Abstract
Background
Mechanical Ventilation (MV) is an essential mechanism of life support in the clinic. It may also lead to ventilator-induced acute lung injury (VILI) due to local alveolar overstretching and/or repeated alveolar collapse. However, the pathogenesis of VILI is not completely understood, and its occurrence and development may be related to physiological processes such as the inflammatory response, oxidative stress, and apoptosis. Some studies have found that the the apelin/APJ axis is an endogenous antagonistic mechanism activated during acute respiratory distress syndrome(ARDS), that can counteract the injury response and prevent uncontrolled lung injury. To indicate that apelin-13 plays a protective role in VILI, an animal model of VILI was established in this study to explore whether apelin-13 can alleviate VILI in rats by inhibiting inflammation, apoptosis and oxidative stress.
Methods
SD rats were divided into four groups: control, high tidal volume, high tidal volume + normal saline and high tidal volume + apelin-13. After tracheotomy, the rats in control maintained spontaneous breathing, and the other rats were connected to the small animal ventilator for 4 h to establish the rat VILI model. The mRNA expression of apelin was measured by real-time quantitative polymerase chain reaction(qRT-PCR), immunofluorescence and Western blotting(WB) were used to detect the expression level of APJ, and WB was used to detect the expression of the apoptotic proteins Bax and bcl-2. The degree of lung injury was evaluated by pathological staining of lung tissue,W/D ratio, and BALF total protein concentration. The expression of inflammatory factors(IL-1β, IL-6, TNF-α) in alveolar lavage fluid was measured using ELISA. The activities of MPO and cat and the content of MDA, an oxidative product, in lung tissue were measured to evaluate the degree of oxidative stress in the lung.
Results
After treatment with apelin-13, the apelin/APJ axis in the lung tissue of VILI model rats was activated, and the effect was further enhanced. The pathological damage of lung tissue was alleviated, the expression of the antiapoptotic protein Bcl-2 and the proapoptotic protein Bax was reversed, and the levels of the inflammatory cytokines IL-1β, IL-6, TNF-α levels were all decreased. MPO activity and MDA content decreased, while CAT activity increased.
Conclusion
The apelin/apj axis is activated in VILI. Overexpression of apelin-13 further plays a protective role in VILI, mainly by including reducing pathological damage, the inflammatory response, apoptosis and antioxidant stress in lung tissue, thus delaying the occurrence and development of VILI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mechanical ventilation(MV) is an important advanced life support mechanism used in clinical practice. However, while saving lives, maintaining respiratory function and improving oxygenation, it can also cause or exacerbate lung injury, which is called ventilator-induced acute lung injury(VILI) [1, 2]. VILI includes volume damage, air pressure damage, shear damage and biological damage, but the main factor is biological damage caused by mechanical stress. Its main features include the following: Mechanical stress causes deformation of the lung cell membrane and its receptors, and organelles sense external abnormal mechanical stimuli, which are converted into biochemical signals and transmitted to cells, activate the intracellular signal transduction system, increase the number of inflammatory cells and secreted inflammatory factors, and produce an inflammatory storm.
Mechanical stress can directly damage alveolar epithelial cells, induce cell apoptosis, destroy the structure and function of alveolar epithelial cells and capillary endothelial cells, destroy the integrity of the basement membrane, cause alveolar hemorrhage, form a hyaline membrane, increase lung permeability, and cause pulmonary edema, thus destroying the integrity of the alveolar barrier and damaging the function of the pulmonary barrier [3, 4]. In addition, mechanical stretching can lead to the release of mediators associated with immune response activation, further increasing damage, and may lead to damage to other distant organs [5]. In recent years, many innovative and protective lung ventilation strategies have been proposed, which avoid VILI mainly by limiting tidal volume and/or plateau pressure and by maintaining lung cell recruitment in alveolar areas with sufficient positive end expiratory pressure (PEEP). Relevant clinical trials have shown that ventilator management can effectively reduce the mortality of patients with acute respiratory distress syndrome (ARDS) [6, 7]. However, a large number of ARDS patients still die of multiple system organ failure (MSOF). Therefore, the prevention and treatment of biological damage is particularly important. In previous studies by our research group, it was been demonstrated that VILI can be reduced by inhibiting inflammation and apoptosis in rats [8]. However, the most critical factors and signaling pathways causing VILI have not yet been identified, and its pathogenesis still needs further study.
