Abstract
Background
Ovarian cancer is the leading cause of gynecological cancer deaths. One of the major challenges in treating ovarian cancer with chemotherapy is managing the resistance developed by cancer cells to drugs, while also minimizing the side effects caused by these agents In the present study, we aimed to examine the effects of a combination of alpha lipoic acid (ALA), with cisplatin and paclitaxel in ovarian cancer(OVCAR-3).
Methods
The cytotoxic effects of ALA, cisplatin and paclitaxel on OVCAR-3 cells were determined. Four groups were formed: Control, ALA, Cisplatin + Paclitaxel, ALA + Cisplatin + Paclitaxel. The effects of single and combined therapy on cell migration, invasion and colony formation were analyzed. Changes in the expression of genes related to apoptosis, cell adhesion and cell cycle were analyzed with Real-time polymerase chain reaction(RT-PCR). The oxidative stress index and The Annexin V test were performed.
Results
The reduction in rapamycin-insensitive companion of mTOR(RICTOR) expression in the ALA + Cisplatin + Paclitaxel group was found statistically significant(p < 0.05). The decrease in MMP-9 and − 11 expressions the ALA + Cisplatin + Paclitaxel group was statistically significant(p < 0.05). The lowest values for mitogen-activated protein kinase(MAPK) proteins were found in the ALA + Cisplatin + Paclitaxel group. No colony formation was observed in the Cisplatin + Paclitaxel and ALA + Cisplatin + Paclitaxel groups. The lowest wound healing at 24 h was seen in the ALA + Cisplatin + Paclitaxel group.
Conclusions
This study is the first one to investigate the combined treatment of ALA, Cisplatin, Paclitaxel on OVCAR-3. While ALA alone was not effective, combined therapy with ALA, has been found to reduce cell invasion, especially wound healing in the first 24 h, along with tumor cell adhesion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although it is the third most prevalent gynecological cancer globally, ovarian cancer stands out for presenting the highest death rate among such cancers. This high mortality is associated with its tendency to progress without symptoms, leading to late detection and a higher chance of recurrence [1,2,3]. Although there has been less than 1% decrease in ovarian cancer incidence over the past two decades, the mortality rate has not changed drastically [4]. In ovarian cancer, there is a pathogenetic process that begins with genotoxic DNA damage, followed by a p53 mutation and loss of progressive cell cycle control, resulting in the development of carcinoma [5]. The PI3K/AKT/mTOR signaling pathway acts as a crucial regulator in cell survival, growth and proliferation, angiogenesis, transcription, translation and regulation of metabolism. Irregularities in the main components of this signaling pathway are common in cancer pathogenesis [6]. The phosphatidylinositol 3-kinase/protein kinase-B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway has been shown to be a frequently changing signaling pathway in ovarian cancer, making it one of the most important signaling pathways for therapeutic targets [6, 7].
The current standard treatment for ovarian cancer involves a combination of optimal cytoreductive surgery, along with chemotherapy using paclitaxel and platinum-based drugs [8]. Cisplatin was one of the first metal-based chemotherapeutics and is still used as the main treatment for ovarian cancer, despite serious side effects and the development of resistance [9, 10]. Cisplatin inhibits the unrestricted replication of cancer cells by forming cross-links with nucleotides of nuclear and mitochondrial DNA [11,12,13]. In addition, cisplatin causes oxidative stress through the production of superoxide anions and hydroxyl radicals [13]. This involves the formation of DNA lesions by interacting with purine bases on DNA and subsequent activation of several signalling pathways leading to apoptosis [10]. Paclitaxel blocks cell division and leads to apoptotic cell death [14]. Paclitaxel treatment promotes tubulin polymerisation and inhibits mitotic progression [15]. However, with the development of chemotherapy-resistant diseases, the sensitivity of these treatment regimens has decreased. For this reason, the development of resistance to chemotherapy for ovarian cancer has reduced the long-term survival rate and increased the recurrence rate [16]. One approach in studies is to evaluate the changes in expression patterns at the gene and protein level that are associated with the phenomenon of drug resistance in ovarian cancer. In a study, patients with stage I-IV ovarian cancer were compared to controls. The analysis showed that the strongest association with drug resistance was found for the mRNAs and the miRNAs [17]. Another important approach is the search for new drugs that are non-toxic and work in an alternative way to these drugs. These new therapies could provide alternative approaches for treating ovarian cancer. Alpha Lipoic acid (ALA) is a natural antioxidant [18]. It is an essential cofactor for mitochondrial enzymes involved in the tricarboxylic acid cycle. The reduced form of ALA, known as Dihydrolipoic acid, is the dominant form that interacts with reactive oxygen species (ROS) [18, 19]. It has been used to treat many disease conditions such as diabetes mellitus, hypertension, Alzheimer’s, Down syndrome, cognitive dysfunction, and certain types of cancer, especially breast cancer [20, 21]. ALA acts on three different levels; the first one inhibits the AKT pathway, causing an increase of pro-apoptotic proteins and suppression of anti-apoptotic proteins. The latter induces the transcription of pro-apoptotic proteins by ROS production. The third activates the AMP-activated protein kinase (AMPK) protein, which negatively regulates the AKT pathway [22]. Thanks to its ability to clear ROS and replenish endogenous antioxidants, it plays an important role in cellular growth. ALA has also been suggested to lead to apoptosis and inhibition of cell proliferation to reduce the high oxidative stress accumulated by cancerous cells [20]. The aim of our study was to investigate the effects of ALA alone and in combination with cisplatin and paclitaxel on migration, invasion, colony formation, apoptosis and PI3K/AKT/mTOR signalling pathway in OVCAR-3 cells.
