Abstract
Background
Chronic kidney disease (CKD) is a serious health threat worldwide. Defective mitophagy has been reported to induce mitochondrial dysfunction, which is closely associated with CKD pathogenesis. Honokiol (HKL) is a bioactive component of Magnolia officinalis that has multiple efficacies. Our study aimed to investigate the effect of HKL on a CKD rat model and explore the possible mechanisms of mitophagy mediated by Bcl-2 interacting protein 3 and BNIP3-like (NIX) (also known as the BNIP3/NIX pathway) and FUN14 domain-containing 1 (the FUNDC1 pathway) and the role of the AMP-activated protein kinase (AMPK) pathway.
Methods
A CKD rat model was established by feeding the animals dietary adenine (0.75% w/w, 3 weeks). Simultaneously, the treatment group was given HKL (5 mg/kg/day, 4 weeks) by gavage. Renal function was assessed by measuring serum creatinine (Scr) and blood urea nitrogen (BUN) levels. Pathological changes were analyzed by periodic acid-Schiff (PAS) and Masson’s trichrome staining. Protein expression was evaluated by Western blotting and immunohistochemistry.
Results
HKL treatment ameliorated the decline in renal function and reduced tubular lesions and interstitial fibrosis in CKD rats. Accordingly, the renal fibrosis markers Col-IV and α-SMA were decreased by HKL. Moreover, HKL suppressed the upregulation of the proapoptotic proteins Bad and Bax and Cleaved caspase-3 expression in CKD rats. Furthermore, HKL suppressed BNIP3, NIX and FUNDC1 expression, leading to the reduction of excessive mitophagy in CKD rats. Additionally, AMPK was activated by adenine, and HKL reversed this change and significantly decreased the level of activated AMPK (phosphorylated AMPK, P-AMPK).
Conclusion
HKL exerted a renoprotective effect on CKD rats, which was possibly associated with BNIP3/NIX and FUNDC1-mediated mitophagy and the AMPK pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) has been acknowledged as a “silent killer” due to the low rate of early diagnosis, high prevalence, costly health care costs and low cure rate. Almost 9.1% of adults worldwide suffer from CKD [1], and the number of cases in Asia increased from 202.4 to 441.2 million between 1990 and 2019 [2]. Nearly 5.4 million patients are projected to receive renal replacement therapy by 2030 [3]. The global death toll due to CKD increased by 98%, the disability-adjusted life years (DALYs) increased by 62% from 1990 to 2016 [1, 4], and CKD is predicted to become the 5th leading cause of death by 2040 [5]. Currently, available treatments with renin-angiotensin system inhibitors (RASIs) fail to prevent end-stage renal disease (ESRD). Therefore, deeper insight and further examination of efficient strategies have become pressing needs.
HKL (C18H18O2) is a small-molecular polyphenol derived from Magnolia officinalis with multiple biological properties [6]. Magnolia officinalis has been widely used as a classic herbal (named “Houpo”) in traditional Chinese medicine for over two thousand years [7]. Accumulating researches discovered various pharmacological effects of HKL including anti-inflammatory [8], anti-cancer [9], antioxidant [10] and antiarrhythmic [11] in diverse disorders [6]. Moreover, accumulating evidence indicates that HKL plays a protective role in mitochondrial function by regulating mitochondrial dynamics, activating Sirtuin 3 (a mitochondrial deacetylase) and modulating mitophagy [10, 12, 13]. Thus, HKL was shown to have renoprotective effects on cisplatin-induced acute kidney injury (AKI) [14] and renal ischemia‒reperfusion (I/R) injury animal models [15]. However, the mechanisms and effects of HKL on CKD remain unclear.
The kidney is a highly metabolic and energy-demanding organ that is rich in mitochondria. Emerging evidence indicates that defective mitophagy correlates with renal diseases [16]. Mitophagy, which is also known as mitochondrial autophagy, is the selective removal of nonfunctional and damaged mitochondria from cells. This type of autophagy affects mitochondrial number, quality control and function and cellular homeostasis, favoring adaptation in response to external challenges [17]. However, under stress or injury, defective mitophagy triggers mitochondrial dysfunction, giving rise to the accumulation of degraded organelles, organismal damage and subsequent pathological disorders [18]. Previous studies revealed that mitophagy is mainly regulated by the Bcl-2 interacting protein 3 (BNIP3)/BNIP3-like (BNIP3L, also named NIX) pathway, FUN14 domain containing 1 (FUNDC1) pathway, and phosphatase with tensin homolog (PTEN)-induced kinase 1 (PINK1)/Parkin pathway [19]. Accumulating evidence indicates that BNIP3/NIX and FUNDC1-mediated mitophagy participate in cell apoptosis and the inflammatory response, which are closely associated with cancer [20], neurodegenerative diseases [21] and cardiovascular diseases [22]. In the kidney, abnormal BNIP3/NIX and FUNDC1-related mitophagy were demonstrated to be linked with renal apoptosis, inflammation and fibrosis in diabetic nephropathy [23] and I/R-AKI [24]. Furthermore, recent studies have shown that activation of the AMP-activated protein kinase (AMPK) pathway, which is the energy metabolism conductor, plays a vital role in mitophagy activation in multiple disorders [25, 26]. However, the role of BNIP3/NIX and FUNDC1-mediated mitophagy and the AMPK pathway in CKD progression remains to be further explored.
