Abstract
Lectins are glycoproteins that are capable of binding reversibly and specifically to sugar moieties, especially the carbohydrate content of glycoproteins and glycoconjugates. Lectin could be a membrane or soluble PRR that has a pivotal role in recognition and eradication of invading microorganisms. Lectins composed of many proteins that may particularly recognize and bind sugars such as lactose, mannose, galactose, N-acetyl galactosamine, and N-acetylglucosamine, resulting in non-covalent interactions. Lectin–carbohydrate interaction is a very important part of immunity which is not only accustomed to detect pathogens, but also employed in several alternative biological processes such as cell adhesion, agglutination, opsonization, complement activation, and phagocytosis. The lectins are classified in different ways. Based on their affinity towards the monosaccharides, lectins have been grouped into five such as Galactose Binding Lectins, Fucose binding lectins, Mannose-Binding Lectins, Sialic acid-binding lectins, and N-acetyl glucosamine binding lectins. On the basis of sources, they can be classified as plant derived lectins, invertebrate lectins and vertebrate lectins.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Lectins are immunoproteins that can bind to carbohydrate moieties, especially to the sugar content of glycoproteins and glycoconjugates (Dong et al. 2004). They constitute a bunch of carbohydrate binding protein molecules having an ability to interact with specific sugar moieties and conglomerate cells by interacting to glycoconjugates on the cell membranes (Saraiva et al. 2011). Lectin could be a membrane or soluble PRR that plays a pivotal role in recognition and eradication of microorganism entering into the living organism (Yu and Kanost 2004). Ricin, purified from the seeds of Ricinus communis and acacia from the Abrus precatorius extract were the first lectins isolated from plants. They are heterodimeric proteins in which two polypeptide chains are linked together by means of disulfide bonds and are ribosome inactivating proteins (RIP). Both of them have an ability to agglutinate blood cells (Olsnes 2004; Bayer et al. 2012).
The term “Lectin” (derived from the Latin “lectus,” meaning “selected”) was first introduced by Boyd and Shapleigh to indicate the category of proteins that have selective characteristics when interacting with carbohydrates. The term has been summarized by Sharon and Lis 1972 to incorporate all proteins from different sources, non-immune sources, and capable of binding carbohydrates, irrespective of whether or not they are peculiar for people red blood cells (Santos et al. 2013). As a non-immune source (glyco) protein, they are reversibly interacts with carbohydrates, using their binding sites, precipitates animal cells, plant cells or glycoconjugates.
Lectins confer with many proteins that may particularly recognize and bind with sugars such as galactose, mannose, lactose, and N-acetyl galactosamine, resulting in non-covalent interactions (Soanes et al. 2004). Lectin–carbohydrate interaction is a very important part of immunity which is accustomed to detect pathogens. Moreover, they can also be employed in several alternative biological activities such as opsonization, phagocytosis, cell adhesion, agglutination, and complement activation (Vasta et al. 2011; Osorio et al. 2011). Lectins are found in wide range of life forms belonging to prokaryotes- like bacteria, fungi, mycoplasma and eukaryotes- like animals, plants and even in viruses (Lakhtin et al. 2011). Lectins from the animal sources typically have a minimum of one carbohydrate recognition domain (CRD) that can specifically interact to varied carbohydrate units found on the cell surfaces of pathogenic microorganisms (Ng et al. 2015).
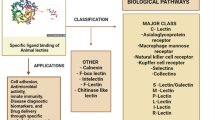
Lectins are categorized mainly into five specific groups, in accordance with their affinity towards the monosaccharide: N-acetylneuraminic acid, galactose/N-acetylgalactosamine, mannose, fucose, and N-acetylglucosamine. Based on CRD structure, pattern, binding properties, and calcium dependence, animal lectins are widely categorized into 11 major families, such as C-type, F-box lectins, F-type, M-type, L-type, I-type, P-type, R-type, calnexin, chitinase such as lectins, intelectins, ficolins, and galectins. These are being classified into several families based on their chemical properties like structure of carbohydrate binding domain, sugar specificity, and requirement of divalent cations (Medzhitov and Janeway 2002). Mainly there are three types of lectins including hololectins, chimerolectins, and merolectins (Peumans and Van Damme 1995) and these lectins are characterized by the presence of several carbohydrate binding sites (Jiang et al. 2010). Studies indicate that almost all lectin genes demonstrate a varying degree of organic phenomenon under abiotic stresses, including heat, cold, salinity, and drought (Hirano et al. 2000, SpadoroTank). All genes involved in the synthesis of lectins in rice, soybean, and Arabidopsis was characterized and identified by Jiang et al. (2015). Plant lectins, like the mannose-binding lectin (MBL), are found to impede the viral attachment at the beginning of their replication (Keyaerts et al. 2007).
Microorganisms such as bacteria, fungi, protozoa, and viruses express lectins thereby providing them with several benefits like the power to bind, infect, and inhibit other microorganisms. Lectins in microorganisms appear to play several important roles, like host–cell interactions, recognition in immune processes, phagocytosis, and cell adhesion. The lectin yield from fresh mushrooms are low, owing to the extremely high water content of fresh mushrooms. For example, extraction of lectins from the fruiting bodies of Pleurocybella porrigens, yielded only 2.6 mg/100 g (Suzuki et al. 2009), whereas edible fruit bodies from Pholiota adipose, Russula lepida, and Inocybe umbrinella yielded 70, 39, and 15 mg of lectins/100 g of fruiting bodies, respectively (Zhang et al. 2009, 2010; Zhao et al. 2009).
Lectins isolated from the phylum porifera are classified into several groups on the basis of their binding activities. It includes F-type, C-type, and tachylectin-like lectins, galectins. Extensive bioactivities are reported for sponge lectins including neuromodulatory, antimicrobial, mitogenic, and anti-tumor activities. Varieties of those activities can be correlated to physiological roles within the sponge, like spiculogenesis, host defense, cell adhesion, and differentiation.
Fish lectins have active role in the identification of pathogens and stimulating the reaction against pathogens by immune cells like phagocytes, facilitating the cell lysis by complement activation and enhancement of the activity of natural CD8 T cells (Hoffmann et al. 1999). Several lectins have been identified from various fishes such as eel (Anguilla japonica) (Tasumi et al. 2002), carp (Cyprinus carpio) (Fujiki et al., 2001), and rainbow trout (Oncorhynchus mykiss) (Zhang et al. 2000) with different specificities towards carbohydrates such as galactose (Vitved et al. 2000), rhamnose (Okamoto et al. 2005), mannose (Ottinger et al. 1999; Konstantina and Ioannis 2006), and fucose (Honda et al. 2000). Latterly, a sequence of lily-type lectins were reported from several fishes including orange-spotted grouper (Epinephelus coioides), large yellow croaker (Larimichthys crocea), Bartail flathead (Platycephalus indicus), and Spotnape ponyfish (Leiognathus nuchalis).
