inducing MMPs in the SOL.
Conclusion
Exercise is a powerful tool not only for maintaining the healthy state of individuals and acting as a poly-pill for disease prevention but also for improving sports performance. While the benefits of exercise are partly due to transient changes in the mRNA expression of genes within the first few hours to 24 h, adaptation to exercise occurs through changes in protein levels during prolonged, repeated acute exercise sessions. Our results suggest that acute treadmill exercise induces muscle-specific responses, with early increases in the mRNA expression of MMPs, TIMPs, Il-1β, and Tnf-α in the SOL and late upregulation of the mRNA expression of MMPs and TIMPs, as well as increased expression of Tgfβ1 in the EDL. These results contribute to our understanding of the role of MMPs, TIMPs, and cytokines in skeletal muscle remodeling in response to acute exercise. Given the molecular adaptations that occur in skeletal muscle in response to exercise, it is important to understand the responses that are fiber type specific.
Limitations of this study and future directions
While these findings provide important insights into exercise-induced changes in different skeletal muscle types, this study was designed to examine the mRNA expression of a limited number of ECM components in both the SOL and EDL. Therefore, it is essential to investigate the roles of proinflammatory cytokines (e.g., Il-6) and anti-inflammatory cytokines (e.g., Il-10) in both skeletal muscle and serum/plasma samples.
Given the complexity of the response to exercise, the incorporation of OMICS-based methods, which are crucial for identifying dynamic molecular networks, could facilitate a thorough characterization of gene and protein expression within skeletal muscle. The complex nature of skeletal muscle makes it difficult to analyze the response to acute exercise because of the mixed muscle fiber composition and resident cells, including satellite cells, fibroblasts, and immune cells. Therefore, single-cell analysis provides a clearer picture of the molecular changes during skeletal muscle remodeling induced by acute exercise compared to mixed muscle analysis. Finally, further research should aim to elucidate the underlying mechanisms involved in the response to exercise training and provide valuable information for long-term skeletal muscle remodeling.
Translational perspective
The skeletal muscle ECM plays a unique role in diverse biological functions, including mechanical transmission, muscle repair, and regeneration. Skeletal muscle plasticity relies on the response of ECM components to physiological and pathophysiological stressors. Moreover, maintaining skeletal muscle integrity without properly functioning ECM components and the ECM itself is challenging. There are physiological, biochemical, and anatomical differences between animal models (e.g., zebrafish and rodents) and humans, making it difficult to generalize findings from animals to humans. However, several aspects of human translation must be considered.
-
1.
The underlying mechanism of ECM remodeling in response to exercise in the SOL and EDL in rodents may better reflect the muscle-specific response due to the difficulty in obtaining biopsies from the SOL and EDL in humans.
-
2.
Differences in the fiber type distribution and function of each muscle fiber have been observed between the skeletal muscles of mammalian species, such as rats and humans. This difference may limit the comparison and translation of the ECM response to exercise across mammalian species [15, 48].
-
3.
Recent studies have shown that the accumulation of ECM (e.g., increased collagen content) during obesity and aging may lead to fibrosis and the impairment of skeletal muscle function [22, 49]. Thus, the adaptive induction of ECM components may play a central role in skeletal muscle function.
-
4.
In addition to its functions, the ECM serves as an environment that transmits mechanical force, allowing the transmission of growth signals to muscle fibers and satellite cells [20]. This signal transduction results in skeletal muscle hypertrophy, directly preventing the loss of skeletal muscle mass during aging [20].
-
5.
Therapeutics targeting ECM components such as MMPs may have great potential and should be considered as exercise mimetics, offering significant health benefits to individuals with metabolic disorders and those with limited physical activity.
Data availability
The datasets can be obtained from the corresponding author upon reasonable request.