In the present study, we explored the effects of HKL on CKD and investigated the underlying mechanisms regarding BNIP3/NIX and FUNDC1-mediated mitophagy and the AMPK pathway by using an adenine-induced CKD rat model.
Materials and methods
Chemicals and antibodies
HKL (HY-N0003) was purchased from MCE (MedChemExpress, NJ, USA). The primary antibodies used were as follows: BNIP3 (1:500; Cell Signaling Technology, no. 3769), BNIP3/Nix (1:500; Cell Signaling Technology, no. 12396), FUNDC1 (1:1000; Cell Signaling Technology, no. 49240), Bax (1:1000; Abcam, no. 32503), Bad (1:1000; Abcam, no. 32445), Caspase-3 (1:2000; Abcam, no. 184787), Cleaved caspase-3 (1:250; Cell Signaling Technology, no. 9664), P-AMPKα (Thr172) (1:1000; Cell Signaling Technology, no. 2535), AMPKα (1:1000; Cell Signaling Technology, no. 2532), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:5000; Proteintech, no. 60004-1-lg). The main reagents used in this study included Signal Stain Boost Detection Reagent (Cell Signaling Technology, no. 2532), antigen retrieval buffer (Abcam, no. 93678), a signal stain diaminobenzidine hydrochloride (DAB) substrate kit (Cell Signaling Technology, no. 8059), and ECL chemiluminescence reagent (Millipore, MA, USA).
Animal models
Eight-week-old male Sprague‒Dawley (SD) rats (n = 18) weighing 220–260 g were obtained from Guangdong Medical Laboratory Animal Center (Foshan, China). All animals were housed under a 12 h/12 h light–dark cycle with steady temperature (24 ± 1 °C) and humidity (55 ± 10%) and had free access to water and food. After 1 week of adaptive feeding, the rats were randomly distributed into three groups: the control group (n = 6), the CKD model group (n = 6), and the HKL treatment group with CKD (n = 6). The rat model of CKD was established by feeding the animals adenine (Sigma-Aldrich, Mo, USA) in a 0.75% w/w adenine-based diet for 3 weeks [27]. Rats in the treatment group received a 5 mg/kg/d dose of HKL by gavage for 4 weeks with concurrent adenine-based feeding [28, 29]. After 28 days, the rats were anesthetized, blood samples were collected via the abdominal aorta, and the rats were euthanized by cervical dislocation with minimum suffering. The kidneys were isolated and prepared for the subsequent Western blotting and histological analysis.
Biochemical analysis
The levels of blood urea nitrogen (BUN) and serum creatinine (Scr) were analyzed using a creatinine serum detection kit (SKT-217; StressMarq Biosciences, British Columbia, Canada) and BUN detection kit (SKT-213; StressMarq Biosciences, British Columbia, Canada) according to the manufacturer’s instructions.
Histological analysis
The kidney tissues were collected, fixed in 4% paraformaldehyde (overnight, 4 °C), dehydrated in a graded series of ethanol and embedded in paraffin. The paraffin-embedded sections (4 μm thick) were sliced and stained with periodic acid-Schiff (PAS) and Masson’s trichrome for general histology. The tubular injury score was evaluated by PAS staining based on tubular dilation, tubular epithelial cell atrophy and shedding. The analysis standard was as follows: 0 = no tubular injury; 1 = < 10%; 2 = 10–25%; 3 = 26–50%; 4 = 51–75%; and 5 = > 75% tubular injury [30]. The collagen volume fraction was used to assess renal tubulointerstitial fibrosis by Masson staining using FIJI/ImageJ software (National Institutes of Health, USA). A total of 3 random microscopic fields (200 ×) in each sample in each group were selected for quantitative analysis in a blinded manner. The calculation method was as follows: collagen volume fraction = collagen area/total area × 100% [31].
Immunohistochemistry
The paraffin-embedded kidney sections used for immunohistochemistry were placed in antigen retrieval solution (4 h, room temperature, RT). Then, the sections were incubated in 3% hydrogen peroxide solution (10 min, RT) and blocked with goat serum (1 h, 37 °C). The primary antibodies were added dropwise and incubated (overnight, 4 °C), and the sections were washed with Tris-buffered saline with 0.1% Tween 20 (TBST) (3 × 5 min/wash). Then, the sections were immersed in signal stain boost detection reagent (30 min, RT) and detected with DAB. The percentage of the DAB-labeled area was calculated and presented as the relative expression of the primary antibodies. Three microscopic fields (200 ×) in each sample and 3 rats in each group were examined by ImageJ software (National Institutes of Health, USA) in accordance with the protocol [32, 33].
