Abstract
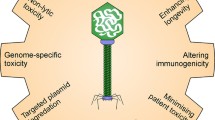
Bacteriophages (phages) are viruses that mainly infect bacteria and are ubiquitously distributed in nature, especially to their host. Phage engineering involves nucleic acids manipulation of phage genome for antimicrobial activity directed against pathogens through the applications of molecular biology techniques such as synthetic biology methods, homologous recombination, CRISPY-BRED and CRISPY-BRIP recombineering, rebooting phage-based engineering, and targeted nucleases including CRISPR/Cas9, zinc-finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs). Management of bacteria is widely achieved using antibiotics whose mechanism of action has been shown to target both the genetic dogma and the metabolism of pathogens. However, the overuse of antibiotics has caused the emergence of multidrug-resistant (MDR) bacteria which account for nearly 5 million deaths as of 2019 thereby posing threats to the public health sector, particularly by 2050. Lytic phages have drawn attention as a strong alternative to antibiotics owing to the promising efficacy and safety of phage therapy in various models in vivo and human studies. Therefore, harnessing phage genome engineering methods, particularly CRISPR/Cas9 to overcome the limitations such as phage narrow host range, phage resistance or any potential eukaryotic immune response for phage-based enzymes/proteins therapy may designate phage therapy as a strong alternative to antibiotics for combatting bacterial antimicrobial resistance (AMR). Here, the current trends and progress in phage genome engineering techniques and phage therapy are reviewed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Bacteriophages (phages) are viruses that specifically infect bacterial hosts either lytically or lysogenically, or both, and are found to be the most ubiquitous microbes on Earth with single-stranded DNA (ssDNA) or double-stranded DNA (dsDNA) or RNA genomes [1,2,3,4,5,6]. Following the discovery of powerful and efficacious antibiotics [7,8,9,10,11,12], phage therapy was lagging even though investigations on bacteriophages were not completely aborted [2, 6, 8]. Unlike antibiotics, phages offer the following advantages: (1) lower developing cost; (2) innocuous to microflora; (3) devoid of side-effects; (4) high-host specificity; (5) sustainability; (6) amenable to nucleic acids manipulation [1, 6, 13, 14]. Although phage therapy for bacterial pathogens control was established for over a century, its potentiality in mitigating bacterial infections had been greatly untapped until the rapid emergence of multidrug-resistant (MDR) bacteria [2, 8, 9, 15,16,17,18,19,20,21,22]. Bacterial antimicrobial resistance (AMR) widespread has recently led to the robust reconsideration of phage therapy for combatting AMR and biocontrol of bacterial contaminants, particularly in wastewater, food, and soil [8, 10, 11, 17, 23,24,25,26]. Therefore, the potential antibacterial properties of phages against pathogenic bacteria could be harnessed for the decontamination of biological agents in food, water, crops, wastewater, and soil, among others [1, 2, 7, 9, 17, 27,28,29,30]. By the United Nations (UN), AMR would account for 10 million deaths of persons per year by 2050 leading to economic disaster likely similar to that of the 2008–2009 global financial crisis with a high probability of dragging nearly 24 million people into extreme poverty by 2030 [17, 27, 31,32,33,34]. As of 2019, global mortality associated with AMR was estimated to be nearly 5 million which surpassed those of HIV/AIDS and malaria [17, 27, 31,32,33,34]. MDR bacteria such as Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa were recently found to be the major leading pathogens attributable to bacterial AMR [17, 27, 31,32,33,34,35,36,37,38]. AMR avails lots of opportunities for reconsideration of phage therapy as a potential tool for alleviating economic burden on public health sector not only as the 2nd -line therapy to AMR cases but also as the 1st -line therapy for biotherapeutics [17, 22, 24, 26, 35]. Due to the increasing demand for combatting AMR, regulatory restrictions on the use of antibiotics in agriculture are currently being enforced and the use of phages as strong alternatives appears to be highly rewarding for the management of AMR in the food chain [15, 17, 21, 24, 37, 39]. Studies on phages are currently being centered at understanding the selection of phage resistance and phage-host interactions to oversimplify the pharmacology of phage therapy [6, 14, 17, 30]. Although monophage therapy appeared to be more effective than multiple doses of one or more antibiotics, cocktails of phage or polyphage therapy was proven to be far more efficacious than that of monophage therapy [7, 9, 17, 27, 30, 40,41,42,43].
Recent advancements in phage genome engineering including synthetic-biology approaches, traditional homologous recombination-based phage, recombineering approaches, rebooting phage-based engineering, CRISPR-Cas (clustered regularly interspaced short palindromic repeats-CRISPR associated proteins) engineering, ZFNs (zinc-finger nucleases) and TALENs (transcription activator-like effector nucleases) appear to overcome the several limitations of phage therapy associated with natural phages as reported elsewhere [6, 14, 17, 44,45,46,47]. CRISPR/Cas9 is currently at the forefront for phage genome engineering owing to its ability to precisely overcome the limitations encountered with the wild-type phages including phage adsorption inhibition, injection blocking of phage genome, restriction-modification, abortive infection, narrow host range, among others [4, 6, 9, 14, 17, 48,49,50,51,52]. Harnessing phage genome engineering especially CRISPR/Cas9 for phage therapy could overcome the limitations of phage therapy exhibited with natural phages as reported elsewhere – thus reducing the economic burden on the public health sector to threat to global mortality due to bacterial AMR before 2050. Here, phage genome engineering approaches and phage therapy are highlighted and presented.
