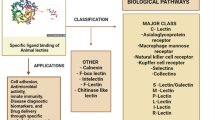
Abstract
The property of lectins to specifically recognize and bind carbohydrates makes them an excellent candidate in biomedical research. Among them are fucose-binding lectins possessing the capacity to bind fucose are taxonomically, evolutionarily and ecologically significant class of lectins that are identified in a wide range of taxa. Purification of fucose-binding lectins dates back to 1967 when L-fucose binding protein from Lotus tetragonolobus was isolated using a dye that contained three α-L-fucopyranosyl residues. Beginning with that, several FBLs were purified from various animals as well as plant sources that were structurally and functionally characterised. This review focuses on fucose-binding lectins, their occurrence and purification with special emphasis on various strategies adopted to purify them followed by molecular and functional characterization. The exclusive ability to recognize and bind to fucose-containing glycans endows these lectins with the potential to act as anti-cancer agents, diagnostic markers and mitogens for immune cells. Though they have been in research focus for more than half a century with their occurrence reported in various taxa, they still need to be explored for their prospective functions to develop them as a biological tool in biomedical research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
From erythrocyte agglutinating proteins to biological recognition molecules that target the saccharine side of the cells, lectins, the carbohydrate-binding proteins of non-immune origin possess a prehistoric legacy. Since the identification of this heterogeneous group of versatile proteins in 1888 by Stillmark [1] from seeds of the castor plant, Ricinus communis, the occurrence of lectins became apparent. The dawn of the 20th century marked a significant leap in lectin research. The inhibition of reactions by sugars envisaged that the phenomenon of haemagglutination could be a result of the interaction of plant proteins with carbohydrates present on the surface of red blood cells, a speculation that formed a major milestone in lectinology. Despite plant lectins, the prevalence of lectins in animals (both vertebrates as well as invertebrates) was also firmly established subsequently. Though there was evidence for the occurrence of haemagglutinins in the body fluids of crustaceans and arachnids, not until the 1960s that, an intricate multicellular vertebrate system was explored. The first mammalian lectin isolated was the galactose-specific asialoglycoprotein receptor by Ashwell and Morell in 1974 [2]. The discovery of macrophage mannan receptors in the early 1980s marked the establishment of the conviction that vertebrate lectins were capable of recognizing specific endogenous ligands [3].
In recent years, there has been a lot of interest in the ability of lectins to discern subtle variations in the carbohydrate structures present on the cell surface that are significant in protein-carbohydrate recognition. Lectins from diverse sources with varied carbohydrate-binding specificities have become increasingly popular as a class of biopharmaceutical products in addition to reagents for drug development and diagnostics [4]. They are known to be excellent anti-proliferative, anti-bacterial, and anti-fungal agents and are discovered to be mitogenic to immune cells. In addition, they are utilised to identify blood types and bacteria, as well as to monitor changes in both normal and cancerous cells [5, 6]. Undoubtedly, their enormous potential is still serving as an impetus for their investigation from various sources, followed by their purification for the intended usage.
Due to their ability to recognise sugars, lectins, particularly fucose-binding lectins have been widely utilised to track alterations in the fucosylation of plasma glycoproteins like anti-freeze proteins (AFP), the most effective and widely used cancer marker for the detection of hepatocellular carcinoma [7]. Numerous serum glycoproteins and cell-surface membrane receptors associated with carcinoma are known to be highly fucosylated serving as an essential diagnostic marker [8]. The fucose-binding lectin from Dicentrarchus labrax (DIFBL) and Aspergillus niger (ANL) are known to possess anti-cancer properties [7, 9]. Fucose-binding lectins are also known to activate B cells since fucosylated epitopes constitute a portion of the B cell receptor (BCR). This is best illustrated by the ability of fucose-binding lectins BambL and LecB from Burkholderia ambifaria and Pseudomonas aeruginosa respectively, to induce B cell activation [10, 11]. These findings are instrumental in showcasing these saccharide-binding molecules way more than a haemagglutinating protein exposing its unravelled potential that can revolutionize modern medicine.
Purification of fucose-binding lectins dates back to 1965 when a dye that contained three α-L-fucopyranosyl residues was used to isolate L-fucose binding protein from Lotus tetragonolobus [12]. Following that, several fucose-binding lectins are isolated from different plant and animal sources, and their structural and functional characteristics were defined. This review focuses on fucose-binding lectins, their occurrence and purification with special emphasis on various strategies adopted to purify FBLs followed by molecular and functional characterization. Despite being the subject of research for decades and having been found in many species, fucose-binding lectins still need to be investigated for their potential activities to be used as biological tools in biomedical research.
Methods
The Science Direct (https://www.sciencedirect.com/) was accessed as a source of literature database and searched for peer-reviewed journal articles with keywords “fucose specific lectin-FSL” or “fucose binding lectins-FBL”. Using these terms as search keywords to find the articles, 6556 research articles were retrieved using the keyword, FSL and 6161 articles were retrieved with the keyword, FBL. As the keyword, FSL retrieved all the articles covering the articles searched under FBL, the FSL keyword was used to display the search results of 100 articles per page. Then the total number of 6556 research articles were filtered to categorize articles pertaining to this topic of review. Finally, the most relevant research articles on the purification, structure and characterization of FSL were filtered as 27 articles between the years 1955 and 1999 and 43 articles between the years 2000 and 2023. The review was mainly concentrated on lectins with fucose specificity from several sources of organisms with emphasis on its detection, various strategies used for purification, and molecular and functional characterizations.
Results
Occurrence and structural features of FBLs
As early as 1967, lectins that bind fucose were identified in the seeds of Ulex europeus and Lotus tetragonolobus [12]. Subsequently, fucose-binding lectins are identified in a broad range of taxa including Proteobacteria, Cyanobacteria, Actinobacteria, Planctomycetes, Bacteroidetes and others [13,14,15,16]. They are also reported to be found in planaria, sea urchins, lobe-finned and ray-finned teleost fishes, horseshoe crabs, insects, Xenopus spp., salamanders and so on.
