Abstract
Background
Bone marrow mesenchymal stem cells (BMSCs) have been widely recognized as a highly promising option for cell-based tissue engineering therapy targeting osteoporosis. However, the osteogenic differentiation of BMSCs is impeded by the limited viability and diminished capacity for bone formation within the osteoporotic microenvironment.
Methods
In this study, the COL6A3 gene was confirmed through an extensive analysis of the preceding single-cell sequencing database. The generation of an inflammatory microenvironment resembling osteoporotic cell transplantation was achieved by employing lipopolysaccharide (LPS). A lentivirus targeting the COL6A3 gene was constructed, and a Western blotting assay was used to measure the marker proteins of osteogenesis, adipogenesis, and mitophagy. Immunofluorescence was utilized to observe the colocalization of mitochondria and lysosomes. The apoptosis rate of each group was evaluated using the TUNEL assay, and the mitochondrial membrane potential was assessed using JC-1 staining.
Results
This investigation discovered that the impaired differentiation capacity and decreased viability of BMSCs within the inflammatory microenvironment were markedly ameliorated upon overexpression of the specific COL6A3 gene. Moreover, the administration of COL6A3 gene overexpression successfully mitigated the inhibitory impacts of LPS on mitophagy and the expression of inflammatory mediators, specifically inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), in BMSCs. To clarify the underlying mechanism, the role of mitophagy during the differentiation of COL6A3 gene-modified BMSCs in the inflammatory microenvironment was evaluated using the mitophagy inhibitor Mdivi-1.
Conclusions
In the context of lipopolysaccharide (LPS) stimulation, COL6A3 enhances the differentiation of BMSCs into osteogenic and adipogenic lineages through the promotion of mitophagy and the maintenance of mitochondrial health. Our findings may provide a novel therapeutic approach utilizing stem cells in the treatment of osteoporosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis, a worldwide age-related disease, is commonly linked to estrogen deficiency following menopause [1, 2]. Currently, the main method employed for the treatment of osteoporosis is systemic anti-osteoporosis drug therapy; however, this approach is associated with certain limitations [3,4,5]. Previous studies have provided evidence supporting the appropriateness of employing bone marrow mesenchymal stem cells (BMSCs) in cell therapy as a viable approach for the treatment of osteoporosis [6,7,8,9,10].
The development of osteoporosis is closely associated with the proliferation and differentiation of BMSCs, which exhibit the capacity for self-renewal and differentiation into various cell types [11]. During the inflammatory phase of bone restoration, the survival rate and osteogenic differentiation of BMSCs are compromised due to the combined effects of various damaging factors, including hypoxia and oxidative stress [12, 13]. While inflammation has traditionally been linked to osteoclast differentiation and subsequent bone loss, recent research has elucidated a crucial connection between inflammation and bone formation [14]. Therefore, it is crucial to undertake further investigation to analyze the methodologies utilized by BMSCs that demonstrate anti-injury, anti-apoptosis, and high viability rates within the framework of bone defects. As evidenced by previous studies [15], the process of mitophagy is of utmost importance in the differentiation of stem cells and the preservation of their stemness. Mitophagy, a distinctive form of autophagy, eliminates damaged mitochondria, thereby facilitating the preservation of normal energy metabolism and cellular function [16]. However, impaired mitochondrial metabolism could lead to mitochondrial dysfunction and the accumulation of compromised mitochondria, ultimately resulting in cell death through pro-apoptotic factors. This finding highlights the significance of mitophagy in stem cells, particularly in the context of preserving their functionality.
In this study, extensive analysis of the preceding single-cell sequencing database was undertaken, revealing a significant upregulation of the COL6A3 gene in BMSCs exhibiting robust osteogenic differentiation potential [17]. Simultaneously, our findings indicate that the COL6A3 gene sustains the stem cell characteristics of BMSCs and enhances the process of osteogenesis via the mechanism of mitophagy. This study provides new insights into the molecular mechanisms underlying BMSC differentiation and their role in bone formation and regeneration. The identification of key genes and pathways involved in cellular processes could lead to the development of more effective treatments for osteoporosis. Additionally, the identification of crucial clusters of BMSCs could help to identify potential therapeutic targets for osteoporosis and contribute to the development of more effective treatments for this disease.
Materials and methods
sc-RNA-seq data analysis
The single-cell dataset GSE147287 contains one scRNA-seq dataset from an osteoporosis patient. The scRNA-seq data underwent the following processing steps: the data were transformed into Seurat objects using the R software package called ‘Seurat’; cells with low-quality counts of mitochondrial or ribosomal genes were excluded; genes present in at least 10 cells were selected; principal component analysis (PCA) and unified flow approximation and projection (Uniform Manifold Approximation and Projection (UMAP)) were used for downsizing and cluster identification based on the selected genes; significant marker genes within different clusters were identified using the ‘Find All Markers’ function, with a log2FC threshold of 0.3 and a minimum value of 0.25. The analysis of cluster annotation was performed using the R software package ‘SingleR’, resulting in a percentage of 0.25. Subsequently, Fisher precision examinations were conducted to detect potentially noteworthy cell categories. The FC values were computed for the identified cell types that had FC values greater than 4 or less than 0.25, and cell types with a p value less than 0.05 were considered significant.