Antibiotics
Discovery and use of antibiotics
Sequel to the scientific discovery of antibiotics particularly penicillin by Alexander Fleming in 1924, the 1940 – 1970 s appeared to be the golden-era of antibiotics [7, 9, 18, 53, 54]. The discovery and development of numerous classes of antibiotics targeting the synthesis of cell-wall, folic acid metabolism, DNA replication and protein biosynthesis of the bacterial cell were successfully used to manage bacterial infections [54,55,56]. Unfortunately, the emergence of resistance to most antibiotics followed such that even the powerful antibiotics – vancomycin marketed in the first quarter of the 1970s developed resistance in the late 1970s [7, 9, 17, 19, 53]. However, the emergence of resistance during the golden era of antibiotics was not challenging due to tremendous-scientific researches being conducted to overcome such resistance [17, 27, 48]. Even though, as antibiotics were widely used as therapeutics and prophylactics in many areas including healthcare, veterinary, agriculture, and industries, the rapid emergence of antibiotic-resistant bacteria is now becoming the most challenging issue particularly, to antibiotic-producing companies [17, 27, 54, 57]. The principled use of antibiotics could be thought of as follows:
-
the first step involves identifying the correct clinical syndrome in patients presenting with signs and symptoms of an infection.
-
identify co-morbidities that will impact etiology and antibiotic choice such as HIV, diabetes, injection drug use, and cancer.
-
recognize common antibiotic resistance patterns at individual levels; carefully screen for antibiotic allergies.
-
initial antibiotic therapy is often empiric and published guidelines help choose the therapy.
-
antibiotic therapy should be narrowed if and when a specific etiology is determined.
-
liberally use local resources to accurately manage infectious diseases and learn appropriate management [20, 54,55,56, 58, 59].
Although novel chemically synthesized drugs are being manufactured and marketed for combatting bacterial AMR, resistance is still emerging against almost all classes of antibiotics [7, 17, 55, 56]. In 2019, AMR caused nearly 5 million deaths associated with bacterial AMR and 1.27 million deaths attributable to bacterial AMR, which accounts for 25.66% [9, 14, 17, 24, 34]. With these drugs’ limitations, phage therapy, being a biological method, can be carefully harnessed to control microbial contaminants as a strong alternative to antibiotics.
Mechanism of actions of antibiotics
The mechanism of action of antibiotics involves targeting the genetic dogma and metabolism of bacterial pathogens (Fig. 1). Antibiotics selectively interferes with various functions of bacterial cell including cell-wall synthesis, DNA replication and transcription, folic acid metabolism, or protein biosynthesis [7, 17, 20, 53,54,55]. The most important members of antibiotics targeting cell-wall are the β-lactam antibiotics and vancomycin [9, 53, 54, 59, 60]. Antibiotics including rifampin, chloramphenicol, macrolides, clindamycins, tetracyclines, and aminoglycosides, among others belong to inhibitors of protein biosynthesis of bacterial cell bacteria – targeting the bacterial RNA-polymerase to a lesser extent or bacterial-ribosome to a large extent [17, 20, 53,54,55,56, 58]. Quinolones especially fluoroquinolones (FQs) are capable of antagonizing bacterial DNA replication by inhibiting the action of DNA gyrase (topoisomerase II) – thus they belong to inhibitors of bacterial DNA replication while Sulfonamides and trimethoprim are inhibitors of folic acid metabolism capable of inhibiting distinctly different steps in folic acid metabolism [55, 56]. The mechanism of action of antibiotics against a pathogenic bacterial cell is diagrammatically depicted as follows:
Multidrug-resistant (MDR) bacteria
Bacterial AMR is globally a major public health problem that is potentially deadlier than HIV/AIDS and malaria; and would account for over 10 million deaths of persons per year by 2050 if care is not taken especially in developing countries including India and Nigeria [17, 24, 31, 32, 34, 36]. A diagram of how bacteria can develop resistance to antibiotics is depicted in Fig. 2. Bacteria are capable of being resistant to a drug if and only if the optimal level of such drug that can be tolerated by the bacterial host does not end up inhibiting their growth [9, 17, 53, 56, 61]. Microbial species, especially gram-negative bacteria, are inherently capable of developing resistance to vancomycin [19, 56]. However, acquired resistance and selection or spontaneous mutation is used by microbial species to generate the microbial strains capable of developing resistance against a particular antibiotics which are by far reported to be responsive to such antibiotics by the microbial species in question [17, 18, 56, 61]. When these microbial strains appeared to be resistant to two or more antibiotics, they are termed MDR strains [17,18,19,20, 55, 56, 58]. The evolution of bacterial cell to antibiotic resistance, which includes antibiotic inactivation, target modification, inhibition of drug uptake, altered permeability, efflux, and “bypass” of the metabolic pathway can be thought of as follows:
Consequent upon above, there has been estimated a disease burden in 2019 based on global mortality attributable to bacterial AMR and associated with bacterial AMR accounting for nearly 1·3 million deaths and 5 million deaths respectively [17, 34, 36]. Therefore, investing in research and development for phage-genome-engineering technologies and phage therapy in silico, in vitro and in vivo will greatly reduce AMR to the barest minimum before 2050.