2 Types of Aquatic Lectins
Lectins are a diverse family of proteins or glycoproteins ubiquitously present in nature and are reported from different groups of living organisms including bacteria, fungi, animals, plants, and even in mycoplasmas and viruses (Barre et al. 2019). Lectins have a characteristic affinity towards carbohydrates(Marques et al. 2018). The lectins can be classified in different ways. Functionally they are classified into five groups such as galactose-binding lectins, mannose-binding lectins, fucose binding lectins, N-acetyl glucosamine binding lectins and Sialic acid-binding lectins, which is based on the lectins affinity towards a monosaccharide (Barre et al. 2019). Based on the source, they can be classified as plant derived lectins, invertebrate lectins, and vertebrate lectins (Fig. 1.1).
2.1 Aquatic Plant Lectins
Plant lectin research started in 1888 by the identification of a highly toxic protein from the castor bean (Ricinus communis) named as Ricin and later turned out to be the first lectin (Stillmark 1888). It is followed by the extensive mining of plants for the identification of lectins and many lectins were identified from terrestrial plants, but very few lectins have been identified in aquatic lectins. It includes the recently identified lectins from Lemna minor or duckweed and Eichornia crassipens or water hyacinth. The duckweed lectin is a sialic acid lectin and the water hyacinth lectin is N-acetyl-glucosamine/N-acetyl-galactosamine binding lectins (Córdoba-Aguilar et al. 2018).
2.2 Algal Lectins
The first report of lectins in algae was made by Boyd et al. (Boyd et al. 1997), Rogers et al. (Rogers et al. 1977) and Matsubara et al. (Matsubara et al. 1996). Algal lectins are often termed as phycolectins and they are similar to plant lectins but there is some difference in terms of their physical and chemical properties and unique carbohydrate specificity (Singh et al. 2015). Phycolectins are identified in the red, green, and brown algal groups. More than 800 lectins were identified from algae among which 61% of them are from red algae, 22% from green algae, and 17% from brown algae. Even though these many lectins have been identified, less than 40 lectins are purified and sequenced yet (Hwang et al. 2018; Singh et al. 2015). Algal lectins share some common attributes such as low molecular weight, thermostability, independence on divalent cations for hemagglutination, and high amount of acidic amino acids. Moreover they have high affinity towards glycoproteins (Hori et al. 1990; Rogers and Hori 1993). Consequently, phycolectins are monomeric proteins having low molecular mass with an isoelectric point (pI) lies in between 4 and 6 and studies on biological models revealed that, they may be less antigenic (Teixeira et al. 2012).
2.3 Sponge Lectins
The exploration of phylum porifera for the identification of lectins begun during the second half of 1980, and about 50 lectins have been isolated and biochemically characterized from this species so far (Sousa et al. 2021). During this period, lectins with a wide variety of biological activities like mitogenic, cytotoxic, antimicrobial, and cytotoxic activities have been isolated. Their involvement in the immune responses in order to defend sponges from their invaders and there active role in the grouping of sponge cells was also described (Prokop et al. 1968; Müller et al. 1979). Lectins belong to the class F-type lectins, galectins, C-type lectins, and unclassified types have been identified in sponges, among which galectins are the widely seen in phylum porifera(Sousa et al. 2021). Interest in the sponge was increased recently, as they represent a stable and permanent replica of a accepted marine microecosystem having an intricate multifariousness of microbes (Gardères et al. 2015).
Even though about 50 sponge lectins were isolated and most of them have partially purified, complete determination of primary structure had been completed for only eight of them. Elucidation of their structure may provide a better understanding of their biological activities (Buck et al. 1992; Carneiro et al. 2013a; Funayama et al. 2005; Gundacker et al. 2001; Pfeifer et al. 1993; Sousa et al. 2021; Ueda et al. 2013; Wiens et al. 2003, 2005). Table 1.1 lists the lectins isolated from the phylum porifera till now.
2.4 Crustacean Lectin/Shellfish Lectins
Different types of lectins, including Chitinase like lectins, F-type, C-type, L-type, I-type, M-type, R-type, P-type, ficolins, galectins, intelectins, and calnexin are reported in aquatic arthropods (Wang and Wang 2013). Among these, the C-type lectins (CTLs) are highly conserved in crustaceans and are well-characterized. They acts as pattern recognition receptors which can recognize and agglutinate pathogens and stimulate their phagocytosis. Lectins role in the neutralization of pathogens, involved in bacterial, fungal, and viral infections have been reported. Aside from C-type, other lectins like L-type lectins and galectins, were also reported as important immune molecules to market phagocytes against viruses and bacteria in crustaceans. However, very few lectins from molluscs were isolated and classified with their defensive roles (Liu et al. 2020).
2.5 Fish Lectins
Different lectins belong to Galectins, F-type, C-type, and Rhamnose-binding lectins were identified from the fishes. Fish lectins are actively involving in the innate and acquired immune reactions against pestilential microorganisms and assisting in the establishment of favorable symbiotic interactions with colonizing microbes (Vasta et al. 2012). In addition to that they also have other functions such as agglutination and eradication of infectious agents (Ewart et al. 2001; Russell and Lumsden 2005). Among the various lectins, C-type lectins are majorly reported in fishes (Elumalai et al. 2019).
3 Functional Aspects of Aquatic Lectins
Lectins are proteins having affinity towards carbohydrates and are identified in prokaryotes, eukaryotes and viruses. These proteins play major role in cell agglutination and can precipitate polysaccharides, glycoprotein, or glycolipid mediating several biological processes that include glycoprotein traffic signal and clearance, cell–cell interaction, induction of apoptosis, mitogenic activity, and anti-tumor activity (Fig. 1.2). Lectins have active roles in the immune system of various aquatic animals, it is believed to arbitrate pathogenic recognition and plays a crucial role in innate immune reaction (Barre et al. 2019). Aquatic lectins have found to play pivotal role in processes such as embryogenesis, fertilization, and morphogenesis. Lectins can be classified into C-type lectins, F-type lectins, Galectins, I-type lectins, etc. on the basis of their structure, calcium dependency, and binding specificities. Several lectins including Calnexin, Galectins, F-type lectins, etc. are inevitable for disease resistance and in innate immunity.
3.1 Antimicrobial Activity
Marine sponge lectins were reported to have extensive range of biological activity. The CaL, a lectin identified in Cinachyrella apion, inhibited HeLa cell development and decreased cell proliferation in a dose-dependent fashion. Another lectin, CvL, from marine sponge Cliona varians, reduced the development of human leukemia cells but, interestingly it had no effect on healthy blood lymphocytes.
