Abstract
The unnotified or undifferentiable early stages of oral squamous cell carcinoma (OSCC) progression are the prime reasons for late-stage detection and poor survival outcomes of oral cancer. This review summarizes the prior research and recent advancements on the influence of dysregulated non-coding RNA (ncRNA) on cell cycle and their employability as diagnostic and prognostic biomarkers of oral cancer. The literature search was performed using the following keywords: ‘serum/saliva non-coding RNAs’ and ‘serum/saliva non-coding RNAs and cell cycle’, ‘serum/saliva dysregulated ncRNAs and cell cycle’, ‘Cdk/CKI and ncRNAs’, ‘tissue ncRNAs’ concerning ‘oral cancer’’. The compiled data focuses mainly on the diagnostic and prognostic significance of MicroRNAs (miRNAs), Circular RNAs (circRNAs), and Long noncoding RNAs (lncRNAs) on oral cancer and all other cancers as well as subject-relevant articles published in languages other than English are beyond the scope of this review and excluded from the study. Moreover, articles focusing on DNA, protein, and metabolite markers are eliminated from the study. While there exist various potential biomolecules such as DNA, RNA, proteins, metabolites, and specific antigens representing predictive biomarkers in body fluids for oral cancer, this review completely focuses on non-coding RNAs restricted to saliva and blood, picking out a few of the reliable ones amongst the recent investigations based on the sophisticated techniques, cohort, and sensitivity as well as specificity, i.e., salivary miR-1307-5p, miR-3928, hsa_circ_0001874 and ENST00000412740, NR_131012, ENST00000588803, NR_038323, miR-21 in circulation. Thus, further studies are required to clinically confirm the usage of these non-invasive biomarkers in oral cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the entire Head and Neck cancer incidence, more than 95% comprises oral squamous cell carcinoma (OSCC) and is placed among the top ten cancer-related mortality worldwide [1]. In India, OSCC and oral cancer remain the most frequently seen, representing nearly one-third of the total incidences worldwide [2]. The common risk factors of OSCC include betel quid chewing with areca nut, excess tobacco usage or smoking, alcohol consumption, poor oral hygiene, and dietary deficiencies. Other factors include environmental factors, sustained viral infections, i.e., human papillomavirus (HPV), and microbial infections. Besides, genetics and epigenetic alteration contribute to the formation of oral cancer. Despite advancements in the knowledge gap and scientific findings over the previous 20 years, an oral cancer patient's 5-year survival rate remains below 50%. Diagnosis of OSCC early on with pharmacological and surgical interventions results in a better prognosis. However, due to unavailable appropriate biomarkers, recognizing at later stages where metastasis would have occurred results in high morbidity and low survival rate [3].
Most biomarkers, including the coding genes, constitute approx. 2% and the rest are non-coding genes that are often ignored. Nearly 98% of the human genome contains non-coding RNA (ncRNA) that does not possess a protein-encoding ability but functions as a master regulators of gene expression by serving as protein scaffolds, transcription factor regulators, post-transcriptional and epigenetic regulators. Alteration in gene expression is identified as a new hallmark in the development of cancer. Among ncRNAs, the long noncoding RNA (lncRNA) started to gain attention due to its underlying mechanism either as a tumor suppressor or an oncogene. Based on its over or under expression lncRNAs are regarded as a biomarkers in oral pre-malignant lesions, associated with tumor initiation, progression, and metastasis, as well as in drug resistance. LncRNAs are analyzed within the saliva and site-specific expression profile helping in the early diagnosis of OSCC and monitoring recurrence post-surgery [4].
In the same way, circular RNA (CircRNA) has also been investigated, perhaps providing a biomarker because of its similar property of miRNA sponging and regulating gene expression. Numerous MicroRNAs (miRNAs) are investigated for their diverse role in hallmarks of cancer and are putative markers for cancer diagnosis and prognosis. It is to be noted that comparatively fewer studies have been conducted on lncRNA and circRNA than miRNA exploiting its clinical utility as a non-invasive diagnostic and prognostic marker in saliva and blood samples.
Initially, the development of pre-malignant lesions is manifested as erythroplakia, leukoplakia, lichen planus, or the mixed forms that are triggered down to oral malignant forms. The incessant progression of the cell cycle is pivotal for tumor growth and thus frequent mutation in the cell cycle checkpoints is commonly seen in OSCC. The mutations in the p53 gene are found in more than 50% of the oral cancer tissues followed by CDKN2A mutations [5]. Surgical biopsy is established to be a gold standard in the diagnosis of OSCC, although there are certain setbacks as it is an invasive technique that targets a single tumor at a specific point in time and does not reveal spatiotemporal heterogeneity of solid tumors [6]. Hence discovering a better strategy to precisely characterize the disease to assist in diagnosis, prognosis, and treatment is of prime necessity.
Methodology
The current review provides comprehensive knowledge on the non-coding RNAs (lncRNA, circRNA, and miRNA) as prospective markers for oral cancer diagnosis and prognosis, considering their viability in saliva, serum, and plasma. The literature search was performed using major search engines like PubMed and Google Scholar between the timeline of 2012 to 2023. Around 2300 articles were obtained from the first keyword combination (“non-coding RNA” AND “oral cancer”), 312 from (“non-coding RNA” AND “oral cancer” AND “cell cycle”), 78 from (“serum non-coding RNA” AND “oral cancer”), 81 from (“plasma non-coding RNA” AND “oral cancer”), 143 from (“salivary non-coding RNA” AND “oral cancer”). The compiled data focuses mainly on the diagnostic and prognostic significance of miRNAs, circRNAs, and lncRNAs in oral cancer and all other cancers. The subject-relevant articles published in a language other than English are excluded from the review. Moreover, DNA, metabolites, protein, and peptide markers are not included in the review.
Overview of cell cycle: CKIs, dysregulated cyclin and Cdks
The cell cycle consists of gap phases G1 and G2, S, and M phases regulated by serine-threonine kinases called Cdks with their regulatory subunit cyclins. The human genome encodes around 20 Cdks and 29 cyclins. Cdk 1,2,4,6 is directly involved in the cell cycle, whereas Cdk 7,8,9 has a major role in the regulation of gene expression. Besides participating in DNA damage and response, stemness, metabolism, and angiogenesis. Upon external stimulus, the Cdk4, 6/ Cyclin D complex initiates phosphorylation of the Rb pocket in the early G1 phase releasing transcription factor E2F which further transcribes genes involved in the cell cycle progression. In the late G1 phase, the Cyclin E/Cdk2 complex completes the phosphorylation of E2F and initiates the S phase. Cyclin A/Cdk2 and cyclin B/Cdk1 fulfil DNA synthesis and mitotic phases. The G0 phase also called the quiescence stage or dormancy period, is mediated by the cyclin C/Cdk3 complex.
Endogenous CKIs (Cyclin-dependent kinase inhibitors) can restrain the activity of specific cyclin-Cdk complexes thus regulating the cell cycle. The different families of CKI include INK4, Cip/Kip, and ribosomal protein-inhibiting Cdks (RPICs). Compounds resembling the action of CKIs are clinically developed to destroy the hyperproliferative cells. These include first-generation pan CKIs Flavopiridol and roscovitine though lacking specificity further led to the invention of selective second-generation CKIs mainly pan-Cdk inhibitor AT7519 and dinaciclib which even failed in the clinical trials. Consequently, it paved the way for third-generation Cdk4/6 selective inhibitors such as Palbociclib, Ribociclib, and Abemaciclib to get clinically approved for breast cancer [7]. A study on Histone deacetylase inhibitors (HDACis) in combination with Cdk4/6 inhibitor Abemaciclib showed better sensitivity and enhanced antitumor activity against OSCC [8].
The dysregulation of cyclins, Cdks, and CKIs are commonly implicated in both pre-cancerous (dysplastic) lesions and oral cancer. Mutations in retinoblastoma (Rb) protein alongside the loss of p16 activity on cyclin D-Cdk4/6 are commonly manifested in oral carcinogenesis. The downregulation of p16, a tumor suppressor and cell cycle controller, by targeting cyclin D1 is associated with the transformation of the dysplastic lesion and poor survival in OSCC patients. Inactivation of tumor suppressor genes via hypermethylation of promoter regions of p14 and p16 is commonly manifested in pre-cancerous lesions and OSCC [9]. A study conducted had [10] identified that overexpression of p53 and EGFR of cytoplasmic and nuclear sublocation decreased the overall survival rate in OSCC patients. The cyclin-dependent kinase inhibitor p27KIP1 internally controls the cell cycle at G0 to S phase by regulating cyclin D/Cdk4 and cyclin D/Cdk6 complexes. p27 is also identified as an anti-oncogene and functions mainly in regulating cell proliferation, and apoptosis and is found to be a therapeutic target and prognostic marker of OSCC. The level of p27 is reported to be reduced in correspondence to the increased expression of cyclin D1 and corresponding Cdks in the early stage of OSCC development [11]. Silencing of Cdk5 slowed down G1 to S phase and vice versa is directly correlated with poor survival and tumor development in tongue squamous cell carcinoma (TSCC) [12].