Apelin, a newly discovered small molecule active polypeptide, is a natural ligand of the orphan G protein coupled receptor angiotensin receptor at-1-related protein (APJ). Currently, active apelin peptides have been described, including apelin-36, apelin-17, apelin-13, and apelin-12. Among these subtypes, apelin-13 and apelin-36 are the main subtypes [9, 10]. Increasing evidence shows that the apelin/APJ axis is widely expressed in the heart, lung and liver [11–14]. Apelin-13 is involved in the regulation of cardiovascular and endocrine systems [12, 15]. And people have gradually realized the importance of the apelin/APJ axis in respiratory diseases [16]. Some studies have confirmed that in the oleic acid (OA) -induced ARDS rat model, OA-induced ARDS is combined with the upregulation and activation of the apelin/APJ axis, and the enhanced apelin/APJ axis plays a functional role. In OA- and LPS- induced ARDS models, posttraumatic treatment with apelin-13 reduced lung injury and improved oxygenation. It is suggested that the apelin/APJ axis can be used as an endogenous anti injury mechanism to protect lung tissue [17]. Moreover, apelin-13 can inhibit the NF-κB pathway, and the NLRP3 inflammasome is activated to prevent LPS-induced acute lung injury. Regulating the gene expression of various inflammatory cytokines, apoptotic proteins and antioxidant stress molecules may be the potential mechanism of apelin-13’s protective effect [18].
However, there is still a lack of research on the effect of VILI on the expression of apelin and APJ receptors and the regulation of VILI through the apelin/APJ axis. Based on the above research background, we hypothesized that apelin-13 delays the occurrence and development of VILI, which may be a new therapeutic strategy for VILI.
Materials and methods
Animal experiment
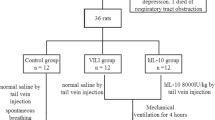
The Changsha Tianqin Biotechnology Co. ltd. (Changsha, China) provided 24 specific pathogen-free male Sprague-Dawley (SD) rats, (age, 6–8 weeks and weight, 200–250 g). The rats were housed in an SPF Animal Experimental Center, under 12-h light/dark cycle, and provided with food and drinking water ad libitum; however, they fasted for 12 h before the experiment but provided free drinking water. Then they were divided into four groups: control (spontaneous breathing), high tide volume (HV), high tide volume + normal saline (NS), and high tide volume + apelin-13 (apelin-13). There were 6 rats in each experimental group. Anesthesia was induced by intraperitoneal injection of pentobarbital (50 mg/kg, narcoren, Merial, Germany) and fentanyl (0.05 mg/kg, Janssen cilag, Neuss, Germany); Anesthesia was supplemented every hour: pentobarbital (5–10 mg/kg per hour) and fentanyl (2.5-5 µg/kg per hour). After complete anesthesia, the control group maintained spontaneous breathing without mechanical ventilation after tracheotomy and intubation. Rats in other groups were subjected to tracheotomy MV for 4 h, and the ventilation parameters were set as follows: LVT was 7 ml/kg, HVT was 40 ml/kg, and respiratory rate was 60 times per minute. Ventilation parameters refer to the study of Roman et al [19]. The endogenous Apelin receptor agonist [pyr1]-apelin-13 (medchemexpress, USA) was injected into apelin-13 group(10nmol/kg, i.p.) 30 min before the experiment; at the same time, equal volume of normal saline was injected intraperitoneally into NS group. For follow-up experiments, rats were euthanized after 4 h of MV or spontaneous breathing. Their bronchoalveolar lavage fluid (BALF) and lung tissue were collected. The experiment was conducted according to the guide for the care and use of laboratory animals of the National Institutes of Health (NIH Publication No. 85-232011). The animal experiment protocol was approved by the animal experiment ethics checklist of Guizhou Medical University (approval number 2,001,132) and followed arrive guidelines.