Western blot analysis
The extracted kidney cortex tissues were homogenized in lysis buffer and then centrifuged at 12,000 rpm (10 min, 4 °C). The supernatant concentrations were determined by a Bradford protein detection assay, and then standards with an equal amount of 4 × SDS sample buffer were denatured (10 min, 100 °C). Then, the processed sample proteins were separated on 10–15% SDS‒PAGE gels and transferred to nitrocellulose membranes (Millipore, MA, USA). The membranes were then blocked with 5% nonfat milk dissolved in TBST buffer (1 h, RT). Next, the primary antibodies were added to the blocked membranes in TBST and incubated (overnight, 4 °C). After primary antibody incubation, the membranes were washed with TBST (3 × 10 min/wash) and then incubated with horseradish peroxidase-conjugated IgG secondary antibodies (Life Technologies, CA, USA) (1 h, RT). Finally, the membranes were visualized and analyzed by a ChemiDoc MP Imaging System (Bio-Rad Laboratories, CA, USA).
Statistical analysis
The data were calculated by GraphPad Prism (GraphPad software 7.0, USA) and are presented as the mean ± SEM for graphical manifestation. Tukey’s multiple comparisons test and ANOVA were used for statistical analysis, and P < 0.05 was considered statistically significant.
Results
Effects of HKL on renal function in CKD rats
Renal function in the groups is shown in Fig. 1. Serum Scr and BUN levels were elevated after adenine-induced kidney injury in all CKD rats. Compared with those in the CKD group, the levels of Scr and BUN were significantly reduced by HKL treatment. These results verified the establishment of the CKD model in rats. In addition, HKL treatment effectively suppressed the decline in renal function, as evidenced by the reduced Scr and BUN levels.
Effects of HKL on renal function in CKD rats. a Serum Scr levels in the control, CKD and HKL treatment group (n = 6 rats/group). Compared with the control group, the level of Scr was severely elevated by adenine-induced kidney injury in CKD rats and significantly reduced by HKL treatment. b The BUN levels in all groups (n = 6 rats/group). HKL treatment depressed the BUN levels of CKD rats. Data are presented as means ± SEM (****P < 0.0001 compared with the control group; #P < 0.05, ####P < 0.0001 compared with the CKD group)
Effects of HKL on renal pathological injury
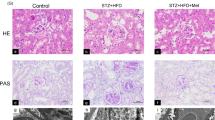
Tubulointerstitial injury is a pathological feature of renal injury in many types of CKD [35] and is characterized by tubulointerstitial atrophy, tubulointerstitial collapse and interstitial fibrosis [33]. As shown in Fig. 2a, c, PAS staining showed extensive tubular collapse, tubular cell shedding and tubular expansion in the CKD group. The Masson staining showed remarkable collagen deposition and interstitial fibrosis in the CKD group (Fig. 2b, d). HKL significantly decreased tubular atrophy, collagen deposition and interstitial fibrosis compared with those in the CKD group.
Effects of HKL on renal pathological injury. a Representative renal histology by PAS staining in all groups. Remarkable tubular cells shedding, tubulo-atrophy, tubulo-collapse were showed in the CKD group, and relieved by HKL treatment. b Renal histology by Masson staining in all groups. Distinctly collagen deposition and interstitial fibrosis were captured in the CKD group, and reduced in the HKL group. Magnification = × 200, bars = 100 μm. c, d Quantitative analysis of the tubular injury score and collagen volume fraction in each group (n = 6 rats/group). Tubular injury score and collagen volume fraction were severely up-regulated in CKD rats compared with the control group, respectively. HKL treatment reduced the up-regulated tubular injury score and collagen volume fraction of CKD rats, respectively. Data are presented as means ± SEM (****P < 0.0001 compared with the control group; ####P < 0.0001 compared with the CKD group)
Effects of HKL on renal fibrosis in CKD rats
Enhanced expression of Col-IV and α-SMA are considered to be key features of renal tubulointerstitial fibrosis during CKD progression. We measured Col-IV and α-SMA expression levels in the groups by immunohistochemistry. As shown in Fig. 3a, b, the expression level of Col-IV was markedly increased in the CKD group compared with the control group, and HKL reduced this increase compared with that in the CKD group. As shown in Fig. 3a, c, the expression of α-SMA increased after adenine-induced CKD induction and was suppressed by HKL treatment. These results indicated the antifibrotic effects of HKL treatment.
Effects of HKL on renal fibrosis in CKD rats. a Representative Immunohistochemical images of Col-IV and α-SMA staining in all groups. Magnification = × 200, bars = 50 μm. b, c Quantification of Col-IV and α-SMA in each group (n = 3 rats/group). Treatment with HKL significantly reduced the Col-IV and α-SMA expression in CKD rats, which were markedly increased by adenine induced kidney injury compared with the control group, respectively. Data are presented as means ± SEM (****P < 0.0001 compared with the control group; #P < 0.05, ###P < 0.001 compared with the CKD group)
Effects of HKL on renal apoptosis in CKD rats
The apoptosis-related proteins Bad, Bax and Cleaved caspase-3 have been reported to be linked with BNIP3/NIX and FUNDC1-mediated mitophagy [20, 34]. As shown in Fig. 4a–g, compared with those in the control group, the expression levels of Bad and Bax were markedly upregulated in the CKD group, similar to the change in the expression levels of Caspase-3, Cleaved caspase-3 and Cleaved caspase-3/Caspase-3. In addition, this effect was reversed by HKL treatment, which significantly decreased the expression levels of Bad, Bax, Cleaved caspase-3 and Cleaved caspase-3/Caspase-3. These data indicated the inhibitory effects of HKL on renal apoptosis in CKD rats.