Materials and methods
Reagents
RPMI 1640 with L-glutamine, with 25mM HEPES (Capricorn Scientific, Germany, Cat No:RPMI-HA), Penicillin-streptomycin (Capricorn Scientific, Germany, Cat No:PS-B) and Dulbecco’s Phosphate buffered saline (PBS) were purchased from CAPRICORN (Capricorn Scientific, Germany, Cat No:PBS-1 A), Fetal Bovine Serum (FBS) was obtained from GIBCO (GIBCO, New Zealand. Ref No:26170-043), Trypsin-EDTA was obtained from Biological Industries (Sartorius, Biological Industries, Israel, Ref No:03-079-IB), TRIzol Reagent was obtained from Invitrogen (Invitrogen™, USA, Ref No:15,596,018, Ethanol (Honeywell, Germany, Cat No:32,221) and Methanol (Honeywell, Germany, Cat No: 34,966) were from Honeywell, the primary antibodies were purchased from Sentebiolab (Sentebiolab,Turkey), Dimethyl sulfoxide was from Roche, Cisplatin and Paclitaxel were purchased from Cayman Chemical Company (Item: 10,461), isopropanol (Sigma, Germany, Cat No:67-63-0 and ALA (Sigma, China, Cat No:107728-7) were from Sigma. All other chemical substances used in the study were obtained from commercial sources in an analytical and high-purity degree.
Cell lines and culture
OVCAR-3 cells (ATCC) have been cultured in RPMI 1640, which contains 10% FBS, 1% L-glutamine, penicillin (100 U/ml) + streptomycin (100 µg/ml). The cells were followed at 37 °C, with 95% humidity and 5% CO2 in the incubator. Every 5–6 days, the cells were subcultured.
XTT assay
ALA, cisplatin and paclitaxel, at varying doses, were administered to OVCAR-3 cells and XTT cell proliferation testing was used to determine time and dose-dependent cell viability and to determine the dose (IC50) at which 50% (50%) of the cells were alive. OVCAR-3 cells were cultured in 100 µl RPMI to be 1 × 104 cells/wells into 96-well plates. The cells were kept in the incubator with 5% CO2 at 37 °C for 24 h to adhere to the surface. At the end of 24 h, the medium was aspirated. For ALA, 25 µM, 50 µM, 100 µM, 125 µM, 250 µM, 500 µM, 750 µM, 1 mM [23]for Cisplatin, 0.0625 µM, 0.125 µM, 0.25 µM, 0.5 µM, 1 µM, 2 µM, 4 µM, 8 µM, 16 µM, 32 µM, 64 µM, 128 µM [24]; for Paclitaxel, 1 nM, 5 nM, 10 nM, 25 nM, 50 nM, 100 nM [25] study concentrations prepared in RPMI (containings 10% FBS). Each dose was studied in triplicates. Also, the dose was given at 24, 48, and 72 h to investigate the time-dependent effect. Untreated cells were used as control. Cell viability was determined using a commercial XTT kit (BI Cell Proliferation Kit XTT based Colourimetric Assay, LOT: 2,046,899) in accordance with the manufacturer’s protocol. At 24-, 48- and 72-hours post-incubation, the absorbance values of the groups were read on the Enzyme linked immunosorbent assay (ELISA) instrument at a wavelength of 450 nm and within the reference range of 630 nm. The percentage of cell viability was calculated by dividing the optical density value measured in each well by the control optical density value and multiplying it by one hundred to determine the IC50 ratio. The XTT assays for combined doses over IC50 doses were then applied 96-well plates for 24, 48, 72 h by culturing 1 × 104 cells per well. The assay groups were as follows: Control, ALA, Cisplatin + Paclitaxel, ALA + Cisplatin + Paclitaxel.
RNA isolation, cDNA synthesis, and real-time PCR (RT-PCR)
In the control and dose groups of OVCAR-3 cancer cells, the RNA isolation by TRIzol Reagent was carried out according to the manufacturer’s instructions. The cells were resuspended with 500 µl of TRIzol and total RNA was extracted. Complementary DNA (cDNA) synthesis was conducted using the cDNA synthesis Kit with RNAse inhibitor (High-Capacity) and oligo (dT) primer and the Reverse Transcriptase enzyme in accordance with the manufacturer’s protocol. After cDNA synthesis, differences in expression levels in mRNA levels were determined using the RT-PCR method and using SYBR green assay. Real-time PCR tests were performed according to the A. B.T™ Universal SYBR® Green Mastermix (Atlas;Q03-01–05) protocol. Threshold cycle values were normalised to beta- actin values for each gene and the 2 - ΔΔCT method was used for quantitative analysis (Table 1).
Enzyme-linked immunosorbent assay (ELISA)
Six-well plaques were cultured with 3 × 105 cell/well with three wells for each group. The next day, when the cells became confluent and adhered to the plates, the drug doses were administered. When the IC50 period of 48 h expired, the cell medium was removed and 350 µl of RIPA solution was put in each well. Cells were removed. In our study, the p-mTOR, p-AKT, MAPK, p- Forkhead box O1 (p-FOXO1) protein amounts of OVCAR-3 cells were evaluated with the ELISA kit (Bioassay Technology Laboratory). In the final phase of the assay, the optical density (OD) of each well was measured using a microplate reader tuned to 450 nm.