At the same time, overexpression of Cdk1 and cyclin B1 are commonly observed in patients with recurrent OSCC or lymph node metastasized OSCC. The cumulative 5-year survival period of Cdk1-positive patients remarkably declined than the negative ones validating its role to be manipulated as a prognostic marker for OSCC survival [13]. The overexpression of cyclin E and B1 is associated with advanced tumor stage and severity level of OSCC and larynx cancer. Cyclin B1 is a reliable indicator of lymph node metastasized tongue carcinoma. Among the D cyclins, Cyclin D1(a proto-oncogene) is commonly overexpressed in OSCC by inappropriate mitogen expression or receptor activation and the commencement of the Rb pathway evading the inhibitory signals. The risk factors of OSCC leave the chromosomal region 11q13 vulnerable to aberrant amplification of genes associated with it predominantly CCND1 coding for cyclin D1 [14]. Owing to the mutation in cyclin D1, it cannot be cleared via the 26S proteasomal ubiquitin mechanism in the cytoplasm leading to its nuclear accumulation. Further altering the Cdks, inhibitory proteins, cell cycle checkpoints, obstinate towards growth factors, and eventually progressing into a pile of abnormal cells [15]. The upregulation of cyclin D1 Cdk4/6 results in the increased severity of dysplasia to cancerous tissue is attributed to its early and late-stage characterization in carcinogenesis and in concordance with the downregulation of tumor suppressor genes that code for the INK family of inhibitors [16].
Non-coding RNAs
All the cyclins, Cdks, and CKIs are regulated by ncRNAs either at the transcriptional or post-transcriptional level. ncRNAs are 98% of human genes that do not encode any proteins. The ncRNAs are mainly categorized according to their size into small and long non-coding RNAs consisting of miRNAs, and circRNAs proven to have a constituent role in oral cancer. Short interfering RNAs (siRNAs), PIWI-interacting RNAs (piRNAs), small nucleolar RNAs (snoRNA), small nuclear RNA (snRNA), repeat-associated RNAs (rasiRNAs), and additional ncRNAs yet to be decoded and characterized [17].
Cell cycle regulatory miRNAs in OSCC
miRNAs and siRNA are short single-stranded RNAs of around 20–22 nucleotides that play a huge role in gene expression and regulation. miRNAs are initially transcribed into primary miRNA (pri miRNA), a stem-loop structure by RNA pol II. If there is perfect complementarity between the sequences of miRNA and mRNA it leads to endonucleolytic cleavage of the target mRNA whereas its partial complementarity leads to translational silencing of the mRNA. miRNAs are involved in both tumor development and suppression. They are involved in regulating a large number of coding and non-coding genes encompassing some of the crucial oncogenes RAS, MYC, and EGFR along with the tumor suppressors TP53, PTEN, and BRCA1. miR21 is identified as an oncomiR since it is overexpressed in progressive leukoplakia and OSCC. miR-184 is overexpressed in TSCC and functions as an anti-apoptotic factor altering the pro-apoptotic factor c-myc and promoting cell proliferation whereas its downregulation has also been reported. MiR24 is studied for its role in regulating CDKN1B gene encoding p27kip1, inevitably cell proliferation and evasion of apoptosis. In addition, miR-196a and miR-10b are also found to be overexpressed in OSCC. Downregulation of miR-125b and miR-100 [18], miR-596, miR-494 [19], miR-145 (targets c-Myc and Cdk6) [20], and miR-195 are involved in the regulation of cell cycle proteins cyclin D1 and Bcl2 [21] in oral cancer indicates its involvement as a tumor suppressor. MiR-137 and miR-193a were demonstrated to be downregulated in OSCC possessing a crucial role in inhibiting the expression of Cdk6 and E2F6 resulting in the arrest of cell cycle progression and apoptosis. miR-503 and miR-15a are involved in the downregulation of cyclin D1 and E and as a consequence, the level of these miRNAs is found to be declining in OC [22]. miR-222-3p a tumor promoter miRNA showed a higher expression in OSCC tissues and is associated with clinical staging and lymph node metastasis while the 5-year survival rate declined in comparison to the low expression group. miR-222-3p modulates its action through downregulation of CDKN1B/p27 [11].
Mir5100 promotes proliferation in OSCC, attenuating the G1-S phase and decreasing the action of SCAI, a suppressor of cancer cell invasion. Consequently, the mir5100 inhibitor inhibited the G1-S transition by lowering the expression of proliferative genes Cdk2 and Cyclin D and upregulating p27 expression. Furthermore silencing of miR5100 suppressed the migration and invasion of OSCC via tuning of SCAI [23]. Upregulation of miR-504 decreased the expression of Cdk6, cyclin D1, and E2F1 implying its function as a tumor suppressor in OSCC [24]. Downregulation of miR-191 silenced the expression of PCNA, CDK4, and other proteins in the downstream Wnt/β-catenin signaling pathway exerting an anti-tumor effect on OSCC cells via. Phospholipase C delta1 (PLCD1) [25]. Oral pre-malignant disorders (OPMDs) are early epithelial lesions seen in the oral cavity possessing a greater chance of transforming into a malignancy. They manifest features such as oral leukoplakia (OLK), oral lichen planus (OLP), and oral submucous fibrosis (OSF). Among a variety of miRNAs altered in OLK, miR-10b-3p is one found in the saliva of mild dysplastic OLK. Eight miRNAs were found to be intensified in progressing OLK notably miR-345/21/181b subset, and therefore miRNAs demonstrate a strong attribute in differentiating between progressing and nonprogressive mild OLK. Chronic inflammation and degeneration of keratinocytes are the underlying pathological causes recognized in OLP. miR-27b and miR-125b are found to be downregulated in OLP whose function is to inhibit keratinocyte proliferation and promote apoptosis. Thus aiming miRNA can be an approach to hinder the transformation of OLP to OSCC [26]. Mir-9 was found to inhibit cell proliferation of OSCC through the downregulation of Cdk6 and Cyclin D1 and stopping the cell from G1/S transition thus functioning as a tumor suppressor [27].
Cell cycle regulatory circular RNAs in OSCC
The other major class of ncRNA is the ones bearing more than 200 nucleotides called lncRNAs processed by RNA pol II which includes long intergenic ncRNAs (lincRNAs), antisense RNAs (asRNAs), pseudogenes, and circRNAs. CircRNAs are ncRNAs with covalently linked 5′3’ends without a 5’polyadenylated tail forming a circular loop mainly because of back splicing of pre mRNA and realignment of exons. circRNA contains more than 200 nucleotides and is resistant to endonucleolytic cleavage. They are also called miRNA sponges since they bind to miRNA and stop its action of silencing mRNA besides the regulatory function. Because of these reasons, circRNAs are used as a therapeutic strategy to alleviate the overexpressed miRNA in cancer. In addition, circRNAs are involved in epithelial-mesenchymal transition (EMT) and, therefore involved in tumor metastasis and invasion [17, 28]. Knockdown of circYap, a circular RNA obtained from the YAP (Yes-associated protein) gene, upregulated PCNA and C-MYC as well as Rb and Akt phosphorylation. Further overexpression of Cyclin D1/Cdk4 and transformation of the G1-S phase specifying circYAP could impede OSCC progression [29].
circ_SEPT9 enhanced OSCC progression via miR‐1225 and PKN2 [30]. Upregulation of circATRNL1 enhanced the sensitivity of OSCC towards radiotherapy through endogenous sponging of miR-23a-3p, alleviating the inhibitory action on PTEN and promoting apoptosis and cell cycle arrest [31]. Upregulation of circ_0000745 in OSCC increased the expression of CCND1(cyclin D1) via the mediatory interaction with miR-488 and RNA-binding protein (HuR) [32]. Expression of circCLK3 is linked with TSCC progression by negatively regulating its target miR-455-5p while positively regulating PARVA a cancer progressive gene associated with CAM, integrins, and cytoskeleton involved in metastasis and angiogenesis. In addition, the knockdown of circCLK3 preceded G0/G1 phase arrest by decreasing the expression of cyclin D1 and cyclin E1 and modulating [33]. Downregulation of hsa_circRNA_102459 and knockdown of upregulated hsa_circRNA_043621 induced apoptosis and arrest of the G0/G1 phase in OSCC cells [34]. The recently identified circRNAs as biomarkers in biological fluids, saliva, and circulation are mentioned in Tables 1 and 2.