The biological activities that glycans elicit are typically reliant on the recognition of specific glycan characteristics by the proteins with which they interact. Proteins rely on stereospecific placement of glycan hydroxyl groups at chiral centres, the use of different linkage sites, and extensive branching to achieve specificity in glycan recognition. Generating a three-dimensional understanding of how proteins recognise glycans is thus critical if we are to understand how glycans are synthesised and recognised in the various physiological and pathological processes they regulate. The quest for the structural basis for protein glycan recognition is not new. Protein crystallography has now advanced to a high level of complexity, accounting for the vast bulk of more than 170,000 structures deposited in the Protein Data Bank (PDB), nevertheless, predicting the structure of protein with ligands is still challenging [16].
There have been numerous reports of the three-dimensional structures of lectins that bind fucose. Based on the PDB structures, the fucose-binding lectins whose structure have been determined thus far were gathered, compared and classified by “pfam”, a protein family database which resulted in six different pfam families [17]. The structure of fucose-binding lectins including U. europeus, Anguilla anguilla, and Pseudomonas aeruginosa were solved in complex with fucose [18,19,20,21,22]. All three proteins demonstrated different modes of binding towards the same monosaccharide [18]. The fucose-binding lectin from Pseudomonas aeruginosa (PA-IIL) in complex with fucose demonstrated a tetrameric structure with a nine-stranded, antiparallel-β-sandwich arrangement with two adjacent calcium cations that mediate fucose binding whereas the structure of A. Anguilla agglutinin (AAA) in complex with fucose displayed no similarity to any other fucose-recognising proteins that are structurally characterised [21]. A new lectin fold and amino acid residues in the carbohydrate recognition domain (CRD) that can recognize the non-reducing terminal fucose and sub-terminal sugar units present in an oligosaccharide ligand was found in the structure of AAA/α-L-fucose. This led to the recognition of the novel ‘F-type’ structural fold that folds as a β-barrel with a jelly roll topology with the bulk of the fold consisting of eight major antiparallel β-strands [20, 22]. Structurally, the cleft that binds αL-Fuc is surrounded by five loops formed by connecting β strands on the top face of the barrel whereas the bottom of the barrel is closed by two short anti-parallel strands [20]. Nevertheless, this jelly roll topology is also found to be present in L-type lectins [23]. Three other proteins are also identified as members of this F-type lectin family (FTL) including spGH98 from Streptococcus pneumoniae, MsaFBP32 from striped bass (Morone sazatilis) and lectinolysin from Streptococcus mitis. The CRD of these proteins demonstrated similarity in the overall structure displaying β-barrel structure with a jelly roll topology [17]. Among closely related organisms, the domain organization of the FTLs is found to be consistent with some exceptions of unusual organization demonstrating its versatility [13] It is also found that bacterial FTL domain-containing proteins differ significantly from their eukaryotic counterparts, implying that FTL domains have evolved to suit distinct niches demonstrating the adaptability of this lectin family [15].
The crystal structure of A. aurantia lectin (AAL), a fungal protein in complex with fucose solved using single-wavelength anomalous diffraction (SAD) revealed that each monomer consisted of a six-bladed-β propeller fold and a small antiparallel two-stranded-β sheet [18]. A six-bladed β propeller architecture very similar to that of the previously mentioned A. aurantia is displayed by the fucose-binding lectin from the bacterium Ralstonia solanacearum. The overall fold of putative fucose binding lectin from Burkholderia ambifaria is also a six-bladed-β propeller formed through oligomerization. These bacterial and fungal fucose-binding six-bladed-β propellers are evolutionarily related although these structures do not display any similarities in the sequence [16].
Another important class of lectins are the C-type lectins that exhibit broad carbohydrate specificity. One group of C-type lectins with EPN (Glu-Pro-Asn) motif can bind L-fucose along with other carbohydrates with a calcium ion directly contributing to the binding of the fucose moiety. Six structures of proteins belonging to the L-type lectin family, a well-studied lectin family is also identified with a fucose moiety which consisted of two antiparallel β-sheets. A lectin from Sambucus nigra is known to bind fucose moiety with two identical β- trefoil domains consisting of an arrangement of three subdomains containing four β-strands [25].
Coprinopsis cinerea lectin 2 (CCL2) is a fucoside-binding lectin which also assumes a β-trefoil dimeric structure. Some of the lectins are found to be metal ion-dependent for fucose binding. For instance, both magnesium and calcium are required for carbohydrate binding in the case of fucose-binding lectin from the L-type lectin family. By selecting for precise stereochemistry of hydroxyl groups, calcium ions are known to contribute to lectin’s specificity [16]. In contrast to this, no metal ions are needed for fucose binding to the members of FBL belonging to the R-type lectin family. The structures of fucose-binding lectins are composed mainly of β-strands and the diversity in the structural features arises from the arrangement of β-strands. The relationship between the structure of fucose-binding lectins and glycan recognition preference thus remains obscure.
Purification and characterization of FBLs
Purification of lectins began decades back to decipher the carbohydrate constituents of cell surface glycoproteins with their broad usage in the affinity purification of glycoproteins (Table 1). Goldstein and his colleagues pioneered affinity chromatography for the capture of lectins using crosslinked dextran gels, which is contemplated to be a breakthrough in lectin purification [26]. Specifically, the purification of FBLs though referred to as fucose binding protein can be traced back to 1965 when L-fucose binding protein from L. tetragonolobus demonstrating haemagglutinating (HA) activity against human-O red blood cells was isolated using a dye that contained three α-L-fucopyranosyl residues with a yield of 45 mg per 100 g of seeds [12]. Here, the inhibition of HA activity by L-fucose served as the basis for the use of the dye with three α-L-fucopyranosyl residues. Further, it was found that agglutinating activity was inhibited only by L-fucose and not by D-fucose establishing the stereospecificity exhibited by the lectin. Following this, Blumberg et al. [27] instead adopted the method of affinity chromatography on a column of N-(c-aminopropyl)-P-D-fucopyranosylamine coupled to agarose gel to isolate the lectin. Despite this, insoluble polyleucyl hog A + H blood-group substance was also used for the lectin isolation [28]. Apart from the aforementioned strategies, lectin specific to L-fucose was purified using affinity chromatography from the seeds of U. europeus and L. tetragonolobus. Here, to isolate L-fucose binding lectin by affinity chromatography, divinyl sulfone is utilized to crosslink L-fucose to Sepharose 6B. This newly developed method could bypass multi-step procedures that include organic syntheses for preparing various carbohydrate-containing ligands appropriate for coupling to insoluble matrices that were adopted earlier. A yield of 45 mg/100 g of Ulex seeds and 394 mg/100 g of Lotus seeds were obtained from the affinity column. The yield per 100 g of the protein obtained by affinity chromatography here was much higher than the previously adopted methods [29]. The method according to Fornstedt and Porath [30] was adopted to couple L-fucose to Sepharose 6B instead of Vretblad [31] which utilises epoxy-activated Sepharose 6B. Further, in 1985, Ogata et al. [32] re-examined the isolectin components of fucose binding proteins of L. tetragonolobus. The binding (FBP-M) and the non-binding components (FBP-F) obtained by affinity chromatography on porcine gastric mucin Sepharose differed both in their structure and activity.
