Abstract
Objective
Sperm freezing is considered as an effective way in assisted reproductive technology (ART) programs, it has detrimental effects on sperm function, due to the production of reactive oxygen species (ROS). This study aimed to investigate the potential of Mitoquinone (MitoQ) in inhibiting the production of mitochondrial ROS during sperm freezing.
Methods
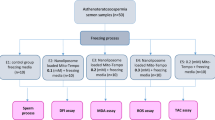
A total of 20 human normozoosperm samples were collected for this study. The samples were divided into four groups, each containing different concentrations of MitoQ (0, 0.2, 2, and 20 nM), and then subjected to the freezing process. After thawing, the sperm suspensions were evaluated for parameters including motility, morphology, acrosome integrity, adenosine triphosphate (ATP) level, intracellular ROS, viability, chromatin packaging, DNA denaturation, DNA fragmentation, as well as the expression of antioxidants (GPX, SOD) and apoptotic (Bax, Bcl2) genes.
Results
The results showed that total and progressive mobility of sperms significantly increased in the 2 nM group, while significantly decreased in the 20 nM group (p ≤ 0.05). Sperm morphology did not significantly improve across all the tested concentrations (p ≥ 0.05). Intracellular ROS levels showed a significant decrease and increase in the concentrations of 2 and 20 nM, respectively (p ≤ 0.05). Furthermore, a significant increase was observed in viability, ATP, acrosome integrity, chromatin packaging, and non-denatured and non-fragmented DNA after treatment with 2 nM of MitoQ, compared with the control group (p ≤ 0.05). Regarding gene expressions, the relative expressions of oxidative stress genes were increased in the 2 nM group and decreased in the 20 nM group (p ≤ 0.05), while no significant difference was observed in the expressions of apoptotic genes compared with the control group (p ≥ 0.05). All the comparisons were made with respect to the control group.
Conclusion
Adding the optimal concentration of MitoQ (2 nM) to the sperm freezing medium not only improves sperm functional parameters and reduces DNA damages, but also stimulates the expression of antioxidant genes, leading to even greater benefits for sperm cryopreservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sperm freezing is a commonly used method in assisted reproductive technology (ART), for male factor infertility, fertility preservation in cancer patients, and hypogonadotropic disorders [1,2,3]. Sperm freezing plays a useful role in infertility treatment protocols, however, it is important to be aware that it can increase oxidative stress [4]. By affecting the sperm membrane, which contains high levels of unsaturated fatty acids, oxidative stress can lead to peroxidation damage and a decrease in sperm fertilization capability by mitigating membrane integrity and motility [5,6,7]. Freezing-induced damage has been found to increase the production of reactive oxygen species (ROS). Sperms are particularly susceptible to producing excessive ROS due to their limited cytoplasmic content, low antioxidant capacity, and high number of mitochondria [8, 9].
A study has reported that ROS can diminish sperm functional parameters and cause DNA damage by affecting the DNA structure, which in severe cases triggers apoptosis and cell death, by inducing caspase factors [10]. Recent studies have suggested various antioxidants to mitigate ROS-induced damage during sperm freezing. However, most of the suggested antioxidants cannot cross and enter the inner membrane of mitochondria, which is one of the most important sources of uncontrolled ROS production during the freezing process [11]. Recent studies have shown the ability of certain molecules to specifically target mitochondria and provide protection against oxidative damage [12]. Mitoquinone (MitoQ) is one of these molecules which has been suggested as a mitochondrial-targeting antioxidant [13]. This compound is composed of a triphenyl phosphonium (TPP) molecule attached to the Q10 coenzyme (Ubiquinone), allowing it to enter the mitochondria 800 to 1200 times more than CoQ10 [14]. By transferring electrons through the mitochondrial respiratory chain, CoQ10 protects mitochondria with its antioxidant activity. Freezing-induced ROS can lead to the degradation of the cardiolipin in the mitochondrial membrane, making mitochondrial ROS more hazardous than other sources of ROS [15].