Main reagents
We obtained DMEM/F12 medium (cat #KGM12500-500) from KeyGEN BioTECH (Nanjing, China) and fetal bovine serum (cat #CM1002L) from CELL COOK (Guangzhou, China). Cell Signaling Technology (Massachusetts, USA) was the source of anti-COL6A3 antibody (cat #4912S), anti-RUNX2 antibody (cat #12556S), anti-COL1A1 antibody (cat #72026S), anti-LC3B antibody (cat #3868S), anti-TOMM20 antibody (cat #42406S), anti-FABP4 antibody (cat #50699S), and anti-PPARγ antibody (cat #2435S). The anti-COX-2 antibody (cat #27308-1-AP) and anti-iNOS antibody (cat #22226-1-AP) were purchased from Proteintech (Chicago, USA). Abcam (Cambridge, England) provided the following antibodies: anti-cytochrome C antibody (ab133504), anti-LAMP1 antibody (ab24170), and anti-SQSTM1/p62 antibody (ab109012). We purchased lipopolysaccharide (LPS) (cat #S1732-25 mg) from Beyotime Biotechnology (Shanghai, China). It was obtained from Servivebio in Nanjing, China, and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) was used. The induction medium for osteogenic and adipogenic differentiation of rat BMSCs was obtained from Procell Life Science & Technology (Wuhan, China).
Cell isolation and culture
As reported in previous studies [18], the successful isolation of BMSCs obtained from the femurs and tibia of male Sprague‒Dawley rats (4 weeks old, 100–150 g, n = 10) and expansion of the cells was successful between passages 2 and 8. Briefly, the epiphysis was cut, and the bone marrow cavity was repeatedly flushed with a 5 ml syringe containing DMEM/F12 complete medium containing 15% fetal bovine serum (FBS) and 1% penicillin/streptomycin. A 70-mm stainless steel mesh was used to filter the suspension to eliminate clots and cell clumps before BMSCs were cultured in a humidified incubator (5% CO2, 37 °C). The unattached cells were removed after 3 h by replacing the complete medium with fresh complete medium. This was repeated every 8 h for up to 2 days. BMSCs were then trypsinized and expanded at 80 to 90% confluence. BMSCs treated with LPS (200 µg/ml) were used as a model to identify the role of COL6A3 in the inflammation associated with osteoporosis. The expression of marker proteins for osteogenesis and adipogenesis of BMSCs was detected after LPS (200 µg/ml) treatment for 48 h.
Flow cytometry for BMSC characteristics
To assess the expression of surface markers, we detached BMSCs from passage 3 and subjected them to staining following the protocol outlined in the Rat BMSC Analysis Kit (Cat No. RAXMX-09011). The resulting flow cytometry data were then analyzed using FlowJo V10 software, utilizing a FACSAriaII instrument (BD, USA).
Cell viability assay
We tested cell viability using the Cell Counting Kit-8 (CCK-8). We cultivated BMSCs at 2000 cells per 96-well plate. Then, CCK-8 reagent was added to each well and incubated for 4 h at 37 °C and 5% CO2 in an incubator. The absorbance value of each well at 450 nm was determined using a microplate reader to calculate cell viability.
Osteogenic differentiation of BMSCs
Various substances were used to treat BMSCs, which were then placed into 6-well plates at a cell density of 2 × 104 cells per well. After the cell confluence reached 90%, the medium was substituted with the osteogenic induction differentiation medium of rat BMSCs, and the new culture medium was replaced every 3 d for 15 d. Subsequently, alizarin red staining was performed to observe calcium nodule formation under the microscope, and pictures were captured.
Adipogenic differentiation of BMSCs
BMSCs (2 × 104) were seeded into 6-well plates and treated with different compounds in each well. Unlike osteogenic induction, adipogenic differentiation induction is more complex. After the cell confluence reached 100%, adipogenic differentiation of rat bone marrow mesenchymal stem cells was induced by ADP1 complete medium for 3 d. Then, ADP2 complete medium was used to induce adipogenic differentiation of rat bone marrow mesenchymal stem cells for 1 d. The above procedure was repeated four times, and finally, the ADP2 medium was continued for 3–6 d. A microscope was used to capture images of the formation of lipid droplets stained with Oil Red O.
