Abstract
Background
Ovarian cancer is one of the most lethal gynecological cancers among women worldwide. Cisplatin (Cis) is an effective chemotherapeutic agent used to treat several types of cancer. Silymarin (SLM) is an extract of medicinal plant Silybum marianum (milk thistle) with anti-inflammatory, anti-angiogenesis, antioxidant, and anticancer properties used alone or in combination with other drugs.
Objective
This study aimed to explore the effects of co-treatment with SLM and Cis on A2780 human ovarian cancer cell lines.
Methods
In this study, A2780 cells were treated with various concentrations of SLM and Cis, separately and in combination. Cell cytotoxicity, scratch, clonogenic, and flow-cytometry assays were accomplished to estimate cell viability, migration, colony formation, and apoptosis, respectively. Real-time PCR was utilized to determine the expression levels of miR-155 and miR-27a.
Results
SLM significantly reduced the proliferation of A2780 cells in a concentration- and time-dependent manner. Combination treatment with SLM and Cis was more potent than either single treatment in reducing viability, suppressing migration, inhibiting colony formation, and promoting the induction of apoptosis. Additionally, gene expression analysis revealed a significant decline in the expression levels of miR-155 and miR-27a in response to all separate and combined treatments, and co-treatment was more effective than individual treatments in altering miRNAs expression.
Conclusion
Based on our findings, SLM boosts the anticancer activity of Cis and mitigates its side effects. Thus, the co-treatment of SLM and Cis can be proposed as a promising therapeutic strategy for further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a lethal disease caused by dysregulation in cell proliferation and cell death. This disease has turned into a serious health risk worldwide and in addition to the physical health, it also overshadows the mental health of the patient Cancer cell genotypes cause malignant and unlimited growth in cells in response to changes including self-sufficiency in growth signals, avoidance of apoptosis, insensitivity to anti-growth signals, stable angiogenesis, tissue invasion, and metastasis. Cancer treatment methods depend on the type and stage of cancer, and based on this, conventional methods such as surgery, chemotherapy, radiotherapy, or recent methods such as immunotherapy, targeted therapy, hormone therapy, and photodynamic therapy may be prescribed for the patient [1]. Anticancer drugs are often associated with adverse reactions and may produce an unfavorable effect in the cancer treatment due to drug resistance, since chemotherapy drugs usually target a single pathway and in addition to cancer cells, they also target normal cells [2]. Cisplatin (Cis) is a well-known chemotherapeutic drug used to treat all types of solid cancers, including bladder, lung, ovarian, and testicular cancers. Its mode of action involves covalent binding to DNA, causing cell death through a cascade of biochemical mechanisms including oxidative stress, DNA damage, and interference with several signal transduction pathways [3, 4]. Cisplatin activates apoptosis through two pathways: one involves p53 protein, which is a tumor suppressor, and the other is mediated by p73 protein related to p53 [5]. Side effects and drug resistance are two important challenges for cisplatin, which limit its application and efficacy for cancer treatment [6]. Due to the emergence of these problems during cancer treatment with cisplatin, multiple studies have demonstrated alternatives to boost the bioactivity of cisplatin and to reduce or eliminate its adverse effects. This is why the combination of cisplatin with other drugs, especially herbal products, is a better choice for the treatment of cancer cells [7]. The significance of combination treatment with chemotherapy is that it enhances the antitumor immune response, reduces the possibility of developing chemotherapy-resistant cancer cells, increases the sensitivity of cancer cells to chemotherapy, and decreases the side effects of chemotherapeutic drugs on normal cells [8].