With very few exceptions, fucose-binding lectins often agglutinated human-O RBC type and can be attributed to the carbohydrate chemistry of human-O red blood cells with exposed fucose residues on its surface (Table 1). In some cases, instead of native erythrocytes, enzyme-treated erythrocytes were used as indicator RBCs. Papain-treated human-O erythrocytes were used as indicator RBCs for the isolation of Ulva lectin from Ulva lactuca [33]. The indicator RBC used for the isolation of EPL-1 (Enteromorpha prolifera Lectin 1) and EPL-2 (Enteromorpha prolifera Lectin 2) were trypsin-treated rabbit erythrocytes. Enzyme treatment of erythrocytes demonstrated an increased degree of agglutination [34].
Among the L and D enantiomers of fucose, most of the lectins isolated were found to be L-fucose specific (Table 1). The configurational stereochemistry where the L isomers with hydroxy group positioned on the left of the asymmetric carbon and D isomers with hydroxy group positioned on the right side is an important feature that can ascertain the specificity. In the case of AAA, the primary binding site was found to be the 4-OH group of L-fucose [20]. Owing to the high selectivity and high resolution of separation, affinity chromatography was adopted as the method of isolation of fucose-binding lectins. And in most of the cases L-fucose, which inhibited the haemagglutinating activity was coupled with Sepharose. For instance, L-fucose coupled with Sepharose 4B was used to isolate FBP-M (porcine gastric mucin-Sepharose binding protein) and FBP-F (porcine gastric mucin-Sepharose non-binding protein) from L. tetragonolobus. The molecular characterization by polyacrylamide disc electrophoresis revealed the presence of isolectins, with a major lectin fraction having an apparent molecular weight of 35,000 [29]. In some cases, gel filtration was also combined with affinity chromatography. U. lactuca as a source of anti-H lectin capable of agglutinating papain-treated erythrocytes was indicated by Gilboa-Garber et al. in 1988. Later, this lectin was isolated by integrating affinity chromatography on porcine stomach mucin-Sepharose 6B and gel filtration method using Sephadex G-100 column with a yield of 69% and a specific activity of about 34,717 units/mg protein (Table 2). This divalent cation-dependent lectin was found to be stable over a broad range of temperatures and pH. The SDS-PAGE revealed only one single band. Acidic and hydroxyl amino acids were found to be predominant [35].
The haemagglutinin from the culture filtrate of a strain of Streptomyces sp. strongly agglutinated human-O erythrocytes and the activity was inhibited by L-fucose. This agglutinin with an estimated molecular weight of 70,000 was isolated using affinity chromatography and affinity absorbent was prepared according to the method put forth by Matsumoto and Osawa [36]. Accordingly, the high specificity rendered by affinity chromatography formed the impetus for the isolation of fucose-binding lectins from variegate sources (Table 1). A highly thermostable fucose-binding lectin with a molecular weight of about 121,000 consisting of four sub-units from the serum of A. anguilla was isolated by affinity chromatography on A-peptone-Sepharose column integrated with affinity chromatography using α-L-fucose-agarose (Table 2). Further, it was characterized using isoelectric focusing which identified 17–20 bands between pH 5.5 and 6.2. Interestingly, the fucose-binding lectin isolated was found to be more thermostable than the MBL (mannan-binding lectin) that was isolated from the same serum [37]. Later studies on AAA/α-L-fucose demonstrated the presence of a novel F-type fold. Fucose-binding lectins are also found to be prevalent among different fungal species including Aleuria aurantia and Rhizopus stolonifera and so on [38, 39]. The fucose-binding lectin from A. aurantia was isolated using the H-active glycopeptide of desialyzed porcine sub-maxillary mucine coupled to Sepharose 4B and characterized using PAGE which revealed a dimer with a molecular weight of 72,000 [38]. Besides, MsaFBP32, a lectin isolated using affinity chromatography from the liver extracts of M. saxatilis did not possess signature motifs of the CTLs or any lectin families reported at that time but demonstrated two 140-amino acid tandemly arrayed domains on sequencing. A crucial outcome of the study led to the recognition of a novel lectin type (F-type lectins). Apart from the above-mentioned sources, fucose-binding lectins were isolated from various sources with affinity chromatography as the major method of isolation (Table 1). Ion exchange chromatography was employed for the isolation of fucose-binding lectin from the mycelial mats of Aspergillus fumigatus [40]. The source, method of isolation, column/matrix used and elution strategy adopted for the purification of reported FBLs are provided in Table 1. Most recently, Ca ++ - independent fucose binding protein was isolated from the Antarctic fish Trematomus bernacchii by affinity chromatography. The molecular weight was found to be 32 and 30 kDa under reducing and non-reducing conditions, respectively (Table 2). The two CRD-FBLs displayed by the lectin further authenticated it as a member of the fucose-binding lectins family [41].
FBLs: an in-silico approach
With the advent of bioinformatics in the 1960s, computational tools that could analyse and process biological information became popular. Through in silico experiments, FBLs from diverse sources of organisms were characterized functionally. The computational models and simulations produced were used to make predictions regarding the possible interactions of FBLs. The interaction of U. europaeus isolectin I specific for fucose-containing oligosaccharide was studied using homology modelling and molecular docking [42]. Legume lectins including Lathyrus ochrus, Lens culinaris and Pisum sativum specific for mannose and glucose showed higher affinity for fucosylated glycan than for glycans that are without fucose whereas ConA was not able to differentiate between the two. The ability of the former to discriminate between the two glycans is attributed to the presence of a secondary binding site [43].
