Abstract
Ventilator-induced lung injury (VILI) is one of the most serious complications of mechanical ventilation (MV) and can increase the mortality of patients with acute respiratory distress syndrome (ARDS). This work aimed to test the hypothesis that the anti-inflammatory properties of human interleukin-10 (hIL-10) can reduce VILI. Thirty-six healthy male Sprague-Dawley rats were randomly assigned into three groups (n = 12) as follows: a control group, a VILI group, and a hIL-10 group. Lung function was evaluated by oxygenation index and pulmonary edema, and morphological changes associated with lung injury were assessed by HE staining and quantitative histological lung injury score. Malondialdehyde (MDA) and Superoxide dismutase (SOD) were measured, and the levels of various inflammatory cytokines were assessed in BALF and plasma. The oxygenation index in the VILI group decreased significantly relative to the control group and improved substantially in the hIL-10 group (P < 0.01). Compared to the control group, MDA production was stimulated (P < 0.01), and SOD activity rapidly declined (P < 0.01) in the VILI group. After hIL-10, MDA content was lower than that seen in the VILI group (P < 0.01), and SOD activity was enhanced (P < 0.01). The VILI group had the highest cytokine levels, compared to either the hIL-10 group or the control group (P < 0.05). High tidal volume MV can induce VILI. hIL-10 may regulate the inflammatory response in the lung tissue, improve lung tissue oxygenation, and inhibit oxidative stress, therefore reducing VILI in rats. These experiments reveal a potential new treatment option for VILI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Mechanical ventilation (MV) is widely used in clinical anesthesia and critical care as an important means of respiratory support. However, MV is an external force and can induce or aggravate lung injury by interfering with the natural process of respiration [1]. Clinical studies have shown that patients who received MV were subjected to excessive dilation of the alveoli by mechanical traction which induced inflammation and caused acute lung injury (ALI), specifically defined as ventilator-induced lung injury (VILI) [2, 3]. VILI is the most serious complication of MV, and the high mortality of patients with acute respiratory distress syndrome (ARDS) is due in part to this complication [4].
Previous studies have indicated that various pathways are involved in the causal mechanisms and devastating processes of VILI, including both the direct mechanical damage and indirect biological injury [5]. Mechanical damage can mediate biological damage through multiple pathways. During MV, the lungs are subjected to mechanical stretch and direct damage to cell walls, leading to the release of a large number of cytokines in the alveolar and systemic circulatory systems, leading to both local and systemic inflammation. The main pathophysiological changes included alveolar structural damage, increased pulmonary vascular permeability, activation of various inflammatory cells and inflammatory factors, and an imbalance between oxidation and antioxidation [6].
Although a small tidal volume with positive end expiratory pressure (PEEP) has been confirmed to effectively reduce VILI related to mechanical damage [7, 8], the extensive application of MV coupled with an in-depth understanding of the pathogenesis of VILI had led more researchers to focus on biological injury [9] in the search for safe and effective options for MV.
Interleukin-10 (IL-10) is an anti-inflammatory immunosuppressive cytokine, known to play an important role in immune regulation and relevant to both inflammatory and infectious diseases [10]. An important feature of ALI is the excessive expression of inflammatory mediators such as tumor necrosis factor (TNF-α) and interleukin-8 (IL-8) [11]. A recent endotoxin study of ALI in a rat model found that exogenous IL-10 reduced pro-inflammatory cytokines in pulmonary tissue and minimized lung injury [12].
MV initiates systemic inflammation as well as aggravated pulmonary inflammation [13, 14]. The injury is not confined to the lungs, but also enhances the systemic inflammation reaction, increases the incidence of secondary beat, and facilitates multiple organ dysfunction syndromes (MODS).
The effect of IL-10 on ALI has not been investigated sufficiently given the common application of MV for patients with critical illness or under general anesthesia. Here, we designed a VILI model using high tidal volume MV and intravenously injected hIL-10 before MV application in order to investigate the protective role of hIL-10 and probe its potential mechanisms for the prevention of VILI.