Western blot analysis
The Total Protein Extraction Kit (KeyGen, China) was used to extract protein from each group of BMSCs. Samples were separated on a 10% or 12% sodium dodecyl sulfate‒polyacrylamide gel and transferred to a polyvinylidene fluoride (PVDF) membranes. Then, the membranes were incubated with diluted primary antibody overnight at 4 °C. The next day, the secondary antibody was added to the membranes at the appropriate concentration for 1 h at room temperature. Finally, the detection and quantification of protein signals from immunoreactive bands were performed using ImageJ software v1.8.0 in conjunction with an ultrasensitive chemiluminescence (ECL) system (Minichemi; Beijing, China).
Transfection
GemmaPharma (Shanghai, China) provided lentiviruses that overexpressed the COL6A3 gene as well as a negative control lentivirus. The second generation cells were placed in a 6-well dish and allowed to grow for 24 h at a moderate concentration (1 × 106) before transfection. The following day, lentivirus transfection of BMSCs was performed according to the product instructions. An 88% transfection rate was confirmed 48 h after adding lentivirus and HiTransG A to BMSCs (viral titer: 1 × 108 TU/ml). Lentivirus was transfected for 24 h at an MOI = 15. The stably expressed cells were filtered using puromycin (5 mg/ml).
TUNEL assay
Apoptosis in each experimental group was detected using the One Step TUNEL Apoptosis Assay Kit (Beyotime; Shanghai, China) following the guidelines provided by the manufacturer. Different compounds were used to treat BMSCs, which were seeded at a density of 2 × 104 cells per well in 6-well plates. TUNEL test solution was prepared, and 100 µl TUNEL test solution was added to each well. The mixture was then incubated at 37 °C for 60 min. Samples were observed under a fluorescence microscope after being sealed with the anti-fluorescence quenching sealing solution.
Colocalization of LAMP1 and TOMM20
BMSCs were subjected to treatment in 12-well plates at a density of 1 × 104 cells per well. Following the application of LPS to the cells, they were subsequently treated with 4% paraformaldehyde for 20 min and then permeabilized using 0.5% Triton X-100 for 10 min. Afterward, the cells were cultured with the anti-LAMP1 antibody and anti-TOMM20 antibody overnight at a temperature of 4°C, followed by incubation with the goat anti-rabbit IgG Cy3/488 antibody (1:200; Servicebio) for 1 h at room temperature. DAPI was used to counterstain the nuclei, and a fluorescence microscope (Olympus; Japan) was utilized to observe the localization of LAMP1 and TOMM20.
Mitochondrial membrane potential measurement
A Mitochondrial Membrane Potential Assay Kit with JC-1 (Beyotime; China) was used according to the manufacturer’s instructions. Normal mitochondria emit red fluorescence due to the aggregation of JC-1. Within impaired mitochondria, JC-1, on the other hand, penetrates the cytoplasm to generate individual units that produce a green glow. In short, BMSCs were cultured in six-well dishes and exposed to LPS. Afterward, 1 ml of JC-1 solution was introduced into each well and the plate was placed in an incubator (37 °C, 5% CO2) for 15 min. Then, the staining was examined under a fluorescence microscope after washing with JC-1 staining buffer. The v1.8.0 version of ImageJ was used to determine and quantify the green fluorescence intensity.
Statistical analysis
All data were obtained from three independent replicates. The statistical analysis was conducted using SPSS 25.0 software (IBM, USA). The quantitative data of the study are presented as the mean ± standard deviation (\(\stackrel{-}{\text{x}}\pm \text{s}\)). T tests were employed to compare differences between two groups, while ANOVAs were utilized to compare differences among multiple groups followed by Tukey’s post hoc test. Statistics were considered significant if p < 0.05.
Results
Cell clustering and annotation of scRNA-Seq for BMSCs in osteoporosis
First, the single-cell data went through preprocessing, where the scaledata function was used to scale the selected highly variable genes. Afterward, the anchor points were determined using PCA downsizing. Following this, the data pertaining to the top 10 principal components (PCs) were selected for downscaling (Fig. 1a). Afterward, we employed BMSC markers (LEPR, NGFR, CD44, ENG, NT5E, and THY1) to ascertain the presence of BM-MSCs in individuals with osteoporosis (Fig. 1b). We annotated the cellular samples using SingleR and visualized them through t-distributed stochastic neighbor embedding (tSNE) and uniform manifold approximation and projection (UMAP) downscaling techniques. Ultimately, we identified distinct clusters comprising a total of 6 cells, and the spatial arrangement of BMSCs in patients was visualized (Fig. 1c). To conduct a more comprehensive examination of the attributes of each BMSC in the context of osteoporosis, an analysis of the marker genes within each subgroup was performed (Fig. 1d). The findings revealed that Cluster 3 exhibited a notable enrichment in terms of osteogenic differentiation, with the COL6A3 gene displaying the highest expression levels (Fig.S1).