Cell cycle regulatory long non-coding RNAs in OSCC
Lack of consistent results in identifying miRNAs as a promising tumor biomarker shifted the paradigm of research towards lncRNAs which in the past was considered junk gene. LncRNAs are involved in the regulation of gene expression at the post-transcriptional level via binding to the chromatin-modifying complexes and transcription factors. Besides participating in epigenetic regulation, lncRNAs play a direct role in cell cycle regulation and differentiation. Also involved in inhibiting transcription by targeting RNA pol II and preventing translational silencing by masking the miRNA binding site i.e., miRNA sponging. Based on an initial study conducted on lncRNA expression profiling of oral mucosa, nearly 325 lncRNA was expressed, and more than half of it is altered in dysplasia of the oral cavity. Prominent lncRNAs such as HotairR (Hox Transcript Antisense RNA), Neat1(Nuclear Enriched Abundant Transcript (1), Uca1 (urothelial cancer associated (1), LncHIFCAR are found to be elevated. While Meg-3(Maternally expressed gene (3) possesses a suppressive action on Wnt/β-catenin signaling in tumors and functions as a regulatory element of enzyme DNA methyltransferase 3B is reported to be downregulated in saliva and tissue samples from OSCC and TSCC patients orchestrating poor patient survival. Hotair and Taurine upregulated gene 1 (Tug1) is overexpressed in lymph node metastasized OSCC. High expression of Uca1 is associated with migratory ability and regional lymph node metastasis in tongue squamous cell carcinoma. ZEB1-AS1, HOXA11-AS, LINC01116, and LEF1-AS1 were also found to have a role in the development of OSSC and showed a poor prognosis [35, 36]. HOTAIR performs its role by employing polycomb repressive complex 2 (PRC2) subunit, a chromatin remodeling complex whose function is in gene silencing or transcriptional repression and thus binding to the promoter region of tumor suppressor gene PTEN inactivates its expression and condensation of chromatin resulting in tumor progression [37]. HOTTIP (HOXA distal transcript antisense RNA)is involved in the carcinogenesis and migration of OSCC cells and acts as a competing endogenous (ceRNA) of miR-206 [38].
One among the frequently overexpressed lncRNAs in OSCC includes DLEU1 (deleted in lymphocytic leukemia 1) is associated with a low survival rate in HNSCC patients and is linked with the expression of multiple oncogenes demonstrating it as a possible target for therapeutics and prognosis [39]. HOXA-AS3 negatively regulates miR-218-5p a tumor suppressor that inhibits COL1A1 and LPCAT1 in the proliferation and migration of OSCC [40]. A study on integrative profiling of lncRNA identified the role of DUXAP8 in oral carcinoma (OC). DUXAP8 localized in the nucleus of OC cells binds to histone methyltransferase enhancer of zeste homolog 2 (EZH2) a commonly dysregulated methyltransferase enzyme. It represses the transcription of tumor suppressor gene KLF2 resulting in tumor formation although its complete underlying mechanism is unclear [41]. SOX21AS1 suppresses the growth and invasion of cancer cells either by acting as a decoy preventing the binding of the transcription factor to the DNA and transcription of oncogenes or by enhancing other tumor suppressors. Thus downregulation of lncRNA SOX21AS1 is associated with a poor prognosis of OSCC [42]. Overexpressed GASL1 induces the arrest of the G0/G1 phase by inhibiting cyclin D/CDK4 and apoptosis of OSCC cells [43]. LUCAT-1 (lung cancer-related transcript 1) is upregulated in OSCC and positively correlated with the expression of PCNA (proliferating cell nuclear antigen) [44]. lncRNA GACAT1(Gastric cancer-associated transcript 1) is involved in OSCC proliferation and migration, negatively targeting miR-149, as a result, the knockdown of GACAT1 promoted autophagy and apoptosis of the cell [45] Fig. 1.
In normal cells, MALAT1 (metastasis-associated lung adenocarcinoma transcript (1) participates in alternative splicing of pre mRNA by functioning along with various pre-mRNA splicing complexes such as the Ser/Arg (SR) splicing factor. It is a transcript of Neat 2 and is accepted to be involved in the metastasis of OSCC. The knockdown of MALAT1 downregulated N-cadherin and Vimentin while upregulation of E-cadherin. Moreover, the nuclear and cytoplasmic levels of β-catenin and NF-κB are suppressed elucidating the role of MALAT1 lncRNA in EMT and metastasis of OSCC through modulation of β-catenin and NF-κB pathway [46]. Enhanced expression of lncRNA TIRY from Cancer-associated fibroblasts (CAF)-derived exosomes notably reduced miR-14 expression and downstream activation of Wnt/β-catenin and EMT expression displayed in OSCC tissues [40]. ZEB1-AS1 and RBM5-AS1 are highly expressed in tumor tissues and sponging of its corresponding miRNA, miR-23a, and miR-1285-3p respectively contributed to the invasion, migration, and proliferation of OSCC [47, 48]. BRAF-activated long non-coding RNA (BANCR) overexpression is seen in OSCC correlated with its carcinogenic property [48]. LOLA1 (lncRNA oral leukoplakia progressed associated (1) is found to help predict the transformation stage from OL to high-risk dysplasia to early-stage OSCC via AKT/GSK‐3β pathway. It is involved in EMT transition [49]. Expression of RPSAP52 was identified to be negatively involved in the expression of miR‐423‐5p and promoted tumor growth,TNM staging of TSCC, and release of cytokines such as IL-1β,6, 8, 10 and TGF-β via MYBL2 [50]. LncRNA ZFAS1 promoted OSCC development through the sponging of miRNA-6499-3p and activation of CCL5 [51]. LncRNA PHACTR2-AS1 through ceRNA of miR-137 activates EMT transcription factor Snail as a result of TSCC migration [52]. The schematic illustration of various cell cycle regulatory ncRNAs is put together in Fig. 2.
LncRNAs, miRNAs, and CircRNAs displaying resistance to chemo regimen
Enhanced expression of CASC2 increased the chemosensitivity of OSCC cells resistant to cisplatin through the expression KANK1 and sponging of miR-31-5p [53]. Transcription factor FOXD1 transcribes lncRNA CYTOR sponges miR-1252–5p and miR-3148 and upregulation of lipoma-preferred partner (LPP) expression is positively linked with cisplatin chemoresistance [54]. CEBPA-DT overexpression augmented the cisplatin-induced chemoresistance in OSCC cells via CEBPA/BCL2 axis [55] Overexpression of chemotherapy-induced lncRNA 1(CILA1) via Wnt/β-catenin signaling involved in chemoresistance of TSCC [56]. One more factor implicated in resistance against cisplatin-induced death of OSCC is Medkine, a heparin-binding growth factor released by CAFs that elevates the expression of lncRNA ANRIL. Restriction of ANRIL in tumor cells had an impact on increasing the sensitivity towards cisplatin by blocking the action of drug transporters MRP1 and ABCC2 and therefore enhancing the cisplatin cytotoxicity, apoptosis, and inhibiting proliferation of tumor cells [57] Uca1 demonstrated enhanced chemoresistance in cisplatin-induced OSCC through the sponging of miR-184 and inhibiting its role in tumor suppression [58]. Overexpression of GAS5 in conjunction with downregulated GAS5 boosted cisplatin chemosensitivity in oral cancer cell lines and OSCC tissues [59]. KCNQ1OT1 sponges miR-211-5p and upregulation of Ezrin/Fak/Src signaling leading to cisplatin resistance in TSCC [60].
Inhibitory miRNA-485-5p attenuated keratin 17 gene and its signaling pathway involving integrin/FAK/Src/ERK/β-catenin through which OSCC cells attain the property of stemness, EMT, cisplatin resistance, and poor outcome of survival [61]. Under expression of miR-15b, upregulation of TRIM14, and further activation of ERCC1 and YAP led to chemoresistance on TSCC [62]. Intratumoral transfer of miR-155 inhibitor in cisplatin-resistant OSCC reduced the stemness, and EMT expression, declined the expression of efflux pump protein, and upregulated the expression of FOXO3a, thereby reducing the tumor [63]. In vitro and in vivo transfection of exosomal mir200c significantly sensitized the Docetaxel-resistant TSCC cells by targeting TUBB3 and PPP2R1B [64]. ZEB1 attaches to the promoter region of carbonic anhydrase IX (CA9) positively regulating its activity of chemoresistance, preventing the decrease in the pH and caspase 3-mediated apoptosis induced by chemotherapy [65]. miR-1224-5p bound with NSD2 partially neutralized the action of lncRNA APCDD1L-AS1 in 5-fluorouracil induced OSCC resistance [66]. Loss of expression of miR-132 upregulated TGF-β1/Smad2/3 signaling as a result of proliferation, invasiveness, and cisplatin resistance in OSCC [67]. The expression of circANKS1B and TGF-β are closely associated, and thus, targeting TGF-β through the modulation of circANKS1B is presumed to prevent cisplatin resistance in OSCC [68].