The mechanisms of action of natural products in combination with chemotherapy drugs can be briefly explained by three conditions: Reversing chemo-resistance by reducing drug efflux or overcoming other accumulation mechanisms to enhance chemotherapy drugs in cancer cells, directly boosting the effect of tumor killing by sensitizing cancer cells to respond more to chemotherapy drugs, and reducing the toxicity of chemotherapy drugs by promoting the repair mechanism in normal cells against the damage of chemotherapy drugs [9]. Flavonoids are a class of nature-derived compounds with a polyphenolic structure. Flavonoids enhance the potential of chemotherapy through depletion of the cellular oxidative system, resulting in disruption of mitochondrial function and subsequent apoptosis [10, 11]. Flavonoids possess several pharmacological and biological properties, including antibacterial, antifungal, antipyretic, antiparasitic, and anticancer activities. Several studies have demonstrated that various nature-derived compounds lower the resistance of cancer cells to cisplatin and the side effects of cisplatin in normal organs as well as systems, such as the liver, kidneys, together with cardiovascular, hematopoietic, reproductive, and nervous systems [12]. Silymarin (SLM) is one of the flavonoid compounds with anti-inflammatory and anti-cancer effects confirmed in various studies. Silymarin derived from milk thistle contains the flavonolignan silybin, which protects normal cells against various toxic molecules or the harmful effects of chemotherapy agents on normal cells [13, 14]. In addition, silymarin and its key bioactive compounds inhibit organic anion transporters (OATs) and ATP-binding transporters (ABC), thereby assisting in combating chemoresistance. Thus, silymarin acts as a chemical sensitizer and reduces the drug resistance of cancer cells [15]. The present study aimed to enhance the anticancer activity of Cis against A2780 cells by combining it with SLM. In addition, to determine the effect of these compounds on epigenetic processes in cells, the expression of miR-27a and miR-155 was also measured in cell treatments. Various studies have shown that nutraceuticals and phytochemicals can act as molecular inhibitors for miRNAs [16, 17]. The regulation of miRNAs by phytochemicals can be effective on tumor progression and development, and in combination with other drugs, it can produce a synergistic effect on the expression level of miRNAs [18]. As a result, it leads to an increase in the sensitivity of some cancer cells to drugs. miR-27a and miR-155 play an oncogenic role in various cancers [19]. Thus, in this study, in addition to determining the effect of simultaneous silymarin and cisplatin treatment on cancer cells, the expression of miR-155 and miR-27a was also measured.
Materials and methods
Cell lines and cell cytotoxicity assay
In this study, A2780 cell line derived from ovarian endometrioid adenocarcinoma tumor was used. The Ovarian cancer cell line was obtained from the Iranian Biological Resources Center. It was cultured in T25 flasks (37 °C, 5% CO2) containing RPMI640 (L-Glutamine) medium with 10% fetal bovine serum (FBS), and 1% antibiotics. The cells were used for further experiments after reaching a density of 70–80%. The MTT colorimetric assay was used to investigate the effect of silymarin and cisplatin (individually and in combination) on growth inhibition and cytotoxicity and also to calculate the survival rate of A2780 cells. First, the cells were cultured at the number of 1 × 104 cells/well, and after 24 h, Cells were exposed to various concentrations of cisplatin (0–6 µg/ml) and silymarin (0–500 µM/ml) separately for 48 h. After determining the effect of single treatment on the cells, four concentrations of silymarin (0, 10, 30 and 100) were combined with a concentration gradient of cisplatin (0–100µM/ml) and the cells were treated with the prepared combinations for 48 h. After the completion of the treatment time, MTT solution (5 mg/ml PBS) was added to each well and the cells were incubated for 4 hours at 37 °C in the dark. Next, to dissolve the formazan crystals, the 100 µl of DMSO was added to the wells and the optical density was measured using a 96-well plate-reader at 490 nm. The percentage of cell survival after both separate and combined treatments was calculated using the following formula:
Synergistic effect of silymarin and cisplatin
The combined effect of SLM and Cis was analyzed using the CompuSyn software. Combination index (CI) is an important factor in pharmacology and combination drug therapy. This index measuring the effect of the combination of two or more than two drugs. CI <, =, and > 1 represented synergistic, additive, and antagonistic combined effects, respectively. In the present study, to determine this effect, the effective value for each specific treatment was first entered into the software, which then calculated the amount of CI.
The rate of cell migration
In the scratch test, the attached cells were scratched at the middle of each well and a cell-free area was created. The migration rate from around the scratched site towards the center was analyzed by taking photos 0, 24, 48, and 72 h after scratching. Briefly, cells were cultured in the 6-well plate with a density of 7 × 105 cells per well. After 24 h of cell culture, the cell density reached about 95% and a vertical scratch could be created in the middle of the well. Then the cells were washed twice with PBS, and then exposed to medicinal medium containing silymarin, cisplatin and the combination of cisplatin and silymarin at a concentration of IC30. The amount of cell migration was investigated by imaging at the time intervals of 0, 24, 48, and 72 h and then the scratch distance was calculated using Image J software.