Hematoxylin-eosin (HE) staining
Following the establishment of the model, the lung tissue was fixed in formalin solution, stored at 4 °C for 24 h, dehydrated with gradient ethanol, and paraffin embedded. The sample was sliced into 4 μm thick sections, and stained with Hematoxylin-eosin (Solarbio, China). After soaking with gradient ethanol, the section was mounted with a neutral balsam and cover glass. Observing the morphology of alveoli under an optical microscope, and using the Smith scoring method to assess pulmonary edema, alveolar and interstitial bleeding, atelectasis, and hyaline membrane formation, the lung tissues of rats in each group were scored. A semi quantitative analysis based on a score of 0–4 was performed to determine the degree of lung tissue damage: 0 point = normal lung tissue; 1 point = minor injury, < 25% of lung injury; 2 points = moderate injury, 25–50% of lung injury; 3 points = severe injury, 50–75% of lung injury; 4 points = extremely severe injury, > 75% of lung injury. Observe 10 high-power fields of view per rat(×200), take its average value [20].
Immunofluorescence staining
The expression levels of APJ(1:800; Proteintech, China) proteins were determined using immunofluorescence. After drying at 60◦C for 1 h, dewaxing and hydrating the 4-um thick paraffin sections, sealing them with goat serum at 37 °C for 30 min, and incubating them with a specific primary antibody overnight. Rewarming at 37 °C for 30 min, and incubated with a fluorescent secondary antibody (1:500, Proteintech, Beijing, China) for 1 h in a wet box. After the addition of DAPI (Solarbio, Beijing, China) in the dark the tablets were re-dyed for 10 min. Finally, the slides were sealed with an anti-fluorescence quenching agent, and images were obtained using a positive fluorescence microscope.
Lung wet/dry (W/D) weight ratio detection
After euthanization, rats were cleaned with PBS to separate the right upper lung and then the lung weighed. Wet lungs were dried at 65 °C for 48 h and referred to as dry lungs. We calculated the lung wet weight /dry weight ratio.
BALF analysis
The left lung was lavaged three times with 0.8 ml PBS. The collected lavage fuid was subjected to centrifugation for 10 min at 1000 g and 4 °C. The supernatant was collected for use as BALF, and the total protein content was measured using a BCA kit (Solarbio, China).
Enzyme-linked immunosorbent assay (ELISA)
The BALF was subjected to centrifugation at 1500 rpm for 10 min and the supernatant collected. In order to determine the expression of interleukin-1β(IL-1β), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) in BALF, we used ELISA reagents (Cusabio Biotech, Wuhan, China), as instructed by the manufacturer IL-1β(CSB-E08053h), IL-6 (Ab100712) and TNF-α (CSB-E11987r). In less than five minutes, the optical density value of each sample was determined with a spectrophotometer at 450 nm.
Western blotting analysis
The pre-cryolysis solution containing PMSF and phosphatase inhibitor was added to the tissue, ground, and cleaved thoroughly using an ultrasonic crusher. Thereafter, 5× loading buffer was added to the protein sample. Proteins were estranged via SDS-PAGE and moved onto a polyvinylidene fluoride (PVDF) membrane, which was stuck down with 5% skim milk at 25 °C for 1 h and incubated overnight with a particular primary antibody APJ (1:800;Proteintech, China) or Bax (1:10, 000; Proteintech, China) or Bcl-2(1:3,000; Proteintech, China) at 4 °C. The membrane was then incubated with a secondary antibody (IgG, 1:5000 Proteintech, Chicago, USA) at 25 °C for 1 h. Finally, imprinting was observed using enhanced chemiluminescence (ECL, P0018AM, Shanghai, China).
Real-time quantitative polymerase chain reaction (qRT-PCR)
Sum RNA was obtained from rat lung tissue using the TRIzol kit (Thermo Fisher, USA). By using the PrimeScriptTM RT reagent kit (RR047A, Takara, Dalian, China), complementary DNA (cDNA) was synthesized from the extracted RNA. mRNA expression levels were measured in strict compliance with TB Green® Premix ExTaqTM II instructions from Takara (RR820A, Takara, Dalian, China). GAPDH served as the internal control for Apelin. All primers (Fig. 1a) were synthesized by Shenggong Bioengineering Co. ltd. (Shanghai, China). The relation expression of the genes was deliberated using the 2-ΔΔCT manner.