Stem cell biomarkers of cell cycle
Cancer stem cells (CSCs) are initiated from a cancer cell with the property of self-renewal and differentiation showing metastatic potential and resistance towards chemo and radiotherapy as well as tumor recurrence. Hence targeting specific biomarkers expressed on the CSCs can aid towards therapeutic innovation. The most frequently expressed CSC biomarkers in HNSCC are CD44, CD24, CD98, c-MET, CD133, CD166, Notch 1, ALDH1, SOX2, Nanog, OCT4. CD147 inhibits p53 signaling pathway and its higher expression is associated with a poor prognosis of OSCC. The expression of CD147 was in concurrent with the severity and progression of dysplasia from OLK to OSSC [69]. The same was observed on the expression of aldehyde dehydrogenase (ALDH1/2) differentiating dysplastic and non-dysplastic OSCC. Anterior gradient protein 2 (AGR2), is involved in the suppression of p53, regulating cyclin D1, and EMT Transition that is directly correlated with the stemness of OSCC [70].
The impact of lncRNAs on cell cycle
Around 42% of the genes dysregulated in the cell cycle belonged to ncRNAs, specifically lncRNAs. Most of it was overexpressed in the G1 phase demonstrating its role during G1 to S transition. LncRNAs are expressed upon DNA damage and regulate the transcription of the CCND1 gene. The tumor-suppressive lncRNA growth arrest specific 5 (GAS5) inhibits Cdk6 and upregulates p27kip1 restraining the proliferation of tumor cells. Besides the lncRNAs, ANRIL, p15AS, or CDKN2B-AS1 are involved in the development of tumors by regulating CKI via epigenetic repression of the INK4/ARF locus. MALAT 1, LAST (LncRNA-Assisted Stabilization of Transcripts), and MIR100HG are overexpressed in the G1/S transition. The cancer stem cells attain resistance to antiproliferative drugs by undergoing quiescence or entry into the G0 phase and the property of stemness is contributed by various lncRNAs such as GAS5 and DANCR however, the complete mechanism needs to be investigated further. lncRNA viz SUNO1 plays a role in S phase progression by its interaction between the helicases of DNA replication in tumor cells. The lack of lncRNA-RI, APAL, and OIP5-AS1 produced mitotic defects linking lncRNA in regulating mitotic kinases [22]. Downregulation of MALAT1 appeared to inhibit cell cycle proliferation arresting the G2/M phase and ceasing tumor growth of different cancer types in both in vitro cell lines and in vivo tumor xenograft nude mice models [71]. Knockdown of SLC16A1-AS resulted in decreased expression of Cyclin D1 and ceased OSCC cell proliferation at the G0/G1 phase[72]. CCAT1 aberrant expression downregulated Cdk4 and cyclin D1 while suppressing miR-181, a tumor suppressor, and activating Wnt signaling [73]. Overexpression of oncogenic lncRNA SLC16A1-AS prevented G0/G1 arrest, decreased cyclin D1, and proliferation of OSCC cells [72]. Identifying the function of the fifth genetic element within the INK4 locus ANROC (associated negative regulation of cell proliferation) is presumed to raise the expression of p16INK4A, p15INK4B, and ARF whereas inhibited cyclin B1 and arrest of G2 to M phase. Thus, denoting the action of lncRNA ANROC in ceasing the cell cycle by modulating cell cycle-related proteins [74].
Salivary miRNA, a biomarker for oral cancer
The saliva forms the environment in which the oral tissues are constantly immersed in and hence identifying a biomarker within the salivary secretions could be used as a non-invasive, easily accessible, storable, and safe to handle diagnostic and prognostic tool in oral cancer patients. Although saliva consists of various enzymes such as ribonucleases that degrade RNA, some protective measures are utilized by miRNA to remain stable within the harsh conditions of biological fluids with the aid of exosomes. The ncRNA miR-31 was found to be more highly upregulated in salivary specimens than that of plasma, implying its significant local contribution via salivary contents, and its secretion even from minute tumors illustrates its sensitivity in detection. This sensitivity remains the same when it comes to the case of advanced tumors as well. Following surgical excision of the tumor, the level of miR31 declined significantly, inferring its contribution to the salivary RNA pool specifically by the tumor tissue within the oral cavity [75]. Salivary levels of miRNA-21 were significantly increased in OSCC compared to the normal and diseased controls while there was no significant difference between the dysplastic PMD suggesting miRNA-21 could be an early molecular marker during the pathogenesis of oral cancer. Moreover, this study also revealed that miRNA-184 can be used to distinguish between OSCC and dysplastic PMD. Besides salivary miRNA -145 results demonstrated its decrease whose restoration in OSCC influenced cell apoptosis by arresting the G1 phase and hindering the activity of Cdk6 and c-Myc [76].
In a study on miRNA expression in saliva conducted within OSCC, OLP, OSCC-remission patients, and the healthy ones, miRNA-27b showed an increased expression in OSCC patients compared to the latter groups presuming it to be a promising marker in the detection of OSCC which has also been reported to be an oncogenic miRNA [77]. Upregulation of salivary miR-21 and miR-31 is observed in OPMD patients when compared to the healthy ones, whereas miR-31 showed a significantly high expression in OPMD transformed to malignant forms and on relapse of post-initial treatment as well. The oncogenic characteristic of miR-31 is attributed to targeting FIH-HIF-VEGF regulatory cascade and blockage of DNA repair mechanism, concomitant cell proliferation, and genomic instability. Moreover, previous studies reported an upregulated miR-31 in tumors of the tongue and OSCC of buccal mucosa [78]. miR-139-5p was found to be downregulated in the salivary samples of TSCC patients in contrast to healthy individuals. Subsequently, the resection of the tumor or post-operative analysis of TSCC saliva samples after 4–6 weeks demonstrated the restoration of salivary miR-139–5-p expression back to normal. Thus, specifying the downregulation of miR-139 was by the tumor and has been suggested to be a diagnostic and prognostic biomarker [79]. The recent OSCC salivary miRNA biomarkers are compiled in Table 1.
Circulatory miRNA, a biomarker for oral cancer
The stability of miRNA within the whole blood, serum, or plasma and their altered expression is utilized in the diagnosis and management of cancer and thus miRNA is demonstrated to be a non-invasive biomarker. A study comparing the miRNA expression in whole blood of OSCC, and healthy patients showed aberrant expression of miR-186, miR-3651, and miR-494. Upregulation of miR-3651, miR-494, and downregulation of miR-186 conclude its prominence in carcinogenesis and progression in OSCC [80]. The serum miR-9 expression is compared in patients with OLK and OSCC with healthy individuals. The results stipulated the significant downregulation of miR-9 expression, consequently reducing Cdk6 and Cyclin D1 compared to the healthy lots specifying it to be a tumor suppressor. It is correlated with advanced stages of OSCC and is a potential prognostic marker [81]. One of the works done on circulatory miRNA involves comparing miRNA expression from pooled plasma of around 5 patients with GSCC (gingival squamous cell carcinoma) versus healthy individuals. The highly expressed miRNA includes miR-26a, miR-126, miR-223, and miR-21, and due to the abundant expression of miR-223 was further investigated that revealed its upregulated expression not only within the plasma but by the noncancerous tissue as well. This implies that the plasma miR-223 is released by the healthy tissue as a part of a biological defense mechanism against tumor growth which correlates with its tumor suppressive action designating its potential as a diagnostic as well as therapeutic marker. The receiver operating characteristic (ROC) analysis curve produced an AUC value of 0.73 and a moderate level of specificity and sensitivity [82]. Plasma miR-196a/b was found to be highly expressed during the initial stages of tumorigenesis and its progression while miR-196a showed better specificity whereas mir-196b showed better sensitivity thus usage of multiple biomarkers complemented the efficacy of these two molecules in detecting oral cancer [83]. It was noted that plasma miR-187 level was 6.4-fold high in pre-operative OSCC patients(n = 63) whose level dropped by threefold following the tumor resection compared to the healthy controls (n = 26). Thus suggesting miR-187 as a marker for survival chances post-surgery [84]. Another study proved the metastatic potential of OSCC contributed by miR-296 in plasma in contrast to miR-130b, which is over-expressed in non-metastatic plasma samples [85]. A study conducted by Xu Huanxi and his colleagues on miRNA expression in the sera of OSCC patients (n = 101) before the surgery without any history of radio or chemotherapy identified significant upregulation of miR-483-5p especially of TNM staging III and IV category exhibiting AUC = 0.85 and sensitivity, specificity (0.853,0.746) correlating with lymph node metastasis [86]. The recent OSCC miRNA biomarkers in circulation are listed in Tables 2 and 3.