Determination of colony formation
Cells were seeded in a 6-well plate (1 × 103 cells/well) and incubated for 24 h at 37 °C in 5% CO2. Next, Cells were treated with IC30 of SLM/Cis individually and in combination with IC30 of Cis and incubated for 48 h. Then, cells were washed with PBS and re-cultured with a complete medium for 14 days. Cells were analyzed for colony formation. Colonies were fixed using methanol and acetic acid. After 20 min, 0.5% crystal violet (Sigma-Aldrich) was used to stain colonies for 1 h. Subsequently, colonies were washed twice with distilled water and after the plate was dried, the colonies were counted using image J software. The ratio of the number of colonies to the number of cultured cells was calculated.
Cellular apoptosis test
Cells were cultured in 6-well plates at 80% density and keep for 24 h at 37 °C in 5% CO2. Subsequently, cells were treated with IC10 of SLM and Cis individually and in combination with IC10 of Cis. After 48 h, cell death was investigated using a Mab-Tag apoptosis kit based on AnnexinV-FITC and PI according to the manufacturer’s guidelines. Briefly, cells were harvested and centrifuged at 300 g for 5 min. The pellets of the cells were washed with PBS twice and resuspended in 1X AnnexinV buffer. Then, 5 µL of AnnexinV-FITC and 5 µL of Propidium iodide were added to each sample and incubated in a dark at 37 °C for 20 min. Next, 400µL of AnnexinV binding buffer was added to each sample separately and centrifuged at 400 g for 5 min. Finally, the samples were analyzed using a flow cytometer (BD Bioscience, Ex. 488 mm and Em. 530 nm) and Flowjow 7.6.1 software.
Gene expression assay
qPCR was used to evaluate the effect of separate and co-treatment of cisplatin and silymarin on the expression of miR-155 and miR-27a in A2780 cell line. The cells were cultured at 1 × 106 cells/well and after 24 h, the treatment with the IC30 of each of the compounds was done separately and in combination. 48 h after treatment, total cell RNA was extracted using TRIzol solution. cDNA synthesis was done by stem loop primers of miRNA with the help of MMLV enzyme. The real-time PCR reaction was performed by specific primers and SYBR green mastermix, and the U6 gene was used as the internal control of the reaction. Finally, the 2−ΔΔCT method was used to analyze the results.
Data analysis
GraphPad Prism v.9.0 software was used to investigate the statistical results of colony formation, migration, and flow cytometry tests, as well as to calculate and compare the expression level in control and treated samples. In order to do comparisons, student t-test and ANOVA were used for more than 2 groups. P-value less than 0.05 was considered as significance level. All experiments were performed in triplicates for statistical reliability.
Results
Cell proliferation assay (separate and combination treatments)
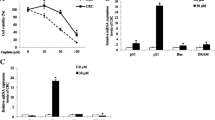
The effect of separate and combined treatments of SLM and Cis on A2780 cells were evaluated using MTT assay. A2780 cells were treated with different concentrations of SLM (0–500 µM/ml) and Cis (0–100 µM/ml) separately for 24 and 48 h. The results indicated that separate treatment with SLM and Cis decreased the viability of A2780 cells in a dose- and time-dependent manner. As shown in Fig. 1A, reduction in cell viability after treatment with Cis occurred at concentrations above 1 µM, which was more after 48 h of treatment compared to 24 h. The IC50 (concentration required for 50% cell death) of Cis calculated to be 2.7 ± 0.22 and 1.76 ± 0.36 µM after 24 and 48 h, respectively. Figure 1B also shows that separate treatment with SLM decreased the viability of A2780 cells in a concentration- and time-dependent manner. The IC50 value of SLM calculated to be 347.18 ± 0.81 and 207.82 ± 0.21 µM after 24 and 48 h of treatment, respectively.
To understand the anti-proliferative effect of SLM and Cis co-treatment on A2780 cells, four concentrations of SLM (0, 10, 30, and 100 µM) were selected and combined with various concentrations of Cis. As shown in Fig. 1C, after 48 h of co-treatment with SLM and Cis, the cell viability decreased significantly. Based on the results of this experiment, the reduction in cell viability was visible with a combination of 0.5 and 1 µM Cis with 30 and 100 µM SLM. The IC50 value of Cis in combination with 10, 30, and 100 µM of SLM was calculated to be 1.06 ± 0.85, 0.91 ± 0.87, and 0.59 ± 0.98 µM, respectively. Therefore, combination treatment with Cis and high concentrations of SLM led to a decrease in the consumption of Cis. CompuSyn software was used to evaluate the combined effects of SLM and Cis. The Fa-CI curve demonstrated a CI of less than one, indicating synergistic effects (Fig. 1D).