The mechanism of action of MitoQ includes the attraction of positively-charged TPP to the negatively-charged mitochondrial inner membrane; this facilitates the entry of MitoQ into the matrix and activates the CoQ10 antioxidant [14]. Although some studies have investigated the effects of MitoQ on some functional parameters of the sperm, there is a need for its effects on the chromatin packaging and DNA integrity of human sperm to be evaluated [16]. Therefore, this study aimed to investigate the effects of MitoQ on the motility, intracellular ROS level, ATP level, viability, morphology, acrosome reaction, chromatin packaging, DNA fragmentation, and levels of glutathione peroxidase (GPX) and apoptotic (Bax, Bcl2) after human sperm freezing.
Methods
This study was approved by the Ethics Committee of the Islamic Azad University of Hamedan (Project number 68424–12). Consent was obtained from men referred to the Omid Infertility Center for using their samples in the study.
All the used chemicals were purchased from Sigma Aldrich (USA), except where noted otherwise. In the present study, the inclusion criteria were ejaculate samples containing at least 20 × 106 sperm/ml, with motility of 40% or more and morphology of 4%.
Sperm collection and preparation
Semen samples were collected from 20 healthy men by masturbation after 2 to 4 days of sexual abstinence according to the World Health Organization guidelines (WHO, 2021). Samples were prepared using the swim-up method. In this method, after centrifugation, sample pellets were diluted with human tubal fluid (HTF) and 1% human serum albumin (HSA). The mixture then incubated for 1 h at 37°C and 5% CO2. Finally, the supernatant was separated and sperm analysis was performed.
Sperm freezing and thawing
After preparing the sperms we used rapid freezing method. First, sperm samples were divided into four aliquots in cryovials containing various concentrations of MitoQ (0, 0.2, 2, and 20 nM). Second, to each 100 µl of sperm samples, 70 µl sperm freezing medium (Vitrolife) was added. After a 10-min incubation at room temperature, we ensured that the cryovials were kept at a distance of 15–20 cm from liquid nitrogen, exposed to nitrogen vapor for 20 min, and then immersed in liquid nitrogen.
Cryovials were first thawed at room temperature and then transferred to a water bath at 37°C. Subsequently, the samples were mixed with pre-heated Hams F10 medium, centrifuged for 10 min, at a speed of 200 × g, and finally resuspended with an adequate amount of medium [8].
Assessment of sperm parameters
Sperm motility
The total and progressive motility of sperms were measured. In addition, other motility characteristics were evaluated using computer-assisted sperm analysis (CASA, labomed, LX400; Labomed Inc., Culver City CA, USA). Pre-heated slides (at 37°C) were prepared using 5 µl of semen samples in order to analyze the sperms (at least ten random fields were observed at × 100 magnification) [17, 18].
Viability and morphology
For assessing the sperm viability, eosin–nigrosine method was performed. We diluted 10 µl of the sperm suspension with 10 µl of eosin–nigrosine and placed it on a dry and clean slide. After the slides have completely dried at room temperature [19], 200 sperms in 5 microscopic fields were evaluated at × 400 magnification by phase-contrast microscopy, and the results were reported (WHO 2021). Red or pink-colored sperms and non-stained sperms were reported as non-viable and viable, respectively.
Papanicolaou Staining method was employed to evaluate sperm morphology according to the WHO 2021 guidelines.
Intracellular ROS assessment
Dihydroethidium (DHE) was applied to evaluate intracellular ROS production (O2−). In this method, the samples were first centrifuged and second mixed with 1 ml of phosphate-buffered saline (PBS). Then 100 µl of sperm sample (5 × 106 concentration) was mixed with 1.25 μM DHE in the dark (37°C, 20 min). Finally, the produced ROS was measured using a spectrophotometer (570 nm) [20].
Assessing adenosine triphosphate (ATP) concentration
According to the method of Sun et al., ATP concentration was measured [21]. ATP assay kit was first used for this purpose. We encountered semen samples with ice to lyse ATP and then centrifuged the samples for 5 min at a speed of 12,000 × g (4 °C). 40 µl of the collected supernatant was mixed with 100 µl of a working dilution containing concentrations of 10 nM and 10 M. The absorbance was then measured using a spectrophotometer at a wavelength of 636 nm.