Bacteriophages
Discovery and use of phages
Although the discovery and use of phages against Vibrio cholerae, Bacillus subtilis and Staphylococcus aureus was established for over a century by scientists such as Ernest Hanbury Hankin (1896), Nikolay Gamaleya (1898), Frederick Twort (1915) and Felix d’Herelle (1917), the golden-era of antibiotics had led to a drastic decrease in the employment of phage therapy [2, 4, 6, 17, 40]. However, researches on bacteriophages were not completely aborted in the West due to the discovery and characterization of the viral nature of phage in 1917 and the establishment of the International Bacteriophage Institute in Tbilisi, Georgia in 1923 all together by d’Herelle [17, 27]. Lytic activities of phages were largely reported by d’He’relle; phage λ was isolated in 1951 by Esther Lederberg; the study of Smith and Huggins in 1982 revitalized researches on phages in the West leading to culture-independent approaches to phage λ in 1982 by Sanger; phage lysins’ activity was demonstrated in 2001 by Fischetti; approvals of phage used in agricultural plants, food industry, and live animals were granted by United States, Environmental Protection Agency (EPA) or Food and Drug Administration (FDA) in 2005, 2006 and 2007 respectively; clearance of phage products by FDA and their phase I and II clinical trials in the USA were also granted in 2011 and 2013 respectively; in 2017, successful phage therapy for MDR bacterial infections in USA was followed [2, 8, 17, 40, 41]. The landmarks in phage history are diagrammatically depicted (Fig. 3) as follows:
Mechanism of actions of phages
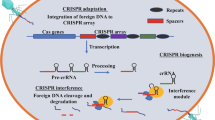
Following the discovery of phages, it was known that two types of the life cycle namely: the lytic cycle and lysogenic cycle were reported [17, 27]. The mechanism of action of the lytic cycle comprises six steps namely: (1) adsorption; (2) genome entry; (3) DNA replication; (4) gene expression; (5) phage assembly; (6) host cell lysis; in contrast to the lysogenic cycle which involves (1) adsorption; (2) genome entry; (3) prophage formation [2, 6, 17, 27]. Broadly speaking, phages are similar to nanoparticles owing to their inability to replicate and divide independently. However, the ubiquity of phages in an ecosystem that supports bacterial growth initiates their adsorption to bacterial cells which harbour dsDNA, ssDNA or RNA of phage genome with energy, proteins and other accessory machinery to complete their lytic or lysogenic development [6, 14, 17]. Adsorption allows the phage-host interaction by random collision so that phage-host receptors which are in close proximity and right orientation interact with one another thereby injecting the phage genome into the host bacterial cell [6, 14, 17]. Phages, being the obligatory intracellular parasites of bacteria, hijack the bacterial cell machinery via either the lytic cycle or lysogenic cycle [8, 6, 14, 17]. However, the host bacteria can develop resistance against phage infection as well as at every crucial step of the lytic development by different mechanisms including phage adsorption inhibition, injection blocking, restriction-modification, abortive infection, and CRISPR-Cas systems [6, 14, 17]. Phages, equally, have evolved mechanisms for resisting countermeasures displayed by bacterial hosts [6, 14, 17]. In the lytic cycle, the phage genome does not get integrated into the bacterial host genome. Rather, it uses the cellular proteins, enzymes, and energy, among other host machinery of bacterial host cells to undergo DNA replication followed by gene expression so that the products of gene expression are assembled leading to the bacterial host cell lysis [17, 27]. In contrast, the lysogenic cycle leads to the integration of the phage genome into the bacterial host chromosome notably for horizontal gene transfer or lytic cycle depending upon the circumstance [2, 17, 27]. The mechanism of action of phages is elucidated (Fig. 4) as follows:
Methods of phage genome engineering
Biochemistry, molecular biology and genetic engineering of phages
The gigantic pieces of information culminated through culture-independent methods in the phage genome can be harnessed as a key to designing novel antimicrobial agents. Phage engineering involves nucleic acids manipulation of phages for an enhanced antimicrobial activity for pathogen control through the applications of molecular biology techniques including recombination-based techniques, CRISPR-Cas-based engineering, rebooting phages using assembled phage genomic DNA, among others [17, 23, 43, 44, 48, 50, 52, 62, 63]. Culture-independent approaches revealed that the phage genomes could be either dsDNA, ssDNA or RNA in nature with different sizes and different ORFs (open reading frames) with or without tRNA [16, 17, 27, 50, 64,65,66,67]. Analysis of the phage genome has resulted in a dramatic understanding of phage evolution and is becoming invaluable for basic molecular biology techniques as well as for diagnosis of bacterial pathogens using luminescent phage-based, syringed-based biosensor, nanoluc-based reporter phage, among others [6, 14, 17, 57, 68,69,70]. The diversity of phages was estimated to be 1031 and phages capable of infecting and killing Escherichia coli species or strains appeared to be employed as models to understand the biochemistry, molecular biology, and genetic engineering of phages via electron microscopy, culture-dependent and culture-independent approaches [2, 4, 6, 14, 17, 27]. T1 – T7 phages and bacteriophage lambda appeared to be the most prevalent ones [6, 14, 17, 44, 51, 69, 71, 72]. Although phage genome engineering can be employed for antibacterial activity of wild-type phages, engineering phage genome with CRISPR/Cas9 is currently being incorporated to overcome the limitations of phage therapy such as phage narrow host range, phage resistance and any potential eukaryotic immune stimulation [6, 7, 14, 17, 41, 42, 72, 73]. Phage genome engineering strategies that involved CRISPR/Cas9 are now incorporated for phage therapy elsewhere reported to have the aforementioned limitations of phage therapy [6, 14, 42, 44]. As such, robust phage genome engineering methods designate phage therapy as a strong alternative to antibiotics for combatting bacterial AMR [17, 24, 33, 57, 69].