Colony formation assay
Six-well plates were cultured with 103 cells per well for control and dose groups. For 14 days, the cells were incubated at 37oC, 5% CO2, with a change of culture medium every 3 days. Fourteen days later, they were fixated with cold methanol for 10 min at -20oC. The cells were stained with crystal violet and a colony count was performed.
The wound healing assay
OVCAR-3 cells were cultured into 6-well plates with 100% confluence. After the cells were observed to have adhered to the surface of the plaque, the medium was removed. It was washed 3 times with PBS. With the help of a 200 µl pipette tip, a drawing in the form of “+” was made on the plate bottom. After that, they were washed again with PBS and dosed groups were administered in the medium. At 0, 16, 24 and 48 h, images were recorded as magnified by 10X on the invert microscope and analyzed to assess the migration of cells to the drawn area.
Matrigel-invasion assay
In the OVCAR-3 cell line, the invasion capacity of experimental groups was examined using a matrigel matrix coated upper and lower transwell chamber with pores with a diameter of 8 μm. Cells with a serum-free RPMI 1640 (500 µL) medium were cultured in upper compartments with a density of 25 × 103 cell/well, and an RPMI 1640 with 10% FBS (750 µL) was added to the lower compartments. After the materials were administered to the dosing groups, they were kept in the incubator with 5% CO2 at 37 °C for 48 h. After the invasive cells in the matrigel matrix were fixed with methanol, they were stained with crystal violet and counted as magnified by 40X under the microscope. Invasion (%) = the number of cells in the matrigel matrix basement membrane/the number of cells in control membrane X 100.
Total antioxidant status (TAS) – total oxidant status (TOS) and oxidative stress index (OSI) measurements
The TAS and TOS of all groups were determined using the Rel Assay commercial kits (Rel Assay Kit Diagnostics, Gaziantep, Turkey). TAS and TOS values were measured using a microplate reader. The OSI value was calculated using the formula “OSI = TOS (µmol H2O2 Eq/l)/TAS (mmol Trolox Eq/l) X 100”. For all dose groups TAS and TOS assays were studied in triplicates.
Cell apoptosis assay
ALA, Cisplatin + Paclitaxel and ALA + Cisplatin + Paclitaxel doses were administered to OVCAR-3 cells and the cells were collected after an incubation of 48 h. The washed cells were re-centrifuged, the supernatant was discarded, and the cells were resuspended in the annexin binding buffer. For every 100 µL cell suspension, 5 µL of Fluorescein isothiocyanate (FITC) annexin V and 1 ~ 2 µL of potassium iodide (PI) were added. The cells were incubated for 15 min in the dark at room temperature. After incubation, 400 µL 1X was slightly mixed with the addition of annexin binding buffer (specimens are kept on ice). Once the cells were loaded onto the slides, PI fluorescence and Annexin V were simultaneously measured in the Arthur Image-Based Cytometer using appropriate filters and analyzed using the instrument’s operating software.
Statistical analysis
The analysis of the data was quantified by computer program using the 2-ΔΔCT method. The groups were compared using the Volcano Plot analysis featured in the “RT² Profiler; PCR Array Data Analysis” program. The groups were statistically evaluated by the “Student t-test” analysis. In addition, parametric and non-parametric analyses of all groups were evaluated with SPSS 17.0 statistical analysis program. In the analysis of the data in the study, the Shapro-Wilk test was used to determine whether the group distributions were normal (p < 0.05 indicates statistical significance).
Results
XTT assay
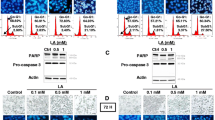
According to the XTT result, the IC50 dose of ALA was determined to be 83.1 μm at 48 h (Fig. 1A). In the results of the 24-hour cell viability test, the dosing rate at 48 h was considered IC50 as cell proliferation did not fall below 50%. At 48 h, IC50 doses of Cisplatin and Paclitaxel were found 10.6 µM and 19 µM, respectively (Fig. 1B and C). Then a combined XTT assay was conducted with these doses. Selected combined doses included Cisplatin IC50 + Paclitaxel IC50 and ALA IC50 + Cisplatin IC50 + Paclitaxel IC50; and the effective time in combined doses was also found to be 48 h (Fig. 1D).
(A) The effect of alpha lipoic acid (ALA) on the viability of ovarian cancer (OVCAR-3) cells. The cells were treated with ALA at different concentrations and time intervals, and their proliferation was assessed by 2,3-bis (2-methoxy- 4 nitro-5-sulfophenyl)–2 H-tetrazolium-5-carboxanilide ( XTT) assay. The data represent the average results of three independent experiments. IC50 dose of ALA in OVCAR-3 cells was detected as 83.1 µM/ml at 48 h. (B) Effect of Cisplatin on the viability of OVCAR-3 cells. IC50 dose of Cisplatin in OVCAR-3 cells was detected as 10.6 µM/ml at 48 h. (C) The effect of Paclitaxel on the viability of OVCAR-3 cells. IC50 dose of Paclitaxel in OVCAR-3 cells was detected as 19 nM/ml at 48 h. (D) The effect of groups on the viability of OVCAR-3 cells at 48 h
Real-time PCR
The expression of the p53 and Bcl-2 genes increased in the Cisplatin + Paclitaxel group and decreased in the ALA and ALA + Cisplatin + Paclitaxel groups. Bax expression increased in the ALA and Cisplatin + Paclitaxel groups and decreased in the ALA + Cisplatin + Paclitaxel group. The expression of TNF-α, was reduced in the Cisplatin + Paclitaxel and ALA + Cisplatin + Paclitaxel groups. But the changes in this group of genes were not statistically significant. In the ALA group, PI3K, AKT regulatory-associated protein of mTOR (RAPTOR), RICTOR gene expressions were reduced, while an increase in AKT gene expression was particularly observed in the Cisplatin + Paclitaxel group. However, it was not significant. Decreased expressions of PI3K, RAPTOR, RICTOR were found in the ALA + Cisplatin + Paclitaxel group. The reduction in RICTOR gene expression in the ALA + Cisplatin + Paclitaxel group was found statistically significant (p < 0.05). Of the genes associated with tumor cell adhesion, MMP-3 expression was decreased and MMP-9 expression was increased in the ALA group and Cisplatin + Paclitaxel group (p < 0.05); MMP-2, MMP-3, MMP-9, MMP-11 expressions were decreased in the ALA + Cisplatin + Paclitaxel group, and the reduction in MMP-9 and MMP-11 gene expressions in this group were found to be significant (p < 0.05) (Table 2).