Furthermore, lectins from marine sponges have a role in the organism’s self-defense, as certain lectins may identify, agglutinate, and prevent the formation of bacterial cells and biofilms. Study on the antibiofilm activity of the lectin, AlL purified from Aplysina lactuca, revealed that it can agglutinate a wide range of Gram positive and Gram negative bacterial cells and thereby reduce bacterial biofilm biomass in a dose-dependent fashion (Gardères et al. 2015). A lectin isolated from Cliona varians (CvL), on the other hand, has shown a selective cytotoxicity against Gram positive bacteria without any effect on Gram negative bacteria.
Some lectins have cytotoxic activity against parasites and microorganisms. Lectins are agglutinating the cells by directly binding with them and in contrast, it is possible for them to act as part of a complex to exert its biological functions, as shown by the mammalian lectins. In fact lectins can bind to pathogens, by which they can either inhibit growth of the pathogen and/or kill pathogens directly. Many lectins are reported to have antibacterial activities and as the analysis used in these studies were unable to distinguish between the growth inhibition and killing, the exact mechanism of antibacterial activity is yet to be elucidated. The bacterial growth inhibitory activity of lectin is also important; whereas, further studies to characterize lectins should aim to determine whether it will kill pathogens. For example, this can be done with a fluorescent dye (like propidium iodide), which exclusively stains the cells having pores in their cell membrane.
Catfish (Ictalurus dotatus) have 12 galectin genes and majority of them are actively expressed in mucous tissue. Interestingly, galectin expression profiling of fish challenged with two different gram negative bacterial pathogens revealed that peculiar change has occurred in a different tissues in response to different pathogens.
3.2 Hemagglutination
Agglutination is the process by which cells and virus stick together or gather together. Antibodies are classic lectins and are crucial as part of both the acquired and innate immune systems. Lectins are the chief player in the innate immune mechanisms (Barre et al. 2019). Most of the lectins secreted by the mucosa have the ability to agglutinate the bacteria and exogenous red blood cells, which is termed as hemagglutination. Binding of foreign bodies (bacteria, virus, parasites, and yeasts) to lectins can prompt agglutination, which can lead to destruction (by activating complement or through endocytosis/phagocytosis uptake) or by lectin mediated direct destruction. Mechanisms like agglutination are important in preventing the absorption of pathogens through the mucosal surface. In addition to lectins, mucus also contains many immune-related molecules which can agglutinate bacteria and exogenous red blood cells. In mucus, different mucosal proteins can work in conjunction to achieve the agglutination, but in the case of recombinantly expressed or isolated lectins, they can initiate the agglutination without the aid of other proteins, which suggests that a single lectin can cause agglutination and hemagglutination. Ability of lectin to agglutinate depends on its confirmation, which is affected by the pH, temperature and presence of ions. Hence, treating the lectin in an elevated temperature can hamper the agglutination and agglutination also depends on the presence of Ca2+. Lectins possess a carbohydrate recognition domain (CRD) essentially forms a dimer or higher structure to agglutinate.
The Pufflectin, a lectin isolated from the puffer fish has no homology with other animal lectins, interestingly it has homology with plant derived mannose-binding lectins. They have been expressed from the mucosa of the oral cavity walls, esophagus, gills, and skin, etc. and reported to bind with the fluke Heterobothrium okamotoi. This finding suggested their active role in parasite defense. Their interactions with other bacterial species have not been tested. Hemagglutination is also very common. The mannose used in Sebastes schlegelii binds SsLTL lily-type lectin, the mannose of Atlantic cod (Gadus morhua) binds natterin-like protein, and it has been used in a calcium-dependent manner and from the flat head (Platycephalus indicus) mannose-binding lectin homologous to kallikrein.
3.3 Antibiofilm Activity
Lectins can reduce the growth and inhibit biofilm formation by several pathogenic bacterial species and thus they can have promising applications as an alternative to antibiotics to combat the increasing number of infections associated with multidrug-resistant pathogens.
Lectins identified in the marine sponge Chondrilla caribensis (CCL) is found to have antibiofilm activity. The analysis of antibiofilm activity of CCL showed significantly reduced total biomass on Staphylococcus epidermidis, Staphylococcus aureus, and Escherichia coli biofilms, but it could not reduce the viability of cells trapped in the biofilm. Biofilms constitutes sticky microbial communities covered by a macromolecular extracellular matrix produced by the microorganism itself. The resistance of the biofilm will be up to 10 ± 1000 times greater than that of the plankton cells, the protection provided by the biofilm’s matrix polymers. Biofilm biomass includes not only the bacterial cells, but also biofilm substrates, which constitute for more than 90% of total biomass in most biofilms.
Chondrilla caribensis lectin activity was hampered by α-lactose, which suggests that antibiotic activity of the lectin is conferred by the carbohydrate recognition domain or CRD. Lectin exerts their antibacterial activity by the specific recognition of molecules present on the bacterial cell surface. Based on several studies, it is proven that, lectin can impede the formation of biofilms by interacting with the biofilm microbiota and altering the expression of genes involved not only in biofilm formation but also in virulence. Lectin from the haemolymph of Metapenaeus dobsoni (Md-Lec) prevents the entry and multiplication of pathogenic Gram negative organisms present in the aquatic environment. This is mediated by the inhibition of biofilm formation and agglutination of bacteria in a dose-dependent fashion.
3.4 Antitumour Activity
The lectins have an appreciable ability to target cancer cells which could be applied not only to kill cancers but also for the targeted delivery of anti-tumor drugs to these cells. Lectin plays central role in several processes including tissue growth, sugar storage, cell–cell interaction and communication, and other processes and pathways for cell survival and activation of immune system. Most of the endogenous lectins are involving both in physiological and pathological processes.
Eucheuma serra agglutinin (ESA), a well-characterized mannose-binding lectin isolated from the homonymous red macroalga, is capable of promoting apoptosis not only in various cell lines but also in animal cancer models. ESA consists of four tandem repeat units in its amino acid structure, which represents a binding site for the carbohydrate, mannose. All these repeated motifs specifically interact to the high mannose N glycan with minimal tetra or pentasaccharide size, such as Human (alpha13) Human (alpha1 6) Human (beta14) GlcNAc (beta14) GlcNAc.
Sialic acid-binding lectin (SBLc), alternatively termed as leczyme, purified from the egg cells of Rana catesbeiana is multifunctional with both lectin activity and RNAse activity. This dual nature provides them with interesting anti-tumor properties. SBLc is unique in terms of their properties and is no homologous proteins were identified yet. This is a monomeric protein having 111amino acids and contains no covalently linked carbohydrates.