Cell viability analysis. A2780 cells were exposed to different concentrations of Cis A, SLM B, or their combination C. Combination treatment exerted a greater inhibitory effect on cell growth compared with SLM or Cis separate treatment. D CI Value-Fa (Fa: fraction affected level) curve for SLM and Cis derived from CompuSyn, indicating a synergistic effect with CI < 1
Migration rate
A wound-healing assay was performed to determine the effect of SLM and Cis, separately and in combination, on the migration of A2780 cells. To this end, A2780 cells in scratched wells were treated with IC30 of SLM and Cis separately and in combination. Figure 2 illustrates the scratch width ratio and cellular migration under a light microscope after 24 and 48 h of treatment. Based on these results, separate treatment with SLM and Cis inhibited the migration of A2780 cells after 24 and 48 h compared to that in the control group. Furthermore, co-treatment with SLM and Cis was more effective in inhibiting migration than both separate treatments, and the scratched area was larger, confirming the synergistic effect of SLM and Cis.
Colony formation assay
In the colony formation assay, the number of colonies is considered as an indicator of cell survival. A clonogenic assay was used to investigate the effect of SLM and Cis, separately and in combination, on the colony formation ability of A2780 cells. Figure 3A shows colony formation under treatment with SLM and Cis separately and in combination compared to the control group. Separate treatment with SLM and Cis decreased the number of colonies compared with the untreated control group, and co-treatment with SLM and Cis led to a significant reduction compared with both separate treatments, indicating the synergistic effect of SLM and Cis. Figure 3B also shows the ratio of the number of colonies to the number of cultured cells as colony formation ratio, which decreased significantly with the combination of SLM and Cis compared to both separate treatments (p-value < 0.01). Based on the diagram, the ratio of colony formation in the control group was calculated to be 48%, in the SLM- and Cis-treated cells was 35% and 27.5%, respectively, and in the co-treated cells was 19.5%.
determination of colony formation inhibition after separate and combined treatments with SLM and Cis in A2780 cell line. A Clonogenic assay was performed after 2 weeks of treatment, and colonies were then stained with crystal violet 5%. B the ratio number of colonies to the number of cultured cells. Experiment was conducted in triplicates. Co-treatment was significantly more effective than separate treatments (* <0.05, ** <0.001)
Apoptosis induction
The extent of apoptosis after treatment with IC10 of SLM and Cis separately and in combination was evaluated using Annexin V-FITC/PI dual staining. As shown in Fig. 4A, separate treatments with SLM and Cis induced apoptosis in A2780 cells. Co-treatment with SLM and Cis significantly increased apoptosis induction compared with both separate treatments. In calculating the percentage of these cells according to the division of different parts of the diagram based on the percentage of positive and negative cells for each color, the percentage of cells undergoing apoptosis in the separate treatments with SLM and Cis was 17.25% and 28.3%, respectively. However, co-treatment with SLM and Cis increased the apoptosis rate to approximately 38% (Fig. 4B). Collectively, these results indicate the synergistic pro-apoptotic effects of SLM and Cis combination treatment in A2780 cells.
Cell death analysis using Mab-tag apoptosis kit and Annexin-V/PI dual staining. A Apoptosis rate after treatment with IC10 of SLM and Cis, separately and in combination. Q3 (the lower right; Annexin V+/PI−), Q2 (the upper right; Annexin V+/PI+), and Q1 (the upper left; Annexin V−/PI+) indicate the percentage of cells undergo early apoptosis, late apoptosis, and necrosis, respectively. Q4 (lower left; Annexin V−/PI-) shows the percentage of live cells. B Co-treatment with SLM and Cis resulted in ~ 38% apoptosis in the A2780 cell population, which was much greater than that observed with SLM (17.25%) or Cis (28.3%)
qPCR analysis
The effects of separate and combined treatments with SLM and Cis on the expression levels of miR-155 and miR-27a in A2780 cells were evaluated using qPCR. In this study, the expression of both miRNAs significantly decreased under co-treatment with SLM and Cis. In the comparison between the effect of SLM and Cis separatly on the expression of miR-155 and miR-27a, a significant difference was observed for miR-155 (p < 0.001). Regarding miR-27a, the expression difference between SLM, Cis and control was not significant and no difference was observed (Fig. 5).