Effects of HKL on renal apoptosis in CKD rats. a Representative Western blot pictures of Bad, Bax, Caspase-3 and Cleaved caspase-3. b–g Quantitative analysis of the Bad, Bax, Caspase-3, Cleaved caspase-3, Caspase-3/Cleaved caspase-3 and Cleaved caspase-3/Caspase-3 expression levels in each group, GAPDH, Cleaved caspase-3 and Caspase-3 were used as respective loading control (n = 4–6 rats/group). Assessment of Bad, Bax, Caspase-3, Cleaved caspase-3 and Cleaved caspase-3/Caspase-3 expression levels were severely enhanced, while Caspase-3/Cleaved caspase-3 was significantly reduced in CKD rats compared with the control group, respectively. HKL treatment could reduce the up-regulated Bad, Bax, Cleaved caspase-3 and Cleaved caspase-3/Caspase-3 expression levels in varying degrees. Data are presented as means ± SEM (**P < 0.01, ***P < 0.001, ****P < 0.0001 compared with the control group; #P < 0.05, ##P < 0.01, ###P < 0.001 compared with the CKD group)
Effects of HKL on renal mitophagy in CKD rats
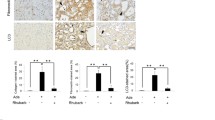
The BNIP3/NIX and FUNDC1 signaling pathways control receptor-mediated mitophagy. To further examine mitophagy in CKD and the effects of HKL treatment, the expression levels of BNIP3, NIX and FUNDC1 were assessed by Western blotting (Fig. 5a–d) and immunohistochemistry (Fig. 5e–h). The Western blot results showed that BNIP3 and NIX protein levels were prominently increased in the CKD group (Fig. 5a–c), similar to the change in FUNDC1 expression levels (Fig. 5a, d). Moreover, HKL treatment significantly reduced BNIP3, NIX, and FUNDC1 expression to varying degrees. Consistent results were verified via IHC analysis (Fig. 5e–h). These results indicated that an increase in BNIP3/NIX and FUNDC1-mediated mitophagy participated in CKD progression and that HKL inhibited excessive mitophagy in CKD rats.
Effects of HKL on renal mitophagy in CKD rats. a Representative Western blot images of BNIP3, NIX and FUNDC1. b–d Quantitative analysis of the BNIP3, NIX and FUNDC1 protein expression in each group standardized to GAPDH content (n = 5–6 rats/group). The expression of BNIP3, NIX and FUNDC1 were all markedly up-regulated in CKD rats. HKL treatment reversed the overexpression trend and down-regulated the BNIP3, NIX and FUNDC1 expression to varying extents. e IHC staining images of BNIP3, NIX and FUNDC1 in different groups. Magnification = × 200, bars = 50 μm. f–h Quantification of BNIP3, NIX and FUNDC1 expression in all groups (n = 3 rats/group). The BNIP3, NIX and FUNDC1 expression levels were all significantly raised compared with the control group, respectively. HKL treatment markedly reduced the BNIP3, NIX and FUNDC1 overexpression in variable ranges. Data are presented as means ± SEM (**P < 0.01, ***P < 0.001, ****P < 0.0001 compared with the control group; #P < 0.05, ###P < 0.001, ####P < 0.0001 compared with the CKD group)
Effects of HKL on AMPK in CKD rats
Recent studies have demonstrated that activated AMPK (phosphorylated AMPK, P-AMPK) is a modulator of BNIP3/NIX-mediated mitophagy [35]. Western blot analysis demonstrated a significant increase in P-AMPK induced by adenine in CKD rats (Fig. 6a, b). In addition, consistent results were observed by IHC staining (Fig. 6c, d). Moreover, compared to that in the CKD group, P-AMPK expression was notably decreased by HKL treatment, and IHC staining showed consistent results. These results demonstrated that the AMPK pathway was associated with adenine-induced CKD and that HKL significantly reduced AMPK activation in CKD rats.
Effects of HKL on AMPK in CKD rats. a Representative Western blot images of P-AMPK and AMPK. b Quantitative analysis of the P-AMPK expression normalized to AMPK content in each group (n = 5–6 rats/group). The P-AMPK expression level was significantly activated in CKD rats. Treatment with HKL significantly reduced the up-regulated P-AMPK expression. c, d IHC images and quantitation of P-AMPK expression in different groups (n = 3 rats/group). Magnification = × 200, bars = 50 μm. P-AMPK was significantly activated by adenine induced kidney injury compared with the control group. HKL treatment markedly reduced the P-AMPK expression level in CKD rats. Data are presented as means ± SEM (***P < 0.001, ****P < 0.0001 compared with the control group; #P < 0.05, ##P < 0.01 compared with the CKD group)
Discussion
In this study, we demonstrated the renoprotective effect of HKL, which improved renal function, ameliorated pathologic injury and reduced renal fibrosis in CKD rats. The underlying mechanism might be associated with the inhibitory effects of HKL on excessive mitophagy and apoptosis via BNIP/NIX- and FUNDC1-mediated mitophagy and the AMPK signaling pathway.