Identification and characterization of BMSCs in osteoporosis patients. a PCA downscaling was employed to identify anchor points, and the top 10 principal PCs were selected for subsequent analysis. b Visualization of BMSC distribution in patients using tSNE and UMAP downscaling techniques, showing the expression levels of the identified markers. c Distinct clusters of BMSCs were identified in individuals with osteoporosis using BMSC markers, resulting in the identification of a total of six cell clusters. d Analysis of marker gene expression within each BMSC cell cluster in the context of osteoporosis. BMSCs: Bone Marrow Mesenchymal Stem Cells; PCA: Principal Component Analysis; PCs: principal components; tSNE: t-distributed Stochastic Neighbor Embedding; UMAP: Uniform Manifold Approximation and Projection
COL6A3 is associated with osteogenic differentiation and viability of BMSCs
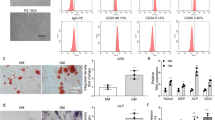
We analyzed the typical surface markers of BMSCs through flow cytometry (Fig. 2a). BMSCs strongly express typical surface antigens (CD73, CD90, CD105); moreover, they are negative for specific surface antigens (CD45, CD34). A positive correlation was observed between the osteogenic differentiation ability and cell viability of BMSCs. The findings from the cell viability assay indicated that the 3rd-generation (p3) BMSCs exhibited significantly higher cell viability than the 8th-generation (p8) BMSCs (Fig. 2b). Concurrently, the calcium nodule composition in p3 BMSCs exhibited a marked increase in comparison to that in p8 BMSCs (Fig. 2c). Correspondingly, COL6A3 expression was significantly lower in p8 BMSCs than in p3 BMSCs (Fig. 2d). These findings indicate that the proliferation activity and osteogenic differentiation of BMSCs may be correlated with the function of the COL6A3 gene.
Reduced expression of COL6A3 was associated with osteogenic differentiation of BMSCs. a Fluorescence-activated cell sorting analysis of BMSCs revealed strong expression of typical surface antigens of stem cells, including CD90, CD105, and CD73; in contrast, the cells were negative for surface antigens not contained in stem cells (CD45, CD34). b The proliferative activity of p3- and p8-generation BMSCs was detected by CCK-8 assay. c Osteogenic differentiation of BMSCs in the p3 and p8 generations was induced. d: The protein expression of COL6A3 in the p3 and p8 generations of BMSCs was detected by Western blotting and quantified. *: p < 0.05; OS: osteogenic stimulation; BMSC: bone marrow mesenchymal stem cell
COL6A3 alleviated the inhibitory effect of LPS on the osteogenic and adipogenic differentiation of BMSCs
Given the detrimental role of inflammation in osteoporotic bone repair, LPS was employed to mimic the inflammatory microenvironment of osteoporosis to further investigate the biological function of COL6A3 on the differentiation capacity of BMSCs. Lentivirus targeting overexpression of the COL6A3 gene was transfected into BMSCs. The expression of the COL6A3 protein was detected by Western blotting(Fig.S1). In comparison to the control group, the LPS group exhibited a notable decrease in the development of calcium nodules, while the LPS + COL6A3-ov group displayed more calcium nodules than the LPS group (Fig. 3a). Then, the expression of marker proteins for osteogenesis (COL1A1, RUNX2) in each group was detected by Western blotting. The protein expression of COL1A1 and RUNX2 in the COL6A3-ov group was significantly increased compared to that in the LPS group. (Fig. 3b). Next, the effect of COL6A3 on the adipogenic differentiation of BMSCs was further evaluated. Compared with the control group, the formation of lipid droplets in the LPS group was reduced, but COL6A3 overexpression reversed the effect of LPS on adipogenic differentiation (Fig. 3c). The expression of signature proteins (PPAR-γ, FABP4) of adipogenesis was reduced in the LPS group compared to the control group. Overexpression of COL6A3 enhanced the adipogenic capacity of BMSCs in the LPS microenvironment (Fig. 3d).
Overexpression of the COL6A3 gene maintained the osteogenic capability and adipogenic capability of BMSCs in the LPS microenvironment. a Representative Alizarin red staining of BMSCs in different groups. b The expression and quantification of COL1A1 and RUNX2 in BMSCs were determined by Western blotting. c Representative oil red O staining of BMSCs in each group. d The protein expression and quantification of PPAR-γ and FABP4 in BMSCs were detected by Western blotting. *: p < 0.05; OS: osteogenic stimulation; AS: adipogenic stimulation; LPS: lipopolysaccharide; ov: overexpression; NC: transfection control
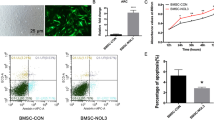
COL6A3 ameliorates apoptosis and inflammation of BMSCs induced by LPS
Limited anti-inflammatory potential and decreased cell viability have posed a challenge for BMSCs in the context of osteogenesis bone repair. Consequently, additional investigations were conducted to evaluate the viability of BMSCs overexpressing COL6A3 in the inflammatory microenvironment. In line with this speculation, the levels of apoptosis-associated proteins (Bax and cleaved caspase-3) were decreased in the LPS + COL6A3-ov group compared to the LPS group (Fig. 4a). The LPS + COL6A3 group exhibited a significantly reduced apoptosis rate of BMSCs compared to the LPS group (Fig. 4b). Moreover, the LPS + COL6A3-ov group exhibited a decrease in the levels of inflammatory mediators (iNOS and COX-2) compared to the LPS group (Fig. 4c). At the same time, COL6A3 effectively limits the release of cytochrome C (Cyt-c) from the mitochondria to the cytoplasm, which is induced by LPS (Fig. 4d). The decrease in ΔΨm is a landmark event in the early stage of apoptosis. The reduction in ΔΨm was obvious in the LPS group compared to the control group; however, the ΔΨm of COL6A3-ov BMSCs treated with LPS was not significantly reduced (Fig. 4e).