References
Hawley JA, Hargreaves M, Joyner MJ, Zierath JR (2014) Integrative biology of exercise. Cell 159(4):738–749
Egan B, Sharples AP (2022) Molecular responses to acute exercise and their relevance for adaptations in skeletal muscle to exercise training. Physiol Rev. https://doi.org/10.1152/physrev.00054.2021
Csapo R, Gumpenberger M, Wessner B (2020) Skeletal muscle extracellular matrix—what do we know about its composition, regulation, and physiological roles? Narrat Rev Front Physiol 11:253
Kyriakopoulou K, Piperigkou Z, Tzaferi K, Karamanos NK (2023) Trends in extracellular matrix biology. Mol Biol Rep 50(1):853–863
Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK (2016) Extracellular matrix structure. Adv Drug Deliv Rev 97:4–27
Cui N, Hu M, Khalil RA (2017) Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci 147:1–73
Nissinen L, Kahari VM (2014) Matrix metalloproteinases in inflammation. Biochim Biophys Acta 1840(8):2571–2580
Manicone AM, McGuire JK (2008) Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol 19(1):34–41
Snyman C, Niesler CU (2015) MMP-14 in skeletal muscle repair. J Muscle Res Cell Motil 36(3):215–225
Carmeli E, Moas M, Reznick AZ, Coleman R (2004) Matrix metalloproteinases and skeletal muscle: a brief review. Muscle Nerv 29(2):191–197
Chen X, Li Y (2009) Role of matrix metalloproteinases in skeletal muscle: migration, differentiation, regeneration and fibrosis. Cell Adh Migr 3(4):337–341
Koskinen SO, Wang W, Ahtikoski AM, Kjaer M, Han XY, Komulainen J, Kovanen V, Takala TE (2001) Acute exercise induced changes in rat skeletal muscle mRNAs and proteins regulating type IV collagen content. Am J Physiol Regul Integr Comp Physiol 280(5):R1292-1300
Koskinen SO, Ahtikoski AM, Komulainen J, Hesselink MK, Drost MR, Takala TE (2002) Short-term effects of forced eccentric contractions on collagen synthesis and degradation in rat skeletal muscle. Pflugers Arch 444(1–2):59–72
Peck BD, Murach KA, Walton RG, Simmons AJ, Long DE, Kosmac K, Dungan CM, Kern PA, Bamman MM, Peterson CA (2022) A muscle cell-macrophage axis involving matrix metalloproteinase 14 facilitates extracellular matrix remodeling with mechanical loading. FASEB J 36(2):e22155
Bloemberg D, Quadrilatero J (2012) Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS ONE 7(4):e35273
Contarteze RVL, Manchado FB, Gobatto CA, De Mello MAR (2008) Stress biomarkers in rats submitted to swimming and treadmill running exercises. Comp Biochem Physiol A Mol Integr Physiol 151(3):415–422
Oliveira GP Jr, Porto WF, Palu CC, Pereira LM, Petriz B, Almeida JA, Viana J, Filho NNA, Franco OL, Pereira RW (2018) Effects of acute aerobic exercise on rats serum extracellular vesicles diameter, concentration and small RNAs content. Front Physiol 9:532
Poole DC, Copp SW, Colburn TD, Craig JC, Allen DL, Sturek M, O’Leary DS, Zucker IH, Musch TI (2020) Guidelines for animal exercise and training protocols for cardiovascular studies. Am J Physiol Heart Circ Physiol 318(5):H1100–H1138
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4):402–408
Brightwell CR, Latham CM, Thomas NT, Keeble AR, Murach KA, Fry CS (2022) A glitch in the matrix: the pivotal role for extracellular matrix remodeling during muscle hypertrophy. Am J Physiol Cell Physiol 323(3):C763–C771
Zhang Q, Joshi SK, Lovett DH, Zhang B, Bodine S, Kim HT, Liu X (2014) Matrix metalloproteinase-2 plays a critical role in overload induced skeletal muscle hypertrophy. Muscles Ligaments Tendons J 4(4):446–454