ELISA assay results
P-AKT significantly decreased in the Cisplatin + Paclitaxel group compared to others, and there was a significant reduction in P-FOXO1 expression in all treatment groups compared to the control group (p < 0.05). The lowest levels for MAPK proteins were found in the ALA + Cisplatin + Paclitaxel triple dosage group and were statistically significant (p < 0.05) (Fig. 2).
Colony formation assay
At the end of day 14, the average number of colonies counted in the control group was 192 ± 11.7; the number of colonies in the ALA group was 370 ± 29.1. It was notable that the colony formation increased in the ALA group relative to the control group (p < 0.05) (Fig. 3A). No colony formation was observed in the Cisplatin + Paclitaxel and ALA + Cisplatin + Paclitaxel groups.
(A) Representative images for colony formation after 14 days in which colonies were stained with crystal violet. The comparison of colony formation rates of OVCAR-3 cells cultured with control and alpha lipoic acid (ALA) (83.1 µM) groups. The data are expressed as mean ± SD, n = 3, *p < 0.05 versus control. (B) Invading cells in Control, ALA, Cisplatin + Paclitaxel, ALA + Cisplatin + Paclitaxel treated groups
Wound healing assay results
At 16, 24 and 48 h, a significant increase was observed in the wound healing experiment in the control group. (p < 0.005). A comparison of the treatment groups among themselves at16 hours showed that the ALA group had significantly higher levels of wound healing than the Cisplatin + Paclitaxel and ALA + Cisplatin + Paclitaxel groups (p < 0.0001, respectively 50.7%, 13.5%, 18.1%). At 24 and 48 h, the ALA group alone presented a significant level of wound healing than all the others, including the control group. The lowest wound healing at 24 h was in the ALA + Cisplatin + Paclitaxel group, while in the ALA group, wound healing was found to increase significantly at 48 h (p < 0.005) (Fig. 4).
(I) The wounded area was calculated and plotted in the graph. The values are presented as mean ± S.D. (n = 3) with the p-values being calculated using Student’s t-test. A p value of < 0.05 was considered statistically significant. * p < 0.005, ** p < 0.0001. (II) Wound healing images of Control (A), alpha lipoic acid (ALA) (B), Cisplatin + Paclitaxel (C) and ALA + Cisplatin + Paclitaxel (D) treated groups at 0, 16, 24, 48 h
Matrigel invasion test results
As a result of counting in the matrix basal membrane setting, it was determined that an average of 1199 ± 10.2 cells in the control group, an average of 1256 ± 17.9 cells in the ALA group, 8 ± 0.8 cells in the Cisplatin + Paclitaxel group and 36 ± 1.6 cells in the ALA + Cisplatin + Paclitaxel group were invasive and passed to the other side of the control chamber (Fig. 3B).
Determination of oxidative stress level
TAS level increased in the ALA + Cisplatin + Paclitaxel group compared to the control. TOS was reduced in the ALA group and Cisplatin + Paclitaxel group compared to the control group but increased in the ALA + Cisplatin + Paclitaxel group. The highest OSI was found in the ALA + Cisplatin + Paclitaxel group. However, the comparisons between the groups were not significant (p > 0.05) (Fig. 5I).
I. Total oxidant status (TOS) (A), The total antioxidant status (TAS) (B) and Oxidative stress index (OSI) (C) values in study groups. II. Percentage of live and apoptotic OVCAR-3 cells of groups after 48 h. The data are represent three independent experiments. H2O2 (200 µM) was used as a positive control. *Significantly different from the control: *p < 0.05
Apoptosis detection with Annexin V
In the Annexin V apoptosis detection assay, the number of living cells in the Control group was significantly higher than in H2O2 and treatment groups. Although treatment groups significantly increased apoptosis compared to Control group, no significant difference was found between the treatment groups (p > 0.05) (Fig. 5II).
Discussion
Relying on just one anticancer agent to treat recurrent ovarian cancer is generally ineffective due to two main challenges: resistance developed by the cancer cells to the agent, and the limitations on dosage imposed by the side effects the agent causes. Therefore, it is crucial to discover new drugs that elevate the therapeutic effectiveness of cisplatin and paclitaxel, enabling the use of lower doses and thereby reducing their toxic side effects. That is why we examined the effect of the combined treatment of ALA on the OVCAR-3 cell line in this study. While ALA therapy alone was not effective, combined therapy with ALA reduced cell invasion, especially wound healing in the first 24 h, along with tumor cell adhesion.