3.5 Phagocytosis
Phagocytosis is an intricate process for the digestion and eradication of pathogens. Moreover it contributes to the fundamental homeostasis of the tissues by the elimination of apoptotic cells. The process of Phagocytosis consists of four main stages including identification of the target which leads to the triggering of signaling activity to initiate the cellular machinery for the development of phagosome, and eventually the maturation of phagolysosome. Carbohydrate–lectin interactions serve as the basis for phagocytic cells to recognize different particles and target cells.
C-type lectins (CTLs) are the well-studied class of lectins and hasn’t undergone any mutations or changes in crustaceans. They serve as receptors for bacterial binding and agglutination or act as opsonin to induce phagocytosis of viruses and bacteria. Different CTLs involved in phagocytosis in crab and crayfish, Litopenaeus vannamei, P. monodon, Fenneropenaeus chinensis, S. paramamosain, Procambarus clarkii, and shrimp have been isolated till the date. PmLec, a lectin isolated from P. monodon was instrumental in recognizing E. coli by binding specifically to the lipopolysaccharide (LPS) present in the bacterial membrane. Furthermore, it acts as an opsonin to augment its ability phagocytize of blood cells. Several lectins with marked level of phagocytic ability against the bacteria have been identified from L. vannamei. Studies on the rate of phagocytosis by V. parahaemolyticus demonstrated that silencing of lectin genes LvLdlrCTL, LvCTLU, and LvCTL5, reduced their ability by 2.5%, 4.5%, and 8.3%, respectively, compared with control groups. From these findings, it is clear that lectin produced by LvCTL5 has prominent role in phagocytosis than that of the other two lectins. Likewise, two lectins of Paramamosain clarkii were also able to promote phagocytic activity of erythrocytes against Vibrio anguillarum.
4 Prospective Applications of Aquatic Lectins
Lectins are ubiquitously found in the living organisms ranging from prokaryotes to eukaryotes and even in higher plants. This large group of protein is characterized by the presence of a one non-catalytic region which has the ability to reversibly bind with particular sugar moieties and their ability to agglutinate red blood cells to a specific sugar moiety is renowned. Lectins are categorized into different groups and among those C-type lectins, F-type lectins, rhamnose-binding lectins, intelectins, and galectins have been isolated from aquatic sources. .
Nowadays, appreciable attention is gained by aquatic lectins, mostly lectins from algae and cyanobacteria, because of their antiviral activity. Lectins can prevent the virus from entering host cells and spreading the virus, in contrast to the conventional antiviral therapy, in which the majority of the antiviral agents are working through the blocking of the viral life cycle after it enters into the cell. N-linked glycans present on the viral envelope has an important role in the proper folding of envelope proteins to attain its tertiary structure folding, which in turn helps the virus to enter the host cells and evasion of the host immune system. Lectins block the receptor–ligand interaction between the virion and host cells, a crucial step in the entry of the virus into the host cells, by binding with the carbohydrate residues on the viral envelope. In addition, lectins also act as surface markers for recognition of tumor cells, transmembrane signal transduction, cell adhesion, mitosis apoptosis, and cytotoxicity. Therefore, it can be used in cancer diagnosis and therapy.
Innate immunity, being the first line of defense mechanisms depends on the pattern recognition on the pathogen surfaces. Each group of microorganisms has unique molecules or patterns, which can be recognized by the patterns recognition receptors found on the macrophages, dendritic cells, and epithelial cells of the host. Studies on HSL, a lectin from sea cucumber (Holothuria scabr) suggests that exposure to variety of bacteria can induce its expression, which involves the interaction of glycoconjugates present on the bacterial cell wall with innate immunity receptors (Sousa et al. 2021). This result establishes the role of lectins in innate immunity. A lipopolysaccharide-binding protein named L6 isolated from limulus red blood cells, which has the characteristics of a lectin, shows agglutinating activity against the tested bacteria. Agglutination was initiated by the recognition of carbohydrate moieties present in the bacterial cell wall. It also inhibits the growth of E. coli, Klebsiella pneumoniae, and Salmonella minnesota.
Fungal agglutination is often studied during the characterization of lectin, as agglutination may assist its elimination through phagocytosis. Lectin’s expression levels are upregulated when encountered with fungi and they normally attach to and agglutinate fungi. They act in a comparable fashion as in the eradication of invading pathogenic bacteria. Ec-CTL, A C-type lectin purified from Epinephelus coioides (orange-spotted grouper) also showed antifungal activity. Its expression is unregulated when stimulated with Saccharomyces cerevisiae and the expressed lectin bind with the fungal cells, which leads to its aggregation. The aggregation happened in a Ca2+ dependent manner. An extra cellular, serum lectin belonging to interlectins was identified in Lampetra japonica (lamprey) by Xue et al. (2013). It has glycan-binding receptors that attach to epithelial glycan of invading pathogens in the host system. In vivo stimulation using bacteria induced its expression and it showed agglutinative activity against opportunistic pathogenic yeast Candida albicans. The results suggests that it has a crucial role in the innate immune response against yeast in lamprey.
GANL, a lectin purified from the gills of bighead carp (Aristichthys nobilis) was tested for anti-tumor activity (Yao et al. 2003). The anti-tumor activity against six human cancer cell lines were tested, and concluded that among these, it has strong anti-tumor activity against HeLa cell lines. The IC50 value of GANL against HeLa cell line was 11.86 μg/mL. Rabelo et al. (2012) studied the apoptosis-inducing activity of sponge agglutinin in human cancer cells. They isolated CaL from the sea sponge Cinachyrella apion and examined its anti-proliferative effect on three different human cancer cell lines. The result demonstrated that CaL had the highest anti-proliferative activity on HeLa cells, and it was in a dose-dependent fashion. It was also reported that the lectin induced the expression of apoptotic regulator Bax, initiating the apoptosis by the activation of caspase cascade, halting the cell cycle during the S phase and improving the permeability of the mitochondrial membrane.