Martinez-Huenchullan S, McLennan SV, Verhoeven A, Twigg SM, Tam CS (2017) The emerging role of skeletal muscle extracellular matrix remodelling in obesity and exercise. Obes Rev 18(7):776–790
Carmeli E, Moas M, Lennon S, Powers SK (2005) High intensity exercise increases expression of matrix metalloproteinases in fast skeletal muscle fibres. Exp Physiol 90(4):613–619
Zimowska M, Brzoska E, Swierczynska M, Streminska W, Moraczewski J (2008) Distinct patterns of MMP-9 and MMP-2 activity in slow and fast twitch skeletal muscle regeneration in vivo. Int J Dev Biol 52(2–3):307–314
Rullman E, Norrbom J, Stromberg A, Wagsater D, Rundqvist H, Haas T, Gustafsson T (2009) Endurance exercise activates matrix metalloproteinases in human skeletal muscle. J Appl Physiol 106(3):804–812
Rullman E, Rundqvist H, Wagsater D, Fischer H, Eriksson P, Sundberg CJ, Jansson E, Gustafsson T (2007) (2007) A single bout of exercise activates matrix metalloproteinase in human skeletal muscle. J Appl Physiol 102(6):2346–2351
Egan B, Carson BP, Garcia-Roves PM, Chibalin AV, Sarsfield FM, Barron N, McCaffrey N, Moyna NM, Zierath JR, O’Gorman DJ (2010) Exercise intensity-dependent regulation of peroxisome proliferator-activated receptor coactivator-1 mRNA abundance is associated with differential activation of upstream signalling kinases in human skeletal muscle. J Physiol 588(Pt 10):1779–1790
Kovanen V, Suominen H, Risteli J, Risteli L (1988) Type IV collagen and laminin in slow and fast skeletal muscle in rats-effects of age and life-time endurance training. Coll Relat Res 8(2):145–153
Kovanen V, Suominen H, Heikkinen E (1984) Collagen of slow twitch and fast twitch muscle fibres in different types of rat skeletal muscle. Eur J Appl Physiol Occup Physiol 52(2):235–242
Takala TE, Myllyla R, Salminen A, Anttinen H, Vihko V (1983) Increased activities of prolyl 4-hydroxylase and galactosylhydroxylysyl glucosyltransferase, enzymes of collagen biosynthesis, in skeletal muscle of endurance-trained mice. Pflugers Arch 399(4):271–274
Hadler-Olsen E, Solli AI, Hafstad A, Winberg JO, Uhlin-Hansen L (2015) Intracellular MMP-2 activity in skeletal muscle is associated with type II fibers. J Cell Physiol 230(1):160–169
Staron RS, Hagerman FC, Hikida RS, Murray TF, Hostler DP, Crill MT, Ragg KE, Toma K (2000) Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem 48(5):623–629
Gusev E, Zhuravleva Y (2022) Inflammation: a new look at an old problem. Int J Mol Sci. https://doi.org/10.3390/ijms23094596
Chen SE, Jin B, Li YP (2007) TNF-alpha regulates myogenesis and muscle regeneration by activating p38 MAPK. Am J Physiol Cell Physiol 292(5):C1660–C1671
Cannon JG, Fielding RA, Fiatarone MA, Orencole SF, Dinarello CA, Evans WJ (1989) Increased interleukin 1 beta in human skeletal muscle after exercise. Am J Physiol 257(2 Pt 2):R451–R455
Fielding RA, Manfredi TJ, Ding W, Fiatarone MA, Evans WJ, Cannon JG (1993) Acute phase response in exercise. III. neutrophil and IL-1 beta accumulation in skeletal muscle. Am J Physiol 265(1 Pt 2):R166–R172
Isanejad A, Saraf ZH, Mahdavi M, Gharakhanlou R, Shamsi MM, Paulsen G (2015) The effect of endurance training and downhill running on the expression of IL-1beta, IL-6, and TNF-alpha and HSP72 in rat skeletal muscle. Cytokine 73(2):302–308
Plomgaard P, Penkowa M, Pedersen BK (2005) Fiber type specific expression of TNF-alpha, IL-6 and IL-18 in human skeletal muscles. Exerc Immunol Rev 11:53–63
Mourkioti F, Rosenthal N (2008) NF-kappaB signaling in skeletal muscle: prospects for intervention in muscle diseases. J Mol Med (Berl) 86(7):747–759