Synthetic biology approaches to phage genome
Synthetic biology methods of phage genome can be employed to overcome the challenges of phage resistance and phage narrow host range in vitro by manipulating the phage genome which was known to be amenable to nucleic acid manipulation with high fidelity [5, 44][17]. It involves the use of rational design, de novo synthesis and transformation tools to dramatically improve the efficiency of phage infection and to overcome the limitations associated with natural phages [44] [17]. Rationally designing and straightforward construction of phages in vitro with smaller genome size either circular or linear can produce genetically engineered phages with the potential of infecting and killing either Gram-positive or Gram-negative bacteria, or both [17, 44, 74]. Efficacy and safety of engineered phages can be tested via culture-dependent and culture-independent approaches for various applications [17, 42, 44, 49, 69, 71]. Phage genomes capable of infecting and killing the members of bacteria belonging to genera namely Pseudomonas, Klebsiella, Bacillus, Lactococcus, and Mycobacterium have been engineered using synthetic biology approaches of phage genome engineering [17, 44, 75, 76]. Owing to having orthogonal parts by phages, synthetic biology genome engineering of phages is now amenable [17, 44, 49, 74, 76]. However, the drawback of these approaches is that phages with a large, dsDNA, ssDNA, or RNA genome cannot be employed for engineering via synthetic biology [2, 4, 17, 40, 44, 49, 69].
Traditional homologous recombination/recombineering based phage engineering
While homologous recombination (HR) is traditionally a naturally-occurring type of genetic recombination by which nucleotide sequences are exchanged between molecules that share similar or identical sequences [6, 17, 44, 49, 51, 72], recombineering is a genetic engineering mediated-recombination approach developed to enhance the frequency of HR [2, 4, 17, 50]. However, the frequency of recombination appears to be unenhanced especially for Gram-positive bacteria. Infection is desperately needed for both HR and recombineering methods. In contrast to HR, recombineering requires electroporation as well as recombination proteins such as Gam, RecT, RecE, Exo, and Beta (which protect the donor dsDNA or ssDNA from bacterial hosts exonucleases) for enhancing the frequency of recombination thereby repairing DNA double-strand breaks (DSBs) in the phage genomic DNA via HR-dependent manner [17, 27, 44, 50, 63]. While the donor DNA (dsDNA or ssDNA) for HR with a single phage is incorporated in the donor plasmid, the donor dsDNA or ssDNA for recombineering approaches is not incorporated in the donor plasmid [17, 27, 44]. The donor DNA is co-electroporated with phage genome-bacterial cell after infection [27, 44, 50]. The concept of HR with two-parent phages and a single phage is depicted (Fig. 5) as follows:
CRISPY-BRED and CRISPY-BRIP recombineering approaches
Bacteriophage recombineering with electroporated DNA (BRED) and bacteriophage recombineering with infectious particles (BRIP) are recombineering techniques associated with high chances of success (4–60%) when compared with traditional HR [17, 50, 77, 78]. The dramatic improvement of BRED and BRIP recombineering technologies compared to traditional HR was known to be due to coliphage lambda proteins including Exo, Beta, and Gam as well as proteins of mycophage Che9c particularly gp60 and gp61 [38, 43, 44, 70]. CRISPR/Cas9 has been recently incorporated with BRED and BRIP technologies to enhance the efficiency and accuracy of phage genome editing via recombineering [17, 50, 78]. These powerful combinations of CRISPR/Cas9 with BRED and BRIP are termed as CRISPY-BRED and CRISPY-BRIP recombineering methods respectively. They are by far the most efficient recombineering approaches ever identified with a success rate of nearly 100% and are considered as the mutations generating systems currently developed in lytically replicating bacteriophages capable of recombineering with electroporated DNA or with infectious particles [17, 38, 50, 77, 78]. Although CRISPY-BRED appears to be more efficient than that of CRISPY-BRIP, relatively large phage genomes cannot be employed for bacterial host transfection using CRISPY-BRED technology [27, 50, 77, 78]. Thus, the need to employ CRISPY-BRIP for editing of relatively large phage genomes efficiently and precisely. Phages capable of infecting and/or killing the bacterial hosts belonging to genera particularly Klebsiella, Escherichia, Salmonella, and