Abbreviations
- CRD:
-
Carbohydrate recognition domain
- CTLs:
-
C-type lectins
- MBL:
-
Mannose-binding lectin
- PRR:
-
Pattern recognition receptor
- SBL:
-
Sialic acid-binding lectin
References
Atta A, Barral-Netto M, Peixinho S, Sousa-Atta M (1989) Isolation and functional characterization of a mitogenic lectin from the marine sponge Cinachyrella alloclada. Braz J Med Biol Res 22:379–385
Atta A, Menezes EP, Peixinho S, Sousa-Atta ML (1990) Isolation of a lectin from the marine sponge Desmapsama anchorata by affinity chromatography on raffinose-sepharose 6B. Braz J Med Biol Res Rev Bras Pesqui Medicas E Biol 36(4):447–457
Barre A, Simplicien M, Benoist H, Van Damme EJM, Rougé P (2019) Mannose-specific lectins from marine algae: diverse structural scaffolds associated to common virucidal and anti-cancer properties. Mar Drugs 17:440. https://doi.org/10.3390/md17080440
Bayer H, Ey N, Wattenberg A, Voss C, Berger MR (2012) Purification and characterization of riproximin from Ximenia americana fruit kernels. Protein Expr Purif 82:97
Boyd MR, Gustafson KR, McMahon JB, Shoemaker RH, O’Keefe BR, Mori T, Gulakowski RJ, Wu L, Rivera MI, Laurencot CM, Currens MJ, Cardellina JH, Buckheit RW, Nara PL, Pannell LK, Sowder RC, Henderson LE (1997) Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother 41:1521–1530. https://doi.org/10.1128/AAC.41.7.1521
Bretting H, Kabat EA, Liao J, Pereira MEA (2002) Purification and characterization of the agglutinins from the sponge Aaptos papillata and a study of their combining sites [WWW document]. ACS Publ. https://doi.org/10.1021/bi00668a013
Buck F, Luth C, Strupat K, Bretting H (1992) Comparative investigations on the amino-acid sequences of different isolectins from the sponge Axinella polypoides (Schmidt). Biochim Biophys Acta Protein Struct Mol Enzymol 1159:1–8. https://doi.org/10.1016/0167-4838(92)90067-N
Carneiro RF, de Melo AA, de Almeida AS, da Moura R, Chaves RP, de Sousa BL, do Nascimento KS, Sampaio SS, Lima JPMS, Cavada BS, Nagano CS, Sampaio AH (2013a) H-3, a new lectin from the marine sponge Haliclona caerulea: purification and mass spectrometric characterization. Int J Biochem Cell Biol 45:2864–2873. https://doi.org/10.1016/j.biocel.2013.10.005
Carneiro RF, de Melo AA, Nascimento FEP, Simplicio CA, Nascimento KS, Rocha BAM, Saker-Sampaio S, da Moura R, Mota SS, Cavada BS, Nagano CS, Sampaio AH (2013b) Halilectin 1 (H-1) and Halilectin 2 (H-2): two new lectins isolated from the marine sponge Haliclona caerulea. J Mol Recognit 26:51–58. https://doi.org/10.1002/jmr.2243
Carneiro RF, de Lima PHP, Chaves RP, Pereira R, Pereira AL, de Vasconcelos MA, Pinheiro U, Teixeira EH, Nagano CS, Sampaio AH (2017) Isolation, biochemical characterization and antibiofilm effect of a lectin from the marine sponge Aplysina lactuca. Int J Biol Macromol 99:213–222. https://doi.org/10.1016/j.ijbiomac.2017.02.020
Carneiro RF, Viana JT, Torres RCF, da Silva LT, Andrade AL, de Vasconcelos MA, Pinheiro U, Teixeira EH, Nagano CS, Sampaio AH (2019) A new mucin-binding lectin from the marine sponge Aplysina fulva (AFL) exhibits antibiofilm effects. Arch Biochem Biophys 662:169–176. https://doi.org/10.1016/j.abb.2018.12.014
Córdoba-Aguilar E, Coutiño-Rodríguez R, Giles-Ríos H, Hernández-Cruz PA, Ríos-Cortés P, Montero H (2018) Lectins from Eichornia crassipens and Lemna minor may be involved in Vibrio cholerae El tor adhesion. Epidemiol Mikrobiol Imunol Cas Spolecnosti Epidemiol Mikrobiol Ceske Lek Spolecnosti JE Purkyne 67:24–30
do Nascimento-Neto LG, Cabral MG, Carneiro RF, Silva Z, Arruda FVS, Nagano CS, Fernandes AR, Sampaio AH, Teixeira EH, Videira PA (2018) Halilectin-3, a lectin from the marine sponge Haliclona caerulea, induces apoptosis and autophagy in human breast cancer MCF7 cells through Caspase-9 pathway and LC3-II protein expression. Anticancer Agents Med Chem 18:521–528. https://doi.org/10.2174/1871520617666171114094847
Dogović N, Sladić D, Kljajić Z, Poznanović S, Gašić MJ (1996) Isolation and partial characterization of a lectin from the marine sponge Crambe crambe. J Serb Chem Soc 61:83–88
Dong CH, Yang ST, Yang ZA, Zhang L, Gui JF (2004) A C-type lectin associated and translocated with cortical granules during oocyte maturation and egg fertilization in fish. Dev Biol 265:341e354
Dresch RR, Zanetti GD, Lerner CB, Mothes B, Trindade VMT, Henriques AT, Vozári-Hampe MM (2008) ACL-I, a lectin from the marine sponge Axinella corrugata: isolation, characterization and chemotactic activity. Comp Biochem Physiol Toxicol Pharmacol 148:23–30. https://doi.org/10.1016/j.cbpc.2008.03.003
Dresch RR, Lerner CB, Mothes B, Trindade VMT, Henriques AT, Vozári-Hampe MM (2012) Biological activities of ACL-I and physicochemical properties of ACL-II, lectins isolated from the marine sponge Axinella corrugata. Comp Biochem Physiol B Biochem Mol Biol 161:365–370. https://doi.org/10.1016/j.cbpb.2012.01.001
Elumalai P, Rubeena AS, Arockiaraj J, Wongpanya R, Cammarata M, Ringø E, Vaseeharan B (2019) The role of lectins in finfish: a review. Rev Fish Sci Aquac 27:152–169. https://doi.org/10.1080/23308249.2018.1520191
Engel M, Bachmann M, Schröder HC, Rinkevich B, Kljajic Z, Uhlenbruck G, Müller WE (1992) A novel galactose- and arabinose-specific lectin from the sponge Pellina semitubulosa: isolation, characterization and immunobiological properties. Biochimie 74:527–537. https://doi.org/10.1016/0300-9084(92)90150-d
Ewart KV, Johnson SC, Ross NW (2001) Lectins of the innate immune system and their relevance to fish health. ICES J Mar Sci 58:380–385. https://doi.org/10.1006/jmsc.2000.1020
Funayama N, Nakatsukasa M, Kuraku S, Takechi K, Dohi M, Iwabe N, Miyata T, Agata K (2005) Isolation of Ef silicatein and Ef lectin as molecular markers for sclerocytes and cells involved in innate immunity in the freshwater sponge Ephydatia fluviatilis. Zoolog Sci 22:1113–1122. https://doi.org/10.2108/zsj.22.1113