HKL has exhibited renoprotective properties in renal disease studies. In a renal ischemia/reperfusion (I/R)-injury rat model, Yu et al. found that HKL relieved renal injury by reducing oxidative stress, inducible nitric-oxide synthase, and inflammation and enhancing catalase and superoxide dismutase levels [36]. Another study indicated that HKL protected sepsis-induced mouse survival and relieved acute renal injury by means of its anti-inflammatory and antioxidative effects [37]. In the present study, HKL protected renal function in CKD rats and remarkably suppressed excessive Scr and BUN levels. In addition, renal pathological injury, including tubular collapse, tubular atrophy, and renal fibrosis, in CKD rats was ameliorated by HKL treatment. These results demonstrated that HKL could protect against renal injury in CKD rats.
Mitophagy is a major modulator of mitochondrial quality control by opportunely degrading and eliminating damaged mitochondria to maintain mitochondrial number and function and cellular homeostasis [16]. Under oxidative stress, hypoxia and aging conditions, defective mitophagy is activated, leading to cellular membrane destruction, abnormal organelles and enhanced apoptosis [17]. Defected mitophagy was shown to be a double-edged sword (including deficient and excessive forms) and involved in various disorders, including cancer [20], neurodegenerative diseases [21] and cardiovascular diseases [22]. Mitophagy is generally regulated by mitophagy receptors, which are mediated by ubiquitin-independent mold (also known as direct mold) and ubiquitin-dependent mold. The outer mitochondrial membrane (OMM)-localized receptors BNIP3, NIX (a homolog of BNIP3) and FUNDC1 compose the ubiquitin-independent mitophagy mold. The PINK1/Parkin pathway mainly forms the ubiquitin-dependent mitophagy mold [18, 19]. Based on our previous work, we found that BNIP3/NIX and FUNDC1-mediated mitophagy were more associated with the adenine-induced CKD model in rats than the PINK1/Parkin pathway (data not shown). Initially, due to its proapoptotic activity, BNIP3/NIX-mediated mitophagy was shown to participate in metabolic disorders in cancer mechanism studies [20]. BNIP3 and NIX are activated by hypoxic induction in human tumor cell lines [38], while BNIP3 silencing [39] or NIX knockdown [40] promotes tumor growth. Conversely, in the field of heart disease, high levels of BNIP3 and NIX expression increased cardiac myocyte apoptosis, which caused ischemic cardiomyopathy [22] and hypertrophic cardiomyopathy [41]. The protein FUNDC1 was discovered later and is associated with hypoxia-induced mitophagy [42], and it has been verified that silencing the FUNDC1 gene decreased the pathologic progression of chronic obstructive pulmonary disease [43]. Excessive BNIP3-related mitophagy also promotes neuronal death in chronic cerebral hypoperfusion [44], and BNIP3 might be a promising target for Huntington’s disease therapy [45]. Although the underlying mechanism in various diseases remains unclear, the potential role of BNIP3/NIX and FUNDC1-mediated mitophagy in chronic diseases demands further research. In the present study, we found that BNIP3, NIX and FUNDC1 protein levels were upregulated by adenine in CKD rats. Accordingly, the levels of the proapoptotic proteins Bax and Bad were increased, and there was excessive mitophagy in joint renal injury, which is consistent with previous reports [22]. Inhibiting BNIP3/NIX and FUNDC1-related mitophagy could protect against defective mitochondrial dynamics and function in diabetic nephropathy [23, 46]. In a cisplatin-induced kidney injury rat model, Li et al. demonstrated that BNIP3/NIX-related mitophagy was activated and associated with mitochondrial fission and tubular epithelial cell apoptosis [47]. Thus, mitophagy plays a complex role in various disease models, and how BNIP3/NIX and FUNDC1-related mitophagy affects CKD remains to be further researched.
AMPK acts as a pivotal energy sensor and modulator of cellular bioenergy metabolism. Numerous studies have indicated that AMPK plays a crucial role in sustaining energy homeostasis, mitochondrial function, cellular apoptosis, and autophagy [48]. P-AMPKα, which is phosphorylated at Thr172, serves as the domain for AMPK activation [49]. Recent evidence has indicated that activated AMPK is a major modulator of mitophagy. Li et al. demonstrated that BNIP3 transcription and mitophagy are enhanced by AMPK activation and hypoxia-inducible factor 1 in endothelial cells [50]. In addition, Mao et al. found that mitophagy was regulated by AMPK activation and enhanced FUNDC1 expression in many cells [51]. A recent study by Liang et al. highlighted the crosstalk between phosphorylated BNIP3 and the AMPK pathway, indicating that the AMPK-BNIP3 interaction activated mitophagy and reduced mitochondria-dependent apoptosis in virus-infected insect vectors [35]. Consistent with these studies, we demonstrated that P-AMPK levels were elevated, which was accompanied by BNIP/NIX and FUNDC1 pathway enhancement in adenine-induced CKD injury. Furthermore, P-AMPK expression was downregulated, and the level of BNIP3/NIX and FUNDC1-related mitophagy was decreased by HKL treatment in adenine-induced CKD rats. However, the mechanism by which HKL regulates BNIP3/NIX and FUNDC1-dependent mitophagy in CKD requires further examination.