Salivary lncRNA, a biomarker for oral cancer
Haikuo tang et al. investigated the presence of lncRNA in saliva, a non-invasive sample from lymph node metastasized OSCC patients, and identified HOTAIR to be significantly expressed than other lncRNAs (HULC, MALAT-1, MEG-3, NEAT-1, UCA1) investigated in the study. MALAT-1 was expressed, but no significant difference was seen between metastatic and non-metastatic OSCC. Although NEAT-1 is abundantly present in metastatic tissues, there was no expression of it identified due to its least stability [87]. The recent salivary lncRNA biomarkers of OSCC are mentioned in Table 4.
Circulatory lncRNA, a biomarker for oral cancer
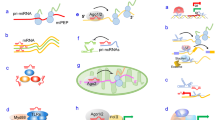
Merdan Fayda and his colleagues looked for three lncRNAs GAS5, lincRNA-p21, and HOTAIR in plasma collected from Head and neck cancer patients (n = 41) pre and post-chemoradiotherapy (CRT) to determine its diagnostic and prognostic relevance in circulation. The level of GAS5 significantly declined post-treatment compared to pre-group, justifying that the decline in the malignant tissues decreased the release of GAS5 from these tissues while patients with the poorer response towards CRT exhibited raised levels in circulation. Thus, GAS5 outlines the progress of CRT in HNSCC patients [88]. There are only limited investigations done on circulatory and salivary lncRNA and hence further research needs to be done in these fields. The recently investigated lncRNA biomarkers of oral cancer are listed in Table 3 and Fig. 1 represents the ncRNA biomarkers in various biological samples.
Result and conclusion
The identification and quantification of non-coding RNAs in biological samples are widely performed owing to the sophisticated molecular detection methods and thus in the near future, there will be a complete shift towards these non-invasive biomarkers. Firstly, the rationale behind adopting a liquid biopsy over tissue marker is its non-invasive property, repeatable and quick sampling, less complexity, and feasibility. Secondly, they provide a broader picture of the tumor than just the snapshot, early detection, real-time monitoring for targeted therapy and personalized medicine, and detection of drug resistance and prediction of recurrence post-treatment [89]. In the present article, we have clubbed all the miRNA, circRNA, and lncRNAs detected within saliva and blood (Serum and plasma) of oral cancer patients and their influence on the cell cycle from 2012 to 2023. While generous amounts of reads were available on miRNA, a few studies are available about circRNA and lncRNA on oral cancer, specifically on the mentioned body fluids. Considering the diverse patient cohort, molecular techniques performed, sensitivity and specificity in detection, and based on the AUC curve we have curated a few of the ncRNA diagnostic panels. salivary miR-1307-5p, miR-3928, hsa_circ_0001874 and plasma ENST00000412740, NR_131012, ENST00000588803, NR_038323, miR-21, miR200b-3p in circulation which needs to be further deciphered on their mechanism of action, regulatory mechanism, and complex pathway through which their oncogenic property is obtained. Hence future investigations are required to identify the therapeutic targets and clinically approve these biomarkers for oral cancer diagnosis and prognosis.
Data availability
No datasets were generated or analyzed during the current study.
Abbreviations
- OSCC:
-
Oral squamous cell carcinoma
- HNSCC:
-
Head and Neck squamous cell carcinoma
- OSMF:
-
Oral submucous fibrosis
- HPV:
-
Human papillomavirus
- ncRNA:
-
Non-coding RNA
- lncRNA:
-
Long non-coding RNA
- CircRNA:
-
Circular RNA
- miRNA:
-
MicroRNA
- CKIs:
-
Cyclin-dependent kinase inhibitors
- RPICs:
-
Ribosomal protein inhibiting Cdks
- HDACis:
-
Histone deacetylase inhibitors
- Rb:
-
Retinoblastoma
- TSCC:
-
Tongue squamous cell carcinoma
- siRNAs:
-
Short interfering RNAs
- piRNAs:
-
PIWI-interacting RNAs
- snRNA:
-
Small nuclear RNA
- snoRNA:
-
Small nucleolar RNAs
- rasiRNAs:
-
Repeat-associated RNAs
- pri miRNA:
-
Primary miRNA
- OPMDs:
-
Oral pre-malignant disorders
- OLK:
-
Oral leukoplakia
- OLP:
-
Oral lichen planus
- OSF:
-
Oral submucous fibrosis
- lincRNAs:
-
Long intergenic ncRNAs
- asRNAs:
-
Antisense RNAs
- EMT:
-
Epithelial-mesenchymal transition
- YAP:
-
Yes-associated protein
- HotairR:
-
Hox Transcript Antisense RNA
- Neat1:
-
Nuclear Enriched Abundant Transcript 1
- Uca1:
-
Urothelial cancer associated 1
- Meg-3:
-
Maternally expressed gene 3
- Tug1:
-
Taurine upregulated gene 1
- PRC2:
-
Polycomb repressive complex 2
- HOTTIP:
-
HOXA distal transcript antisense RNA
- ceRNA:
-
Competing endogenous RNA
- DLEU1:
-
Deleted in lymphocytic leukemia 1
- OC:
-
Oral carcinoma
- EZH2:
-
Enhancer of zeste homolog 2
- LUCAT-1:
-
Lung cancer-related transcript 1
- PCNA:
-
Proliferating cell nuclear antigen
- GACAT1:
-
Gastric cancer-associated transcript 1
- MALAT1:
-
Metastasis-associated lung adenocarcinoma transcript 1
- CAF:
-
Cancer-associated fibroblasts
- BANCR:
-
BRAF activated long non-coding RNA
- LOLA1:
-
LncRNA oral leukoplakia progressed associated 1
- LPP:
-
Lipoma preferred partner
- CILA1:
-
Chemotherapy-induced lncRNA 1
- LPP:
-
Lipoma preferred partner
- GAS5:
-
Growth Arrest Specific 5
- LAST:
-
LncRNA-assisted stabilization of transcripts
- ANROC:
-
Associated negative regulation of cell proliferation
- ROC:
-
Receiver operating characteristic
References
Hyuna S et al (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71:209–249
Swati S et al (2018) Oral cancer statistics in India on the basis of first report of 29 population-based cancer registries. J Oral Maxillofac Pathol 22:18
Purandar S et al (2021) Dysbiosis of Oral Microbiota During Oral Squamous Cell Carcinoma Development. Front Oncol 11:1–15
Ganesan A et al (2017) Expression profiling of long non-coding RNA identifies linc-RoR as a prognostic biomarker in oral cancer. Tumor Biol. https://doi.org/10.1177/1010428317698366
Vasileios R et al (2018) P53 mutations in oral cavity carcinoma. J BUON 23:1569–1572
Ying C et al (2021) Longitudinal detection of somatic mutations in saliva and plasma for the surveillance of oral squamous cell carcinomas. PLoS ONE 16:1–15
Marina B, Le CB, Gerardo F, Volker B, Frédéric L (2021) Cell biology new insights into CDK regulators novel opportunities for cancer therapy. Trends Cell Biol 31:331–344