Overexpression of COL6A3 alleviates LPS-induced apoptosis, inflammation, and mitochondrial damage in BMSCs. a The protein expression of cleaved caspase-3 and Bax was detected by Western blotting and densitometry analysis. b The apoptosis rate of BMSCs in different experimental groups was detected by the TUNEL method and quantified. c The protein expression of COX-2 and iNOS was detected by Western blotting and quantified. d: In each group, Western blots were used to measure cytochrome C levels in BMSC cytoplasm and mitochondria. e The mitochondrial membrane potential of each group was measured by acquiring the results of JC1 staining by fluorescence microscopy and then quantified. *: p < 0.05; LPS: lipopolysaccharide; ov: overexpression; NC: transfection control; CON: untreated group; cyt-c: cytochrome c; cytosol: cytoplasmic lysate; mito: mitochondrial lysate
COL6A3 attenuates the LPS-induced inhibition of mitophagy in BMSCs
Mitophagy, which is the selective separation and degradation of defective mitochondria, is the key to the quality control of mitochondria in the cell [19]. The potential of COL6A3 to induce mitophagy was tested with a comparative analysis between each group. The COL6A3-ov group exhibited significantly reduced protein accumulation of p62 and upregulation of LC3B II (Fig. 5a). The results of immunofluorescence localization of mitochondria (TOMM20) and lysosomes (LAMP1) revealed that the mitochondria and lysosomes of COL6A3-ov BMSCs showed substantial overlap compared to normal cells (Fig. 5b). Compared with the control group, the expression of LC3B II was decreased and the expression of p62 was increased in the LPS group. In contrast, the COL6A3-ov group exhibited an elevation in the expression of LC3B II and a reduction in the expression of p62 compared to the LPS group (Fig. 5c). The results of colocalized mitochondria (TOMM20) and lysosomes (LAMP1) suggested that the aggregation of mitochondria and lysosomes in the LPS + COL6A3-ov group was higher than that in the LPS group (Fig. 5d). Collectively, these findings demonstrate a significant enhancement in the level of mitophagy in COL6A3-ov BMSCs.
Overexpression of the COL6A3 gene relieved the inhibition of mitochondrial autophagy in BMSCs by LPS. a The expression levels of p62, LC3B (LC3B I/LC3B II) and COL6A3 in COL6A3-overexpressing BMSCs were analyzed by Western blotting and quantified. b Colocalization of mitochondria (TOMM20) and lysosomes (LAMP1) in normal and COL6A3-overexpressing BMSCs was assessed by immunofluorescence. c The expression levels of p62 and LC3B (LC3B I/LC3B II) in each group were analyzed by Western blotting and quantified. d Colocalization of mitochondria (TOMM20) and lysosomes (LAMP1) in each group was observed by immunofluorescence. *: p < 0.05; LPS: lipopolysaccharide; ov: overexpression; NC: transfection control; CON: untreated group
COL6A3 enhances osteogenic and adipogenic differentiation of BMSCs in the LPS microenvironment by promoting mitophagy
To confirm whether the effect of COL6A3 on osteogenesis and adipogenesis of BMSCs in the inflammatory microenvironment was related to mitophagy, the mitophagy inhibitor Mdivi-1 (25 µM) was used to block mitophagy. As expected, the formation of calcium nodules and lipid droplets was significantly reduced in the LPS + COL6A3-ov + Mdivi-1 group compared with the LPS + COL6A3-ov group in the LPS-inflammatory microenvironment (Fig. 6a and c). Compared with that in the LPS + COL6A3-ov group, the protein expression of osteogenesis markers (COL1A1 and RUNX2) and adipogenesis markers (PPAR-γ and FABP4) was decreased in the LPS + COL6A3-ov + Mdivi-1 group (Fig. 6b and d). To further determine the role of COL6A3 in the differentiation of BMSCs in the inflammatory microenvironment, we targeted the COL6A3 gene of p8 BMSCs to further observe their osteogenesis and adipogenesis capacities. Calcium nodule and lipid droplet formation was increased in the LPS + COL6A3-ov group compared with the LPS group (Fig. Figure 6e and g). Simultaneously, the protein expression of osteogenesis markers (COL1A1 and RUNX2) and adipogenesis markers (PPAR-γ and FABP4) was decreased in the LPS + COL6A3-ov group (Fig. 6f and h).