Discussion
Several therapeutic strategies have been developed to target various cancer pathways. Combination therapies have provided the most effective results in the field of anticancer effects. The superiority of combination therapy is due to its ability to target several pathways. This minimizes drug resistance, because cancer cells treated with combination therapy are often unable to adapt to the simultaneous toxic effects of the two treatment agents [20]. In many cases, when the tumor size increases or cancer cells metastasize, combined chemotherapy can prolong patient survival. In addition, combining chemotherapy with natural products such as flavonoids, as the largest class of phytonutrients and secondary polyphenolic metabolites, has provided promising results in inhibiting cancer progression, metastasis, and sensitizing chemotherapy-resistant phenotypes [21]. Polyphenols are among the substances that have antioxidant and anti-inflammatory properties and modulate cell signaling pathways. Numerous in vitro and in vivo tests have shown the effectiveness of combining polyphenols with chemotherapy drugs compared to conventional antineoplastic drugs and have highlighted the possible use of these compounds in clinical settings [22]. Silymarin (SLM) is one of these phenolic compounds, extracted from the seed of Silybum marianum. SLM possesses several biological and pharmacological properties, including anti-inflammatory, antiangiogenic, and antioxidant properties. SLM also exerts anticancer activities by inhibiting proliferation and invasion, suppressing migration and metastasis, inducing apoptosis and autophagy, and arresting cell cycle in various types of cancers such as hepatocellular carcinoma, skin, breast, cervical, colon, lung, bladder, prostate, and renal cancers [23, 24]. In this study, we found that separate treatment with SLM inhibited proliferation, migration, and colony formation and induced apoptosis in A2780 ovarian cancer cells. These results are consistent with those of Kala et al., who reported that SLM inhibited proliferation and enhanced apoptosis in MCF7 breast cancer cells and NCI-H23 lung cancer cells, owing to alterations in the expression levels of apoptotic genes such as caspase-3 and p53 [25]. Kim et al. also indicated that SLM inhibits proliferation, and migration and induces apoptosis by modulating the MAPK signaling pathway in AGS gastric adenocarcinoma cell [26], MCF7 and MDA-MB-231 breast cancer cells [27]. It has been also reported that the active substance of silymarin (silybin) increases the cytotoxicity of chemotherapeutic drugs, such as doxorubicin, cisplatin (Cis), 5-FU, and carboplatin [28,29,30,31]. SLM can also exert protective effects against chemotherapy-induced toxicity such as nephrotoxicity, neurotoxicity, cardiotoxicity, and hepatotoxicity [32,33,34,35]. In addition, the effect of silymarin in competition with membrane transporters, including P- glycoprotein (P-gp), Breast Cancer Resistant-Protein (BCRP), and Multi Drug Resistant-Proteins (MRPs), can cause a decrease in resistance to chemotherapy, as silymarin inhibits membrane transporters [30, 36]. This type of drug use can target different pathways, thus reducing the chances of cancer cells becoming malignant and incurable. Thus, the main purpose of the current study was to examine the combination of SLM and Cis in enhancing the sensitivity of A2780 ovarian cancer cells to Cis and to reduce its consumption dosage. According to our findings, co-treatment with SLM and Cis effectively inhibited cell viability in a concentration- and time-dependent manner in A2780 cells. In addition, co-treatment with SLM and Cis significantly boosted the lethal effect of Cis on ovarian cancer cells at lower concentrations. Separate treatment with 2.7 ± 0.22 and 1.76 ± 0.36 µM Cis resulted in 50% cell death after 24 and 48 h, respectively. However, 0.59 ± 0.98 µM of Cis was required for 50% cell death when combined with 100 µM of SLM. We also showed that co-treatment with SLM and Cis significantly reduced the number and size of colonies, and migration rate of A2780 cells compared with both SLM and Cis separate treatments, confirming the effect of SLM on enhancing the chemotherapeutic potential of Cis against A2780 cells. We then evaluated the effect of the co-treatment of SLM and Cis on apoptosis. Flow cytometry analysis with Annexin V-FITC and PI double staining revealed that separate treatments with SLM and Cis resulted in a 17.25% and 28.3% increase in the apoptotic rate of cancer cells, respectively, and co-treatment with SLM and Cis resulted in a 38% increase in the apoptotic rate, suggesting the synergistic pro-apoptotic effects of the SLM and Cis cotreatment on cancer cells. To confirm the synergistic effect of the combination of SLM and Cis, the CI was then calculated using the Compusyn software. A CI of less than one indicates a synergistic effect of SLM and Cis. The results of the present study were also in line with those of Ninsontia et al.., who reported that SLM at concentrations up to 200 µM dramatically enhanced the cytotoxic effect of Cis against human lung cancer epithelial cells (H460) and that the addition of SLM to Cis-treated human melanoma cells (G361) significantly augmented the cytotoxicity of Cis in a concentration-dependent manner and induced apoptosis [37].