Mycobacteria are currently being employed for phage genome editing in vivo using CRISPY-BRED and CRISPY-BRIP recombineering methods [50, 78,79,80]. While the CRISPY-BRED recombineering approach requires co-electroporation of the phage genome and donor DNA (dsDNA or ssDNA) directly at no additional cost to infection, CRISPY-BRIP recombineering technology requires infection of a bacterial host cell with phage genome and electroporation of donor DNA similar to that in CRISPY-BRED [4, 7, 27, 50, 77, 78]. A striking feature of CRISPY-BRED and CRISPY-BRIP recombineering methods is that they are virtually similar to recombineering approaches except that infection is not required for the former and CRISPR-plasmid is not needed for the latter [27, 50]. The drawback of CRISPY-BRED and CRISPY-BRIP recombineering methods are limited to bacteria associated with extremely efficient transformation implying that host bacteria which are Gram-positive in nature do not apply to these technologies [4, 7, 17, 50, 77, 78].
Targeted nucleases for precise genome editing/engineering
CRISPR/Cas9, ZFNs, and TALENs promote genome editing by stimulating a DSB at a target genomic locus [17, 43, 44, 48, 49, 51, 52, 63]. Upon cleavage by the just mentioned targeted nucleases, the target locus typically undergoes one of two major pathways for DNA damage repair: the error-prone NHEJ (nonhomologous end joining) or the high-fidelity HDR (homology-directed repair) pathway, both of which can be used to achieve a desired editing outcome in vivo or in vitro [17, 44, 52, 63]. Of these targeted nucleases, CRISPR/Cas9 engineering appears to be the most important genome engineering tool for phages which is an adaptive immune system of bacteria gifted to fight phage genomes and other invading DNA elements on entering the bacterial cell [4, 9, 17, 38, 44, 48, 50, 51, 63]. CRISPR systems function by acquiring genetic records of invaders to facilitate robust interference upon reinfection [4, 17, 40, 44]. In CRISPR/Cas9-based phage engineering, the bacterial host cell is not only modified with the donor dsDNA or ssDNA being incorporated in the donor plasmid but also it is modified with the CRISPR-Cas9 plasmid devoted to generating the CRISPR-Cas9 complex that once formed is capable of specifically getting bound to the target site in the phage genome and thus creates a DSB during phage infection [4, 17, 44, 52, 63]. The mutations are introduced into the donor dsDNA or ssDNA being incorporated in the donor plasmid capable of repairing the DSB by recombination thereby generating mutants of interest [4, 17, 44, 51]. The concept of CRISPR/Cas9-based phage engineering (Fig. 6) is fully elucidated as follows:
Rebooting phage-based engineering
Phage rebooting entails the acquisition of activated virions from the phage genome. This approach involves assembling phage genomes with desired mutations either in vivo or in vitro followed by their transformation into the bacterial host cells through electroporation [2, 4, 17, 27, 44, 49, 50]. The replication and gene expression of genomic DNA in the host cells bring about the assembly of infectious phages [4, 17, 27, 44, 49]. Rebooting phage-based engineering is akin to recombineering-based engineering approaches except that the process is conducted without the additional cost of infection between the phage genome and bacterial host cell [2, 4, 17, 44]. The concept of rebooting phages via phage genomic DNA assembly (Fig. 7) is shown as follows:
Phage-based enzybiotics or proteins therapy
The concomitant need for the implementation of new approaches for combatting bacterial AMR has indeed justified phage therapy as a strong alternative to antibiotics. Phage therapy could now be incorporated as a powerful tool for poverty eradication due to an exponential increase in global mortality associated with AMR [17, 24, 34, 36, 38, 42, 81]. E. coli, S. aureus, K. pneumoniae, S. pneumoniae, A. baumannii, and P. aeruginosa, among others MDR bacteria were reported to be the leading pathology of global mortality attributable to bacterial AMR [6, 7, 9, 13, 14, 17, 40, 50, 55,56,57, 72, 81]. Phage therapy is proven to be effective particularly in tandem with antibiotic-resistant infections both in vitro and in vivo [4, 6, 9, 14, 17, 42, 57]. Phages having desirable features for phage therapy can be administered orally, locally, or by dropping a phage suspension into different parts of the body including the eye, ear, or nose to effectively treat drug-resistant disease in humans [2, 4, 17, 33, 41, 49]. These features include particularly host