Conclusion
In conclusion, the present study demonstrated that HKL exerted renoprotective effects against renal function decline, tubular injury and fibrosis to varying degrees in an adenine-induced CKD rat model. The mechanism might be associated with the reduction in excessive BNIP3/NIX and FUNDC1-related mitophagy and the inhibition of AMPK activation. Further investigation of the effect of HKL on mitophagy and energy metabolism would be worthwhile in future CKD studies.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AKI:
-
Acute kidney injury
- AMPK:
-
AMP-activated protein kinase
- BNIP3:
-
Bcl-2 interacting protein 3
- BUN:
-
Blood urea nitrogen
- CKD:
-
Chronic kidney disease
- Col-IV:
-
Type IV collagen
- DALYs:
-
Disability-adjusted life years
- ESRD:
-
End-stage renal disease
- FUNDC1:
-
FUN14 domain containing 1
- HKL:
-
Honokiol
- I/R:
-
Ischemia–reperfusion
- NIX:
-
BNIP3-like
- PAS:
-
Periodic acid-Schiff
- PINK1:
-
Phosphatase with tensin homolog (PTEN)-induced kinase 1
- RASIs:
-
Renin-angiotensin system inhibitors
- Scr:
-
Serum creatinine
- TCM:
-
Traditional Chinese medicine
References
Belousov DM, Mikhaylenko EV, Somasundaram SG et al (2020) Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395:709–733. https://doi.org/10.1016/S0140-6736(20)30045-3
Aashima M, Nanda RS, Jani C (2022) The burden of chronic kidney disease in Asia, 1990–2019: examination of estimates from global burden of disease 2019 study. Nephrology 27:610–620. https://doi.org/10.1111/nep.14051
Liyanage T, Ninomiya T, Jha V et al (2015) Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 385:1975–1982. https://doi.org/10.1016/S0140-6736(14)61601-9
Xie Y, Bowe B, Mokdad AH et al (2018) Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int 94:567–581. https://doi.org/10.1016/j.kint.2018.04.011
Foreman KJ, Marquez N, Dolgert A et al (2018) Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 392:2052–2090. https://doi.org/10.1016/S0140-6736(18)31694-5
Rauf A, Olatunde A, Imran M et al (2021) Honokiol: a review of its pharmacological potential and therapeutic insights. Phytomedicine 92:153769. https://doi.org/10.1016/j.phymed.2021.153647
Fried LE, Arbiser JL (2009) Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid Redox Signal 11:1139–1148. https://doi.org/10.1089/ars.2009.2440
Debsharma S, Pramanik S, Bindu S, Bandyopadhyay U et al (2023) Honokiol, an inducer of Sirtuin 3, protects against NSAID-induced gastric mucosal mitochondrial pathology, apoptosis and inflammatory tissue injury. Br J Pharmacol 2023:14765381. https://doi.org/10.1111/bph.1607010
Huang K-J, Kuo C-H, Chen S-H et al (2018) Honokiol inhibits in vitro and in vivo growth of oral squamous cell carcinoma through induction of apoptosis, cell cycle arrest and autophagy. J Cell Mol Med 22:1894–1908. https://doi.org/10.1111/jcmm.13474
Pillai VB, Zhou Y, Tang J et al (2023) Honokiol alleviated neurodegeneration by reducing oxidative stress and improving mitochondrial function in mutant SOD1 cellular and mouse models of amyotrophic lateral sclerosis. Acta Pharm Sin B 13:577–597. https://doi.org/10.1016/j.apsb.2022.07.019
Chen C, Zhang Q-W, Ye Y, Lin L-G (2021) Honokiol: a naturally occurring lignan with pleiotropic bioactivities. Chin J Nat Med 19:481–490. https://doi.org/10.1016/S1875-5364(21)60047-X
Yi X, Guo W, Shi Q et al (2019) SIRT3-dependent mitochondrial dynamics remodeling contributes to oxidative stress-induced melanocyte degeneration in vitiligo. Theranostics 9:1614–1633. https://doi.org/10.7150/thno.30398
Ye J-S, Chen L, Lu Y-Y et al (2019) Honokiol-mediated mitophagy ameliorates postoperative cognitive impairment induced by surgery/sevoflurane via inhibiting the activation of NLRP3 inflammasome in the hippocampus. Oxid Med Cell Longev 2019:8639618. https://doi.org/10.1155/2019/8639618
Mao R, He S, Lan J, Zhu W (2022) Honokiol ameliorates cisplatin-induced acute kidney injury via inhibition of mitochondrial fission. Br J Pharmacol 179:3886–3904. https://doi.org/10.1111/bph.15837
Park EJ, Dusabimana T, Je J et al (2020) Honokiol protects the kidney from renal ischemia and reperfusion injury by upregulating the glutathione biosynthetic enzymes. Biomedicines 8:352. https://doi.org/10.3390/biomedicines8090352