MATERIALS AND METHODS
We used high VT MV to develop an animal model of VILI in 40 healthy male Sprague-Dawley rats. The rats were purchased from Shanghai Slack Laboratory Animal Co., LTD, and the administration of the experimental animals was guided by the principles of the Fujian Medical University Ethics Committee. hIL-10 was obtained from the American PeproTech Corporation, and the DHX-150 rodent ventilator was purchased from Chengdu Instrument Factory. The rat TNF-α ELISA kit and the rat IL-8 ELISA kit were purchased from Fuzhou’s Biotech Corp. The rat MDA and SOD test kit were obtained from Nanjing Institute of Bioengineering.
Study Design
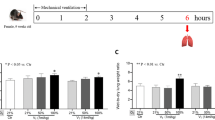
During developing an animal model of VILI, three rats died of respiratory depression and one died of respiratory tract obstruction. The remaining 36 male Sprague-Dawley rats weighing 220~300 g were randomly divided into three groups (n = 12). Tail vein catheterization and tracheal intubation were performed after inducing anesthesia with 10% chloral hydrate (0.3 ml/100 g) transperitoneal injection for all rats. Spontaneous breathing was maintained in the control group, while both the VILI group and hIL-10 group were mechanically ventilated for 4 h with the following parameters: tidal volume (VT) = 30 ml/kg, respiratory rate (RR) = 40/min, respiratory ratio (I:E) = 1:3, PEEP = 0, fraction of inspired oxygen (FiO2) = 21%. Thirty minutes before ventilation, the hIL-10 group received 8000 U/kg hIL-10 by intravenous administration, while the control and VILI groups received saline solution by intravenous administration. Anesthesia was maintained with 10% chloral hydrate (0.2 ml/100 g) transperitoneal injection for a 1-h interval in the control group. Anesthesia was maintained with 10% chloral hydrate (0.2 ml/100 g) transperitoneal injection and rocuronium (0.6 mg/kg) intravenous administration for a 1-h interval in the VILI group and hIL-10 group. During anesthesia and ventilation, laboratory temperature was kept at 22 ± 2 °C. Rectal temperature was maintained at 37 °C with the aid of servo-controlled warming blanket (TCAT-2LV, Physitemp instruments Inc., Clifton, NJ). Heart rate and SpO2 were measured continuously during anesthesia with a MouseOx™ Pulse Oximeter (Harvard Apparatus, Holliston, MA). After experiments were completed, all animals were killed by overdose of chloral hydrate followed by transection of the abdominal aorta and vena cava. Lungs were then extracted for histology and molecular biology analysis (Fig. 1).
Collection and Processing of Blood Samples
All rats were euthanized after 4 h of ventilation and laparotomy. Blood samples were drawn from the abdominal aorta into a sterile syringe containing heparin. Some were used for blood gas analysis and the calculation of arterial oxygenation index (PaO2/FiO2), and the others were transferred to procoagulant tubes and immediately placed in a pre-cooled 4 °C low temperature centrifuge and spun at 3000 rpm/min for 10 min. After centrifugation, the supernatant aliquots were dispensed into 0.6 ml EP tubes, marked, and stored at − 80 °C in an ultralow temperature refrigerator for subsequent ELISA detection.
Collection and Treatment of Bronchoalveolar Lavage Fluid
The rat lung tissue and trachea were isolated, and a silk thread was tied to the right lung. A sterile syringe was used to extract 2 ml of cold saline for bronchoalveolar lavage through an endotracheal tube. The normal saline was absorbed after a brief incubation in the left lung, and the process was repeated three times. The subsequent processing and storage for ELISA of the bronchoalveolar lavage fluid was the same that described above for serum.