Gardères J, Bourguet-Kondracki M-L, Hamer B, Batel R, Schröder HC, Müller WEG (2015) Porifera lectins: diversity, physiological roles and biotechnological potential. Mar Drugs 13:5059–5101. https://doi.org/10.3390/md13085059
Gardères J, Domart-Coulon I, Marie A, Hamer B, Batel R, Müller WEG, Bourguet-Kondracki M-L (2016) Purification and partial characterization of a lectin protein complex, the clathrilectin, from the calcareous sponge Clathrina clathrus. Comp Biochem Physiol B Biochem Mol Biol 200:17–27. https://doi.org/10.1016/j.cbpb.2016.04.007
Gundacker D, Leys SP, Schröder HC, Müller IM, Müller WE (2001) Isolation and cloning of a C-type lectin from the hexactinellid sponge Aphrocallistes vastus: a putative aggregation factor. Glycobiology 11:21–29. https://doi.org/10.1093/glycob/11.1.21
Hasan I, Ozeki Y (2019) Histochemical localization of N-acetylhexosamine-binding lectin HOL-18 in Halichondria okadai (Japanese black sponge), and its antimicrobial and cytotoxic anticancer effects. Int J Biol Macromol 124:819–827. https://doi.org/10.1016/j.ijbiomac.2018.11.222
Hirano K, Teraoka T, Yamanaka H, Harashima A, Kunisaki A, Takahashi H, Hosokawa D (2000) Purification, crystallization and preliminary X-ray analysis of adenylylsulfate reductase from Desulfovibrio vulgaris Miyazaki F. Plant Cell Physiol 41:258–267
Hoffmann JA, Kafatose FC, Janeway CA, Ezekwitz RA (1999) Phylogenetic perspectives in innate immunity. Science 284:1313e1318
Honda S, Kashiwagi M, Miyamoto K, Takei Y, Hirose S (2000) Multiplicity,structures, and endocrine and exocrine natures of eel fucose-binding lectins. J Biol Chem 275:e33157
Hori K, Miyazawa K, Ito K (1990) Some common properties of lectins from marine algae. Hydrobiologia 204:561–566. https://doi.org/10.1007/BF00040287
Hung LD, Ly BM, Hao VT, Trung DT, Trang VTD, Trinh PTH, Ngoc NTD, Quang TM (2018) Purification, characterization and biological effect of lectin from the marine sponge Stylissa flexibilis (Lévi, 1961). Comp Biochem Physiol B Biochem Mol Biol 216:32–38. https://doi.org/10.1016/j.cbpb.2017.11.008
Hwang H-J, Han J-W, Jeon H, Han JW (2018) Induction of recombinant lectin expression by an artificially constructed tandem repeat structure: a case study using Bryopsis plumosa mannose-binding lectin. Biomol Ther 8:146. https://doi.org/10.3390/biom8040146
Jiang S-Y, Ma Z, Ramachandran S (2010) Evolutionary history and stress regulation ofthe lectin superfamily in higher plants. BMC Evol Biol 10:79. https://doi.org/10.1186/1471-2148-10-79
Jiang X, Yao F, Li X, Jia B, Zhong G, Zhang J, Hou LJG (2015) Molecular cloning,characterization and expression analysis of the protein arginineN-methyltransferase 1 gene(As-PRMT1) from Artemia sinica. Gene 565:122–129
Kawagishi H, Yamawaki M, Isobe S, Usui T, Kimura A, Chiba S (1994) Two lectins from the marine sponge Halichondria okadai. An N-acetyl-sugar-specific lectin (HOL-I) and an N-acetyllactosamine-specific lectin (HOL-II). J Biol Chem 269:1375–1379
Kawsar SMA, Fujii Y, Matsumoto R, Ichikawa T, Tateno H, Hirabayashi J, Yasumitsu H, Dogasaki C, Hosono M, Nitta K, Hamako J, Matsui T, Ozeki Y (2008) Isolation, purification, characterization and glycan-binding profile of a d-galactoside specific lectin from the marine sponge, Halichondria okadai. Comp Biochem Physiol B Biochem Mol Biol 150:349–357. https://doi.org/10.1016/j.cbpb.2008.04.004
Keyaerts E, Vijgen L, Pannecouque C, Van Damme E, Peumans W, Egberink H, VanRanst M (2007) Plant lectins are potent inhibitors of coronaviruses byinterfering with two targets in the viral replication cycle. Antivir Res 75:179–187. https://doi.org/10.1016/j.antiviral.2007.03.003
Konstantina N, Ioannis KZ (2006) Molecular cloning and characterization of two homologues of mannosebinding lectin in rainbow trout. Fish Shellfish Immunol 21:305e314
Lakhtin V, Lakhtin M, Alyoshkin V (2011) Lectins of living organisms. The overview. Anaerobe 17:452–455. https://doi.org/10.1016/j.anaerobe.2011.06.004
Liu S, Zheng S-C, Li Y-L, Li J, Liu H-P (2020) Hemocyte-mediated phagocytosis in crustaceans. Front Immunol 11:268. https://doi.org/10.3389/fimmu.2020.00268
Marques DN, de Almeida AS, de Sousa AR, Pereira R, Andrade AL, Chaves RP, Carneiro RF, de Vasconcelos MA, do Nascimento-Neto LG, Pinheiro U, Videira PA, Teixeira EH, Nagano CS, Sampaio AH (2018) Antibacterial activity of a new lectin isolated from the marine sponge Chondrilla caribensis. Int J Biol Macromol 109:1292–1301. https://doi.org/10.1016/j.ijbiomac.2017.11.140
Matsubara K, Sumi H, Hori K (1996) Platelet aggregation is inhibited by phycolectins. Experientia 52:540–543. https://doi.org/10.1007/BF01969724
Mebs D, Weiler I, Heinke HF (1985) Bioactive proteins from marine sponges: screening of sponge extracts for hemagglutinating, hemolytic, ichthyotoxic and lethal properties and isolation and characterization of hemagglutinins. Toxicon 23:955–962. https://doi.org/10.1016/0041-0101(85)90388-5
Medeiros DS, Medeiros TL, Ribeiro JKC, Monteiro NKV, Migliolo L, Uchoa AF, Vasconcelos IM, Oliveira AS, de Sales MP, Santos EA (2010) A lactose specific lectin from the sponge Cinachyrella apion: purification, characterization, N-terminal sequences alignment and agglutinating activity on Leishmania promastigotes. Comp Biochem Physiol B Biochem Mol Biol 155:211–216. https://doi.org/10.1016/j.cbpb.2009.10.016
Medzhitov R, Janeway CA Jr (2002) Decoding the patterns of self and nonself by the innate immune system. Science 296:298e300
Miarons PB, Fresno M (2000) Lectins from tropical sponges: purification and characterization of lectins from genusaplysina*. J Biol Chem 275:29283–29289. https://doi.org/10.1074/jbc.M001366200