Evaluation of acrosome integrity
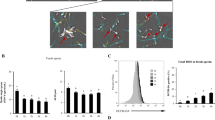
Pisum sativa agglutinin lectin (PSA) conjugated to fluorescein isothiocyanate (FITC) was utilized to evaluate acrosome damage during sperm freezing. In this test, 20 µl of sperm suspensions was washed with Dulbecco’s phosphate-buffered saline (DPBS; Gibco, USA) and then centrifuged. The resulting pellets were resuspended in PBS and subsequently fixed with 4% paraformaldehyde at room temperature. Following this, PSA-FITC solution was applied to each slide and incubated for 20 min (37°C, 5% CO2). The slides were then washed with PBS and left to dry. The slides were evaluated by fluorescence microscopy. Nearly 200 sperms were examined on each slide. The microscopic observations revealed bright fluorescence with a clear acrosome cap in the acrosome region, indicating intact acrosomes, while no fluorescence in the equatorial part with irregular acrosome cap were considered as damaged acrosome (Fig. 1) [22].
Sperm chromatin packaging
Aniline blue (AB)
Aniline blue (AB) staining was used to assess sperm chromatin packaging. AB is an acidic cytochemical dye, binds to alkaline amino acids in histones. Initially, sperm suspension smears were fixed with 3% glutaraldehyde in 0.2 M buffer (pH 7.2) for 30 min. Subsequently, smears were stained with AB in 4% acetic acid and examined using light microscopy (× 1000). Mature and immature sperms were distinguished by their light blue and dark blue colors, respectively (Fig. 2) [8].
Toluidine blue (TB)
TB staining was used to assess sperm chromatin density. TB binds to the exposed phosphate groups of DNAs: higher TB binding indicates lower chromatin density. For this test, sperm smears were fixed in 96% ethanol, immersed in 0.1 N HCl at 4°C, washed with distilled water, and stained with 0.05% TB. The evaluation was performed using light microscopy (× 1000), categorizing dark-blue sperms as immature and light-blue sperms as mature [8].
DNA fragmentation
Sperm chromatin dispersion (SCD)
To assess DNA fragmentation, 30 µl of each sperm sample was mixed with 1% agarose. Subsequently, a slide covered with 0.65% agarose and 50 µl of this solution was poured on it. Afterward, a dry cover slip was added, and the slides were incubated at 4°C for 4–5 min. Cover slips were then removed, and slides were immersed in 0.08 N HCl for 7 min in the dark. This process was followed by placing the slides in lysing solution for 25 min and washing with distilled water for 5 min. Slides were sequentially immersed in 70%, 90%, and 100% ethanol and stained with Wright’s dye. Light microscopy results indicated the presence of a halo around some of the sperms. Medium and large halo presence were considered to indicate absent to moderate DNA damage; while the absence of a halo or a small halo was considered an indication of severe DNA fragmentation [23].
DNA denaturation
The acridine orange (AO) test was used to assess the level of DNA denaturation. To perform this test, the sperm suspension smears were prepared and fixed with Carnoy’s solution (methanol glacial acetic acid) (3:1 ratio) at 4°C for 3 h, and stained with AO in a dark room. The slides were evaluated using a fluorescence microscope with a 460 nm filter at × 1000 magnification. Microscopic observations indicated the presence of green (normal) and orange to red spectrum (abnormal)-stained sperms. The higher the degree of DNA damage, the more the sperm color shifted from orange to red. The results were expressed as percentage (Fig. 3) [24].