Inhibition of mitophagy decreased the osteogenesis and adipogenesis capacities of COL6A3-overexpressing BMSCs in the LPS microenvironment. a Representative alizarin red staining of the LPS group, LPS + COL6A3-ov group, and LPS + COL6A3-ov + Mdivi-1 group was obtained. b The protein expression of COL1A1 and RUNX2 was detected by Western blotting and quantified. c Representative oil red O staining of the LPS group, LPS + COL6A3-ov group, and LPS + COL6A3-ov + Mdivi-1 group. d The protein expression of PPAR-γ and FABP4 in BMSCs was detected by Western blotting and quantified. Representative alizarin red staining of p8 BMSCs in the LPS group and LPS + COL6A3-ov group. f The protein expression of COL1A1 and RUNX2 was detected by Western blotting and quantified. g Representative oil red O staining of p8 BMSCs in the LPS group and LPS + COL6A3-ov group. h The protein expression of PPAR-γ and FABP4 in BMSCs was measured by Western blotting and quantified. *: p < 0.05; LPS: lipopolysaccharide; ov: overexpression; NC: transfection control; OS: osteogenic stimulation; AS: adipogenic stimulation; LPS: lipopolysaccharide
Discussion
Osteoporosis is characterized by low osteoblast activity and activation of osteoclasts, increased bone fragility, a high risk of fracture, and difficulty in repairing bone defects [20]. Studies on BMSCs have demonstrated a correlation between osteoporosis and the proliferation of BMSCs, as well as the degeneration of osteogenic differentiation capacity [21]. Animal and in vitro studies indicated that heightened levels of inflammatory cytokines facilitate bone resorption through various mechanisms, such as the differentiation, activation, and survival of osteoclasts, as well as the hindrance of osteoblast survival [22]. Due to insufficient oxygen supply and excessive activation of the inflammatory response, osteoblasts are insufficient, and the osteogenic differentiation of BMSCs is deficient [16]. In this study, it was found that COL6A3-ov BMSCs exhibited enhanced resilience within the osteoporosis microenvironment, thereby offering a novel therapeutic approach for addressing osteoporosis.
The gene COL6A3 encodes the collagen VIα3 chain, a crucial component involved in the process of osteogenesis. Collagen VI, a fibrous protein predominantly present in connective tissues such as bones, cartilage, and skin, plays a significant role in preserving the structural integrity and stability of these tissues [23, 24]. Specifically, during osteogenesis, the assembly of collagen VIα3 chains and collagen VIα1 chains results in the formation of collagen VI fibers, which establish a network-like structure within bone tissue. This network structure provides essential support and anchoring for bone cells, ultimately contributing to the maintenance of bone strength and stability. Consequently, the close association between COL6A3 and osteogenesis is evident [25].
Mitochondria facilitate bone cell differentiation and mesenchymal stem cell tolerance to environmental factors in bone marrow [16]. The quantity and integrity of mitochondria are essential for maintaining the physiological function of cells [26]. Mitochondrial dysfunction was manifested by the depolarization of ΔΨm, increasing ROS, the release of the proapoptotic molecule cytochrome c, and increased mitophagy. It is involved in various bone metabolic diseases, such as osteoporosis and OA [27,28,29,30]. Fan demonstrated that mitophagy mediated by PINK1-PARKIN inhibits the senescence of BMSCs in the oxidative stress microenvironment and enhances the survival rate of BMSCs in intervertebral discs treated by BMSC transplantation [31]. Guo found that Sirt3 prevents AGE-induced senescence of BMSCs and senile osteoporosis by mediating mitophagy [32]. Therefore, it is necessary to improve the level of mitophagy to clear dysfunctional mitochondria to maintain the normal physiological function of BMSCs. Gratifyingly, the present study reveals that the level of mitophagy in COL6A3-ov BMSCs was upregulated, which mitigates the inhibitory effects of LPS on mitophagy and ameliorates mitochondrial damage.