Collection and Treatment of the Lung Tissue
The right upper lobe of the lung was quickly and gently dissected. Samples were fixed by 4% paraformaldehyde, and then dehydrated, cleared until transparent, dipped in wax, embedded, sliced, and made into paraffin sections for hematoxylin and eosin (HE) staining and immunohistochemistry. The right middle lobe was clipped and weighed after excess liquid was gently absorbed with clean gauze. After removal of the soft connective tissue, the lower lobe of the right lung was arranged in a 1.5-ml sterile centrifuge tube and frozen at – 80 °C in an ultralow temperature refrigerator for assays of malondialdehyde (MDA) content and superoxide dismutase (SOD) activity. MDA is an intermediate product of oxidation after cell damage resulting from inadequate oxygen scavenging activity, which reflects the degree of membrane lipid peroxidation and the extent of the damage. SOD is a scavenger of oxygen free radicals that plays a protective role in cells and tissue.
Detection of each Index
Plasma and BALF were collected to detect the density of TNF-α, IL-8, and intercellular cell adhesion molecule-1 (ICAM-1) by enzyme-linked immunosorbent assay (ELISA). The right upper lung was removed for HE staining in order to observe the morphological changes in the lung tissue; the right middle lung was dried to constant weight in a 65 °C oven in order to determine the wet dry weight ratio (W/D), and the right lower lobe of the lung was homogenized to detect malondialdehyde (MDA) content and superoxide dismutase (SOD) activity.
Histology Analysis
The right upper lung was removed for HE staining in order to observe the morphological changes in the lung tissue. Photomicrographs at magnifications of × 100 were obtained by a light microscope (Olympus BX51, Olympus Latin America Inc., Brazil). Lung injury was evaluated by an independent pathologist who was blinded to the grouping under light microscopy, taking into account hemorrhage in the lung tissue, alveolar congestion, edema, infiltration of macrophages and neutrophils, and morphological changes in the alveolar wall. Lung injury was quantified by two independent pathologists who did not participate in the study. Quantitative histological lung injury score was described [15]. They scored lung injury on a scale from 0 to 4, where 0 represented minimum damage; 1 represented mild damage; 2 represented moderate damage; 3 represented severe damage; and 4 represented maximum damage.
Statistical Analysis
Sample size was calculated based on a previous study [6], which showed that a sample size of 12 rats in each group was required to detect a 20% increase in PaO2/FiO2 with an alpha of 0.05 and a beta of 10%. The success rate of our pre-experiment model is about 90%. Thus, the total rats were 40.
The data on lung injury score are given as median (range), whereas the other data are expressed as mean ± S.E.M. Differences were evaluated by using ANOVA followed by the Student-Newman-Keul’s post hoc analysis. The lung injury score among three groups was analyzed using the Kruskall-Wallis rank test. The lung injury score between groups was analyzed using the Mann-Whitney test. P < 0.05 was accepted as significant. Data was analyzed using SPSS16.0 (SPSS, Chicago, IL, USA).
RESULTS
Oxygenation Index, Wet/Dry Ratios, and BALF Total Protein
We generated a VILI model using 4 h of high tidal volume mechanical ventilation. Pulmonary function and morphologic changes were observed to confirm the occurrence of acute lung injury and evaluate the effect of hIL-10 pretreatment.
Arterial oxygenation index is an important indicator reflecting respiratory function. We performed arterial blood gas analysis and calculated oxygenation index after ventilation. The oxygenation index in the VILI group decreased significantly relative to the control group and improved substantially in the hIL-10 group (280.95 ± 7.60, 375.79 ± 6.56 vs. 457.94 ± 6.04 mmHg, P < 0.01, Fig. 2a).
Pulmonary function of rats by group (n = 12, mean ± SEM). a Arterial oxygenation index. b Wet/dry ratio. c Total protein content in bronchoalveolar lavage fluid. Control: non-ventilated after intubation; VILI: high VT ventilation; hIL-10: high VT ventilation plus hIL-10. **P < 0.01 vs. control group; ##P < 0.01 vs. VILI group.