range, lytic activity and lack of temperate growth. Bacteria capable of producing biofilm are poorly accessible to both antibiotics and antiseptic agents, however, phages and phage-derived enzymes appeared to be the most powerful antibiofilm agents [7, 17, 72, 73]. Importantly, coliphage K29, coliphage T4, and Listeria phage possess enzymes capable of degrading the biofilm matrix and thus leading to lytic infection [3, 7, 17, 72, 82, 83]. In addition, phages in tandem with quaternary ammonium compounds appeared to confer a synergistic effect in degrading biofilms [2, 73]. Furthermore, phage lysins against biofilms of S. aureus and S. epidermidis were shown to be a powerful therapeutics for the control of pathogenic bacteria [2, 7, 17, 73, 84]. T7 phage via genetic engineering appeared to dramatically lower biofilm cell counts of E. coli by more than 99% [2, 4, 71]. This implies that phage engineering is a powerful tool in degrading biofilms by either the engineered phages or phage-encoded enzymes [2, 4, 17, 49, 72, 73]. Indeed, a lot of researches are being carried out worldwide on phages as strong alternatives to antibiotics in a wide scope of applications particularly in medicine, agriculture, food processing, and the environment for quality control monitoring of bacterial infections [9, 17, 33, 42, 50, 62, 71, 83, 85, 86]. The antibacterial proteins encoded by phages most notably peptidoglycan hydrolases and polysaccharide depolymerases are currently at the forefront for phage therapy [2, 8, 16, 17, 28, 33]. These phage-enzymes are capable of degrading bacterial peptidoglycan layer and extracellular polysaccharides including biofilm matrix, slime layers, bacterial capsules, lipopolysaccharides, among other bacterial surface polysaccharides [17, 28, 41, 83, 87, 88].
Several limitations associated with phage therapy including phage narrow host range, phage resistance and any potential eukaryotic immune response are currently being addressed following the incorporation of cocktails of phages ranging from two (2) monophages to ten (10) monophages, engineering of phage genome with CRISPR/Cas9, and encapsulation of phage enzybiotics/proteins with nano-emulsions as well as optimizing the pharmacology of phage therapy using combination therapy and selective administration [6, 14, 17, 38, 42, 57, 83, 84, 87, 89]. Therefore, a proper understanding of pharmacokinetics and pharmacodynamics with promising efficacy and safety of bacteriophage-based proteins/enzymes therapy could offer an everlasting solution to bacterial AMR.
Conclusion
Taken together, phage genome engineering especially CRISPR/Cas9 appears to be the subject of renewed molecular biology techniques towards overcoming the narrow host range of phages and the rapid emergence of resistant mutants developed by monophage therapy. Similarly, it is the most powerful and suitable molecular biology tool for developing phage cocktails or polyphages which are by far proven effective for combatting MDR bacteria. A single dose of phage-based enzyme or protein appeared to be more effective than several doses of antibiotics, even though cocktails of phage or polyphage therapy was proven to be not only more efficacious than that of phage-based enzyme or protein monotherapy but also proved to render pathogenic bacteria blind to developing resistance against phages. Enzybiotics or proteins-derived from lytic phages for phage therapy could be used as the potential resources for poverty eradication before 2050 by saving not only over 10 million deaths of persons from AMR but also safeguarding ≥ 24 million people out of extreme poverty by 2030. Therefore, harnessing phage-based proteins/enzymes therapy and CRISPR/Cas9-based phage genome engineering for phage therapy elsewhere reported with narrow host range or phage resistance would dramatically combat global mortality due to bacterial AMR before 2050.
Data Availability
Not applicable.
Abbreviations
- AIDS:
-
Acquired immunodeficiency syndrome
- AMR:
-
Antimicrobial resistance
- CRISPY-BRED:
-
CRISPR/Cas9-bacteriophage recombineering with electroporated DNA
- CRISPY-BRIP:
-
CRISPR/Cas9-bacteriophage recombineering with infectious particles
- CRISPR-plasmid:
-
Clustered regularly interspaced short palindromic repeats-plasmid
- CRISPR-Cas:
-
Clustered regularly interspaced short palindromic repeats-CRISPR associated proteins
- CRISPR/Cas9:
-
Clustered regularly interspaced short palindromic repeats/Cas9
- DNA:
-
Deoxyribonucleic acid
- DSBs:
-
DNA double-strand breaks
- dsDNA:
-
Double-stranded DNA
- EPA:
-
Environmental protection agency