Apelin-13 increases the expression of apelin mRNA and its receptor APJ: The qRT-PCR Primer sequence (a Table 1),and its results showed the expression of apelin mRNA in the lung tissue of rats in each group(b); representative WB images of APJ expression of lungs(c); the ratio of APJ/GAPDH in WB(d); immunofluorescence showed the APJ protein in the lung tissue of rats in each group(e); *P < 0.05 vs. C, # P < 0.05 vs. HV, ### P < 0.001 vs. HV
MPO and CAT activity
MPO activity in supernatant of lung tissue was determined using MPO and CAT kit (Nanjing Institute of Bioengineering, Nanjing, China). CAT activity was measured at 405 nm and MPO activity was measured at 460 nm by microplate reader.
MDA content
MDA content in supernatant of lung tissue was determined using MDA kit (Nanjing Institute of Bioengineering, Nanjing, China). MDA content was measured at 532 nm by microplate reader.
Statistical analysis
All experiments were conducted with different batches of rats at least thrice. Data are expressed as mean ± standard deviation (X ± SD). Plots and statistical analyses were performed with GraphPad Prism 8.3 and ImageJ 1.51. Comparing two groups was done via a T test. Comparing more than two groups was done via an ANOVA followed by a Tukey post hoc test. P<0.05 was considered statistically significant.
Result
Apelin-13 increases the expression of apelin mRNA and its receptor APJ in rat lung tissue
To verify whether the apelin/APJ axis is activated during the occurrence and development of VILI and to investigate the effects of apelin 13 intervention on apelin mRNA and its receptor APJ in rat lung tissue. We used qRT-PCR to detect apelin mRNA expression in the lung tissue of rats in each group. The results showed that apelin mRNA expression was increased in the HV and NS groups compared with the control group; Western blot analysis and immunofluorescence showed that compared with that in the control group, the expression level of APJ in the injured lung tissue was increased after MV, indicating that VILI upregulated the expression of apelin and its receptor in the lung tissue. After apelin-13 intervention, the apelin-13 group showed a significant increase in apelin-13 mRNA and its receptor APJ. This result indicated that apelin and its receptor APJ were expressed in normal lung tissue, while VILI led to its increased expression in injured lung tissue. The use of the receptor agonist apelin-13 further upregulated the expression of apelin mRNA and its receptor APJ in lung tissue, and enhanced the signal transduction of the apelin/APJ axis. (Fig. 1).
Apelin-13 relieving VILI
To determine whether apelin-13 further enhances the role of the apelin/APJ axis in alleviate VILI. In the experiment, we measured the W/D ratio of lung tissue and the total protein level in BALF, and observed the changes in lung tissue with HE staining. The results showed that the lung w/d ratio and BALF protein levels in the HV and NS groups were significantly higher than those in the control and apelin-13 groups. After he staining, the alveolar structure in the control group was normal, and the perivascular space edema, alveolar and interstitial neutrophil infiltration, alveolar hemorrhage and hyaline membrane formationwere observed in the HV and NS groups; The severity of inflammatory cell infiltration and hemorrhage in the apelin-13 group was significantly lower than that in the HV and NS groups. According to the Smith scoring system, the lung injury score showed that the degree of injury in the apelin-13 group was significantly lower than that in the HV and NS groups (Fig. 2).
Apelin-13 relieving VILI. Lung dry/wet weight ratio (W/D) level (a); Protein levels in BALF(b); Representative appearance and micrograph of HE(c) stained lung sections (enlarged ×200 and ×400): Severe lung injury occurred in the HV and NS groups, while the apelin-13 group had significantly less lung injury than the HV and NS groups. The histological characteristics of the lung are perivascular edema, infiltration of white blood cells in the interstitium and alveoli, and significant heterogeneity in alveolar dilation; The organizational credit value of the experimental group(d);***P < 0.001 vs. Control, ****P < 0.0001 vs. Control, #P < 0.05 vs. HV, ####P < 0.0001 vs. HV
Apelin-13 alleviates pulmonary inflammatory response in VILI rats
We used ELISA to measure the IL-1β, IL-6 and TNF-α levels in BALF (Fig. 3) to determine the degree of inflammation in the lung tissues. Compared with the control group, the IL-1β, IL-6 and TNF-α levels in BALF of the HV and NS groups were significantly increased. After apelin-13 treatment, the IL-1 β, IL-6 and TNF-α levels were significantly decreased, and the difference was statistically significant. In summary, these data indicate that apelin-13 can reduce the pulmonary inflammatory response of VILI rats.