The mode by which fucose binding legume lectins viz., UEA 1 (U. europeus agglutinin 1) and LTA.
(L. tetragonolobus) recognize L-fucose was studied with the help of structure-based sequence alignment of the different loop regions of legume lectins with distinct monosaccharide specificities and other biophysical data. Four loops (A, B, C & D) form the combining site of legume lectins [44,45,46]. Aspartate (loop A), glycine or arginine (loop B) and asparagine (loop C) play a crucial role in the recognition of carbohydrates. In addition to these, a residue with a backbone NH group is found to hydrogen bond with the hydroxyl group of the carbohydrate. The conserved triad of residues including asparagine 137, glycine 105, and aspartate 87 was found to contribute invariantly to the binding of L-fucose with all the legume lectins [46]. From the crystal structure of the complex of AAA with α-L-fucose, the presence of a novel fucose recognition fold, a characteristic lectin fold (β-barrel with jelly roll topology) of the entire lectin family was determined at 1.9 Å resolution [20]. Several in silico studies were based on the search for fucose-binding domains from diverse sources. MsaFBP32, a fucose-binding lectin, isolated from M. saxatilis comprised two tandem domains with an F-type sequence motif [14]. Modelling of CRDs from MsaFBP32 to the crystalline model of AAA suggested the possibility of gene duplication in the bass and stickleback lineage [47, 48]. Similarly, 3-D structure prediction and structure analysis of the interaction between the pentraxin of Xenopus laevis and α-L-fucose through homology modelling and molecular docking revealed that the fucose-binding triad (His52, Arg79 and Arg86) of A. anguilla agglutinin was found to be conserved in the pentraxin of X. laevis [49]. In Xenopus spp., this lectin is composed of triplicate or quintuple tandem F-type domains [50].
Fucose-binding domains similar to the one present in the bacterial fucolectin of Pseudomonas aeruginosa were identified in three hypothetical proteins from bacteria (Chromobacterium violaceum, Burkholderia cepacia and Photorhabdus luminiscens) and in another protein from archaea (Methanopyrus kandleri) with conserved calcium binding loops [51]. Calcium appears to play a pivotal role in structural stabilization rather than in direct carbohydrate interactions unlike C-type lectins [50]. These phylogenetically widespread FLDs were identified in diverse domain organizations including prokaryotes, viruses and eukaryotes with a report of 437 FLD sequence clusters [15]. The three-dimensional structures of fucose-binding lectins have been reported and deposited in the Protein Data Bank (PDB). Studies on the recognition of fucosylated human blood group determinants by Burkholderia ambifaria lectin (BambL) revealed that Arg15, Glu26 and Trp79 are involved in fucose monosaccharide recognition through hydrogen bonding interactions. The outcome could probably help in the development of fucomimetic molecules that could bind to BambL [52].
Fucose forms a key component of oligosaccharides that are crucial in many biological interactions that include pathogenic bacterial infection of host cells, cell adhesion and glycosylated binding proteins. The S protein of SARS-CoV2 is found to be a highly glycosylated structure. The S protein from ancestral and Omicron strains was found to bind with Pholiota squarrosa lectin (PhoSL) a 40 amino acid chemically synthesised peptide and resulted in the inhibition of infection at sub-micromolecular level demonstrated PhoSL as a potent broad-spectrum inhibitor of infection [53].
FBLs: as biological recognition molecules
The ‘unusual sugar’, fucose (6-deoxy hexose) is ubiquitously present in diverse glycoproteins and glycolipids that are produced by mammalian cells. They are found to be present in 7.2% of oligosaccharides as a common component of glycan modifications that are seen in proteins and lipids. Many serum glycoproteins and receptors found on the cell surface are highly fucosylated and serve as an important diagnostic marker in carcinoma (Fig. 1). This can be attributed to the differential expression of fucosyltransferases in transformed cells leading to the increased fucosylation patterns characteristic of cancer cells [7, 54, 55]. For instance, alpha-fetoprotein (AFP)-L3 fraction, a fucosylated AFP is used as a tumour marker for hepatocellular carcinoma (HCC) clinically [56]. Thus, fucose-binding lectins that could identify aberrant or altered glycosylation patterns are being extensively studied for their potential clinical applications [57].
Schematic illustration of FBL (fucose binding lectin) induced MAPK (mitogen-activated protein kinase) signalling pathway FBL binds to the B cell receptor (fucose residues on the B cell receptor) on the B cell membrane. This binding activates the tyrosine-based activation motifs (ITAMs) found in the CD79, a transmembrane protein that complexes with B cell receptor (BCR). This results in the activation of SYK (spleen tyrosine kinase). This in turn activates the signalosome that comprises Bruton’s tyrosine kinase (BTK), phospholipase Cγ2 (PLCγ2), VAV (a group of tyrosine phosphorylation-regulated signal transduction molecules) and the growth factor receptor bound protein-2 (GRB2). This is followed by the activation of genes of RAS (rat sarcoma) which further activates RAF (rapidly accelerated fibrosarcoma). The phosphorylation and activation of RAF is followed by phosphorylation of MEK (mitogen-activated protein kinase), which in turn phosphorylates ERK (extracellular signal-regulated kinase). With numerous targets inside the nucleus, this transmits the mitogen signals required for cell proliferation
A novel recombinant A. aurantia lectin with core fucose binding was developed that could identify various glycoprotein markers associated with hepatocellular carcinoma whose native form possesses five binding pockets that can bind to fucose linkages, including α-1,2, α -1,3, α -1,4 and core α-1,6-fucose [18, 58]. Similarly, an L-fucose-specific lectin from the pathogenic fungus, A. niger (ANL) displayed strong binding to human pancreatic adenocarcinoma cells (PANC-1) and could be blocked by L-fucose. It also demonstrated a time and dose-dependent inhibitory effect on PANC-1 cells [59]. This lectin was also able to detect tumour-associated glycoproteins linked to human hepatocellular and colon cancer cells. In addition to its role as a diagnostic agent, ANL was also found to increase the apoptosis level in both hepatocellular and colon cancer cell lines indicative of its anti-cancer potential [7]. This effect could be attributed to the capability of ANL to recognize fucosylated glycoproteins associated with various types of carcinomas. Another important lectin with L-fucose binding from Fenneropenaeus indicus manifested an antiproliferative effect against breast cancer cells [60]. In addition to its inhibitory effects on cancer cells, ANL also possessed potent antibacterial activity against Klebsiella pneumoniae, Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli. Fucose-binding lectins from teleost fishes known to function as opsonins, played a crucial role in the clearance of microbial pathogens. This is evidenced by the fucose binding lectin from Antarctic teleost fish Trematomus bernacchii with bacterial agglutination activity underpinning its role in host-pathogen interactions [41]. A novel fucose-specific lectin from the fruiting body of Coprinopsis cinerea was found to exhibit nematotoxic activity as a part of their defence of fruiting bodies of agaricomycete against nematodes (Table 3).