- FDA:
-
Food and drug administration
- FQs:
-
Fluoroquinolones
- HDR:
-
Homology-directed repair
- HIV:
-
Human immunodeficiency virus
- HR:
-
Homologous recombination
- MDR Bacteria:
-
Multidrug-resistant bacteria
- NHEJ:
-
Nonhomologous end joining
- ORFs:
-
Open reading frames
- RNA:
-
Ribonucleic acid
- ssDNA:
-
Single-stranded DNA
- TALENs:
-
Transcription activator-like effector nucleases
- tRNA:
-
Transfer RNA
- UN:
-
United Nations
- USA:
-
United States of America
- ZFNs:
-
Zinc-finger nucleases
References
Abdelsattar AS, Dawoud A, Makky S, Nofal R, Aziz RK, El-Shibiny A (2021) Bacteriophages: from isolation to application. Curr Pharm Biotechnol 22. https://doi.org/10.2174/1389201022666210426092002
Coffey A (2016) Bacteriophage and their lysins for elimination of infectious bacteria: review article bacteriophage and their lysins for elimination of infectious bacteria. no May 2009. https://doi.org/10.1111/j.1574-6976.2009.00176.x
Scattolini S et al (2021) Characterization and in vitro efficacy against listeria monocytogenes of a newly isolated bacteriophage, ɸizsam-1. Microorganisms 9(4). https://doi.org/10.3390/microorganisms9040731
Tao P (2019) Genetic Engineering of Bacteriophages against Infectious Diseases. no May 10:1–12. https://doi.org/10.3389/fmicb.2019.00954
Mutalik VK, Arkin AP (2022) A phage Foundry Framework to systematically develop viral countermeasures to combat antibiotic-resistant bacterial pathogens. iScience 25(4):104121. https://doi.org/10.1016/j.isci.2022.104121
Shaidullina A, Harms A (2023) Toothpicks, logic, and next-generation sequencing_ systematic investigation of bacteriophage-host interactions. Curr Opin Microbiol 70:102225. https://doi.org/10.1016/j.mib.2022.102225
Hassan AY, Lin JT, Ricker N, Anany H (2021) The age of phage: friend or foe in the new dawn of therapeutic and biocontrol applications? Pharmaceuticals 14(3):1–36. https://doi.org/10.3390/ph14030199
Ryu S (2021) “Grand Challenges in Phage Biology,” Front. Microbiol, vol. 12, no. July, pp. 2019–2022, https://doi.org/10.3389/fmicb.2021.715039
Azam AH (2021) “Bacteriophage Technology and Modern Medicine,”
Ganesh SK, Subathra Devi C (2023) Molecular and therapeutic insights of rapamycin: a multi-faceted drug from Streptomyces hygroscopicus. Mol Biol Rep 50(4):3815–3833. https://doi.org/10.1007/s11033-023-08283-x
Alebouyeh M et al (2023) Intestinal colonization of vancomycin-resistant Enterococcus in children admitted to Mofid children’s hospital intensive care unit at admission and at discharge. Mol Biol Rep 3271–3281. https://doi.org/10.1007/s11033-022-08196-1
Jain H, Chahal S, Singh I, Sain SK, Siwach P (2023) The rising threat of geminiviruses: molecular insights into the disease mechanism and mitigation strategies. Mol Biol Rep 50(4):3835–3848. https://doi.org/10.1007/s11033-023-08266-y
Cui Z, Xu Z, Wei Y, Zhang Q, Qin K, Ji X (2021) Characterization and genome analysis of a Novel Mu-like phage VW-6B isolated from the Napahai Plateau Wetland of China. Curr Microbiol 78(1):150–158. https://doi.org/10.1007/s00284-020-02277-9
Tesson F, Bernheim A (2023) Synergy and regulation of antiphage systems: toward the existence of a bacterial immune system ? Curr Opin Microbiol 71:102238. https://doi.org/10.1016/j.mib.2022.102238
John AM, Mercer DK (2023) Antimicrobial resistance: a biochemical society position statement. no February:33–38
Dewanggana MN, Evangeline C, Ketty MD, Waturangi DE, Yogiara, Magdalena S (2022) Isolation, characterization, molecular analysis and application of bacteriophage DW-EC to control enterotoxigenic Escherichia coli on various foods. Sci Rep 12(1):1–10. https://doi.org/10.1038/s41598-021-04534-8
Strathdee SA, Hatfull GF, Mutalik VK, Schooley RT (2023) Ll phage therapy: from biological mechanisms to future directions. Cell 186(1):17–31. https://doi.org/10.1016/j.cell.2022.11.017
Roszak M, Jabłon J (2022) “Bacteriophage – Ciprofloxacin Combination Effectiveness,” vol. 28, no. 6, pp. 613–622, https://doi.org/10.1089/mdr.2021.0324
Lopez-luis BA, Ponce-de-leo A, Ortiz-brizuela E, Leal-vega FJ, Tovar-caldero YE (2022) Bobadilla-del-valle, “Risk factors Associated with failure of Linezolid Therapy in Vancomycin-Resistant Enterococcus faecium bacteremia. 28(6):744–749. https://doi.org/10.1089/mdr.2021.0333
Chen J et al (2022) Different Effects of Antibiotics on Klebsiella pneumoniae and Escherichia coli Resistance Induced by Antibiotics. 28(6):660–669. https://doi.org/10.1089/mdr.2021.0326