Effect of apelin-13 on apoptosis in rat lung tissue
To study whether apelin-13 plays a role in VILI apoptosis, we used WB to measure the expression of the antiapoptotic protein Bcl-2 and proapoptotic protein Bax and analyzed their expression ratio. The results (Fig. 4) showed that compared with the HV and NS groups, the intraperitoneal injection of apelin-13 significantly increased the ratio of Bcl-2/Bax in lung tissues. This shows that apelin-13 activates Bcl-2, promotes its binding to Bax, and thus inhibits the apoptosis process.
Apelin-13 alleviates oxidative stress in the lungs of VILI rats
MPO activity, as a marker of neutrophil infiltration, is positively correlated with the survival of neutrophils and is also an enzyme index related to oxidative damage. In addition, we also detected the content of MDA in the oxidation products to evaluate the degree of oxidative damage during VILI. Since the oxidative system and antioxidant system jointly maintain the homeostasis of the body, in order to clarify the state of the oxidative system in rats, we further chose to detect the activity of the antioxidant enzyme cat. In our experimental results (Fig. 5a,b,c), MPO activity and MDA content in the HV and NS groups were significantly higher than those in the control group, while CAT activity decreased. This indicates that there is oxidative stress damage and antioxidant system imbalance in the process of VILI. After apelin-13 intervention, MPO activity and MDA content in the apelin-13 group were significantly lower than those in the HV and NS groups, and CAT activity was effectively reversed by apelin-13. These results suggest that apelin-13 can delay oxidative stress.
Discussion
VILI triggers a wide range of biological reactions, namely, biological injury, in which proinflammatory cytokines and injury-related signaling cascades are activated. TNF-α plays a very important role in the occurrence and development of VILI and is a key link in the activation and expansion of the inflammatory response [21]. Inflammasome activation in both the lung parenchyma and resident immune cells generates inflammatory factor, such as IL-1β is promoted by increasing capillary permeability, which leads to the cascading of inflammatory responses and exacerbates tissue damage and necrosis [22]. Increasing evidence shows that the apelin/APJ signaling pathway is closely related to the development of respiratory diseases. High concentrations of apelin mRNA and APJ mRNA were observed in rat lung tissues [23, 24]. Early studies reported that apelin and/or APJ receptors were upregulated by during tissue injury [25–27]. Fan [17] found that the ARDS induced by OA was related to the upregulation and activation of the apelin/APJ axis. In the ARDS model induced by OA and LPS, treatment with the receptor agonist apelin-13 reduced lung injury and improved oxygenation. The apelin/APJ axis is an endogenous anti-injury mechanism, and the receptor agonist apelin-13 can reduce the stimulation of apelin/APJ axis, further playing a functional role and alleviating inflammation and the injury response. In this study, we found that in the rat VILI model with large tidal volume ventilation, after 4 h of ventilation, the inflammatory response in rat lung tissues eventually led to pathological damage to lungs and the formation of pulmonary edema. After intraperitoneal administration of the receptor agonist apelin-13, the experimental data showed that in the intervention group, the expression of apelin and APJ receptor in lung tissue was significantly increased compared with that in the control group, the HV and NS groups. The levels of the inflammatory cytokines IL-1β, IL-6, and TNF-α in BALF during VILI was significantly decreased, and the inflammatory response of the rat VILI model was reduced or inhibited, indicating that the severity of VILI in rats was alleviated after treatment with apelin-13. The mechanism may be related to the anti-inflammatory effect. It has been verified that apelin and/or APJ receptors are upregulated during VILI tissue injury, but further activation of apelin-13 results in tissue protection. In summary, the apelin/APJ signaling pathway is also an important endogenous mechanism that protects against injury during VILI. The anti-inflammatory mechanism of apelin-13 may also be related to the inhibition of the classical NF-κB signaling pathway and NF-κB activation. Zhang [18] found that in an LPS-induced acute lung injury animal model, the administration of apelin-13 inhibited ROS formation, the NF-κB pathway and the activation of NLRP3 inflammasome in the lung. In addition to the NF- κB pathway, apelin-13 can activate the ERK1/2 pathway through PTX-sensitive G protein and inhibit forskolin-induced intracellular cAMP production, which may be involved in regulating different biological responses [28].