Borui Z et al (2019) Enhancement of histone deacetylase inhibitor sensitivity in combination with cyclin-dependent kinase inhibition for the treatment of oral squamous cell carcinoma. Cell Physiol Biochem 53:141–156
Maria G et al (2021) Understanding the complex pathogenesis of oral cancer: A comprehensive review. Oral Surg Oral Med Oral Pathol Oral Radiol 132:566–579
Monteiro Luís Silva et al. 2012 Combined cytoplasmic and membranous EGFR and p53 overexpression is a poor prognostic marker in early stage oral squamous cell carcinoma. J Oral Pathol Med 41: 559–567
Guangzhao G, Bakr Mahmoud M, Norman F, Love RM (2018) Expression of cyclin D1 correlates with p27KIP1 and regulates the degree of oral dysplasia and squamous cell carcinoma differentiation. Oral Surg Oral Med Oral Pathol Oral Radiol 126:174–183
Yixuan Li et al (2022) Cyclin-dependent kinase 5 promotes the growth of tongue squamous cell carcinoma through the microRNA 513c–5p/cell division cycle 25B pathway and is associated with a poor prognosis. Cancer 128:1775–1786
Xin C et al (2015) The clinical signifcance of cdk1 expression in oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal 20:e7–e12
Palareti G et al (2016) Comparison between different D-Dimer cutoff values to assess the individual risk of recurrent venous thromboembolism: Analysis of results obtained in the DULCIS study. Int J Lab Hematol 38:42–49
Sana F et al (2022) Immunohistochemical analysis of expression of cyclin D1 in different grades of oral squamous cell carcinoma. J Pharm Res Int. https://doi.org/10.9734/jpri/2022/v34i30B36072
Qiu-shi P et al (2020) CircRNA _ 0000140 suppresses oral squamous cell carcinoma growth and metastasis by targeting miR-31 to inhibit Hippo signaling pathway. Cell Death Dis. https://doi.org/10.1038/s41419-020-2273-y
Irimie Alexandra Iulia et al. (2017) A Looking-Glass of Non-Coding RNAs in Oral Cancer. Int J Mol Sci Doi: https://doi.org/10.3390/ijms18122620
Viviana V, Monica R (2018) miR-100 and miR-125b regulate epithelial-mesenchymal transition and drug resistance in tumors. Non-coding RNA Investig 2:57–57
Libório-Kimura Tatiana N, Min JH, Chan Edward KL (2015) miR-494 represses HOXA10 expression and inhibits cell proliferation in oral cancer. Oral Oncol 51:151–157
Shao Yuan Qu, Yiping DS, Bowen Y, Meiju Ji (2013) MiR-145 inhibits oral squamous cell carcinoma (OSCC) cell growth by targeting c-Myc and Cdk6. Cancer Cell Int 13:1
Ling-fei J, Su-bi W, Kai G, Gan Ye-hua Y, Guang-yan (2013) Prognostic Implications of MicoRNA miR-195 expression in human tongue squamous cell carcinoma. PLoS ONE 8:1–11
Manasa VG, Kannan S (2017) Impact of microRNA dynamics on cancer hallmarks: An oral cancer scenario. Tumor Biol. https://doi.org/10.1177/1010428317695920
Zicheng W, Beili L, Deqiang H, Xiaoming L (2021) Mir-5100 Mediates Proliferation, migration and invasion of oral squamous cell carcinoma cells via targeting SCAI. J Investig Surg 34:834–841
Xiaotang W et al (2020) MicroRNA-504 functions as a tumor suppressor in oral squamous cell carcinoma through inhibiting cell proliferation, migration and invasion by targeting CDK6. Int J Biochem Cell Biol 119:105663
Zekun W et al (2023) MicroRNA-191 regulates oral squamous cell carcinoma cells growth by targeting PLCD1 via the Wnt/β-catenin signaling pathway. BMC Cancer 23:668
Huang Fei et al. Noncoding RNAs in oral premalignant disorders and oral squamous cell carcinoma.
Anquan S et al (2018) miR-9 induces cell arrest and apoptosis of oral squamous cell carcinoma via CDK 4/6 pathway. Artif Cells, Nanomed Biotechnol 46:1754–1762
He Z, da Jiang Lin hong, Sun Da wei, Hou Jun chen, Ji Zhen ling (2018) CircRNA: a novel type of biomarker for cancer. Breast Cancer. https://doi.org/10.1007/s12282-017-0793-9
Zhang Xiao Yun et al. 2022 Circyap inhibits oral squamous cell carcinoma by arresting cell cycle. Acta Odontol Scand 80(2): 117–124
Yilong Ai et al (2020) circ_SEPT9, a newly identified circular RNA, promotes oral squamous cell carcinoma progression through miR-1225/PKN2 axis. J Cell Mol Med 24:13266–13277
Guanhui C et al (2020) Upregulation of Circular RNA circATRNL1 to sensitize oral squamous cell carcinoma to irradiation. Mol Ther Nucleic Acids 19:961–973
Kuangzheng L et al (2021) Circ _ 0000745 strengthens the expression of CCND1 by functioning as miR - 488 sponge and interacting with HuR binding protein to facilitate the development of oral squamous cell carcinoma. Cancer Cell Int. https://doi.org/10.1186/s12935-021-01884-1
Yu Huiming Y, Zhifen WX, Dazhao W (2021) Circular RNA circCLK3 promotes the progression of tongue squamous cell carcinoma via miR-455–5p / PARVA axis. Biotech and App Biochem. https://doi.org/10.1002/bab.2120
Wei D et al (2019) Microarray profile of circular RNAs identifies hsa_circRNA_102459 and hsa_circRNA_043621 as important regulators in oral squamous cell carcinoma. Oncol Rep 42:2738–2749
Soudeh G, Hamed S, Tondro AF (2020) The role of non-coding RNAs in controlling cell cycle related proteins in cancer cells. Front Oncol. https://doi.org/10.3389/fonc.2020.608975
Hongcheng J, Xuan W, Zheng S (2021) Screening and validation of plasma long non-coding RNAs as biomarkers for the early diagnosis and staging of oral squamous cell carcinoma. Oncol Lett 21:1–8
Luka B, Metka RG, Damjan G (2017) Long Noncoding RNAs as Biomarkers in Cancer. Dis Markers. https://doi.org/10.1155/2017/7243968
Na Li, Hongbo D, Qing X, Xuezhen W (2021) Long-chain non-coding RNA HOTTIP enhances oral cancer cell proliferation and migration capacity by down-regulating miR-206. J BUON 26:762–768
Koyo N et al (2018) Screening for long noncoding RNAs associated with oral squamous cell carcinoma reveals the potentially oncogenic actions of DLEU1. Cell Death Dis. https://doi.org/10.1038/s41419-018-0893-2
Yue Z, Rui Y (2021) Long non-coding RNA HOXA-AS3 promotes cell proliferation of oral squamous cell carcinoma through sponging microRNA miR-218-5p. Bioengineered 12:8724–8737
Mingwei C, Yanliang Z, Jingfang X, Enming Z, Xiaoqing Z (2020) Integrative profiling analysis identifies the oncogenic long noncoding RNA DUXAP8 in oral cancer. Anticancer Drugs 8:792–798
Yang Cheng Mei et al. Aberrant DNA hypermethylation-silenced SOX21-AS1 gene expression and its clinical importance in oral cancer. Clin Epigenetics 2016; 8: 129.