ALA has been shown to inhibit the growth of tumorigenic ovarian epithelial cells, while not inhibiting normal ovarian surface epithelial cells. The potential mechanism is the inhibition of cell growth through inhibition of Tumor necrosis factor α (TNFα)-mediated inflammatory signaling pathways and p27 stabilization [26]. According to our study, only ALA treatment had no effect on TNFα in OVCAR-3 cells, while TNFα expression, which is effective in tumour formation, decreased in Cisplatin + Paclitaxel and ALA + Cisplatin + Paclitaxel groups.
Previous studies have shown that pre-treatment with ALA before radiotherapy makes MCF7 breast cancer cells sensitive to radiotherapy and strengthens the effect of irradiation on the inhibition of proliferation [27, 28]. Deveci et al. [29] showed that increased apoptosis, mitochondrial membrane depolarization, and ROS levels decreased with ALA therapy through activation of transient receptor potential-TRP ankyrin 1 (TRPA1) channels by hypoxia induction in glioblastoma cells [29]. That is, ALA inhibited mitochondrial ROS production, causing antioxidant and anti-inflammatory effects, while also causing anti-apoptotic effect by inhibition of TRPA1 channel [29]. Another study showed that after 18 days of oral administration of ALA in xenograft mice, compared with control mice treated with normal saline, ALA significantly reduced tumor nodule counts and tumor burden in the lungs of mice and reduced cell viability in lung cancer A549 cell line [30]. Bax/Bcl-2 ratio increased in A549 cells treated with ALA, suggesting that the cells may undergo apoptosis after ALA treatment [30]. Previous studies have shown that ALA induces apoptosis by activating mitochondrial O2 production, Akt inhibition, and p27Kip-induced cell cycle stoppage in human colon cancer, hepatoma and squamous cell carcinoma cells [31, 32]. In contrast to these observations, A study of A549 cells treated with ALA reported increased expression of Akt and Akt activation in cell cycle-related proteins (c-Myc and Cyclin D1) [30]. It has also been suggested that ALA induces caspase-3 activity, causing cell cycle stoppage, and plays a vital role in increasing p21, p27, and p53 protein expressions mediating apoptosis [20]. The anti-tumor effects of ALA have been noticed with the stoppage of the cell cycle in the G1 phase through the increase of protein p53 [18, 20]. p53 has been associated with ROS production and ROS-induced oxidative stress. Because tumor cells are generally known to be deficient in p53, ALA has been shown to selectively increase p53 gene expression in tumor cells compared to neighboring normal cells [20]. In our study, by contrast, both groups given ALA presented decreased p53 expression. The expression of p53 in the Cisplatin + Paclitaxel group was very high. In leukemia and breast cancer, the Bax/Bcl2 ratio increased significantly with increased caspase-3 activity after ALA treatment [22]. It has been shown that in hepatoma cancer cells ALA triggers the intrinsic apoptotic pathway by activation of caspase-3 and caspase-9 [22]. Kafara et al. [18] showed that in two human ovarian cancer cell lines, ALA reduced Mcl-1 and Bcl-xL expression, suppressing proliferation and inducing cell death. However, they did not investigate Bcl-2, which is not expressed in the cells [18]. Those studies explained the mechanism of reduced Bcl-xL expression by causing the inhibition of Akt and NF-κB [18, 22]. In our study, Bax expression from the pro-apoptotic gene group in OVCAR-3 cells also increased in the Cisplatin + Paclitaxel group in parallel with p53 and decreased in the ALA + Cisplatin + Paclitaxel group. In the ALA and ALA + Cisplatin + Paclitaxel groups, a decrease was observed in Bcl-2 gene expression. This result suggests that ALA performs apoptosis through a mechanism of reduction in Bcl-2 gene expression. In the Annexin V apoptosis assay, apoptosis significantly increased compared to the control group in administration with Cisplatin + Paclitaxel and ALA + Cisplatin + Paclitaxel groups, but no significant difference was found between these treatment groups.
ALA has been found to suppress thyroid cancer cell proliferation and growth through inhibition of the mTOR-S6 signaling pathway. ALA also inhibited migration and invasion of metastatic breast cancer cells by influencing the ERK1/2 and AKT signaling pathway [33]. It is well known that tumor proliferation is caused by the over-expression of different tyrosine kinase receptors such as EGFR and leads to the activation of PI3K/AKT, ERK and mTOR [22]. ALA activates AMPK. It has been shown to inhibit tumor progression by inhibiting the ACT effector mTOR protein complex [22]. PI3K signaling has been shown to play an important role in ovarian tumorigenesis, the emergence of aggressive phenotypes, and chemo-radiotherapy resistance [6, 7]. Our PCR results indicated decreased in AKT and RAPTOR expressions in the group where ALA was administered alone and decreased PI3K, RAPTOR and RICTOR expressions in the ALA + Cisplatin + Paclitaxel group. The reduction in RICTOR gene expression in the ALA + Cisplatin + Paclitaxel group was found statistically significant (p value < 0.05). Interestingly, in the Cisplatin + Paclitaxel group, there was an increase in AKT, RAPTOR expressions. On the other hand, p-FOXO1 and MAPK protein levels were significantly reduced in the ALA + Cisplatin + Paclitaxel group.