In addition to the aforementioned functional characterizations, the fucose-binding lectins can also function as super stimulatory antigens (Fig. 2). The capacity of lectins to bind fucose (fucosylated epitopes in B cells) is known to initiate polyclonal activation of B cells. The bacterial lectins, BambL isolated from B. ambifaria and LecB isolated from P. aeruginosa are known to activate B cells (Table 3) in a carbohydrate-dependent manner [11]. Doubtlessly, the ability of these lectins to act as mitogens that can stimulate B cells to proliferate has a significant impact on human physiology.
Schematic illustration of the mechanism of FBL (fucose binding lectin) induced apoptosis or autophagy in cancer cells In lectin-induced apoptosis, the binding of FBL with the fucosylated receptor on the cell surface triggered the loss of transmembrane potential of the cell, disruption of the mitochondrial membrane and release of cytochrome-c through the mitochondrial pathway. It is hypothesized that fucose-binding lectins which are soluble pattern recognition molecules able to recognize mitochondria through the lectin-mitochondrial pathway once internalized. This is speculated to cause a decrease in the mitochondrial membrane potential and an increase in cytoplasmic calcium release resulting in mitochondrial bioenergetic imbalance leading to caspase activation and apoptosis
Conclusion
Among the different monosaccharides relevant for glycan motifs, fucose in particular is found as terminal sugar frequently and is known to be involved in interactions that are pertinent in biological systems. For instance, in the pathological conditions of cancer and inflammation, variations in the expression of fucosylated oligosaccharides are observed with several types of biomarkers associated with them. Owing to the ability of fucose-binding lectins to discern the overexpressed fucosylated epitopes (glycan-based cancer biomarkers) present on the cancer cell surfaces, they are extensively studied for their anti-cancer potential as well as for diagnostic purposes. In addition to this, some of the fucose-binding lectins reported are found to be involved in the polyclonal activation of B cells that are associated with their sole capacity to bind fucose as the receptors of immune cells are found to be highly glycosylated. The property of these lectins to recognize and specifically bind fucose-containing glycans makes them an excellent tool in biomedical research as chemotherapeutic agents, diagnostic agents, mitogens and drug delivery agents. Though FBLs have been in research for more than several decades, most of the identified fucose-binding lectins across various taxonomic groups are still not functionally characterized. The exclusive property of FBLs to activate immune cells, to be cytotoxic to cancer cells, and to act as a diagnostic tool in identifying cancer cells through various mechanisms needs to be explored further. This review suggests that fucose-binding lectins represent a greater resource to prospect for several therapeutic applications and the enormous potential they endow to develop them as biological tools in biomedical research.
Data Availability
The authors declare that all the data supporting this study are available in the article itself.
References
Stillmark H University of Dorpat; Dorpat, Estonia: 1888. Über Ricin ein giftiges Ferment aus den Samen von Ricinus communis L und einige anderen Euphorbiaceen. MD Thesis
Ashwell G, Morell AG (1974) The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol 41:99–128. https://doi.org/10.1002/9780470122860.ch3
Varki A, Cummings R, Esko JD, Freeze HF, Hart GW, Marth JD (1999) Essentials of glycobiology. Cold Spring Harbor Laboratory Press, Plainview, New York
Nascimento KS, Cunha AI, Nascimento KS, Cavada BS, Azevedo AM (2012) Aires-Barros, an overview of lectins purification strategies. J Mol Recognit 25(11):527–541
Sharon N, Lis H (2004) History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology 14(11). https://doi.org/10.1093/glycob/cwh122. 53R-62R
Tsaneva M, Van Damme EJ (2020) 130 years of plant lectin research. Glycoconj J 37:533–551
Jagadeesh N, Belur S, Campbell BJ, Inamdar SR (2021) The fucose-specific lectin ANL from Aspergillus niger possesses anti‐cancer activity by inducing the intrinsic apoptosis pathway in hepatocellular and colon cancer cells. Cell Biochem Funct 39(3):401–412. https://doi.org/10.1002/cbf.3605
Hutchinson WL, Du MQ, Johnson PJ, Williams R (1991) Fucosyltransferases: differential plasma and tissue alterations in hepatocellular carcinoma and cirrhosis. Hepatology 13:683–688
Yau T, Dan X, Ng CCW, Ng TB (2015) Lectins with potential for anti-cancer therapy. Molecules 20(3):3791–3810
Frensch M, Jäger C, Müller PF, Tadić A, Wilhelm I, Wehrum S, Römer W (2021) Bacterial lectin BambL acts as a B cell superantigen. Cell Mol Life Sci 78(24):8165–8186
Wilhelm I, Levit-Zerdoun E, Jakob J, Villringer S, Frensch M, Übelhart R, Römer W (2019) Carbohydrate-dependent B cell activation by fucose-binding bacterial lectins. Sci Signal 12(571):eaao7194
Yariv J, Kalb AJ, Katchalski E (1967) Isolation of an L-fucose binding protein from Lotus tetragonolobus seed. Nature 215(5103):890–891. https://doi.org/10.1038/215890a0