Rui Z, Tao WanjunLei Yu (2023) Overexpression of long non-coding RNA GASL1 induces apoptosis and G0/G1 cell cycle arrest in human oral cancer cells. Acta Biochim Pol 70:271–276
Ce X, Shou-gang S, Zhi-quan Y, Feng B (2021) Biomedicine & pharmacotherapy role of lncRNA LUCAT1 in cancer. Biomed Pharmacother 134:111158
Jingxin C et al (2021) LncRNA GACAT1 targeting miRNA-149 regulates the molecular mechanism of proliferation, apoptosis and autophagy of oral squamous cell carcinoma cells. Aging (Albany NY) 13:20359–20371
Jun L, Lizhong L, Kexiong O, Zhiqiang Li, Xianping Yi (2017) MALAT1 induces tongue cancer cells’ EMT and inhibits apoptosis through Wnt/β-catenin signaling pathway. J Oral Pathol Med 46:98–105
Chunyu W, Qiang W, Guangqi Y (2021) Long noncoding RNA ZEB1-AS1 downregulates miR-23a, promotes tumor progression, and predicts the survival of oral squamous cell carcinoma patients. OncoTargets and Therapy 14:2699–2710
Chenxi Li et al (2020) Biomedicine & pharmacotherapy Long non-coding RNA RBM5-AS1 promotes the aggressive behaviors of oral squamous cell carcinoma by regulation of miR-1285-3p / YAP1 axis. Biomed Pharmacother 123:109723
Wei L, Yilin Y, Linjun S, Tang GuoyaoLan W (2021) A novel lncRNA LOLA1 may predict malignant progression and promote migration, invasion, and EMT of oral leukoplakia via the AKT/GSK-3β pathway. J Cell Biochem 122:1302–1312
Xiaozhen Wu, Zuode G, Long M, Qibao W (2021) lncRNA RPSAP52 induced the development of tongue squamous cell carcinomas via miR-423-5p/MYBL2. J Cell Mol Med 25:4744–4752
Xiaoyong Q, Chenxi L, Hao C (2021) Long Noncoding RNA ZFAS1 Promotes Progression of Oral Squamous Cell Carcinoma Through Targeting miR-6499-3p/CCL5 Axis. In Vivo (Brooklyn) 35:3211
Fenqian Y et al (2020) Long non-coding RNA PHACTR2-AS1 promotes tongue squamous cell carcinoma metastasis by regulating Snail. J Biochem 168:651–657
Wang J, Jia J, Zhou L (2020) Long non-coding RNA CASC2 enhances cisplatin sensitivity in oral squamous cell cancer cells by the miR-31-5p/KANK1 axis. Neoplasma 67:1279–1292
Shuwei C, Muwen Y, Chunyang W, Ying O (2021) Forkhead box D1 promotes EMT and chemoresistance by upregulating lncRNA CYTOR in oral squamous cell carcinoma. Cancer Lett 503:43–53
Xue Q et al (2021) Long noncoding RNA CEBPA-DT promotes cisplatin chemo-resistance through CEBPA/BCL2 mediated apoptosis in oral squamous cellular cancer. Int J Med Sci. https://doi.org/10.7150/ijms.64253
Zhaoyu L et al (2018) Chemotherapy-Induced Long Non-coding RNA 1 Promotes Metastasis and Chemo-Resistance of TSCC via the Wnt/b -Catenin Signaling Pathway. Mol Ther 26:1494–1508
Dongya Z et al (2017) Midkine derived from cancer-associated fibroblasts promotes cisplatin-resistance via up-regulation of the expression of lncRNA ANRIL in tumour cells. Sci Rep 7:1–11
Zheng F et al (2017) LncRNA UCA1 promotes proliferation and cisplatin resistance of oral squamous cell carcinoma by sunppressing miR-184 expression Medicine. Cancer Med. https://doi.org/10.1002/cam4.1253
Xuguang Y et al (2022) GAS5 alleviates cisplatin drug resistance in oral squamous cell carcinoma by sponging miR-196a. J Int Med Res. https://doi.org/10.1177/03000605221132456
Shanyi Z et al (2018) LncRNA KCNQ1OT1 regulates proliferation and cisplatin resistance in tongue cancer via MIR-211–5p mediated Ezrin/Fak/Src signaling. Cell Death Dis. https://doi.org/10.1038/s41419-018-0793-5
Te Hsuan J et al (2022) MicroRNA-485-5p targets keratin 17 to regulate oral cancer stemness and chemoresistance via the integrin/FAK/Src/ERK/β-catenin pathway. J Biomed Sci 29:1–20
Xijun W, Hongmei G, Banjamin Y, Julia H (2017) miR-15b inhibits cancer-initiating cell phenotypes and chemoresistance of cisplatin by targeting TRIM14 in oral tongue squamous cell cancer. Oncology Reports 5:2720–2726
Sayyed Adil Ali et al. 2021 MiR-155 Inhibitor-Laden Exosomes Reverse Resistance to Cisplatin in a 3D Tumor Spheroid and Xenograft Model of Oral Cancer. Mol Pharm 18(8): 3010–3025
Jun C et al (2020) Exosomal miR-200c suppresses chemoresistance of docetaxel in tongue squamous cell carcinoma by suppressing TUBB3 and PPP2R1B. Aging (Albany NY) 12:6756–6773
Guopei Z et al (2015) ZEB1 transcriptionally regulated carbonic anhydrase 9 mediates the chemoresistance of tongue cancer via maintaining intracellular pH. Mol Cancer 14:1–12
Shen Li et al (2021) Exosomal-mediated transfer of APCDD1L-AS1 induces 5-fluorouracil resistance in oral squamous cell carcinoma via miR-1224-5p/nuclear receptor binding SET domain protein 2 (NSD2) axis. Bioengineered 12:7188–7204
Liqiang C, Zhu Qingli Lu, Lingwei LY (2020) MiR-132 inhibits migration and invasion and increases chemosensitivity of cisplatin-resistant oral squamous cell carcinoma cells via targeting TGF-β1. Bioengineered 11:91–102
Yan JiaweiHongyan Xu (2021) Regulation of transforming growth factor-beta1 by circANKS1B/miR-515-5p affects the metastatic potential and cisplatin resistance in oral squamous cell carcinoma. Bioengineered 12:12420–12430
Vasileios Zisis et al (2023) Preliminary Study of the Cancer Stem Cells’ Biomarker CD147 in Leukoplakia: Dysplasia and Squamous Cell Carcinoma of Oral Epithelial Origin. Cureus. https://doi.org/10.7759/cureus.38807
Vasileios Z, Konstantinos P, Poulopoulos A, Prashanth P, Andreadis D (2023) Altered Presence of Cancer Stem Cell ALDH1/2 in Oral Leukoplakias and Squamous Cell Carcinomas. Cureus 15:1–8
Rajakishore M (2013) Cell cycle-regulatory cyclins and their deregulation in oral cancer. Oral Oncol 49:475–481
Hao F, Xiaoqi Z, Wenli L, Jian W (2020) Long non-coding RNA SLC16A1-AS1: its multiple tumorigenesis features and regulatory role in cell cycle in oral squamous cell carcinoma. Cell Cycle 19:1641–1653
Guang-hui Li, Zhong-hui Ma, Xi W (2019) Long non-coding RNA CCAT1 is a prognostic biomarker for the progression of oral squamous cell carcinoma via miR-181a-mediated Wnt / β -catenin signaling pathway. Cell Cycle 18:2902–2913
Yojiro K, Takeshi T (2020) Long noncoding RNA ANROC on the INK4 locus functions to suppress cell proliferation. Cancer Genomics Proteomics 17:425–430
Ji LC, Chun LS, Chieh YC, Wen CH, Wei CK (2012) Exploiting salivary miR-31 as a clinical biomarker of oral squamous cell carcinoma. Head Neck 34:219–224
Zahran F, Ghalwash D, Shaker O, Al-Johani K, Scully C (2015) Salivary microRNAs in oral cancer. Oral Dis 21:739–747
Momen-Heravi F, Trachtenberg AJ, Kuo WP, Cheng YS (2014) Genomewide Study of Salivary MicroRNAs for detection of oral cancer. J Dent Res 93:86S-93S
Kai-feng H et al (2016) MicroRNA-31 upregulation predicts increased risk of progression of oral potentially malignant disorder. Oral Oncol 53:42–47
Duz Mehmet Bugrahan et al. (2016) Identification of miR-139–5p as a saliva biomarker for tongue squamous cell carcinoma a pilot study. Cell Oncol 39(2): 187–193
Ries Jutta et al. 2014 MiR-186 miR-3651 and miR-494 Potential biomarkers for oral squamous cell carcinoma extracted from whole blood. 31(3): 1429–1436.
Qiuqin W (2016) Association of decreased expression of serum miR-9 with poor prognosis of oral squamous cell carcinoma patients. Med Sci Monit 22:289–294
Hirohiko T, Ri S, Yuji T, Xuhong Z, Yukie Y (2016) Circulating miR-223 in oral cancer its potential as a novel diagnostic biomarker and therapeutic target. PLoS ONE. https://doi.org/10.1371/journal.pone.0159693
Lu Ya Ching et al. 2015 Combined determination of circulating miR-196a and miR-196b levels produces high sensitivity and specificity for early detection of oral cancer. Clin Biochem 48(3): 115–121
Chung-ji Liu et al (2016) Plasma miR-187 is a potential biomarker for oral carcinoma. Clin Oral Investig. https://doi.org/10.1007/s00784-016-1887-z
Patricia S et al (2015) Small RNAs in metastatic and non-metastatic oral squamous cell carcinoma. BMC Med Genomics. https://doi.org/10.1186/s12903-015-0084-9
Huanxi X, Yuqi Y, Hongmei Z, Xuguang Y, Luo Y (2015) Serum miR-483–5p : a novel diagnostic and prognostic biomarker for patients with oral squamous cell carcinoma. Tumor Biol. https://doi.org/10.1007/s13277-015-3514-z
Hidenori T et al (2015) Genome-wide analysis of long noncoding RNA turnover. Methods Mol Biol 1262:305–320
Merdan F et al (2016) Do circulating long non-coding RNAs (lncRNAs) (LincRNA-p21, GAS 5, HOTAIR) predict the treatment response in patients with head and neck cancer treated with chemoradiotherapy? Tumor Biol 37:3969–3978
Chae Young Kwang et al. 2016 Concordance between genomic alterations assessed by next-generation sequencing in tumor tissue or circulating cell-free DNA. Oncotarget 7(40): 65364-65373.
Patel Aditi et al. Salivary exosomal miR-1307–5p predicts disease aggressiveness and poor prognosis in oral squamous cell carcinoma patients. bioRxiv 2022; 2022.07.13.499918.