Moura RM, Queiroz AFS, Fook JMSLL, Dias ASF, Monteiro NKV, Ribeiro JKC, Moura GEDD, Macedo LLP, Santos EA, Sales MP (2006) CvL, a lectin from the marine sponge Cliona varians: isolation, characterization and its effects on pathogenic bacteria and Leishmania promastigotes. Comp Biochem Physiol A Mol Integr Physiol 145:517–523. https://doi.org/10.1016/j.cbpa.2006.08.028
Müller WE, Kurelec B, Zahn RK, Müller I, Vaith P, Uhlenbruck G (1979) Aggregation of sponge cells. Function of a lectin in its homologous biological system. J Biol Chem 254:7479–7481
Müller WE, Zahn RK, Kurelec B, Lucu C, Müller I, Uhlenbruck G (1981) Lectin, a possible basis for symbiosis between bacteria and sponges. J Bacteriol 145:548–558. https://doi.org/10.1128/jb.145.1.548-558.1981
Ng TB, Fai Cheung RC, Wing Ng CC, Fang EF, Wong JH (2015) A review of fish lectins. Curr Protein Pept Sci 16:337–351. https://doi.org/10.2174/138920371604150429160850
Okamoto M, Tsutsui S, Tasumi S, Suetake S, Kikuchi K, Suzuki Y (2005) Tandem repeat L-rhamnosebinding lectin from the skin mucus of ponyfish, Leiognathus nuchalis. Biochem Biophys Res Commun 333:463e469
Olsnes S (2004) The history of ricin, abrin and related toxins. Toxicon 44:361
Osorio F, Reis e Sousa C (2011) Myeloid C-type lectin receptors in pathogen recognition and host defense. Immunity 34:651–664. https://doi.org/10.1016/j.immuni.2011.05.001
Ottinger CA, Johnson SC, Ewart KV, Brown LL, Ross NW (1999) Enhancement of anti-Aeromonas salmonicida activity in Atlantic salmon (Salmo salar) macrophages by a mannose-binding lectin. Comp Biochem Physiol Part C 123:53e59
Pajic I, Kljajic Z, Dogovic N, Sladic D, Juranic Z, Gasic MJ (2002) A novel lectin from the sponge Haliclona cratera: isolation, characterization and biological activity. Comp Biochem Physiol Toxicol Pharmacol 132:213–221. https://doi.org/10.1016/s1532-0456(02)00068-6
Peumans WJ, Van Damme E (1995) Lectins as plant defense proteins. Plant Physiol 109:347. https://doi.org/10.1104/pp.109.2.347
Pfeifer K, Haasemann M, Gamulin V, Bretting H, Fahrenholz F, Müller WEG (1993) S-type lectins occur also in invertebrates: high conservation of the carbohydrate recognition domain in the lectin genes from the marine sponge Geodia cydonium. Glycobiology 3:179–184. https://doi.org/10.1093/glycob/3.2.179
Prokop O, Uhlenbruck G, Köhler W (1968) A new source of antibody-like substances having anti-blood group specificity. A discussion on the specificity of helix agglutinins. Vox Sang 14:321–333. https://doi.org/10.1111/j.1423-0410.1968.tb01722.x
Rabelo L, Monteiro N, Serquiz R, Santos P, Oliveira R, Oliveira A, Rocha H, Morais AH, Uchoa A, Santos E (2012) A lactose-binding lectin from the marine sponge Cinachyrella apion (Cal) induces cell death in human cervical adenocarcinoma cells. Mar Drugs 10(4):727–743
Ratheesh S, Rauf AA (2018) Screening of antifungal activity of a lectin isolated from marine sponge Axinella donnani. Trends Biosci 11:1174–1176
Rogers D, Hori K (1993) Marine algal lectins: new developments. Hydrobiologia 260:589–593. https://doi.org/10.1007/BF00049075
Rogers DJ, Blunden G, Evans PR (1977) Ptilota plumosa, a new source of a blood-group B specific lectin. Med Lab Sci 34(3):193–200
Russell S, Lumsden JS (2005) Function and heterogeneity of fish lectins. Vet Immunol Immunopathol 108:111–120. https://doi.org/10.1016/j.vetimm.2005.08.004
Santos AFS, Napoleão TH, Bezerra RF, Carvalho EVMM, Correia MTS, Paiva PMG, Coelho LCBB (2013) In: Berhardt LV (ed) Advances in Medicine and Biology, vol 63. Nova, New York, p 33
Saraiva TS, Grund LZ, Komegae EN, Ramos AD, Conceição K, Orii NM, Lopes-Ferreira M, Lima C (2011) Nattectin a fish C-type lectin drives Th1 responses in vivo:licenses macrophages to differentiate into cells exhibiting typical DC function. Int Immunopharmacol 11:1546e1556
Sharon N, Lis H (1972) Lectins: cell-agglutinating and sugar-specific proteins. Science 177:949
Schröder HC, Kljajić Z, Weiler BE, Gasić M, Uhlenbruck G, Kurelec B, Müller WEG (1990) The galactose-specific lectin from the sponge Chondrilla nucula displays anti-human immunodeficiency virus activity in vitro via stimulation of the (2′-5′) oligoadenylate metabolism. Antivir Chem Chemother 1:99–105. https://doi.org/10.1177/095632029000100204
Schröder HC, Ushijima H, Krasko A, Gamulin V, Thakur NL, Diehl-Seifert B, Müller IM, Müller WEG (2003) Emergence and disappearance of an immune molecule, an antimicrobial lectin, in basal metazoa. A tachylectin-related protein in the sponge Suberites domuncula. J Biol Chem 278:32810–32817. https://doi.org/10.1074/jbc.M304116200
Schröder HC, Boreiko A, Korzhev M, Tahir MN, Tremel W, Eckert C, Ushijima H, Müller IM, Müller WEG (2006) Co-expression and functional interaction of Silicatein with galectin: matrix-guided formation of siliceous spicules in the marine demosponge suberites domuncula *. J Biol Chem 281:12001–12009. https://doi.org/10.1074/jbc.M512677200
Singh RS, Thakur SR, Bansal P (2015) Algal lectins as promising biomolecules for biomedical research. Crit Rev Microbiol 41:77–88. https://doi.org/10.3109/1040841X.2013.798780
Soanes KH, Figuereido K, Richards RC, Mattatall NR, Ewart KV (2004) Sequence and expression of C-type lectin receptors in Atlantic salmon (Salmo salar). Immunogenetics 56:572–584. https://doi.org/10.1007/s00251-004-0719-5
Sousa ARO, Andrade FRN, Chaves RP, Sousa BL, Lima DB, Souza RODS, da Silva CGL, Teixeira CS, Sampaio AH, Nagano CS, Carneiro RF (2021) Structural characterization of a galectin isolated from the marine sponge Chondrilla caribensis with leishmanicidal potential. Biochim Biophys Acta Gen Subj 1865:129992. https://doi.org/10.1016/j.bbagen.2021.129992