Gene expression profile
Quantitative real-time polymerase chain reaction (qRT-PCR) was used to evaluate GPX, Bax, and Bcl2 expressions. First, total RNA was extracted using TRIzol reagent according to the manufacturer's instructions, and RNA purity (260:280 ratio) and quantity (260 nm) were evaluated using a Nano Drop spectrophotometer (PhotoBiometer, Eppendorf, Germany). Then, cDNA was synthesized from the extracted RNA and stored at 20°C. SYBR green master mix, and specific primers were used to accomplish the qRT-PCR and glyceraldehyde 3-phosphate (GAPDH) was employed as the housekeeping gene (Table 1). The qRT-PCR steps were carried out according to Table 2. The relative changes in the targeted genes were analyzed using the Livak method (2−ΔΔCT) and normalized compared to the GAPDH expression level.
Statistical analysis
Kolmogorov–Smirnov, one-way analysis of variance (ANOVA), and Tukey's tests were used respectively to assess the normal distribution of values and making comparisons between groups. The results were reported as mean ± standard deviation (SD) and a value of p less than 0.05 was considered statistically significant.
Results
Total and progressive motility percentages significantly increased in the presence of 2 nM MitoQ and decreased in 20 nM of that compared with the control group (p ≤ 0.05; Table 3). However, there was no significant increase in motility with the addition of 0.2 nM (p ≤ 0.05; Table 3). Moreover, the addition of 0.2 and 2 nM MitoQ improved the motility characteristics (VCL, VSL, VAP, LIN, BCF) compared with the control group (p ≤ 0.05; Table 3).
According to the Table 4, only 2 nM MitoQ showed a significant increase in viability percentage compared with the control group (p ≤ 0.05). None of the MitoQ concentrations showed a significant improvement in morphology compared with the control group (p ≥ 0.05). The highest and the lowest level of ATP and intracellular ROS were observed in the 2 nM group compared with the control group (p ≤ 0.05). In addition, the acrosome activity percentage was significantly increased in 0.2 and 2 nM groups, while 20 nM mitoQ caused a significant decrease in ATP level compared with the control group. (p ≤ 0.05, Table 4).
Table 5 highlights that 2 nM MitoQ supplementation significantly improved chromatin packaging in AB and TB tests (p ≤ 0.05). Furthermore, the amounts of non-denatured and non-fragmented DNA in 2 nM MitoQ group increased significantly compared with the control group (p ≤ 0.05, Table 5).
As shown in Fig. 5, it is evident that the addition of 2 nM MitoQ to sperm freezing extender led to a significant increase in GPX and SOD expressions compared with the control group (p ≤ 0.05). A similar result was obtained for the concentration of 0.2 nM MitoQ, though this change was not significant (p ≥ 0.05). Conversely, the supplementation of 20 nM MitoQ was associated with a decrease in the expression of these genes compared with the control group (p ≤ 0.05, Fig. 4).
The relative expressions of Bax and Bcl2 genes showed that the addition of 0.2 and 2 nM MitoQ to sperm freezing medium resulted in a non-significant decrease and increase of Bax and Bcl2 genes, respectively, compared with the control group (p ≥ 0.05), while the presence of 20 nM MitoQ led to an increase and decrease in Bax and Bcl2 genes expression, respectively, compared with the control group (Fig. 5).
Discussion
The freeze and thaw process can have detrimental effects on sperm viability and fertility percentage. Sperms are sensitive to oxidative stress and lipid peroxidation, because of high levels of membrane unsaturated fatty acids and trace amounts of cytoplasmic antioxidants [25]. High levels of ROS caused by oxidative stress can lead to structural damages to sperm proteins, lipids, DNAs, and functions [26, 27]. To mitigate these damages, antioxidants such as MitoQ are being explored in sperm freezing protocols. Specifically, the effects of MitoQ on sperm functional parameters, including motility and DNA structure, are of interest. This study investigated the effects of MitoQ on sperm functional parameters and its DNA structure. One of the key factors in sperm functioning is motility, which plays a crucial role in successful fertilization. Disruptions in normal motility patterns can lead to fertilization failure.