Aditi P et al (2023) A novel 3-miRNA network regulates tumour progression in oral squamous cell carcinoma. Biomark Res 11:1–14
Nikolay M et al (2021) Salivary miR-30c-5p as potential biomarker for detection of oral squamous cell carcinoma. Biomedicines 9:1–14
Chiara R et al (2021) Genome-wide study of salivary miRNAs identifies miR-423-5p as promising diagnostic and prognostic biomarker in oral squamous cell carcinoma. Theranostics 11:2987–2999
Cheng Ann-joy et al. Systemic Investigation Identifying Salivary miR-196b as a Promising Biomarker for Early Detection of Head-Neck Cancer and Oral Precancer Lesions. 2021; 1–13.
Koopaie Maryam, Manifar Soheila, Lahiji Shahab Shokouhi. 2021 Assessment of MicroRNA-15a and MicroRNA-16–1 Salivary Level in Oral Squamous Cell Carcinoma Patients. MicroRNA 10: 74–79.
Lihong He et al (2020) Salivary exosomal miR-24-3p serves as a potential detective biomarker for oral squamous cell carcinoma screening. Biomed Pharmacother 121:109553
Tar Ildik, Kiss Csongor. Biomarkers in Patients with Oral Squamous Cell Carcinoma. 2022; 1–13.
Hasan ASM, Mohamed GS, Gamil SO, El AS, Omar ZS (2018) Evaluating the accuracy of microRNA27b and microRNA137 as biomarkers of activity and potential malignant transformation in oral lichen planus patients. Arch Dermatol Res 310:209–220
Fadhil Rushdi S, Wei Ming Q, Dimitrios N, David G, Nair RG (2020) Salivary microRNA miR-let-7a-5p and miR-3928 could be used as potential diagnostic bio-markers for head and neck squamous cell carcinoma. PLoS One 15:1–12
Masoumeh M et al (2023) Salivary level of microRNA-146a and microRNA-155 biomarkers in patients with oral lichen planus versus oral squamous cell carcinoma. BMC Oral Health 23:1–9
Zhao Si Y, Jun W, Bo OS, Kun HZ, Lan L (2018) Salivary Circular RNAs Hsa-Circ-0001874 and Hsa-Circ-0001971 as novel biomarkers for the diagnosis of oral squamous cell carcinoma. Cell Physiol Biochem 47:2511–2521
Jianbo S et al (2019) Serum miR-626 and miR-5100 are promising prognosis predictors for oral squamous cell carcinoma. Theranostics 9:920–931
Yi-an C, Shun-long W, Shun-fa Y, Chih-hung C (2018) A Three – MicroRNA signature as a potential biomarker for the early detection of oral cancer. Int J Mol Sci. https://doi.org/10.3390/ijms19030758
Yan Y et al (2017) Circulating miRNAs as biomarkers for oral squamous cell carcinoma recurrence in operated patients. Oncotarget 8:8206–8214
Chen Ching Mei et al. 2021 Exosome-derived microRNAs in oral squamous cell carcinomas impact disease prognosis. Oral Oncol 120: 105402
Singh Pooja et al. 2018 Circulating MicroRNA-21 Expression as a Novel Serum Biomarker for Oral Sub-Mucous Fibrosis and Oral Squamous Cell Carcinoma. 19: 1053–1058.
Chen Liang et al. Diagnostic and prognostic value of serum miR-99a expression in oral squamous cell carcinoma un co rre ct pr oo f v er si on co rre ct ed pr oo. 2018; 1: 1–7.
Karimi Abbas, Bahrami Naghmeh, Sayedyahossein Amirsalar, Derakhshan Samira. Evaluation of circulating serum 3 types of microRNA as biomarkers of oral squamous cell carcinoma ; A pilot study. 2019; 1–6.
Farzaneh B et al (2021) Early diagnosis of oral squamous cell carcinoma (OSCC) by miR-138 and miR-424–5p expression as a cancer marker. Asian Pac J Cancer Prev 22:2185–2189
Wang Long-long et al. 2018 MiR-31 is a potential biomarker for diagnosis of head and neck squamous cell carcinoma. 11: 4339–4345
Jutta R et al (2017) Prognostic significance of altered miRNA expression in whole blood of OSCC patients. Oncol Rep 37:3467–3474
Sun Guan et al. 2018 Mir-200b-3p in plasma is a potential diagnostic biomarker in oral squamous cell carcinoma. Biomarkers 0: 137–141
Tao He et al (2021) Plasma-derived exosomal microRNA-130a serves as a noninvasive biomarker for diagnosis and prognosis of oral squamous cell carcinoma. J Oncol. https://doi.org/10.1155/2021/5547911
Elisabetta B et al (2022) Extracellular vesicles miR- 210 as a potential biomarker for diagnosis and survival prediction of oral squamous cell carcinoma patients. J Oral Pathol Med 51(4):350–357
Lili W, Hongguang S, Shiming Y (2021) MicroRNA-206 has a bright application prospect in the diagnosis of cases with oral cancer. J Cell Mol Med 25:8169–8173
Sajjad B et al (2021) Role of miR153 and miR455-5p Expression in Oral Squamous Cell Carcinoma Isolated from Plasma. Asian Pacific J Cancer Prev 22:157–161
Zhiyuan Lu et al (2019) miR-31-5p Is a Potential Circulating Biomarker and Therapeutic Target for Oral Cancer. Mol Ther - Nucleic Acids 16:471–480
Hung Kai Feng et al. 2022 Identification of plasma hsa_circ_0000190 and 0001649 as biomarkers for predicting the recurrence and treatment response of patients with oral squamous cell carcinoma. J Chin Med Assoc 85: 431–437
Yanwei Luo, Fengxia Liu, Jie Guo, Rong Gui (2020) Upregulation of circ _ 0000199 in circulating exosomes is associated with survival outcome in OSCC. Sci Rep. https://doi.org/10.1038/s41598-020-70747-y
Fan Chun Mei et al. 2019 CircMAN1A2 could serve as a novel serum biomarker for malignant tumors. Cancer Science 11(7): 2180–2188.
Bing X, Tao H, He Xin H, Xinlan GY (2019) A circular RNA derived from MMP9 facilitates oral squamous cell carcinoma metastasis through regulation of MMP9 mRNA stability. Cell Transplant 28:1614–1623
Yao Y et al (2018) Circulating Long Noncoding RNAs as Biomarkers for Predicting Head and Neck Squamous Cell Carcinoma. Cell Physiol Biochem 50:1429–1440
Xinyu Z et al (2020) Up-regulation of plasma lncRNA CACS15 distinguished early-stage oral squamous cell carcinoma patient. Oral Dis 26:1619–1624
Le Fei Ou, Yangqian LP, Xiaoming Z (2020) LncRNA NCK1-AS1 in plasma distinguishes oral ulcer from early-stage oral squamous cell carcinoma. J Biol Res 27:1–7
Chunmei F et al (2020) Upregulation of long non-coding RNA LOC284454 may serve as a new serum diagnostic biomarker for head and neck cancers. BMC Cancer 20:917
Panpan Z et al (2019) LncRNA PAPAS promotes oral squamous cell carcinoma by upregulating transforming growth factor-β1. J Cell Biochem 120:16120–16127
Huan Shen et al (2020) MIR4435 - 2HG regulates cancer cell behaviors in oral squamous cell carcinoma cell growth by upregulating TGF - β1. Odontology. https://doi.org/10.1007/s10266-020-00488-x
Shieh Tzong Ming et al. 2021 Lack of salivary long non‐coding rna xist expression is associated with increased risk of oral squamous cell carcinoma a cross‐sectional study. J Clin Med Doi: https://doi.org/10.3390/jcm10194622
Acknowledgements
The authors thank Yenepoya Research Centre, Yenepoya (Deemed to be University) for providing the infrastructure required for the research and University Library facility. R. C. Koumar acknowledges the receipt of the Seed grant (YU/Seed Grant/ 090-2020) from Yenepoya (Deemed to be University). Asrarunissa Kalmatta acknowledges the support from Srinivas Institute of Physiotherapy, Mangaluru, Karnataka and Yenepoya (Deemed to be University), Mangaluru, Karnataka.
Funding
This review/study did not receive any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Ethical approval
This article does not contain any studies with animals/humans performed by any of the authors.
Informed consent
There is no recruitment of subjects/patients for this study, hence not included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kalmatte, A., Rekha, P.D. & Ratnacaram, C.K. Emerging cell cycle related non-coding RNA biomarkers from saliva and blood for oral squamous cell carcinoma. Mol Biol Rep 50, 9479–9496 (2023). https://doi.org/10.1007/s11033-023-08791-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08791-w