Tang C, He L, Liu J, Dong Z (2015) Mitophagy: basic mechanism and potential role in kidney diseases. Kidney Dis 1:71–79. https://doi.org/10.1159/000381510
Belousov DM, Mikhaylenko EV, Somasundaram SG et al (2020) The dawn of mitophagy: what do we know by now?”. CN 19:170–192. https://doi.org/10.2174/1570159X18666200522202319
Narendra DP (2021) Managing risky assets—mitophagy in vivo. J Cell Sci 134:jcs240465. https://doi.org/10.1242/jcs.240465
Tang C, Livingston MJ, Liu Z, Dong Z (2020) Autophagy in kidney homeostasis and disease. Nat Rev Nephrol 16:489–508. https://doi.org/10.1038/s41581-020-0309-2
Kubli DA, Ycaza JE, Gustafsson AB (2007) Bnip3 mediates mitochondrial dysfunction and cell death through Bax and Bak. Biochem J 405:407–415. https://doi.org/10.1042/BJ20070319
Wang D-X, Yang Y, Huang X-S et al (2021) Pramipexole attenuates neuronal injury in Parkinson’s disease by targeting miR-96 to activate BNIP3-mediated mitophagy. Neurochem Int 146:104972. https://doi.org/10.1016/j.neuint.2021.104972
Lampert MA, Orogo AM, Najor RH et al (2019) BNIP3L/NIX and FUNDC1-mediated mitophagy is required for mitochondrial network remodeling during cardiac progenitor cell differentiation. Autophagy 15:1182–1198. https://doi.org/10.1080/15548627.2019.1580095
Huang C, Yi H, Shi Y et al (2021) KCa3.1 mediates dysregulation of mitochondrial quality control in diabetic kidney disease. Front. Cell Dev. Biol 9:573814. https://doi.org/10.3389/fcell.2021.573814
Tang C, Han H, Liu Z et al (2019) Activation of BNIP3-mediated mitophagy protects against renal ischemia–reperfusion injury. Cell Death Dis 10:677. https://doi.org/10.1038/s41419-019-1899-0
Lu Y, Zhang C, Chen J et al (2022) Hypothermia preconditioning improves cardiac contractility after cardiopulmonary resuscitation through AMPK-activated mitophagy. Exp Biol Med (Maywood) 2022:153537022210815. https://doi.org/10.1177/15353702221081546
Iorio R, Celenza G, Petricca S (2021) Mitophagy: molecular mechanisms, new concepts on parkin activation and the emerging role of AMPK/ULK1 axis. Cells 11:30. https://doi.org/10.3390/cells11010030
Wei X, Wang Y, Weng J et al (2022) Combination of perindopril erbumine and Huangqi-Danshen decoction protects against chronic kidney disease via Sirtuin3/mitochondrial dynamics pathway. Evid Based Complement Altern Med 2022:5812105. https://doi.org/10.1155/2022/5812105
Elfeky MG, Mantawy EM, Gad AM et al (2020) Mechanistic aspects of antifibrotic effects of honokiol in Con A-induced liver fibrosis in rats: emphasis on TGF-β/SMAD/MAPK signaling pathways. Life Sci 240:117096. https://doi.org/10.1016/j.lfs.2019.117096
Liu X, Gao L, Huang X, Deng R et al (2022) Lipidomics reveals the potential mechanism of honokiol against adenine-induced chronic kidney disease. Front Pharmacol 13:1019629. https://doi.org/10.3389/fphar.2022.1019629
Cortes AL, Gonsalez SR, Rioja LS et al (2018) Protective outcomes of low-dose doxycycline on renal function of Wistar rats subjected to acute ischemia/reperfusion injury. Biochim Biophys Acta (BBA) Mol Basis Dis 1864:102–114. https://doi.org/10.1016/j.bbadis.2017.10.005
Zhao W, Zhao T, Chen Y et al (2018) Differential expression of hypertensive phenotypes in BXD mouse strains in response to angiotensin II. Am J Hypertens 31:108–114. https://doi.org/10.1093/ajh/hpx144
Schindelin J, Arganda-Carreras I, Frise E et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Dumont L, Levacher N, Schapman D et al (2021) IHC_Tool: an open-source Fiji procedure for quantitative evaluation of cross sections of testicular explants. Reproduct Biol 21:100507. https://doi.org/10.1016/j.repbio.2021.100507
Su L, Zhang J, Gomez H et al (2022) Mitochondria ROS and mitophagy in acute kidney injury. Autophagy 2022:1–14. https://doi.org/10.1080/15548627.2022.2084862
Liang Q, Wan J, Liu H et al (2022) A plant nonenveloped double-stranded RNA virus activates and co-opts BNIP3-mediated mitophagy to promote persistent infection in its insect vector. Autophagy 2022:1–16. https://doi.org/10.1080/15548627.2022.2091904
Yu Y, Li M, Su N et al (2016) Honokiol protects against renal ischemia/reperfusion injury via the suppression of oxidative stress, iNOS, inflammation and STAT3 in rats. Mol Med Rep 13:1353–1360. https://doi.org/10.3892/mmr.2015.4660