MiRNA dysregulation is associated with the initiation and progression of several malignancies such as cancer. Previous studies have demonstrated that miR-155 and miR-27a are two oncomiRs that are commonly upregulated in several types of cancers and have the potential to be considered as biomarkers for cancer diagnosis and treatment [38,39,40,41,42]. Hence, we decided to investigate the effect of separate and combined treatments with SLM and Cis on the expression levels of miR-155 and miR-27a in A2780 ovarian cancer cells. Real-time PCR analysis indicated that the expression level of miR-155 and miR-27a decreased significantly in response to SLM treatments. However, co-treatment with SLM and Cis was more potent in downregulating miR-155 and miR-27a than either treatment alone. These results were consistent with those of previous studies that reported the effect of SLM, its derivation, or other drugs on miR-155 and miR-27a expression. As Maleki Zadeh et al. reported, silibinin decreased the expression of miR-155 in MCF7 cells compared to that in untreated cells [43]. Abtin et al. also reported that oleuropein downregulated miR-155, resulting in migration retardation, invasion suppression, and apoptosis induction in MCF7 breast cancer cells [44]. Xia et al. reported that genistein significantly decreased the expression of miR-27a in treated pancreatic cancer cells compared to untreated control cells, leading to inhibition of growth, suppression of invasion, and induction of apoptosis [45]. Toden et al. also discovered that curcumin and boswellic acid downregulated miR-27a in colorectal cancer cells [39].
Conclusion
In summary, the results of our study specify that SLM boosts the anticancer activities of Cis in A2780 ovarian cancer cells with regard to proliferation reduction, migration and colony formation inhibition, as well as apoptosis induction through the downregulation of miR-27a and miR-155. Thus, the combined treatment of SLM and Cis can be potentially a favorable treatment for the chemoprevention and treatment of ovarian cancer.
Data availability
The data generated during and/or analyses during the current study are available from the corresponding author on reasonable request.
Code availability
Not Applicable.
References
Zugazagoitia J, Guedes C, Ponce S, Ferrer I, Molina-Pinelo S, Paz-Ares L (2016) Current challenges in cancer treatment. Clin Ther 38(7):1551–1566
Bukowski K, Kciuk M, Kontek R (2020) Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci 21(9):3233
Brown A, Kumar S, Tchounwou PB (2019) Cisplatin-based chemotherapy of human cancers. J cancer Sci Ther 11(4):97
Rottenberg S, Disler C, Perego P (2021) The rediscovery of platinum-based cancer therapy. Nat Rev Cancer 21(1):37–50
Anand U, Dey A, Chandel AKS, Sanyal R, Mishra A, Pandey DK et al (2022) Cancer chemotherapy and beyond: current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis 10(4):1367–1401
Tchounwou PB, Dasari S, Noubissi FK, Ray P, Kumar S (2021) Advances in our understanding of the molecular mechanisms of action of cisplatin in cancer therapy. J Exp Pharmacol. https://doi.org/10.2147/JEP.S267383
Bishayee A, Sethi G (eds) (2016) Bioactive natural products in cancer prevention and therapy: progress and promise. Seminars in cancer biology. Elsevier.
Pusuluri A, Wu D, Mitragotri S (2019) Immunological consequences of chemotherapy: single Drugs, combination therapies and nanoparticle-based treatments. J Controlled Release 305:130–154
Lin SR, Chang CH, Hsu CF, Tsai MJ, Cheng H, Leong MK et al (2020) Natural compounds as potential adjuvants to cancer therapy: preclinical evidence. Br J Pharmacol 177(6):1409–1423
Kachadourian R, Leitner HM, Day BJ (2007) Selected flavonoids potentiate the toxicity of cisplatin in human lung adenocarcinoma cells: a role for glutathione depletion. Int J Oncol 31(1):161–168
Li Y, Wang X, Lin J, Wang R, Zhang B, Zhang X et al. (2023) Natural flavonoid sinensetin inhibits cisplatin-induced pyroptosis and attenuates intestinal injury. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 1869(3): 166637.