ALA is known to contribute to the conversion of oxidized glutathione (GSSG) back into its reduced form (GSH), which plays an important role in ROS regulation, in order to protect cells from oxidative stress [11]. ALA has been shown to play a central role in protecting cells from ROS-induced apoptotic cell death through efficient regulation of GPX [11]. Although the TAS, TOS and OSI results in our study were not statistically significant (p > 0.05), the highest OSI level in the ALA + Cisplatin + Paclitaxel group showed that this treatment was superior to other treatments. After irradiation in MCF7 breast cancer cells, ALA pretreatment significantly inhibited the colony formation ability of these cells [27]. In OVCAR-3 cells, ALA alone significantly increased the formation of colonies. However, no colony formation was observed in the ALA + Cisplatin + Paclitaxel group. In MCF7 breast cancer, migration of breast cancer cells has been effectively prevented at all doses with ALA pre-treatment [27]. In our study, we morphologically observed that ALA alone increased the migration of OVCAR-3 cells after 24 and 48 h compared to the controls in the wound healing assay. There was no difference in wound healing between Cisplatin + Paclitaxel and ALA + Cisplatin + Paclitaxel groups, and both groups inhibited cell migration to a significantly higher extent than the control and ALA groups.
ALA has been shown to significantly inhibit the activity of radiation-induced MMPs in breast cancer cells [27, 28]. In particular, they suggested that reducing the expression of MMP-2 and MMP-9 by ALA could reduce the migration and invasion of breast cancer cells to distant areas after radiation [23, 27]. In our PCR study, of the genes associated with tumor cell adhesion, MMP-3 expression was decreased and MMP-9 expression was increased in the ALA group and Cisplatin + Paclitaxel group; MMP-2, MMP-3, MMP-9, MMP-11 expressions were decreased in the ALA + Cisplatin + Paclitaxel group, and the reduction in MMP-9 and 11 gene expressions in this group were found to be significant (p < 0.05). In another assay we conducted to evaluate invasion, ALA alone did not reduce the invasion of OVCAR-3 cells compared to the control group; other treatment groups were more effective in preventing invasion.
Interestingly, ALA presents a double-edged effect when it comes to purifying ROS. Depending on its concentration and the redox modulation, ALA can function as either an antioxidant or a pro-oxidant [34]. At low doses, it eliminates ROS to protect normal cells; and it induces apoptosis and cytotoxicity in cancer cells at high doses, [20, 34]. Notably, research suggests that ALA demonstrates significantly enhanced anti-cancer properties when administered in combination with chemotherapy or radiation therapy [28]. ALA increased paclitaxel activity in breast and lung cancer cells by inhibiting NF-κB signaling and integrin β1/β3, respectively [22]. A similar study showed that ALA and Docetaxel therapy increased apoptosis in breast cancer cells compared to single drug protocols [22]. ALA has been shown to strengthen the cytotoxicity of 5-fluorouracil and Temozolomide used in colorectal cancer, and to overcome gefitinib resistance of ALA by reducing the activation of growth factor receptors in non-small cell lung cancer [22, 28]. In our study, ALA has been found to increase Cisplatin and Paclitaxel effectiveness by reducing the MMP expression, which is important for cell adhesion and MAPK activation, which is important for cell invasion, metastasis, and proliferation.
One of the main limitations of our study is the use of a single cell line. Another limitation is the lack of experiments on a mouse xenograft tumour model. Different ovarian cell lines and experimental mouse model studies will increase the value of our study.
Conclusions
According to our study, the use of ALA alone was not effective on the OVCAR-3 cell line. The results of the wound healing assay also showed that, although the ALA + Cisplatin + Paclitaxel group was effective at 24 h, ALA’s tumor-inhibiting effect disappeared at 48 h. The clear effect of ALA in combined therapies was on MMP proteins, which are important in tumor adhesion. Future studies may focus on in vivo studies to develop drugs based on cell-adhesion preventive effects of ALA and its half-life.
Data availability
The data generated and included in this study are available from the corresponding author on reasonable request.
Abbreviations
- ALA:
-
Alpha Lipoic acid
- OVCAR-3:
-
ovarian cancer
- XTT:
-
2-methoxy- 4 nitro-5-sulfophenyl)–2 H-tetrazolium-5-carboxanilide
- RT-PCR:
-
Real-time polymerase chain reaction
- RICTOR:
-
rapamycin-insensitive companion of mTOR
- MMP:
-
Matrix metalloproteinase
- MAPK:
-
Mitogen-activated protein kinase