To investigate the potential impact of COL6A3 on the osteogenic and adipogenic differentiation of BMSCs in an inflammatory microenvironment via mitophagy, Mdivi-1 was employed to inhibit COL6A3-induced mitophagy to assess BMSC differentiation. The experimental induction of BMSC differentiation revealed that the overexpression of COL6A3 augmented the formation of calcium nodules and lipid droplets in BMSCs within the LPS microenvironment. Simultaneously, the expression of osteogenesis and adipogenesis marker proteins detected in all experimental groups further demonstrated that COL6A3 could enhance the differentiation of BMSCs. Concurrently, the capacity for osteogenic and lipogenic differentiation in p8 BMSCs was notably augmented after overexpression of the COL6A3 gene. However, the application of mitophagy inhibition by Mdivi-1 demonstrated that inhibition of mitophagy attenuated the role of COL6A3 in ameliorating the differentiation capacity of BMSCs induced by LPS. Excitingly, the apoptosis rate and the expression of inflammatory mediators in COL6A3-ov BMSCs were reduced in the LPS microenvironment. The decrease in ΔΨm is a hallmark event of early apoptosis and mitochondrial damage. The overexpression of COL6A3 significantly prevented the LPS-induced reduction in ΔΨm in BMSCs. Overall, the above results demonstrate that COL6A3 promotes mitophagy and prevents BMSCs from losing their differentiation potential, which is associated with LPS. The enhanced environmental resilience and differentiation capacity exhibited by COL6A3-ov BMSCs present a novel therapeutic approach for addressing osteoporosis. (Fig. 7).
Data availability
The datasets produced and examined throughout the study can be obtained from the corresponding author upon reasonable request.
References
Holmes D (2017) Non-union bone fracture: a quicker fix. Nature 550:S193–S193
Peck W (1993) Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med 94:646–650
Yang Y-S, Xie J, Chaugule S, Wang D, Kim J-M, Kim J, Tai PW, Seo S-k, Gravallese E, Gao G (2020) Bone-targeting AAV-mediated gene silencing in osteoclasts for osteoporosis therapy. Mol Therapy-Methods Clin Dev 17:922–935
Trombetta RP, Knapp EK, Awad HA (2021) A mouse femoral ostectomy model to assess bone graft substitutes. Skeletal Dev Repair. https://doi.org/10.1007/978-1-0716-1028-2_5
Wu T, Sun J, Tan L, Yan Q, Li L, Chen L, Liu X, Bin S (2020) Enhanced osteogenesis and therapy of osteoporosis using simvastatin loaded hybrid system. Bioactive Mater 5:348–357
Hu Y, Li X, Zhi X, Cong W, Huang B, Chen H, Wang Y, Li Y, Wang L, Fang C (2021) RANKL from bone marrow adipose lineage cells promotes osteoclast formation and bone loss. EMBO Rep 22:e52481
Cui J, Li X, Wang S, Su Y, Chen X, Cao L, Zhi X, Qiu Z, Wang Y, Jiang H (2020) Triptolide prevents bone loss via suppressing osteoclastogenesis through inhibiting PI3K-AKT‐NFATc1 pathway. J Cell Mol Med 24:6149–6161
Liu H, Li M, Zhang T, Liu X, Zhang H, Geng Z, Su J (2022) Engineered bacterial extracellular vesicles for osteoporosis therapy. Chem Eng J 450:138309
Zeng Z-L, Xie H (2022) Mesenchymal stem cell-derived extracellular vesicles: a possible therapeutic strategy for orthopaedic diseases: a narrative review. Biomater Transl 3:175
Yuan J, Maturavongsadit P, Zhou Z, Lv B, Lin Y, Yang J, Luckanagul JA (2020) Hyaluronic acid-based hydrogels with tobacco mosaic virus containing cell adhesive peptide induce bone repair in normal and osteoporotic rats. Biomater Transl 1:89
Berebichez-Fridman R, Montero-Olvera PR (2018) Sources and clinical applications of mesenchymal stem cells: state-of-the-art review. Sultan Qaboos University Medical Journal 18:e264
Yu X, Quan J, Long W, Chen H, Wang R, Guo J, Lin X, Mai S (2018) LL-37 inhibits LPS-induced inflammation and stimulates the osteogenic differentiation of BMSCs via P2X7 receptor and MAPK signaling pathway. Exp Cell Res 372:178–187
Yang Y, Sun Y, Mao W-w, Zhang H, Ni B, Jiang L (2021) Oxidative stress induces downregulation of TP53INP2 and suppresses osteogenic differentiation of BMSCs during osteoporosis through the autophagy degradation pathway. Free Radic Biol Med 166:226–237
Adamopoulos IE (2018) Inflammation in bone physiology and pathology. Curr Opin Rheumatol 30:59–64. https://doi.org/10.1097/bor.0000000000000449
Zhang H, Menzies KJ, Auwerx J (2018) The role of mitochondria in stem cell fate and aging. Development 145:dev143420
Wang S, Deng Z, Ma Y, Jin J, Qi F, Li S, Liu C, Lyu F-J, Zheng Q (2020) The role of autophagy and mitophagy in bone metabolic disorders. Int J Biol Sci 16:2675