Li N, Xie H, Li L et al (2014) Effects of honokiol on sepsis-induced acute kidney injury in an experimental model of sepsis in rats. Inflammation 37:1191–1199. https://doi.org/10.1007/s10753-014-9845-x
Sowter HM, Ratcliffe PJ, Watson P et al (2001) HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res 61:6669–6673 (PMID: 11559532)
Okami J, Simeone DM, Logsdon CD (2004) Silencing of the hypoxia-inducible cell death protein BNIP3 in pancreatic cancer. Cancer Res 64:5338–5346. https://doi.org/10.1158/0008-5472.CAN-04-0089
Fei P, Wang W, Kim S et al (2004) Bnip3L is induced by p53 under hypoxia, and its knockdown promotes tumor growth. Cancer Cell 6:597–609. https://doi.org/10.1016/j.ccr.2004.10.012
Koncsos G, Varga ZV, Baranyai T et al (2016) Diastolic dysfunction in prediabetic male rats: role of mitochondrial oxidative stress. Am J Physiol Heart Circ Physiol 311:H927–H943. https://doi.org/10.1152/ajpheart.00049.2016
Nair S, Leverin AL, Rocha-Ferreira E et al (2022) Induction of mitochondrial fragmentation and mitophagy after neonatal hypoxia-ischemia. Cells 11:1193. https://doi.org/10.3390/cells11071193
Wen W, Yu G, Liu W et al (2019) Silencing FUNDC1 alleviates chronic obstructive pulmonary disease by inhibiting mitochondrial autophagy and bronchial epithelium cell apoptosis under hypoxic environment. J Cell Biochem 120:17602–17615. https://doi.org/10.1002/jcb.29028
Su SH, Wu YF, Wang DP, Hai J (2018) Inhibition of excessive autophagy and mitophagy mediates neuroprotective effects of URB597 against chronic cerebral hypoperfusion. Cell Death Dis 9:733. https://doi.org/10.1038/s41419-018-0755-y
Sassone F, Margulets V, Maraschi A et al (2015) Bcl-2/adenovirus E1B 19-kDa interacting protein (BNip3) has a key role in the mitochondrial dysfunction induced by mutant huntingtin. Hum Mol Genet 24:6530–6539. https://doi.org/10.1093/hmg/ddv362
Wei X, Wei X, Lu Z et al (2020) Activation of TRPV1 channel antagonizes diabetic nephropathy through inhibiting endoplasmic reticulum-mitochondria contact in podocytes. Metabolism 105:154182. https://doi.org/10.1016/j.metabol.2020.154182
Zhou L, Zhang L, Zhang Y et al (2019) PINK1 deficiency ameliorates cisplatin-induced acute kidney injury in rats. Front Physiol 10:1225. https://doi.org/10.3389/fphys.2019.01225
Zhang H, Liu B, Li T et al (2017) AMPK activation serves a critical role in mitochondria quality control via modulating mitophagy in the heart under chronic hypoxia. Int J Mol Med 41:69–76. https://doi.org/10.3892/ijmm.2017.3213
Juszczak F, Caron N, Mathew AV, Declèves A-E (2020) Critical role for AMPK in metabolic disease-induced chronic kidney disease. IJMS 21:7994. https://doi.org/10.3390/ijms21217994
Li C, Tan Y, Wu J et al (2020) Resveratrol improves Bnip3-related mitophagy and attenuates high-fat-induced endothelial dysfunction. Front Cell Dev Biol 8:796. https://doi.org/10.3389/fcell.2020.00796
Mao S, Tian S, Luo X et al (2021) Overexpression of PLK1 relieved the myocardial ischemia–reperfusion injury of rats through inducing the mitophagy and regulating the p-AMPK/FUNDC1 axis. Bioengineered 12:2676–2687. https://doi.org/10.1080/21655979.2021.1938500
Funding
This study was supported by the Shenzhen Science and Technology Plan Project (Grant number JCYJ20190812161001790) and Shenzhen Fund for Guangdong Provincial Highlevel Clinical Key Specialties.
Author information
Authors and Affiliations
Contributions
XW: conceptualization, investigation, data curation, writing—original draft. YW: investigation. YL: investigation. JW: investigation. RD: investigation. SL: funding acquisition. JL: resources. SY: supervision, project administration. XL: conceptualization, funding acquisition, supervision, writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
All animal experiments were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978) and approved by the Ethics Committee of the Guangzhou University of Chinese Medicine.
Consent for publication
Not applicable.
Consent for participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, X., Wang, Y., Lao, Y. et al. Effects of honokiol protects against chronic kidney disease via BNIP3/NIX and FUNDC1-mediated mitophagy and AMPK pathways. Mol Biol Rep 50, 6557–6568 (2023). https://doi.org/10.1007/s11033-023-08592-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08592-1