The present study evaluated total and progressive motility along with other motility characteristics. Results showed that the presence of 2 nM MitoQ significantly increased total and progressive motility, while concentrations higher than 20 nM decreased these parameters. These findings were consistent with a previous study conducted by Sun et al., which also demonstrated that 150 nM MitoQ improved total and progressive motility, while a concentration of 200 nM MitoQ decreased motility of sperms [21]. Our results are also in alignment with those of Najafi et al., who showed the lycopene has the potential to be as a freezing extender of rooster sperm [28]. Another study evaluated the effects of MitoQ on bull sperm motility and observed no significant changes after using it [29], this discrepancy could probably be attributed to the different species or freezing protocols used in this article. The freezing process resulted in mitochondrial dysfunction along with defects in oxidative phosphorylation and the citric acid cycle, which is ultimately associated with lower ATP production [30].
The present study showed that MitoQ at an optimal concentration (2 nM) is associated with a decrease in intracellular ROS, however enhancing the concentration, can cause significant increases. The presence of MitoQ in freezing media led to lower ROS production during the freezing process, through ensuring the delivery of sufficient CoQ10 to the mitochondrial inner membrane. Following ROS detoxification, MitoQ quinones were reduced by the respiratory chain, which caused its antioxidant activity [31]. This study, found that the freezing medium containing 2 or 20 nM MitoQ resulted in more ATP production compared with the control group. However, this higher production was only significant in 2 nM MitoQ group. Similar studies have shown addition of 0.02 nM and 150 nM MitoQ to human and rooster freezing media to be associated with higher ATP production [21, 22]; However, both our results and other studies indicated that a high dose of MitoQ is toxic to sperm because it can increase TPP concentration [32, 33]. MitoQ-induced ATP is associated with sperm motility preservation, lower damage to the acrosome, and greater integrity of sperm DNA during sperm freezing. Another feature necessary for successful fertilization is sperm acrosome integrity.
The present study showed a positive effect of MitoQ on acrosome integrity during the freezing process. The 0.2 and 2 nM groups showed more intact acrosomes than the control group. This finding can probably be attributed to the reduction of intracellular ROS in the 0.2 and 2 nM groups and the reduction of its destructive effect on the integrity of the acrosome membrane. Based on these results, another study investigated the effect of Q10 and reported the positive effect of Q10 in preserving the acrosome membrane during freezing [34]. The improvement of sperm viability occurred only at the 2 nM concentration. Increasing the concentration to 20 nM, the viability reduced. This result was probably related to the peroxidation effects of high doses of antioxidants, which can have toxic effects [35].
The toxic effects of high doses of antioxidants which were reported in a study on yellow catfish sperms exposed to 200 nM MitoQ, is in accordance with our results [6]. Our comparison of different concentrations of MitoQ with the control group did not showed any significant differences in sperm morphology. In line with these results, Rezaei et al. reported that the addition of 100 µM Q10 to the freezing medium of sperms did not improve sperm morphology compared with the control group [36].
An important factor tested in this study was the effect of MitoQ on sperm chromatin density, DNA denaturation, and fragmentation. According to the results, the presence of 2 nM MitoQ in the freezing extender dramatically preserved chromatin density and increased non-denatured and non-fragmented DNA. These results confirmed a direct relationship between sperm chromatin density and DNA denaturation and fragmentation rates, which is consistent with the results of other studies [37, 38]. Sperm chromatin structural defects are usually associated with abnormal nuclear protein contents or DNA strands breaks [39]. In normal conditions, there is a balance between the antioxidant system and DNA repair, and a low amount of oxidized DNA is quickly repaired by defense mechanisms. Human sperm freezing causes the oxidation of purine and pyrimidine bases and leads to single- or two-strand breaks [40].