Stalz H, Roth U, Schleuder D, Macht M, Haebel S, Strupat K, Peter-Katalinic J, Hanisch F-G (2006) The Geodia cydonium galectin exhibits prototype and chimera-type characteristics and a unique sequence polymorphism within its carbohydrate recognition domain. Glycobiology 16:402–414. https://doi.org/10.1093/glycob/cwj086
Stillmark H (1888) Ueber Ricin, ein giftiges Ferment aus den Samen von Ricinus comm. L. und einigen anderen Euphorbiaceen: Inaugural-Dissertation (Thesis). Dorpat, Schnakenburg
Suzuki T, Amano Y, Fujita M, Kobayashi Y, Dohra H, Hirai H, Murata T, Usui T, Morita T, Kawagishi H (2009) Purification, characterization, and cDNA cloning of a lectin from themushroom Pleurocybella porrigens. Biosci Biotechnol Biochem 73:702–709. https://doi.org/10.1271/bbb.80774
Tasumi S, Ohira T, Kawazoe I, Suetake H, Suzuki Y, Aida K (2002) Primary structure and characteristics of a lectin from skin mucus of the Japanese eel Anguilla japonica. J Biol Chem 277:27305e27311
Teixeira EH, Arruda FVS, Nascimento KS, Carneiro VA, Nagano CS, da Silva BR, Sampaio AH, Cavada BS (2012) Biological applications of plants and algae lectins: an overview. In: Carbohydrates—comprehensive studies on glycobiology and glycotechnology. IntechOpen, London. https://doi.org/10.5772/50632
Ueda T, Nakamura Y, Smith CM, Copits BA, Inoue A, Ojima T, Matsunaga S, Swanson GT, Sakai R (2013) Isolation of novel prototype galectins from the marine ball sponge Cinachyrella sp. guided by their modulatory activity on mammalian glutamate-gated ion channels. Glycobiology 23:412–425. https://doi.org/10.1093/glycob/cws165
Vasta GR, Nita-Lazar M, Giomarelli B, Ahmed H, Du S, Cammarata M et al (2011) Structural and functional diversity of the lectin repertoire in teleost fish: relevance to innate and adaptive immunity. Dev Comp Immunol 35:1388–1399. https://doi.org/10.1016/j.dci.2011.08.011
Vasta GR, Ahmed H, Nita-Lazar M, Banerjee A, Pasek M, Shridhar S, Guha P, Fernández-Robledo JA (2012) Galectins as self/non-self-recognition receptors in innate and adaptive immunity: an unresolved paradox. Front Immunol 3:199. https://doi.org/10.3389/fimmu.2012.00199
Vitved L, Holmskov U, Koch C, Teisner B, Hansen S, Salomonsen J, Skjødt K (2000) The homologue of mannose-binding lectin in the carp family Cyprinidae is expressed at high level in spleen, and the deduced primary structure predicts affinity for galactose. Immunogenetics 51:955e964
Xue J, Hoorelbeke B, Kagiampakis I, Demeler B, Balzarini J, Liwang PJ (2013) The griffithsin dimer is required for high-potency inhibition of HIV-1: evidence for the manipulation of the structure of gp120 as part of the griffithsin dimer mechanism. Antimicrob Agents Chemother 57:3976–3989
Yao JH, Zhao XY, Liao ZH, Lin J, Chen ZH, Chen F, Song J, Sun XF, Tang KX (2003) Cloning and molecular characterization of a novel lectin gene from Pinellia ternate. Cell Res 13:301–308. https://doi.org/10.1038/sj.cr.7290175
Yu XQ, Kanost MR (2004) Immulectin-2, a pattern recognition receptor that stimulates hemocyte encapsulation and melanization in the tobacco hornworm, Manduca sexta. Dev Comp Immunol 28:891–900
Wang X-W, Wang J-X (2013) Diversity and multiple functions of lectins in shrimp immunity. Dev Comp Immunol 39:27–38. https://doi.org/10.1016/j.dci.2012.04.009
Wiens M, Mangoni A, D’Esposito M, Fattorusso E, Korchagina N, Schröder HC, Grebenjuk VA, Krasko A, Batel R, Müller IM, Müller WEG (2003) The molecular basis for the evolution of the metazoan Bodyplan: extracellular matrix-mediated morphogenesis in marine demosponges. J Mol Evol 57:S60–S75. https://doi.org/10.1007/s00239-003-0008-1
Wiens M, Belikov SI, Kaluzhnaya OV, Krasko A, Schröder HC, Perovic-Ottstadt S, Müller WEG (2005) Molecular control of serial module formation along the apical–basal axis in the sponge Lubomirskia baicalensis: silicateins, mannose-binding lectin and mago nashi. Dev Genes Evol 216:229. https://doi.org/10.1007/s00427-005-0047-2
Wu T, Xiang Y, Liu T, Wang X, Ren X, Ye T, Li G (2019) Oncolytic vaccinia virus expressing Aphrocallistes vastus lectin as a cancer therapeutic agent. Mar Drugs 17:363. https://doi.org/10.3390/md17060363
Xiong C, Li W, Liu H, Zhang W, Dou J, Bai X, Du Y, Ma X (2006) A normal mucin-binding lectin from the sponge Craniella australiensis. Comp Biochem Physiol Toxicol Pharmacol 143:9–16. https://doi.org/10.1016/j.cbpc.2005.11.008
Zhang H, Robison B, Thorgaard GH, Ristow SS (2000) Cloning, mapping and genomic organization of a fish C-type lectin gene from homozygous clones of rainbow trout (Oncorhynchus mykiss). Biochim Biophys Acta 1494:14e22
Zhang GQ, Sun J, Wang HX, Ng TB (2009) A novel lectin with antiproliferative activity from the medicinal mushroom Pholiota adiposa. Acta Biochim Pol 56:415–421
Zhang G, Sun J, Wang H, Ng TB (2010) First isolation and characterization of a novel lectin with potent antitumor activity from a Russula mushroom. Phytomedicine 17:775–781. https://doi.org/10.1016/j.phymed.2010.02.001
Zhao JK, Wang HX, Ng TB (2009) Purification and characterization of a novel lectin from the toxic wild mushroom Inocybe umbrinella. Toxicon 53:360–366. https://doi.org/10.1016/j.toxicon.2008.12.009
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors have no conflict of interest to declare.
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Abraham, A., Rafeeq, C.M., Karim, R., Rubeena, A.S. (2022). Aquatic Lectins: An Overview (A Paradigm). In: Elumalai, P., Vaseeharan, B., Lakshmi, S. (eds) Aquatic Lectins. Springer, Singapore. https://doi.org/10.1007/978-981-19-0432-5_1
Download citation
DOI: https://doi.org/10.1007/978-981-19-0432-5_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-0431-8
Online ISBN: 978-981-19-0432-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)