Previous literature reports, apelin-13 protects neurons by strengthening autophagy and attenuating early-stage postspinal cord injury apoptosis in vitro [29] and prevents apoptosis in the cochlear tissue of noise-exposed rat via Sirt-1 regulation [30]. These results show that the expression rates of apelin-13 related proteins Bax/Bcl-2 are increased. Our research is consistent with previous research results, which prove that VILI significantly upregulates the level of the apoptosis-promoting protein Bax and downregulates the level of the antiapoptotic protein Bcl-2 in lung tissue, and this result is reversed after treatment with the receptor agonist apelin-13.
In addition to anti-inflammatory effects and inhibition of apoptosis, the protective mechanism of apelin may also be related to antioxidant stress responses.Oxidative stress is another important mechanism for the occurrence and development of lung injury [31], which is caused by the imbalance between the oxidative system and antioxidant system in the body [32]. As a marker of neutrophil infiltration, MPO can also mediate oxidative stress by promoting the production of reactive oxygen species(ROS) and reactive nitrogen species(RNs) and regulating polarization and inflammation related signaling pathways in neutrophils. A previous study proposed the important role of MPO in mediating oxidative stress and neuroinflammation during cerebral injury. Targeting MPO with inhibitors or gene deficiency agents can become a therapeutic strategy to attenuate oxidative damage and neuroinflammation in ischemic stroke [33]. Shi et al [34] found that, in LPS-induced lung injury mouse model and an A549 cell model, MPO activity was enhanced, while ganoderic acid B significantly reduced MPO activity. In our results, the activity of MPO in the VILI group was enhanced. In addition, the level of MDA in various biological samples may be a reliable indicator of the degree of oxidative damage to body cells and tissues [35]. When the oxidative system is enhanced or weakened, the organism will be in a state of oxidative stress, the function of biofilm will be damaged, and the oxidation product MDA will be produced, causing damage to the body [36]. CAT, one of the most important oxidoreductases in the body, plays a vital role in catalyzing the removal of reactive ROS, preventing cell membrane damage and inhibiting tumor cell growth [37]. Our experimental results showed that the MDA content of the VILI rat model group was significantly higher than that of the control group, and the CAT activity decreased, suggesting that oxidative stress damage, antioxidant system dysfunction, and cell function damage occurred in the process of VILI. Our results showed that the MPO activity and MDA content in apelin-13 group decreased, while the cat activity increased.
This study shows that apelin-13 (receptor agonist) plays a protective role in VILI. However, due to experimental limitations, this study did not explore the effects of a concentration gradient of apelin-13 and conduct further in vitro cell experiments. These two limitations need to be addressed in future research.
Conclusion
In summary, we believe that during VILI, the apelin/APJ axis plays an endogenous anti-injury role, and overexpression of apelin can significantly reduce the inflammatory response, apoptosis and oxidative stress in the lung tissues of VILI model rats. Therefore, apelin-13 may become a potential target for the treatment of VILI in the future.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- VILI:
-
Ventilator-induced lung injury
- MV:
-
Mechanical ventilation
- ARDS:
-
Acute respiratory distress syndrome
- PEEP:
-
Positive end expiratory pressure
- MSOF:
-
Multiple system organ failure
- APJ:
-
Angiotensin receptor at-1-related protein
- SD:
-
Sprague–Dawley
- SPF:
-
Specific-pathogen-free
- HE:
-
Hematoxylin and eosin
- IHC:
-
Immunohistochemistry
- IL-1β:
-
Interleukin-1β
- IL-6:
-