- PI3K:
-
Phosphatidylinositol 3-kinase
- ROS:
-
reactive oxygen species
- AMPK:
-
AMP-activated protein kinase
- PBS:
-
Dulbecco’s Phosphate buffered saline
- FBS:
-
Fetal Bovine Serum
- ELISA:
-
Enzyme-Linked Immunosorbent Assay
- cDNA:
-
Complementary DNA
- RT:
-
Reverse Transcriptase enzyme
- Fox O1:
-
Forkhead box O1
- OD:
-
Optical density
- TAS:
-
Total antioxidant status
- TOS:
-
Total oxidant status
- OSI:
-
Oxidative stress index
- FITC:
-
Fluorescein isothiocyanate
- PI:
-
Potassium iodide
- RAPTOR:
-
Regulatory-associated protein of mTOR
- TNFα:
-
Tumor necrosis factorα
- TRPA1:
-
Transient receptor potential-TRP ankyrin 1
References
Kuroki L, Guntupalli SR (2020) Treatment of epithelial ovarian cancer. BMJ Nov 9:371:m3773. https://doi.org/10.1136/bmj.m3773
Barani M, Bilal M, Sabir F, Rahdar A, Kyzas GZ (2021) Nanotechnology in ovarian cancer: diagnosis and treatment. Life Sci 1:266:118914. https://doi.org/10.1016/j.lfs.2020.118914
Rojas V, Hirshfield KM, Ganesan S, Rodriguez-Rodriguez L (2016) Molecular characterization of epithelial ovarian Cancer: implications for diagnosis and treatment. Int J Mol Sci 15(12):2113. https://doi.org/10.3390/ijms17122113
Roett MA, Evans P (2009) Ovarian cancer: an overview. Am Fam Physician 15(6):609–616
Kurman RJ, Shih IM (2010) The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol 34(3):433–443. https://doi.org/10.1097/PAS.0b013e3181cf3d79
Ediriweera MK, Tennekoon KH, Samarakoon SR (2019) Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: Biological and therapeutic significance. Semin Cancer Biol 59:147–160. https://doi.org/10.1016/j.semcancer.2019.05.012
Huang TT, Lampert EJ, Coots C, Lee JM (2020) Targeting the PI3K pathway and DNA damage response as a therapeutic strategy in ovarian cancer. Cancer Treat Rev 86:102021. https://doi.org/10.1016/j.ctrv.2020.102021
Bolton KL, Chenevix-Trench G, Goh C, Sadetzki S, Ramus SJ, Karlan BY, Lambrechts D, Despierre E, Barrowdale D, McGuffog L, Healey S, Easton DF, Sinilnikova O, Benítez J, García MJ, Neuhausen S, Gail MH, Hartge P, Peock S, Frost D, Evans DG, Eeles R, Godwin AK, Daly MB, Kwong A, Ma ES, Lázaro C, Blanco I, Montagna M, D’Andrea E, Nicoletto MO, Johnatty SE, Kjær SK, Jensen A, Høgdall E, Goode EL, Fridley BL, Loud JT, Greene MH, Mai PL, Chetrit A, Lubin F, Hirsh-Yechezkel G, Glendon G, Andrulis IL, Toland AE, Senter L, Gore ME, Gourley C, Michie CO, Song H, Tyrer J, Whittemore AS, McGuire V, Sieh W, Kristoffersson U, Olsson H, Borg Å, Levine DA, Steele L, Beattie MS, Chan S, Nussbaum RL, Moysich KB, Gross J, Cass I, Walsh C, Li AJ, Leuchter R, Gordon O, Garcia-Closas M, Gayther SA, Chanock SJ, Antoniou AC, Pharoah PD EMBRACE; kConFab investigators; Cancer Genome Atlas Research Network (2012) Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA 25;307(4):382–390. https://doi.org/10.1001/jama.2012.20
Kakar SS, Jala VR, Fong MY (2012) Synergistic cytotoxic action of cisplatin and withaferin A on ovarian cancer cell lines. Biochem Biophys Res Commun. 2012;423(4):819 – 25. https://doi.org/10.1016/j.bbrc.2012.06.047
Ghosh S (2019) Cisplatin: the first metal based anticancer drug. Bioorg Chem 88:102925. https://doi.org/10.1016/j.bioorg.2019.102925
Kim KH, Lee B, Kim YR, Kim MA, Ryu N, Jung DJ, Kim UK, Baek JI, Lee KY (2018) Evaluating protective and therapeutic effects of alpha-lipoic acid on cisplatin-induced ototoxicity. Cell Death Dis. 1;9(8):827. https://doi.org/10.1038/s41419-018-0888-z
Kleih M, Böpple K, Dong M, Gaißler A, Heine S, Olayioye MA, Aulitzky WE, Essmann F (2019) Direct impact of cisplatin on mitochondria induces ROS production that dictates cell fate of ovarian cancer cells. Cell Death Dis. 7;10(11):851. https://doi.org/10.1038/s41419-019-2081-4
Ayyagari VN, Hsieh TJ, Diaz-Sylvester PL, Brard L (2017) Evaluation of the cytotoxicity of the Bithionol - cisplatin combination in a panel of human ovarian cancer cell lines. BMC Cancer 13(1):49. https://doi.org/10.1186/s12885-016-3034-2
Świerczewska M, Klejewski A, Brązert M, Kaźmierczak D, Iżycki D, Nowicki M, Zabel M, Januchowski R (2018) New and Old Genes Associated with Primary and Established Responses to Paclitaxel Treatment in Ovarian Cancer Cell Lines. Molecules. 12;23(4):891. https://doi.org/10.3390/molecules23040891
Zhu L, Chen L (2019) Progress in research on paclitaxel and tumor immunotherapy. Cell Mol Biol Lett 13:24:40. https://doi.org/10.1186/s11658-019-0164-y
Lim HJ, Ledger W (2016) Targeted therapy in ovarian cancer. Womens Health (Lond) 12(3):363–378. https://doi.org/10.2217/whe.16.4
Opławski M, Średnicka A, Niewiadomska E, Boroń D, Januszyk P, Grabarek BO (2022) Clinical and molecular evaluation of patients with ovarian cancer in the context of drug resistance to chemotherapy. Front Oncol 5:12:954008. https://doi.org/10.3389/fonc.2022.954008