We measured wet/dry ratio to determine the degree of pulmonary edema. W/D increased significantly in the VILI group, but was significantly reduced after hIL-10 pretreatment (5.61 ± 0.28, 5.08 ± 0.19 vs. 4.49 ± 0.30, P < 0.01, Fig. 2b).
We also measured BALF total protein (BALF TP) by BCA to evaluate pulmonary capillary damage. Protein was significantly increased in the VILI group, while the hIL-10 group showed reduced lung capillary damage (0.87 ± 0.06, 0.59 ± 0.04 vs. 0.34 ± 0.05 g/L, P < 0.01, Fig. 2c).
Morphological Changes Related to Lung Injury
We used HE staining to visualize the pathological changes in the lung. As shown in Fig. 3, the structure of the lung tissue in the VILI group was obviously impaired, with widened alveolar fusion, massive hemorrhages, and exudates. Although the alveolar interval was slightly thickened in the hIL-10 intervention group, the injury was less severe than that seen in the VILI group. Compared with the control group, the lung injury score was higher in the VILI group (P < 0.001) and in the hIL-10 group (P < 0.001). Compared to the hIL-10 group, the lung injury score was higher in the VILI group (P = 0.006).
Pathological changes of the lung tissue in rats (HE staining, × 100) control: non-ventilated after intubation; VILI: high VT ventilation; hIL-10: high VT ventilation plus hIL-10. A five-point, semi-quantitative, severity-based scoring system was used: 0 = normal; 1 = 1–25%; 2 = 26–50%; 3 = 51–75%; 4 = 76–100%. Values are median (interquartile range) of animals.
MDA Content and SOD Activity
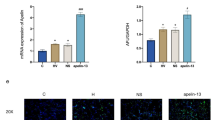
We examined MDA and SOD in the lung tissue to determine oxidative stress after 4 h of mechanical ventilation. Compared to the control group, MDA production was stimulated (3.51 ± 0.31 vs. 1.10 ± 0.21 nmol/mgprot, P < 0.01), and SOD activity rapidly declined (205.91 ± 23.45 vs. 370.09 ± 29.37 U/mgprot, P < 0.01) in the VILI group. After hIL-10, MDA content was lower than that seen in the VILI group (2.20 ± 0.23 vs. 3.51 ± 0.31 nmol/mgprot, P < 0.01), and SOD activity was enhanced (250.84 ± 25.09 vs. 205.91 ± 23.45 U/mgprot, P < 0.01, Fig. 4).
Plasma and BALF Cytokine Levels
Mechanical stretch is another factor contributing to VILI and can cause changes of inflammatory cytokine concentration. We used ELISA to determine TNF-α, IL-8, and ICAM-1 levels in plasma and BALF.
BALF cytokine levels were evaluated to determine the pulmonary inflammatory response to mechanical ventilation. As shown in Fig. 5, the VILI group had the highest cytokine levels, compared to either the hIL-10 group or the control group (TNF-α, 261.10 ± 32.17 (VILI) vs. 210.61 ± 20.51 (hIL-10) and 173.57 ± 41.80 ng/L (control); IL-8, 570.21 ± 56.89 (VILI) vs. 490.36 ± 22.56 (hIL-10) and 455.71 ± 36.12 ng/L (control); ICAM-1, 15.21 ± 2.46 (VILI) vs. 11.47 ± 1.63 (hIL-10) and 8.02 ± 1.29 ng/L (control), P < 0.05).
We measured cytokines in plasma to evaluate systemic inflammation, as shown in Fig. 6. Plasma cytokine levels were significantly increased in the VILI group compared to the other two groups (TNF-α, 200.36 ± 17.83 (VILI) vs. 145.82 ± 16.82 (hIL-10) and 104.95 ± 11.92 ng/L (control); IL-8, 515.54 ± 56.38 (VILI) vs. 440.90 ± 27.05 (hIL-10) and 404.41 ± 36.90 ng/L (control); ICAM-1, 26.19 ± 1.94 (VILI) vs. 21.94 ± 1.41 (hIL-10) and 16.69 ± 1.44 ng/L (control), P < 0.05).