Odom-Crespo EW (2004) F-type lectins: biochemical, genetic, and topological characterization of a novel lectin family in lower vertebrates. University of Maryland, College Park
Odom EW, Vasta GR (2006) Characterization of a binary tandem domain F-type lectin from striped bass (Morone saxatilis). J Biol Chem 281(3):1698–1713. https://doi.org/10.1074/jbc.M507652200
Bishnoi R, Khatri I, Subramanian S, Ramya TNC (2015) Prevalence of the F-type lectin domain. Glycobiology 25(8):888–901. https://doi.org/10.1093/glycob/cwv029
Angulo J, Zimmer J, Imberty A, Prestegard J (2022) Essentials of glycobiology. Structural biology of glycan recognition. Cold Spring Harbor Laboratory Press, Plainview, New York
Makyio H, Kato R (2016) Classification and comparison of fucose-binding lectins based on their structures. Trends Glycosci Glycotechnol 28(160). https://doi.org/10.4052/tigg.1429.1E. E25-E37
Wimmerova M, Mitchell E, Sanchez JF, Gautier C, Imberty A (2003) Crystal structure of fungal lectin: six-bladed β-propeller fold and novel fucose recognition mode for Aleuria aurantia lectin. J Biol Chem 278(29):27059–27067
Audette GF, Olson DJ, Ross AR, Quail JW, Delbaere LT (2002) Examination of the structural basis for O (H) blood group specificity by Ulex europaeus Lectin I. Can J Chem 80(8):1010–1021
Bianchet MA, Odom EW, Vasta GR, Amzel LM (2002) A novel fucose recognition fold involved in innate immunity. Nat Struct boil 9(8):628–634
Mitchell E, Houles C, Sudakevitz D, Wimmerova M, Gautier C, Pérez S, Imberty A (2002) Structural basis for oligosaccharide-mediated adhesion of Pseudomonas aeruginosa in the lungs of cystic fibrosis patients. Nat Struct boil 9(12):918–921
Vasta GR, Amzel LM, Bianchet MA, Cammarata M, Feng C, Saito K (2017) F-type lectins: a highly diversified family of fucose-binding proteins with a unique sequence motif and structural fold, involved in self/non-self-recognition. Front Immunol 8:1648. https://doi.org/10.3389/fimmu.2017.01648
Cummings RD, Etzler ME, Ramya TNC, Kato K, Rabinovich GA, Surolia A (2022) Essentials of glycobiology. Structural biology of glycan recognition. Cold Spring Harbor Laboratory Press, Plainview, New York
Barre A, Bourne Y, Van Damme EJ, Rougé P (2019) Overview of the structure–function relationships of mannose-specific lectins from plants, algae and fungi. Int J Mol Sci 20(2):254
Moradi A, El-Shetehy M, Gamir J, Austerlitz T, Dahlin P, Wieczorek K, Mauch F (2021) Expression of a fungal lectin in Arabidopsis enhances plant growth and resistance toward microbial pathogens and a plant-parasitic nematode, front. Plant Sci 12:657451
Agrawal BB, Goldstein IJ (1965) Specific binding of concanavalin A to cross-linked dextran gels. Biochem J 96(3):23 C. https://doi.org/10.1042/bj0960023c
Blumberg S, Hildesheim J, Yariv J, Wilson KJ (1972) The use of I-amino-L-fucose bound to sepharose in the isolation of L-fucose-binding proteins. Biochim Biophys Acta - Gen Subj 264(1):171–176. https://doi.org/10.1016/0304-4165(72)90128-6
Pereira ME, Kabat EA (1974) Specificity of purified hemagglutinin (lectin) from Lotus tetragonolobus, Biochemistry.13(15. 3184–3192. https://doi.org/10.1021/bi00712a029
Allen HJ, Johnson EA (1977) A simple procedure for the isolation of L-fucose-binding lectins from Ulex europaeus and Lotus tetragonolobus. Carbohydr Res 58(2):253–265. https://doi.org/10.1016/S0008-6215(00)84352-9
Fornstedt N, Porath J (1975) Characterization studies on a new lectin found in seeds of Vicia ervilia. FEBS Lett 57(2):187–191
Vretblad P (1976) Purification of lectins by biospecific affinity chromatography, Biochim. Biophys. Acta - Protein Structure 434(1):169–176. https://doi.org/10.1016/0005-2795(76)90047-7
Ogata SI, Kamachi Y, Arita Y, Sato M, Muramatsu T (1985) A re-examination of the isolectin components of the fucose-binding proteins of Lotus tetragonolobus. Carbohydr Res 144(2):297–304. https://doi.org/10.1016/S0008-6215(00)90677-3
Gilboa-Garber N, Citronbaum R, Levene C, Sela R (1988) H blood group detection by the L-fucose binding lectin of the green marine alga Ulva lactuca. Dev Comp Immunol 12(4):695–705. https://doi.org/10.1016/0145-305X(88)90045-6
Ambrosio AL, Sanz L, Sánchez EI, Wolfenstein-Todel C, Calvete JJ (2003) Isolation of two novel mannan-and L-fucose-binding lectins from the green alga Enteromorpha prolifera: biochemical characterization of EPL-2. Arch Biochem Biophys 415(2):245–250. https://doi.org/10.1016/S0003-9861(03)00232-7
Sampaio AH, Rogers DJ, Barwell CJ (1998) Isolation and characterization of the lectin from the green marine alga Ulva lactuca, L. Bot Mar 41:427–433. https://doi.org/10.1515/botm.1998.41.1-6.427
Matsumoto I, Osawa T (1972) The specific purification of various carbohydrate-binding hemagglutinins. Biochem Biophys Res Commun 46(5):1810–1815. https://doi.org/10.1016/0006-291X(72)90055-1
Gercken J, Renwrantz L (1994) A new mannan-binding lectin from the serum of the eel (Anguilla anguilla L.): isolation, characterization and comparison with the fucose-specific serum lectin. Comp Biochem Physiol - B Biochem 108(4):449–461. https://doi.org/10.1016/0305-0491(94)90098-1