Interleukin-6
- TNF-α:
-
Necrosis fator-α
- W/D:
-
Wet/dry
- ELISA:
-
Enzyme-linked immunosorbent assay
- MPO:
-
Myeloperoxidase
- CAT:
-
Catalase
- MDA:
-
Malondialdehyde
- ROS:
-
Oxygen species
- RNs:
-
Reactive nitrogen species
References
Slutsky AS, Ranieri VM (2013) Ventilator-induced lung injury. N Engl J Med 369(22):2126–2136
Dreyfuss D, Saumon G (1998) Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 157(1):294–323
Chen, Lin (2018) Xia Hai Fa,Shang You et al, Molecular mechanisms of Ventilator-Induced Lung Injury. Chin Med J (Engl) 131(10):1225–1231
Szabari MV, Takahashi K, Feng Y et al (2019) Relation between respiratory mechanics, inflammation, and survival in experimental mechanical ventilation. Am J Respir Cell Mol Biol 60(2):179–188
Curley GF, Laffey JG, Zhang H, Slutsky AS (2016) Biotrauma and Ventilator-Induced Lung Injury: clinical implications. Chest 150(5):1109–1117
Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR (1998) Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338(6):347–354
Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342(18):1301–1308
Zhou L, Xue C, Chen Z, Jiang W, He S, Zhang X (2022) c-Fos is a mechanosensor that regulates inflammatory responses and lung barrier dysfunction during ventilator-induced acute lung injury. BMC Pulm Med 22(1):9
Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M (1998) Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun 251(2):471–476
Cano Martínez LJ, Coral Vázquez RM, Méndez JP, Trejo S, Pérez Razo JC, Canto P (2019) Serum concentrations of apelin-17 isoform vary in accordance to blood pressure categories in individuals with obesity class 3. Clin Exp Hypertens 41(2):168–173
Wang X, Zhang L, Li P, Zheng Y, Yang Y, Ji S (2022) Apelin/APJ system in inflammation. Int Immunopharmacol 109:108822
Gao S, Chen H (2023) Therapeutic potential of apelin and Elabela in Cardiovascular Disease. Biomed Pharmacother 166:115268
Yuan Y, Wang W, Zhang Y, Hong Q, Huang W, Li L, Xie Z, Chen Y, Li X, Meng Y (2022) Apelin-13 attenuates Lipopolysaccharide-Induced inflammatory responses and Acute Lung Injury by regulating PFKFB3-Driven Glycolysis Induced by NOX4-Dependent ROS. J Inflamm Res 15:2121–2139
Ji W, Shi H, Shen H, Kong J, Song J, Bian H, Lv X (2019) Mechanism of KLF4 Protection against Acute Liver Injury via Inhibition of Apelin Signaling. Oxid Med Cell Longev, 2019: p. 6140360
Li C, Cheng H, Adhikari BK, Wang S, Yang N, Liu W, Sun J, Wang Y (2022) The role of Apelin-APJ system in Diabetes and obesity. Front Endocrinol (Lausanne) 13:820002
Yan J, Wang A, Cao J, Chen L (2020) Apelin/APJ system: an emerging therapeutic target for Respiratory Diseases. Cell Mol Life Sci 77(15):2919–2930
Fan XF, Xue F, Zhang YQ, Xing XP, Liu H, Mao SZ, Kong XX, Gao YQ, Liu SF, Gong YS (2015) The Apelin-APJ axis is an endogenous counterinjury mechanism in experimental acute lung injury. Chest 147(4):969–978
Zhang H, Chen S, Zeng M, Lin D, Wang Y, Wen X, Xu C, Yang L, Fan X, Gong Y, Zhang H, Kong X (2018) Apelin-13 Administration protects against LPS-Induced Acute Lung Injury by inhibiting NF-κB pathway and NLRP3 inflammasome activation. Cell Physiol Biochem 49(5):1918–1932
Farre R, Granell S, Rotger M, Serrano-Mollar A, Closa D, Navajas D (2005) Animal model of unilateral ventilator-induced lung injury. Intensive Care Med 31(3):487–490
Smith KM, Mrozek JD, Simonton SC et al (1997) Prolonged partial liquid ventilation using conventional and high-frequency ventilatory techniques: gas exchange and lung pathology in an animal model of respiratory distress syndrome [J]. Crit Care Med 25(11):1888–1897
Ngiam N, Kavanagh BP (2012) Ventilator-induced lung injury: the role of gene activation. Curr Opin Crit Care 18(1):16–22
McVey MJ, Steinberg BE, Goldenberg NM (2021) Inflammasome activation in acute lung injury. Am J Physiol Lung Cell Mol Physiol 320(2):L165–L178