DISCUSSION
MV is a way to maintain airway patency and improve ventilation and oxygenation for patients with respiratory dysfunction or during surgery, making it an important part of clinical anesthesia and critical care. However, MV is a double-edged sword. A large number of studies have shown that MV can also induce ALI, even in patients with no pulmonary disease, leading to inflammation of the lungs [16, 17]. Research has further shown that the primary factor in VILI is volume injury [5]. High tidal volume MV could be used to replicate VILI in an animal model [18]. Therefore, we used 4-h VT at 30 ml/kg to establish a rat model of VILI.
The prevention of VILI is particularly important given the extensive application of MV, and the role of IL-10 as an immune inflammatory mediator in various diseases has become a major research topic in recent years. Here, we established an animal model of VILI and investigated the role of hIL-10 in the alleviation of VILI, acquired differently from the previous model of endotoxin-induced lung injury [19]. As IL-10 synthesis in vivo occurs later than other pro-inflammatory cytokines, we delivered an intravenous injection of hIL-10 in advance, increasing the plasma concentration of IL-10. hIL-10 reduces the severity of lung injury and the expression of various oxidative stress and inflammatory biomarkers. These data indicate a protective role for IL-10 in the prevention of VILI and suggested that oxidative stress and inflammation mechanisms may be involved.
VILI pathogenesis includes a variety of direct and indirect mechanisms [20]. The primary mechanism is likely volutrauma and atelectrauma followed by excessive swelling of the lung tissue at the inspiratory end due to high airway pressure and high capacity, as well as the shear injury caused by the periodic opening and closing of the alveoli. In addition, a large number of inflammatory factors are released and inflammatory pathways are activated after the cells are mechanically disrupted with the increase of alveolar permeability. Animal experiments and clinical studies have found that VILI is primarily stimulated by mechanical stress, triggering local and systemic inflammatory responses by mechanical transduction mechanisms. A primary expression of VILI is lung damage [5], characterized by pulmonary congestion, edema, increased W/D, alveolar rupture, and a large amount of inflammatory cell infiltration observable under the microscope. High tidal volume MV can lead to excessive alveolar expansion and cell membrane damage, which can then lead to pulmonary edema, necrosis, and alveolar capillary injury. In this study, TP content in BALF was detected by the BCA method and indicated an increase in alveolar capillary permeability. Changes in biomarkers are a second major expression of VILI [16]. After high tidal volume MV, the lung structure was destroyed by mechanical stress, and there was diffuse alveolar damage and increased alveolar capillary permeability, prompting the release of a large number of inflammatory mediators, leading to elevated levels of inflammatory mediators such as IL-1 and TNF-α in the lung tissue.
The arterial partial pressure of oxygen is an important indicator of respiratory function. It is less than 300 mmHg after ALI. We found that the PaO2/FiO2 was significantly decreased after 4 h of ventilation. Inflammatory exudation and hemorrhage were observed, and wet/dry ratio and TP content in BALF increased as a result of the mechanical stress damage to the alveoli and capillaries and were correlated with alveolar edema severity [21]. However, pretreatment with hIL-10 resulted in an improvement of oxygenation and pathological injury, consistent with the reports of IL-10-attenuated endotoxin-induced lung injury [22].