Fukumori F, Takeuchi N, Hagiwara T, Ito K, Kochibe N, Kobata A, Nagata Y (1989) Cloning and expression of a functional fucose-specific lectin from an orange peel mushroom, Aleuria aurantia. FEBS Lett 250(2):153–156. https://doi.org/10.1016/0014-5793(89)807094
Oda Y, Senaha T, Matsuno Y, Nakajima K, Naka R, Kinoshita M, Honda E, Furuta I, Kakehi K (2003) A new fungal lectin recognizing α (1–6)-linked fucose in the N-glycan. J Biol Chem 278(34):32439–32447. https://doi.org/10.1074/jbc.M305181200
Kuboi S, Ishimaru T, Tamada S, Bernard EM, Perlin DS, Armstrong D (2013) (Molecular characterization of AfuFleA, an L-fucose-specific lectin from Aspergillus fumigatus. J Infect Chemother 19(6):1021–1028. https://doi.org/10.1007/s10156-013-0614-9
Dara M, Giulianini PG, Manfrin C, Parisi MG, Parrinello D, La Corte C, Vasta GR, Cammarata M (2021) F-type lectin from serum of the Antarctic teleost fish Trematomus bernacchii (Boulenger, 1902): purification, structural characterization, and bacterial agglutinating activity. Comp Biochem Physiol - B Biochem Mol Biol 256:110633. https://doi.org/10.1016/j.cbpb.2021.110633
Gohier A, Espinosa JF, Jimenez-Barbero J, Carrupt PA, Pérez S, Imberty A (1996) Knowledge-based modeling of a legume lectin and docking of the carbohydrate ligand: the Ulex europaeus lectin I and its interaction with fucose. J Mol Graph 14(6):322–327. https://doi.org/10.1016/S0263-7855(97)00010-6
Sokolowski T, Peters T, Pérez S, Imberty A (1997) Conformational analysis of biantennary glycans and molecular modeling of their complexes with lentil lectin. J Mol Graph Model 15(1):37–42. https://doi.org/10.1016/S1093-3263(97)00011-9
Young NM, Oomen RP (1992) Analysis of sequence variation among legume lectins: a ring of hypervariable residues forms the perimeter of the carbohydrate-binding site. J Mol Biol 228(3):924–934. https://doi.org/10.1016/0022-2836(92)90875-K
Sharma V, Surolia A (1997) Analyses of carbohydrate recognition by legume lectins: size of the combining site loops and their primary specificity. J Mol Biol 267(2):433–445. https://doi.org/10.1006/jmbi.1996.0863
Thomas CJ, Surolia A (2000) Mode of molecular recognition of L-fucose by fucose-binding legume lectins. Biochem Biophys Res Commun 268(2):262–267. https://doi.org/10.1006/bbrc.2000.2110
Kostlánová N, Mitchell EP, Lortat-Jacob H, Oscarson S, Lahmann M, Gilboa-Garber N, Chambat G, Wimmerová M, Imberty A (2005) The fucose-binding lectin from Ralstonia solanacearum: a new type of β-propeller architecture formed by oligomerization and interacting with fucoside, fucosyllactose, and plant xyloglucan. J Biol Chem 280(30):27839–27849. https://doi.org/10.1074/jbc.M505184200
Bianchet MA, Odom EW, Vasta GR, Mario L (2005) Crystal structure of Moronis Saxatilis F-lectin. Acta Crystallogr A-Found Adv 61:C269–C269
Majumder S, Roy A, Mandal C (2003) Prediction of 3-D structures of fucose-binding proteins and structural analysis of their interaction with ligands. Glycoconj J 20:545–550. https://doi.org/10.1023/B:GLYC.0000043291.42999.98
Vasta GR, Odom EW, Bianchet MA, Amzel LM, Saito K (2008) H. Ahmed, 31 F-Type lectins: a New Family of Recognition factors. Anim Lectins 465
Majumder S, Patra M, Mandal C (2006) Search for fucose binding domains in recently sequenced hypothetical proteins using molecular modeling techniques and structural analysis. Glycoconj J 23:251–257. https://doi.org/10.1007/s10719-006-7930-6
Dingjan T, Imberty A, Pérez S, Yuriev E, Ramsland PA (2017) Molecular simulations of carbohydrates with a fucose-binding Burkholderia ambifaria lectin suggest modulation by surface residues outside the fucose-binding pocket. Front Pharmacol 8:393. https://doi.org/10.3389/fphar.2017.00393
Yamasaki K, Adachi N, Ngwe Tun MM, Ikeda A, Moriya T, Kawasaki M, Yamasaki T, Kubota T, Nagashima I, Shimizu H, Tateno H, Morita K (2023) Core fucose-specific pholiota squarrosa lectin (PhoSL) as a potent broad‐spectrum inhibitor of SARS‐CoV‐2 infection. FEBS J 290(2):412–427. https://doi.org/10.1111/febs.16599
Fuster MM, Esko JD (2005) The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer 5(7):526–542. https://doi.org/10.1038/nrc1649
Miyoshi E, Moriwaki K, Terao N, Tan CC, Terao M, Nakagawa T, Matsumoto H, Shinzaki S, Kamada Y (2012) Fucosylation is a promising target for cancer diagnosis and therapy. Biomolecules 2(1):34–45. https://doi.org/10.3390/biom2010034
Moriwaki K, Miyoshi E (2010) Fucosylation and gastrointestinal cancer. World J Hepatol 2(4):151. https://doi.org/10.4254/wjh.v2.i4.151
Ohtsubo K, Marth JD (2006) Glycosylation in cellular mechanisms of health and disease. Cell 126(5):855–867. https://doi.org/10.1016/j.cell.2006.08.019
Norton P, Comunale MA, Herrera H, Wang M, Houser J, Wimmerova M, Romano PR, Mehta A (2016) Development and application of a novel recombinant Aleuria aurantia lectin with enhanced core fucose binding for identification of glycoprotein biomarkers of hepatocellular carcinoma. Proteomics 16(24):3126–3136. https://doi.org/10.1002/pmic.201600064
Jagadeesh N, Belur S, Hegde P, Kamalanathan AS, Swamy BM, Inamd SR (2019) An L-fucose specific lectin from Aspergillus niger isolated from mycotic keratitis patient and its interaction with human pancreatic adenocarcinoma PANC-1 cells. Int J Biol Macromol 134:487–497. https://doi.org/10.1016/j.ijbiomac.2019.04.192