Hosoya M, Kawamata Y, Fukusumi S, Fujii R, Habata Y, Hinuma S, Kitada C, Honda S, Kurokawa T, Onda H, Nishimura O, Fujino M (2000) Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J Biol Chem 275(28):21061–21067
Keskin-Aktan A, Kutlay Ö (2023) Exogenous apelin-13 administration ameliorates cyclophosphamide-induced oxidative stress, inflammation, and apoptosis in rat lungs. Protein Pept Lett. Aug 24
Rastaldo R, Cappello S, Folino A, Losano G (2011) Effect of apelin-apelin receptor system in postischaemic myocardial protection: a pharmacological postconditioning tool? Antioxid Redox Signal 14(5):909–922
Apelin-13/APJ (2019) System attenuates early brain injury via suppression of endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation and oxidative stress in a AMPK-dependent manner after subarachnoid Hemorrhage in rats. J Neuroinflammation 16(1):247
Li Y, Guo J, Yu H, Zhou J, Chu X, Hou B, Ge J, Li T, Duan S, Xu H, Yang X (2020) The effect of olmesartan on aortic intimal thickening after balloon injury through Apelin/APJ. Cardiovasc Pathol. Nov-Dec;49:107230
Bai B, Tang J, Liu H, Chen J, Li Y, Song W (2008) Apelin-13 induces ERK1/2 but not p38 MAPK activation through coupling of the human apelin receptor to the Gi2 pathway. Acta Biochim Biophys Sin (Shanghai) 40(4):311–318
Lin T, Zhao Y, Guo S, Wu Z, Li W, Wu R, Wang Z, Liu W (2022) Apelin-13 protects neurons by attenuating early-stage Postspinal Cord Injury apoptosis in Vitro. Brain Sci 12(11):1515
Khoshsirat S, Abbaszadeh HA, Peyvandi AA, Heidari F, Peyvandi M, Simani L, Niknazar S (2021) Apelin-13 prevents apoptosis in the cochlear tissue of noise-exposed rat via Sirt-1 regulation. J Chem Neuroanat 114:101956
Zhou J, Peng Z, Wang J (2021) Trelagliptin alleviates lipopolysaccharide (LPS)-Induced inflammation and oxidative stress in Acute Lung Injury mice. Inflammation 44(4):1507–1517
Qi JH, Dong FX (2021) The relevant targets of anti-oxidative stress: a review. J Drug Target 29(7):677–686
Chen S, Chen H, Du Q, Shen J (2020) Targeting myeloperoxidase (MPO) mediated oxidative stress and inflammation for reducing Brain Ischemia Injury: potential application of Natural compounds. Front Physiol 11:433
Shi J, Wang H, Liu J, Zhang Y, Luo J, Li Y, Yang C, Jiang J (2020) Ganoderic acid B attenuates LPS-induced lung injury. Int Immunopharmacol 88:106990
Tsikas D (2017) Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal Biochem 524:13–30
Koçak H, Oner-Iyidoğan Y, Gürdöl F, Koçak T, Esin D (2005) The relation between serum MDA and cystatin C levels in chronic spinal cord injury patients. Clin Biochem 38(11):1034–1037
Glorieux C, Calderon PB (2017) Catalase, a remarkable enzyme: targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol Chem 398(10):1095–1108
Funding
This work was supported by Science and Technology Support Program of Science and Technology Department of Guizhou Province (NO: Qian Ke He Zhi Cheng [2021] Yi Ban 061); Science and Technology foundation Program of Science and Technology Department of Guizhou Province (NO: Qian Ke He Ji Chu-ZK [2022] Yi Ban 450). The Cultivate project 2021 for National Natural Science Foundation of China, Guizhou Medical University. (20NSP038).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Siyu Lian, Xianming Zhang, Yi Shen, Shuang He, Zongyu Chen, Leilei Zhou, Wenqing Jiang. Performed the experiments: Siyu Lian, Xianming Zhang, Yi Shen,Shuang He, Zongyu Chen, Wenqing Jiang. Analyzed the data:Siyu Lian, Xianming Zhang. Wrote the paper: Siyu Lian, Xianming Zhang. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study approved by the Institutional Animal Care and Use Committee of Guizhou Medical University (no. 2001132).
Consent for publication
Not applicable (animal study).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lian, S., Zhang, X., Shen, Y. et al. Protective effect of apelin-13 on ventilator-induced acute lung injury. Mol Biol Rep 51, 74 (2024). https://doi.org/10.1007/s11033-023-08911-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-023-08911-6