MDA content is related to the membrane lipid peroxidation reaction and degree of damage. Highly reactive oxygen free radicals are produced during mechanical ventilation, attacking the cellular membrane system and enhancing oxidative stress [23, 24], as expressed by substantially increased MDA content. SOD is an oxygen free radical scavenger and broad spectrum antioxidant, widely distributed in the lung tissue, and has an anti-inflammatory and immune regulation effect. In cells undergoing oxidative stress, endogenous antioxidant enzyme synthesis can be increased somewhat, but when the oxidative stress reaction becomes too strong, the endogenous SOD is not enough to scavenge the oxygen free radicals, causing disorders in the oxidative and antioxidant system. Insufficient oxygen scavenging contributes to oxidative damage and presents as notably decreased SOD activity. Our results verify the imbalance between oxidation and the antioxidant system following damage caused by mechanical stress. Cellular mechanisms are abnormally activated by oxidative damage, resulting in a large number of pro-inflammatory cytokines and chemokines in rapid transcription [23]. In this study, MDA content was significantly increased and SOD activity was significantly decreased, indicating that the oxidative stress pathway was activated. Our findings further demonstrate that this pathway can be potently blocked by hIL-10.
The body’s immune system is in a state of equilibrium under normal circumstances. However, pulmonary and systemic inflammatory factors can be synthesized and released in large amounts when triggered by high tidal volume mechanical ventilation. The balance between pro-inflammatory cytokines like TNF-α and IL-8 and anti-inflammatory cytokines such as IL-10 becomes broken [13]. TNF-α is one of the multifunctional factors in the first generation of the immune response and could induce the synthesis and release of other pro-inflammatory factors. IL-8 is an important mediator of many inflammatory diseases and is found to be significantly increased in both local and systemic inflammatory responses. ICAM-1 promotes adhesion to the site of inflammation and also mediates inflammatory responses. Previous studies have shown that mechanical stretch could cause increases of TNF-α, IL-8, and ICAM-1 [14, 16]. The phenomenon was also observed in our study, indicating that MV activated the inflammatory/anti-inflammatory system.
IL-10 is an important anti-inflammatory factor in vivo, can inhibit the expression of inflammatory mediators, and has no effect on the expression of anti-inflammatory mediators [20]. Previous studies found that IL-10 could promote the degradation of TNF-α and IL-8 and directly inhibit the activation of inflammatory cells and the production of inflammatory cytokines [25]. Animal experiments showed that IL-10 could reduce lung injury and play a protective role in systemic inflammatory response syndrome (SIRS). IL-10 can inhibit the inflammatory reaction itself and reduce tissue damage caused by inflammation. It has a dual role related to both inflammatory mediators and anti-inflammatory mediators and has, therefore, attracted attention in the field of ALI research. Previous studies on the protective effect of IL-10 on ALI used the toxin-induced ALI model [19]. In our study, VILI was induced by high tidal volume MV, and the ventilated rats were injected with hIL-10 through the tail vein to increase the plasma concentration of IL-10. After 4 h of ventilation, the lung tissue, serum, and BALF specimens were collected and the results showed that hIL-10 can reduce the degree of pathological damage to the lung tissue and the concentration of each inflammatory cytokine. Inflammation can cause oxidative stress, and oxidative stress can also promote the inflammatory reaction. An imbalance between oxidation and the antioxidant system occurred in the VILI model, as indicated by the increase in oxidative stress and oxidation products. After hIL-10 pretreatment, MDA content was significantly decreased and SOD activity was enhanced. This phenomenon may be related to the inhibition of the inflammatory reaction and reduction of oxidative stress. These results suggest that hIL-10 has a protective effect on VILI in a rat model. The mechanism may be related to the regulation of inflammatory pathways, as well as in the balance of the oxidative and antioxidant systems.
This study has several limitations. We did not analyze the arterial blood gases before the model was established, which would provide a more convincing assessment of VILI. Additionally, the extent of lung injury and hemodynamics were not completely consistent across repetitions of the experiment. Finally, our study provides evidence that hIL-10 alleviates VILI and suggests possible mechanisms, but further research on the specific mechanisms and the link between them is needed.
In summary, we find that hIL-10 attenuates VILI and may act through two primary mechanisms, involving the oxidative stress pathway and the inflammatory reaction pathway. These studies suggest a new strategy for VILI treatment and prevention, useful for critical patients and clinical anesthesia.