Our evaluations showed that MitoQ at a concentration of 2 nM had positive and improving effects, while higher concentrations had opposite effects. The reason behind these results, which were also observed in other sperm parameters, is that excessive amounts of MitoQ can trigger the release of O2− free radicals, which can induce excessive oxidative stress [41, 42]. Therefore, it can be assumed that higher concentrations of MitoQ (20 nM) can lead to higher ROS production. In line with our findings, another study reported positive and negative effects of MitoQ on sperm DNA fragmentation at concentrations of 0.02 and 0.2 µM [22]. Several studies have investigated the effect of different antioxidants on the expression of sperm oxidative and apoptotic genes. Our results showed that the presence of MitoQ in concentrations of 0.2 and 2 nM is associated with a decrease and an increase in expression of Bax and Bcl2 genes, respectively, as well as an increase in expression of GPX and SOD genes. In agreement with these results, Santonastaso et al. demonstrated that 20 µM of curcumin antioxidant in the sperm extender leads to a significant increase in GPX mRNA expression [43]. Mehdipour et al. indicated that the presence of 1 mM of crocin and 100 μM of naringenin can decrease the expression of the caspase 3 gene and increase that of Bcl2 [44]. The increase in the expression of antioxidant genes can be explained by the enhancement of intracellular ROS levels during sperm freezing. Also, increased ROS and decreased antioxidant activity can activate the apoptosis signaling cascade. The decline in viability and DNA denaturation is justified by the increase in apoptosis with 20 nM MitoQ.
Conclusion
This study demonstrated that adding MitoQ at an optimal concentration of 2nM to a human sperm freezing extender can reduce ROS levels, thus improving sperm functional parameters, compensating for mitochondrial damages, restoring ATP balance, and decreasing damage to chromatin and DNA structure. It can also lead to an optimal expression of antioxidant genes. Therefore, using MitoQ in the freezing medium can improve assisted reproductive techniques.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Fraietta R, Zylberstejn DS, Esteves SC (2013) Hypogonadotropic hypogonadism revisited. Clinics 68:81–88
Gandini L, Pallotti F, Paoli D, Lenzi A (2017) Cryopreservation of spermatozoa 42. In: Endocrinology of the testis and male reproduction, vol 1. Springer, Berlin, p 1235
Leroy C, Rigot J-M, Leroy M, Decanter C, Le Mapihan K, Parent A-S et al (2015) Immunosuppressive drugs and fertility. Orphanet J Rare Dis 10:1–15
Rezaei A, Bahmani HR, Mafakheri S, Farshad A, Nazari P (2022) Protective effects of different doses of MitoQ separately and combined with trehalose on sperm function and antioxidative status of cryopreserved Markhoz goat semen. bioRxiv. 2022:2022.08. 22.504802
Nasiri Z, Ghorbani F, Seify M, Sharbati A (2022) Effect of aqueous Nigella sativa extract on the functional parameters of post-thaw human spermatozoa during vitrification. Clin Exp Reprod Med 49(2):110
Fang L, Bai C, Chen Y, Dai J, Xiang Y, Ji X et al (2014) Inhibition of ROS production through mitochondria-targeted antioxidant and mitochondrial uncoupling increases post-thaw sperm viability in yellow catfish. Cryobiology 69(3):386–393
Safa S, Moghaddam G, Jozani RJ, Kia HD, Janmohammadi H (2016) Effect of vitamin E and selenium nanoparticles on post-thaw variables and oxidative status of rooster semen. Anim Reprod Sci 174:100–106
Najafi L, Halvaei I, Movahedin M (2019) Canthaxanthin protects human sperm parameters during cryopreservation. Andrologia 51(10):e13389
Farazmand T, Mansouri F, Koohestanidehaghi Y, Shahandeh E (2023) Human sperm parameter improvement associated with Ceratonia siliqua extract as a cryopreservation supplement after vitrification. Clin Exp Reprod Med 50(2):86