Dasari S, Njiki S, Mbemi A, Yedjou CG, Tchounwou PB (2022) Pharmacological effects of cisplatin combination with natural products in cancer chemotherapy. Int J Mol Sci 23(3):1532
Delmas D (2020) Silymarin and derivatives: from biosynthesis to health benefits. Molecules 25(10):2415
Eita AAB (2021) Milk thistle (Silybum marianum (L.) Gaertn.): an overview about its pharmacology and medicinal uses with an emphasis on oral Diseases. J Oral Biosci. 64(1):71–76
Delmas D, Xiao J, Vejux A, Aires V (2020) Silymarin and cancer: a dual strategy in both in chemoprevention and chemosensitivity. Molecules 25(9):2009
Kim DH, Khan H, Ullah H, Hassan ST, Šmejkal K, Efferth T et al (2019) MicroRNA targeting by quercetin in cancer treatment and chemoprotection. Pharmacol Res 147:104346
Tuli HS, Garg VK, Bhushan S, Uttam V, Sharma U, Jain A et al (2023) Natural flavonoids exhibit potent anticancer activity by targeting microRNAs in cancer: a signature step hinting towards clinical perfection. Transl Oncol 27:101–596
Satari A, Ghasemi S, Habtemariam S, Asgharian S, Lorigooini Z (2021) Rutin: a flavonoid as an effective sensitizer for anticancer therapy; insights into multifaceted mechanisms and applicability for combination therapy. Evidence-Based Complement Altern Med. https://doi.org/10.1155/2021/9913179
Kinose Y, Sawada K, Nakamura K, Kimura T (2014) The role of microRNAs in ovarian cancer. BioMed Res Int. https://doi.org/10.1155/2014/249393
Mokhtari RB, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B et al (2017) Combination therapy in combating cancer. Oncotarget 8(23):38022
Li J, Wang Y, Lei JC, Hao Y, Yang Y, Yang CX et al (2014) Sensitisation of Ovarian cancer cells to cisplatin by flavonoids from Scutellaria Barbata. Nat Prod Res 28(10):683–689
Liskova A, Samec M, Koklesova L, Brockmueller A, Zhai K, Abdellatif B et al (2021) Flavonoids as an effective sensitizer for anti-cancer therapy: insights into multi-faceted mechanisms and applicability towards individualized patient profiles. EPMA J 12(2):155–176
Gazak R, Walterova D, Kren V (2007) Silybin and silymarin-new and emerging applications in medicine. Curr Med Chem 14(3):315–338
Wing Ying Cheung C, Gibbons N, Wayne Johnson D, Lawrence Nicol D (2010) Silibinin-a promising new treatment for cancer. Anti-cancer agents in Medicinal Chemistry (formerly current Medicinal Chemistry-Anti-cancer agents). 10(3):186–195
Kalla PK, Chitti S, Aghamirzaei ST, Senthilkumar R, Arjunan S (2014) Anti-cancer activity of silymarin on MCF-7 and NCIH-23 cell lines. Adv Biol Res 8(2):57–61
Kim SH, Choo GS, Yoo ES, Woo JS, Han SH, Lee JH et al (2019) Silymarin induces inhibition of growth and apoptosis through modulation of the MAPK signaling pathway in AGS human gastric cancer cells. Oncol Rep 42(5):1904–1914
Kim S-H, Choo G-S, Yoo E-S, Woo J-S, Lee J-H, Han S-H et al (2021) Silymarin inhibits proliferation of human Breast cancer cells via regulation of the MAPK signaling pathway and induction of apoptosis. Oncol Lett 21(6):1–10
DiPaola RS et al (2002) To arrest or not to G2-M Cell-cycle arrest: commentary re: AK Tyagi., Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth inhibition, G2-M arrest, and apoptosis. Clin. Cancer Res 8:3512–3519
Tyagi A, Agarwal C, Harrison G, Glode LM, Agarwal R (2004) Silibinin causes cell cycle arrest and apoptosis in human bladder transitional cell carcinoma cells by regulating CDKI-CDK-cyclin cascade, and caspase 3 and PARP cleavages. Carcinogenesis 25(9):1711–1720
Colombo V, Lupi M, Falcetta F, Forestieri D, D’Incalci M, Ubezio P (2011) Chemotherapeutic activity of silymarin combined with doxorubicin or paclitaxel in sensitive and multidrug-resistant colon Cancer cells. Cancer Chemother Pharmacol 67:369–379
AL-Jawad FH, Hussein SM, Maaufe RR (2015) The cytotoxic effect of silymarin and 5-FU on three types of cancer cell lines. AL-yarmouk Journall 1:69–83