References
Tsushima, K., and K. Tatsumi. 2014. Noninvasive mechanical ventilation and neutrophil elastase inhibitor: a new potential approaching to acute hypoxemic failure. Journal of Critical Care 29 (6): 1124–1125.
Moraes, L., C.L. Santos, R.S. Santos, F.F. Cruz, F. Saddy, M.M. Morales, V.L. Capelozzi, P.L. Silva, M.G. de Abreu, C.S. Garcia, P. Pelosi, and P.R. Rocco. 2014. Effects of sigh during pressure control and pressure support ventilation in pulmonary and extrapulmonary mild acute lung injury. Critical Care 18 (4): 474.
Saddy, F., Y. Sutherasan, P.R. Rocco, and P. Pelosi. 2014. Ventilator-associated lung injury during assisted mechanical ventilation. Seminars in Respiratory and Critical Care Medicine 35 (4): 409–417.
Carvalho, N.C., A. Güldner, A. Beda, I. Rentzsch, C. Uhlig, S. Dittrich, P.M. Spieth, B. Wiedemann, M. Kasper, T. Koch, T. Richter, P.R. Rocco, P. Pelosi, and M.G. de Abreu. 2014. Higher levels of spontaneous breathing reduce lung injury in experimental moderate acute respiratory distress syndrome. Critical Care Medicine 42 (11): e702–e715.
Slutsky, A.S., and V.M. Ranieri. 2013. Ventilator-induced lung injury. The New England Journal of Medicine 369 (22): 2126–2136.
Meng, F.Y., W. Gao, and Y.N. Ju. 2017. Parecoxib reduced ventilation induced lung injury in acute respiratory distress syndrome. BMC Pharmacology and Toxicology 18 (1): 25.
Severgnini, P., G. Selmo, C. Lanza, A. Chiesa, A. Frigerio, A. Bacuzzi, G. Dionigi, R. Novario, C. Gregoretti, M.G. de Abreu, M.J. Schultz, S. Jaber, E. Futier, M. Chiaranda, and P. Pelosi. 2013. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology 118 (6): 1307–1321.
Futier, E., J.M. Constantin, C. Paugam-Burtz, J. Pascal, M. Eurin, A. Neuschwander, E. Marret, M. Beaussier, C. Gutton, J.Y. Lefrant, B. Allaouchiche, D. Verzilli, M. Leone, A. De Jong, J.E. Bazin, B. Pereira, and S. Jaber. 2013. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. The New England Journal of Medicine 369 (5): 428–437.
Terragni, P., V.M. Ranieri, and L. Brazzi. 2015. Novel approaches to minimize ventilator-induced lung injury. Current Opinion in Critical Care 21 (1): 20–25.
Ouyang, W., S. Rutz, N.K. Crellin, P.A. Valdez, and S.G. Hymowitz. 2011. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annual Review of Immunology 29: 71–109.
Koch, T. 1998. Origin and mediators involved in sepsis and the systemic inflammatory response syndrome. Kidney International. Supplement 64: S66–S69.
Escofier, N., E. Boichot, N. Germain, P.M. Silva, M.A. Martins, and V. Lagente. 1999. Effects of interleukin-10 and modulators of cyclic AMP formation on endotoxin-induced inflammation in rat lung. Fundamental & Clinical Pharmacology 13 (1): 96–101.
Halbertsma, F.J., M. Vaneker, G.J. Scheffer, and J.G. van der Hoeven. 2005. Cytokines and biotrauma in ventilator-induced lung injury: A critical review of the literature. The Netherlands Journal of Medicine 63 (10): 382–392.
Fu, W., P. Mao, R. Zhang, X.Q. Pang, H.Y. Mo, W.Q. He, X.Q. Liu, and Y.M. Li. 2013. Effects of cyclic stretch on expression of cytokines and intercellular adhesion molecule-1 in human pulmonary artery endothelial cell. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 25 (8): 484–488.