Di Santo M, Tarozzi N, Nadalini M, Borini A (2011) Human sperm cryopreservation: update on techniques, effect on DNA integrity, and implications for ART. Adv Urol 2012:854837
Thomson LK, Fleming SD, Aitken RJ, De Iuliis GN, Zieschang J-A, Clark AM (2009) Cryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosis. Hum Reprod 24(9):2061–2070
Apostolova N, Victor VM (2015) Molecular strategies for targeting antioxidants to mitochondria: therapeutic implications. Antioxid Redox Signal 22(8):686–729
Murphy MP (1997) Selective targeting of bioactive compounds to mitochondria. Trends Biotechnol 15(8):326–330
Smith RA, Porteous CM, Coulter CV, Murphy MP (1999) Selective targeting of an antioxidant to mitochondria. Eur J Biochem 263(3):709–716
Hezavehei M, Sharafi M, Fathi R, Shahverdi A, Gilani MAS (2021) Membrane lipid replacement with nano-micelles in human sperm cryopreservation improves post-thaw function and acrosome protein integrity. Reprod BioMed Online 43(2):257–268
Gonzalez M, Prashar T, Connaughton H, Barry M, Robker R, Rose R (2022) Restoring sperm quality post-cryopreservation using mitochondrial-targeted compounds. Antioxidants 11(9):1808
Ghorbani F, Nasiri Z, Koohestanidehaghi Y, Lorian K (2021) The antioxidant roles of l-carnitine and N-acetyl cysteine against oxidative stress on human sperm functional parameters during vitrification. Clin Exp Reprod Med 316(4):48
Torkamanpari M, Ghorbani F, Lorian K, Koohestanidehaghi Y (2023) The effects of purslane (Portulaca oleracea) and fennel (Foeniculum vulgare Mill) hydroalcoholic extracts on the functional parameters of human spermatozoa after vitrification. Clin Exp Reprod Med 50(2):78
Hosseinmardi M, Siadat F, Sharafi M, Roodbari NH, Hezavehei M (2022) Protective effect of cerium oxide nanoparticles on human sperm function during cryopreservation. Biopreserv Biobank 20(1):24–30
Salih SA, Daghigh-Kia H, Mehdipour M, Najafi A (2021) Does ergothioneine and thawing temperatures improve rooster semen post-thawed quality? Poult Sci 100(10):101405
Sun L, He M, Xu J, Wu C, Zhang S, Zhang D et al (2022) Does antioxidant mitoquinone (MitoQ) ameliorate oxidative stress in frozen-thawed rooster sperm? Animals 12(22):3181
Kumar P, Wang M, Isachenko E, Rahimi G, Mallmann P, Wang W et al (2021) Unraveling subcellular and ultrastructural changes during vitrification of human spermatozoa: effect of a mitochondria-targeted antioxidant and a permeable cryoprotectant. Front Cell Dev Biol 9:672862
Rezaei A, Bahmani HR, Mafakheri S, Farshad A, Nazari P, Masoudi R (2023) Protective effects of different doses of MitoQ separately and combined with trehalose on oxidative stress and sperm function of cryopreserved Markhoz goat semen. Cryobiology 110:36–43
Agarwal A, Sharma R, Ahmad G, Sharma R, Ahmad G (2017) Sperm chromatin assessment. In: Textbook of assisted reproductive techniques, 5th edn. CRC Press: Boca Raton, pp 65–87
Koohestanidehaghi Y, Torkamanpari M, Shirmohamadi Z, Lorian K, Vatankhah M (2021) The effect of cysteine and glutamine on human sperm functional parameters during vitrification. Andrologia 53(1):e13870
Hezavehei M, Mirzaei M, Sharafi M, Wu Y, Gupta V, Fitzhenry M et al (2022) Proteomics study reveals the molecular mechanisms underlying cryotolerance induced by mild sublethal stress in human sperm. Cell Tissue Res 387(1):1–15
Shahandeh E, Ghorbani M, Mokhlesabadifarahani T, Bardestani F (2022) Melatonin and selenium supplementation in extenders improves the post-thaw quality parameters of rat sperm. Clin Exp Reprod Med 49:87–92
Najafi A, Taheri RA, Mehdipour M, Farnoosh G, Martínez-Pastor F (2018) Lycopene-loaded nanoliposomes improve the performance of a modified Beltsville extender broiler breeder roosters. Anim Reprod Sci 195:168–175
Câmara DR, Ibanescu I, Siuda M, Bollwein H (2022) Mitoquinone does not improve sperm cryo-resistance in bulls. Reprod Domest Anim 57(1):10–18
Wiland E, Fraczek M, Olszewska M, Kurpisz M (2016) Topology of chromosome centromeres in human sperm nuclei with high levels of DNA damage. Sci Rep 6(1):31614
Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC et al (2001) Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem 276(7):4588–4596
Cochemé HM, Kelso GF, James AM, Ross MF, Trnka J, Mahendiran T et al (2007) Mitochondrial targeting of quinones: therapeutic implications. Mitochondrion 7:S94–S102
Trnka J, Elkalaf M, Anděl M (2015) Lipophilic triphenylphosphonium cations inhibit mitochondrial electron transport chain and induce mitochondrial proton leak. PLoS ONE 10(4):e0121837
Masoudi R, Sharafi M, Shahneh AZ, Kohram H, Nejati-Amiri E, Karimi H et al (2018) Supplementation of extender with coenzyme Q10 improves the function and fertility potential of rooster spermatozoa after cryopreservation. Anim Reprod Sci 198:193–201
Bouayed J, Bohn T (2010) Exogenous antioxidants—double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev 3(4):228–237
Allahveisi A (2022) Effect of coenzyme Q10 on DNA fragmentation, membrane integrity, and sperm chromatin condensation after thawing of frozen semen. Sci J Kurd Univ Med Sci 26(7):34–44
Golshan-Iranpour F, Zamani Rarani F, Dashti GR (2019) Effect of chromatin condensation on frozen-thawed sperm DNA integrity in normozoospermic men. Sci J Kurd Univ Med Sci 24(3):34–42
Abad C, Amengual M, Gosálvez J, Coward K, Hannaoui N, Benet J et al (2013) Effects of oral antioxidant treatment upon the dynamics of human sperm DNA fragmentation and subpopulations of sperm with highly degraded DNA. Andrologia 45(3):211–216
Fernández JL, Muriel L, Rivero MT, Goyanes V, Vazquez R, Alvarez JG (2003) The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl 24(1):59–66
Len JS, Koh WSD, Tan S-X (2019) The roles of reactive oxygen species and antioxidants in cryopreservation. Biosci Rep 39(8):BSR20191601
Suzuki C, Hatayama N, Ogawa T, Nanizawa E, Otsuka S, Hata K et al (2020) Cardioprotection via metabolism for rat heart preservation using the high-pressure gaseous mixture of carbon monoxide and oxygen. Int J Mol Sci 21(22):8858
Li X, Chen B, Xie H, He Y, Zhong D, Chen D (2018) Antioxidant structure–activity relationship analysis of five dihydrochalcones. Molecules 23(5):1162
Santonastaso M, Mottola F, Iovine C, Colacurci N, Rocco L (2021) Protective effects of curcumin on the outcome of cryopreservation in human sperm. Reprod Sci 28:2895–2905
Mehdipour M, Daghigh Kia H, Najafi A, Mohammadi H, Álvarez-Rodriguez M (2020) Effect of crocin and naringenin supplementation in cryopreservation medium on post-thaw rooster sperm quality and expression of apoptosis associated genes. PLoS ONE 15(10):e0241105
Acknowledgements
We are very grateful to all personnel of the embryology and genetics laboratories. Also, we sincerely thank all the people who helped us in conducting this research.
Funding
The authors declare that no financial aid, grant or other support was received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: HM, TM. Data curation: NT, TM. Formal analysis: SK, ZM. Methodology: HM, FM. Project administration: HM, FM. Visualization: TM, SK. Writing—original draft: NT, ESH. Writing—review and editing: ESH.
Corresponding author
Ethics declarations
Conflict of interest
No competing interests declared.
Ethical approval
Experimental protocols and animal care methods in the experiments were approved by the Ethics Committee of the Islamic Azad University of Hamedan (Project Number 68424-12).
Informed consent
Informed verbal consent was obtained from all individual participants who participated in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moradi Gardeshi, T., Shahandeh, E., Tavakolpoor Saleh, N. et al. Evaluation of the effect of mitoquinone on functional parameters, DNA structure, and genes expression related to the apoptotic and antioxidants of human sperm after freezing–thawing. Mol Biol Rep 51, 183 (2024). https://doi.org/10.1007/s11033-023-09020-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-023-09020-0