Wang Z, Li X, Yang J, Gong Y, Zhang H, Qiu X, Liu Y, Zhou C, Chen Y, Greenbaum J (2021) Single-cell RNA sequencing deconvolutes the in vivo heterogeneity of human bone marrow-derived mesenchymal stem cells. Int J Biol Sci 17:4192
Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L (2009) MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood J Am Soc Hematol 113:6576–6583
Fan P, Xie X-H, Chen C-H, Peng X, Zhang P, Yang C, Wang Y-T (2019) Molecular regulation mechanisms and interactions between reactive oxygen species and mitophagy. DNA Cell Biol 38:10–22
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. The Lancet 377:1276–1287
Pountos I, Georgouli T, Henshaw K, Bird H, Jones E, Giannoudis PV (2010) The effect of bone morphogenetic protein-2, bone morphogenetic protein-7, parathyroid hormone, and platelet-derived growth factor on the proliferation and osteogenic differentiation of mesenchymal stem cells derived from osteoporotic bone. J Orthop Trauma 24:552–556
Maruyama M, Rhee C, Utsunomiya T, Zhang N, Ueno M, Yao Z, Goodman SB (2020) Modulation of the inflammatory response and bone healing. Front Endocrinol 11:386
Marinella G, Astrea G, Buchignani B, Cassandrini D, Doccini S, Filosto M, Galatolo D, Gallone S, Giannini F, Lopergolo D (2022) A Schematic Approach to defining the prevalence of COL VI variants in five years of next-generation sequencing. Int J Mol Sci 23:14567
Bloks N, Dicks A, Adkar S, Timmermans R, Hajmousa G, Ramos Y, Guilak F, Meulenbelt I (2021) A disease-risk mutation in COL6A3 alters the biologic response of chondrocytes to hyper-physiologic mechanical loading. Osteoarthr Cartil 29:S123–S124
Cabrera-Pérez R, Monguió-Tortajada M, Gámez-Valero A, Rojas-Márquez R, Borràs FE, Roura S, Vives J (2019) Osteogenic commitment of Wharton’s jelly mesenchymal stromal cells: mechanisms and implications for bioprocess development and clinical application. Stem Cell Res Ther 10:1–11
Chen L, Shi X, Xie J, Weng S-J, Xie Z-J, Tang J-H, Yan D-Y, Wang B-Z, Fang K-H, Hong C-X (2021) Apelin-13 induces mitophagy in bone marrow mesenchymal stem cells to suppress intracellular oxidative stress and ameliorate osteoporosis by activation of AMPK signaling pathway. Free Radic Biol Med 163:356–368
Guo Y, Yang TL, Liu YZ, Shen H, Lei SF, Yu N, Chen J, Xu T, Cheng Y, Tian Q (2011) Mitochondria-wide association study of common variants in osteoporosis. Ann Hum Genet 75:569–574
Hui W, Young DA, Rowan AD, Xu X, Cawston TE, Proctor CJ (2016) Oxidative changes and signalling pathways are pivotal in initiating age-related changes in articular cartilage. Ann Rheum Dis 75:449–458
Collins JA, Diekman BO, Loeser RF (2018) Targeting aging for disease modification in osteoarthritis. Curr Opin Rheumatol 30:101
Peng X, Wang K, Zhang C, Bao JP, Vlf C, Gao JW, Zhou ZM, Wu XT (2021) The mitochondrial antioxidant SS-31 attenuated lipopolysaccharide-induced apoptosis and pyroptosis of nucleus pulposus cells via scavenging mitochondrial ROS and maintaining the stability of mitochondrial dynamics. Free Radic Res 55:1080–1093. https://doi.org/10.1080/10715762.2021.2018426
Fan P, Yu XY, Chen CH, Gao JW, Xu YZ, Xie XH, Wang YT (2022) Parkin-mediated mitophagy protects against TNF-α-induced stress in bone marrow mesenchymal stem cells. Exp Gerontol 164:111829. https://doi.org/10.1016/j.exger.2022.111829
Guo Y, Jia X, Cui Y, Song Y, Wang S, Geng Y, Li R, Gao W, Fu D (2021) Sirt3-mediated mitophagy regulates AGEs-induced BMSCs senescence and senile osteoporosis. Redox Biol 41:101915. https://doi.org/10.1016/j.redox.2021.101915
Acknowledgements
Special thanks to the editorial team at Home for Researchers (www.home-for-researchers.com) for refining and revising the article.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81902252).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [Kun Wang], [Xin Peng] and [Rui Zhang]. Financial support and supervision were completed by [Xiaotao Wu] and [Lu Mao]. The first draft of the manuscript was written by [Kun Wang] and all authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals was followed for all animal experiments, and the Administration Committee of Experimental Animals of Southeast University approved the study (No. 20210907001). The study was conducted in accordance with ARRIVE guidelines.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, K., Peng, X., Zhang, R. et al. COL6A3 enhances the osteogenic differentiation potential of BMSCs by promoting mitophagy in the osteoporotic microenvironment. Mol Biol Rep 51, 206 (2024). https://doi.org/10.1007/s11033-023-08918-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-023-08918-z