Chatterjee B, Ghosh K, Yadav N, Kanade SR (2017) A novel L-fucose-binding lectin from Fenneropenaeus indicus induced cytotoxicity in breast cancer cells. J Biochem 161(1):87–97. https://doi.org/10.1093/jb/mvw057
Kochibe N, Furukawa K (1980) Purification and properties of a novel fucose-specific hemagglutinin of Aleuria aurantia. Biochemistry 19(13):2841–2846. https://doi.org/10.1021/bi00554a004
Honda S, Kashiwagi M, Miyamoto K, Takei Y (2000) Hirose Multiplicity, structures, and endocrine and exocrine natures of eel fucose-binding lectins. J Biol Chem 275(42):33151–33157. https://doi.org/10.1074/jbc.M002337200
Töpfer-Petersen E, Henschen A (1987) Acrosin shows zona and fucose binding, novel properties for a serine proteinase. FEBS Lett 226(1):38–42. https://doi.org/10.1016/0014-5793(87)80546-X
Töpfer-Petersen F, Friess AE, Nguyen H, Schill WB (1985) Evidence for a fucose-binding protein in boar spermatozoa. Histochemistry 83:139–145
do Nascimento AS, Serna S, Beloqui A, Arda A, Sampaio AH, Walcher J, Ott D, Unverzagt C, Reichardt NC, Jimenez-Barbero J, Nascimento KS, Imberty A, Cavada BS, Varrot A (2015) Algal lectin binding to core (α1–6) fucosylated N-glycans: structural basis for specificity and production of recombinant protein. Glycobiology 25(6):607–616. https://doi.org/10.1093/glycob/cwv002
Mansour MH, Abdul-Salam F (2009) Characterization of fucose-binding lectins in rock-and mud-dwelling snails inhabiting Kuwait Bay. Immunobiology 214(1):77–85. https://doi.org/10.1016/j.imbio.2008.03.002
Salerno G, Parisi MG, Parrinello D, Benenati G, Vizzini A, Vazzana M, Vasta GR, Cammarata M (2009) F-type lectin from the sea bass (Dicentrarchus labrax): purification, cDNA cloning, tissue expression and localization, and opsonic activity. Fish Shellfish Immunol 27(2):143–153. https://doi.org/10.1016/j.fsi.2009.01.004
Kalb AJ (1968) The separation of three L-fucose-binding proteins of Lotus tetragonolobus, Biochim. Biophys. Acta - Protein Structure 168(3):532–536. https://doi.org/10.1016/0005-2795(68)90186-4
Leu RW, Herriott MJ, Worley DS (1985) Characterization of Lotus tetragonolobus fucolectin components for differences in hemagglutinating and macrophage activating activities. Immunobiology 169(3):250–262. https://doi.org/10.1016/S0171-2985(85)80037-1
Nakamura O, Wada Y, Namai F, Saito E, Araki K, Yamamoto A, Tsutsui S (2009) A novel C1q family member with fucose-binding activity from surfperch, Neoditrema ransonnetii. Fish Shellfish Immunol 27(6):714–720. https://doi.org/10.1016/j.fsi.2009.09.010. Perciformes, Embiotocidae
Jensen LE, Thiel S, Petersen TE, Jensenius JC (1997) A rainbow trout lectin with multimeric structure comp. Biochem Physiol - B Biochem Mol Biol 116(4):385–390. https://doi.org/10.1016/S0305-0491(96)00273-8
Argayosa AM, Lee YC (2009) Identification of L-fucose-binding proteins from the Nile tilapia (Oreochromis niloticus L.) serum. Fish Shellfish Immunol 27(3):478–485. https://doi.org/10.1016/j.fsi.2009.06.012
Kobayashi Y, Tateno H, Dohra H, Moriwaki K, Miyoshi E, Hirabayashi J, Kawagishi H (2012) A novel core fucose-specific lectin from the mushroom pholiota squarrosa. J Biol Chem 287(41):33973–33982. https://doi.org/10.1074/jbc.M111.327692
Kumar A, Sýkorová P, Demo G, Dobeš P, Hyršl P, Wimmerová M (2016) A novel fucose-binding lectin from Photorhabdus luminescens (PLL) with an unusual heptabladed β-propeller tetrameric structure. J Biol Chem 291(48):25032–25049. https://doi.org/10.1074/jbc.M115.693473
Cammarata M, Benenati G, Odom EW, Salerno G, Vizzini A, Vasta GR, Parrinello N (2007) Isolation and characterization of a fish F-type lectin from gilt head bream (Sparus aurata) serum, Biochim. Biophys Acta Gen Subj 1770(1):150–155. https://doi.org/10.1016/j.bbagen.2006.09.015
Kameyama T, Oishi K, Aida K (1979) Stereochemical structure recognized by the L-fucose-specific hemagglutinin produced by Streptomyces sp, Biochim. Biophys. Acta - Gen Subj 587(3):407–414. https://doi.org/10.1016/0304-4165(79)90444-6
Saito T, Hatada M, Iwanaga S, Kawabata SI (1997) A newly identified horseshoe crab lectin with binding specificity to O-antigen of bacterial lipopolysaccharides. J Biol Chem 272(49):30703–30708. https://doi.org/10.1074/jbc.272.49.30703
Bleuler-Martinez S, Varrot A, Olieric V, Schubert M, Vogt E, Fetz C, Wohlschlager T, Plaza DF, Wälti M, Duport Y, Capitani G, Aebi M, Künzler M (2022) Structure–function relationship of a novel fucoside-binding fruiting body lectin from Coprinopsis cinerea exhibiting nematotoxic activity. Glycobiology 32(7):600–615. https://doi.org/10.1093/glycob/cwac020
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All the authors equally contributed to the accomplishment of this work.
Corresponding author
Ethics declarations
Declaration of competing interest
The authors declare that they have no known competing interests that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nivetha, R., Meenakumari, M., Peroor Mahi Dev, A. et al. Fucose-binding lectins: purification, characterization and potential biomedical applications. Mol Biol Rep 50, 10589–10603 (2023). https://doi.org/10.1007/s11033-023-08896-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08896-2