Kafara P, Icard P, Guillamin M, Schwartz L, Lincet H (2015) Lipoic acid decreases Mcl-1, Bcl-xL and up regulates Bim on ovarian carcinoma cells leading to cell death. J Ovarian Res 12:8:36. https://doi.org/10.1186/s13048-015-0165-z
Bilska A, Włodek L (2005) Lipoic acid - the drug of the future? Pharmacol Rep 57(5):570–577
Novotny L, Rauko P, Cojocel C (2008) Alpha-lipoic acid: the potential for use in cancer therapy. Neoplasma 55(2):81–86
El Barky AR, Hussein SA, Mohamed TM (2017) The potent antioxidant alpha Lipoic Acid. J Plant Chem Ecophysiol 2(1):1016
Farhat D, Lincet H (2020) Lipoic acid a multi-level molecular inhibitor of tumorigenesis. Biochim Biophys Acta Rev Cancer 1873(1):188317. https://doi.org/10.1016/j.bbcan.2019.188317
Tripathy J, Tripathy A, Thangaraju M, Suar M, Elangovan S (2018) α-Lipoic acid inhibits the migration and invasion of breast cancer cells through inhibition of TGFβ signaling. Life Sci 15:207:15–22. https://doi.org/10.1016/j.lfs.2018.05.039
Karaca B, Atmaca H, Bozkurt E, Kisim A, Uzunoglu S, Karabulut B, Sezgin C, Sanli UA, Uslu R (2013) Combination of AT-101/cisplatin overcomes chemoresistance by inducing apoptosis and modulating epigenetics in human ovarian cancer cells. Mol Biol Rep 40(6):3925–3933. https://doi.org/10.1007/s11033-012-2469-z
Boivin M, Lane D, Piché A, Rancourt C (2009) CA125 (MUC16) tumor antigen selectively modulates the sensitivity of ovarian cancer cells to genotoxic drug-induced apoptosis. Gynecol Oncol 115(3):407–413. https://doi.org/10.1016/j.ygyno.2009.08.007
Vig-Varga E, Benson EA, Limbil TL, Allison BM, Goebl MG, Harrington MA (2006) Alpha-lipoic acid modulates ovarian surface epithelial cell growth. Gynecol Oncol 103(1):45–52. https://doi.org/10.1016/j.ygyno.2006.01.060
Tripathy J, Chowdhury AR, Prusty M, Muduli K, Priyadarshini N, Reddy KS, Banerjee B, Elangovan S (2020) α-Lipoic acid prevents the ionizing radiation-induced epithelial-mesenchymal transition and enhances the radiosensitivity in breast cancer cells. Eur J Pharmacol 15:871:172938. https://doi.org/10.1016/j.ejphar.2020.172938
Choi HS, Kim JH, Jang SJ, Yun JW, Kang KM, Jeong H, Ha IB, Jeong BK (2021) Synergistic Tumoricidal effects of Alpha-Lipoic acid and radiotherapy on human breast Cancer cells via HMGB1. Cancer Res Treat 53(3):685–694. https://doi.org/10.4143/crt.2020.1015
Deveci HA, Akyuva Y, Nur G, Nazıroğlu M (2019) Alpha lipoic acid attenuates hypoxia-induced apoptosis, inflammation and mitochondrial oxidative stress via inhibition of TRPA1 channel in human glioblastoma cell line. Biomed Pharmacother 111:292–304. https://doi.org/10.1016/j.biopha.2018.12.077
Peng P, Zhang X, Qi T, Cheng H, Kong Q, Liu L, Cao X, Ding Z (2020) Alpha-lipoic acid inhibits lung cancer growth via mTOR-mediated autophagy inhibition. FEBS Open Bio 10(4):607–618. https://doi.org/10.1002/2211-5463.12820
Wenzel U, Nickel A, Daniel H (2005) Alpha-lipoic acid induces apoptosis in human colon cancer cells by increasing mitochondrial respiration with a concomitant O2-*-generation. Apoptosis 10(2):359–368. https://doi.org/10.1007/s10495-005-0810-x
Shi DY, Liu HL, Stern JS, Yu PZ, Liu SL (2008) Alpha-lipoic acid induces apoptosis in hepatoma cells via the PTEN/Akt pathway. FEBS Lett 582:1667–1671
Salehi B, Berkay Yılmaz Y, Antika G, Boyunegmez Tumer T, Fawzi Mahomoodally M, Lobine D, Akram M, Riaz M, Capanoglu E, Sharopov F, Martins N, Cho WC, Sharifi-Rad J (2019) Insights on the Use of α-Lipoic Acid for Therapeutic Purposes. Biomolecules. 9;9(8):356. https://doi.org/10.3390/biom9080356
Attia M, Essa EA, Zaki RM, Elkordy AA (2020) An Overview of the Antioxidant Effects of Ascorbic Acid and Alpha Lipoic Acid (in Liposomal Forms) as Adjuvant in Cancer Treatment. Antioxidants (Basel). 25;9(5):359. https://doi.org/10.3390/antiox9050359
Funding
This study was supported by The Pamukkale University Scientific Research Projects Coordination Unit (grant 2021TIPF002).
Author information
Authors and Affiliations
Contributions
HSC and GAM were involved in the design of the experiments and drafted the manuscript. HSC, NC and EO were involved in data analysis and editing of the manuscript. GAM, NC and EO reviewed and edited the manuscript. All authors read and approved the final version of manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This research did not involve in human or animal subjects.
Conflict of interest
The authors declare no conflict of interest regarding this publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Çoban, H.Ş., Çil, N., Önder, E. et al. Anti-cancer effects of alpha lipoic acid, cisplatin and paclitaxel combination in the OVCAR-3 ovarian adenocarcinoma cell line. Mol Biol Rep 51, 485 (2024). https://doi.org/10.1007/s11033-024-09422-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09422-8