Wang Y, Yuan A-J, Wu Y-J, Wu L-M, Zhang L (2023) Silymarin in cancer therapy: mechanisms of action, protective roles in chemotherapy-induced toxicity, and nanoformulations. J Funct Foods 100:105384
Abdelmeguid NE, Chmaisse HN, Zeinab N (2010) Protective effect of silymarin on cisplatin-induced nephrotoxicity in rats. Pak J Nutr 9(7):624–636
Mansour HH, Hafez HF, Fahmy NM (2006) Silymarin modulates cisplatin-induced oxidative stress and hepatotoxicity in rats. BMB Rep 39(6):656–661
Abouzeinab N (2013) Cytoprotective effect and antioxidant properties of silymarin on cisplatin induced hepatotoxicity in rats: a biochemical and histochemical study. Int J Cancer Res 9(1):9–23
Ferreira A, Rodrigues M, Meirinho S, Fortuna A, Falcão A, Alves G (2021) Silymarin as a flavonoid-type P-glycoprotein inhibitor with impact on the pharmacokinetics of carbamazepine, oxcarbazepine and phenytoin in rats. Drug Chem Toxicol 44(5):458–469
Ninsontia C, Pongjit K, Chaotham C, Chanvorachote P (2011) Silymarin selectively protects human renal cells from cisplatin-induced cell death. Pharm Biol 49(10):1082–1090
Li Y, Tian Z, Tan Y, Lian G, Chen S, Chen S et al (2020) Bmi-1-induced miR-27a and miR-155 promote tumor metastasis and chemoresistance by targeting RKIP in gastric cancer. Mol Cancer 19(1):1–14
Toden S, Okugawa Y, Buhrmann C, Nattamai D, Anguiano E, Baldwin N et al (2015) Novel evidence for curcumin and boswellic acid–induced chemoprevention through regulation of miR-34a and miR-27a in colorectal cancer. Cancer Prev Res 8(5):431–443
Lu JJ, Yang WM, Li F, Zhu W, Chen Z (2019) Tunneling nanotubes mediated microRNA-155 intercellular transportation promotes bladder cancer cells’ invasive and proliferative capacity. Int J Nanomed. https://doi.org/10.2147/IJN.S217277
Zhang W, Ji W, Zhao X (2019) MiR-155 promotes anaplastic thyroid cancer progression by directly targeting SOCS1. BMC Cancer 19:1–11
Xu W, Song C, Wang X, Li Y, Bai X, Liang X et al (2021) Downregulation of mir-155-5p enhances the anti-tumor effect of cetuximab on triple-negative breast cancer cells via inducing cell apoptosis and pyroptosis. Aging 13(1):228
Zadeh MM, Motamed N, Ranji N, Majidi M, Falahi F (2016) Silibinin-induced apoptosis and downregulation of microRNA-21 and microRNA-155 in MCF-7 human breast cancer cells. J Breast cancer 19(1):45–52
Abtin M, Alivand MR, Khaniani MS, Bastami M, Zaeifizadeh M, Derakhshan SM (2018) Simultaneous downregulation of miR-21 and miR-155 through oleuropein for breast cancer prevention and therapy. J Cell Biochem 119(9):7151–7165
Xia J, Cheng L, Mei C, Ma J, Shi Y, Zeng F et al (2014) Genistein inhibits cell growth and invasion through regulation of miR-27a in pancreatic cancer cells. Curr Pharm Design 20(33):5348–5353
Funding
Not Applicable.
Author information
Authors and Affiliations
Contributions
MRK and AMC performed most of the experiments and wrote the manuscript. SS performed data analysis and participated in intellectual discussions of the data. SR coordinated the study, designed the experiments, and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We certify that there is no conflict of interest with any financial organization.
Ethical approval
This article does not contain any studies on human or animals.
Consent to participate
This article does not contain any individual person’s data in any form.
Consent for publication
This article does not contain any individual person’s data in any form.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karimzadeh, M.R., Masoudi Chelegahi, A., Shahbazi, S. et al. Co-treatment of silymarin and cisplatin inhibited cell proliferation, induced apoptosis in ovarian cancer. Mol Biol Rep 51, 118 (2024). https://doi.org/10.1007/s11033-023-09026-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-023-09026-8