Kiss, T., P.L. Silva, R. Huhle, L. Moraes, R.S. Santos, N.S. Felix, C.L. Santos, M.M. Morales, V.L. Capelozzi, M. Kasper, P. Pelosi, M. Gama de Abreu, and P.R. Rocco. 2016. Comparison of different degree of variability in tidal volume to prevent deterioration of respiratory system elastance in experimental acute lung inflammation. British Journal of Anaesthesia 116 (5): 708–715.
Kim, D.H., J.H. Chung, B.S. Son, Y.J. Kim, and S.G. Lee. 2014. Effect of a neutrophil elastase inhibitor on ventilator-induced lung injury in rats. J Thorac Dis 6 (12): 1681–1689.
Sutherasan, Y., M. Vargas, and P. Pelosi. 2014. Protective mechanical ventilation in the non-injured lung: Review and meta-analysis. Critical Care 18 (2): 211.
Serpa Neto, A., L. Nagtzaam, and M.J. Schultz. 2014. Ventilation with lower tidal volumes for critically ill patients without the acute respiratory distress syndrome: A systematic translational review and meta-analysis. Current Opinion in Critical Care 20 (1): 25–32.
Inoue, G. 2000. Effect of interleukin-10 (IL-10) on experimental LPS-induced acute lung injury. Journal of Infection and Chemotherapy 6 (1): 51–60.
Dos Santos, C.C., and A.S. Slutsky. 1985. Invited review: Mechanisms of ventilator-induced lung injury: A perspective. Journal of Applied Physiology 89 (4): 1645–1655.
Santiago VR, Rzezinski AF, Nardelli LM, Silva JD, Garcia CS, Maron-Gutierrez T, Ornellas DS, Morales MM, Capelozzi VL, Marini J, Pelosi P, Rocco PR: Recruitment maneuver in experimental acute lung injury: the role of alveolar collapse and edema. Crit Care Med 38(11): 2207–2214.
Zhang, H.X., S.J. Liu, X.L. Tang, G.L. Duan, X. Ni, X.Y. Zhu, Y.J. Liu, and C.N. Wang. 2016. H2S attenuates LPS-induced acute lung injury by reducing oxidative/nitrative stress and inflammation. Cellular Physiology and Biochemistry 40 (6): 1603–1612.
Papaiahgari, S., A. Yerrapureddy, S.R. Reddy, N.M. Reddy, J.M. Dodd-O, M.T. Crow, D.N. Grigoryev, K. Barnes, R.M. Tuder, M. Yamamoto, T.W. Kensler, S. Biswal, W. Mitzner, P.M. Hassoun, and S.P. Reddy. 2007. Genetic and pharmacologic evidence links oxidative stress to ventilator-induced lung injury in mice. American Journal of Respiratory and Critical Care Medicine 176 (12): 1222–1235.
Abbas, O., and J. Bhawan. 2010. Cutaneous perineural inflammation: A review. Journal of Cutaneous Pathology 37 (12): 1200–1211.
Zhang, J., Z.H. Tong, Z.Q. Qin, B.S. Pang, S.J. Niu, and C. Wang. 2010. Expression of intercellular cell adhesion molecule-1, interleukin-10 and the activation of activator protein-1 in ventilator-induced lung injury in rabbits. Zhonghua Jie He He Hu Xi Za Zhi 33 (8): 587–592.
Funding
This work was supported by the Science and Technology Project of Education Department in Fujian Province (Project No. JK2014017), medical innovation program of Fujian Provincial Health Bureau (2016-CX-43).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chen, J., Lin, J., Luo, H. et al. Effects of Human Interleukin-10 on Ventilator-Associated Lung Injury in Rats. Inflammation 42, 538–547 (2019). https://doi.org/10.1007/s10753-018-0911-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-018-0911-7