Chazaud B (2016) Inflammation during skeletal muscle regeneration and tissue remodeling: application to exercise-induced muscle damage management. Immunol Cell Biol 94(2):140–145
Heinemeier KM, Bjerrum SS, Schjerling P, Kjaer M (2013) Expression of extracellular matrix components and related growth factors in human tendon and muscle after acute exercise. Scand J Med Sci Sports 23(3):e150–e161
Gavin TP, Wagner PD (2001) Effect of short-term exercise training on angiogenic growth factor gene responses in rats. J Appl Physiol 90(4):1219–1226
Curzi D, Sartini S, Guescini M, Lattanzi D, Di Palma M, Ambrogini P, Savelli D, Stocchi V, Cuppini R, Falcieri E (2016) Effect of different exercise intensities on the myotendinous junction plasticity. PLoS ONE 11(6):e0158059
Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Munoz-Canoves P (2011) Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle 1(1):21
Zhu J, Li Y, Shen W, Qiao C, Ambrosio F, Lavasani M, Nozaki M, Branca MF, Huard J (2007) Relationships between transforming growth factor-beta1, myostatin, and decorin: implications for skeletal muscle fibrosis. J Biol Chem 282(35):25852–25863
Risinger GM Jr, Updike DL, Bullen EC, Tomasek JJ, Howard EW (2010) TGF-beta suppresses the upregulation of MMP-2 by vascular smooth muscle cells in response to PDGF-BB. Am J Physiol Cell Physiol 298(1):C191-201
Wu L, Derynck R (2009) Essential role of TGF-beta signaling in glucose-induced cell hypertrophy. Dev Cell 17(1):35–48
Schiaffino S, Reggiani C (2011) Fiber types in mammalian skeletal muscles. Physiol Rev 91(4):1447–1531
Kragstrup TW, Kjaer M, Mackey AL (2011) Structural, biochemical, cellular, and functional changes in skeletal muscle extracellular matrix with aging. Scand J Med Sci Sports 21(6):749–757
Acknowledgements
We would like to thank the Amasya University Central Research Laboratory (AUMAULAB) and Gulhane Training and Research Hospital Pathology Laboratory for allowing the use of their facilities. Graphical abstract was created with BioRender.com.
Funding
The authors declare that no funds, grants, or other support was received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Ibrahim Turkel: Conceptualization, Methodology, Investigation, Visualization, Writing—Original Draft. Sema Tahtalioglu: Conceptualization, Methodology, Investigation, Writing—Original Draft. Ertugrul Celik: Methodology, Investigation, Writing—Original Draft. Burak Yazgan: Conceptualization, Methodology, Investigation, Writing—Review and Editing. Gokhan Burcin Kubat: Methodology, Investigation, Writing—Original Draft. Berkay Ozerklig: Conceptualization, Methodology, Writing—Original Draft. Sukran Nazan Kosar: Conceptualization, Methodology, Writing—Review and Editing, Project administration. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
All animal experiments were approved by the local ethics committee of Hacettepe University (protocol number: 2021/06-03), in accordance with the National Guidelines for the Care and Use of Laboratory Animals approved by the institutional committee of Hacettepe University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Turkel, I., Tahtalioglu, S., Celik, E. et al. Time-course and muscle-specific gene expression of matrix metalloproteinases and inflammatory cytokines in response to acute treadmill exercise in rats. Mol Biol Rep 51, 667 (2024). https://doi.org/10.1007/s11033-024-09637-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09637-9