Ahmed BT, Mokhtar B, Yahia B, Wassila C (2023) “Antibiotic resistance: A Global Public Health Crisis and current strategies for Antibiotic Resistance : A Global Public Health Crisis and current strategies for combatting it,” no. April,
Costanzo V, Roviello GN (2023) “The potential role of vaccines in preventing Antimicrobial Resistance (AMR): an update and future perspectives,” pp. 1–21,
Ács N, Gambino M, Brøndsted L (October, 2020) Bacteriophage enumeration and detection methods. Front Microbiol 11. https://doi.org/10.3389/fmicb.2020.594868
Helmy YA et al (2023) “Antimicrobial Resistance and recent Alternatives to Antibiotics for the control of bacterial pathogens with an emphasis on Foodborne Pathogens,”
Pimenta J, Pinto AR, Jos M (2023) “Equine gram-negative oral microbiota: an Antimicrobial Resistances Watcher ?,” pp. 1–11,
Islam S (2023) “A comprehensive review on bacterial vaccines combating Antimicrobial Resistance in Poultry,”
Łobocka M, Dąbrowska K, Górski A (2021) Engineered Bacteriophage therapeutics: Rationale, Challenges and Future. BioDrugs 35(3):255–280. https://doi.org/10.1007/s40259-021-00480-z
Liu R et al (2022) Bacteriophage therapy in aquaculture: current status and future challenges. Folia Microbiol (Praha). https://doi.org/10.1007/s12223-022-00965-6
Choi Y, Ha E, Kong M, Ryu S (2023) A novel chimeric endolysin with enhanced lytic and binding activity against Clostridium perfringens. LWT 181:114776. https://doi.org/10.1016/j.lwt.2023.114776
Ali S, Karaynir A, Salih H, Oncü S, Bozdo B (2023) Characterization, genome analysis and antibiofilm efficacy of lytic Proteus phages RP6 and RP7 isolated from university hospital sewage. vol 326 no October 2022. https://doi.org/10.1016/j.virusres.2023.199049
Release JN (2019) “New report calls for urgent action to avert antimicrobial resistance crisis,”
Franco EG (2020) “Global Risks : An Unsettled World,” Glob. Risks Rep, pp. 8–17, 2020
Dedrick RM et al (2019) Disseminated drug resistant Mycobacterium abscessus. 25(5):730–733. https://doi.org/10.1038/s41591-019-0437-z.Engineered
Collaborators AR (2022) Articles Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. 6736(21). https://doi.org/10.1016/S0140-6736(21)02724-0
Infections B (2023) “Current Promising Strategies against Antibiotic-Resistant Bacterial Infections,”
Jeje O, Ewunkem AJ, Jeffers-francis LK (2023) “Serving two masters: Effect of Escherichia coli Dual Resistance on Antibiotic susceptibility,” pp. 1–20,
Morris C, Wickramasingha D, Abdelfattah EM, Pereira RV, Okello E, Maier G (2023) Prevalence of antimicrobial resistance in fecal Escherichia coli and Enterococcus spp. isolates from beef cow-calf operations in northern California and associations with farm practices. no Febr 1–14. https://doi.org/10.3389/fmicb.2023.1086203
Cristiane R, Leite T, Antônio T, Mendes DO (2023) Potential of the endogenous and artificially inserted CRISPR-Cas system for controlling virulence and antimicrobial resistance of food pathogens. Food Chem Adv 2:100229. no. February10.1016/j.focha.2023.100229
Gahamanyi N, Umuhoza T, Saeed SI, Mayigane LN, Hakizimana JN (2023) “A review of the important Weapons against Antimicrobial Resistance in Sub-Saharan Africa,” pp. 136–156,
Hyman P (2019) Phages for phage therapy: isolation, characterization, and host range breadth. Pharmaceuticals 12(1). https://doi.org/10.3390/ph12010035
Dedrick RM et al (2019) Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med 25(5):730–733. https://doi.org/10.1038/s41591-019-0437-z
Liang S et al (2023) “Bacteriophage therapy as an application for bacterial infection in China,” pp. 1–20,
Mahler M, Costa AR, Brouns SJJ, Van Beljouw SPB, Fineran PC (2023) Trends in Biotechnology Approaches for bacteriophage genome engineering. Trends Biotechnol 41(5):669–685. https://doi.org/10.1016/j.tibtech.2022.08.008
Sun Q et al (2023) Advance on Engineering of Bacteriophages by Advance on Engineering of Bacteriophages by Synthetic Biology. https://doi.org/10.2147/IDR.S402962
Kim K, Shin J, Kang TA, Kim B, Kim WC (2023) CRISPR/Cas9-mediated AtGATA25 mutant represents a novel model for regulating hypocotyl elongation in Arabidopsis thaliana. Mol Biol Rep 50(1):31–41. https://doi.org/10.1007/s11033-022-07926-9
Mathew SM (2023) Strategies for generation of mice via CRISPR/HDR-mediated knock-in. Mol Biol Rep 50(4):3189–3204. https://doi.org/10.1007/s11033-023-08278-8
Yang Y, Wang D, Lü P, Ma S, Chen K (2023) Research progress on nucleic acid detection and genome editing of CRISPR/Cas12 system. Mol Biol Rep 50(4):3723–3738. https://doi.org/10.1007/s11033-023-08240-8
Cameron P et al (2019) Harnessing type I CRISPR–Cas systems for genome engineering in human cells. Nat Biotechnol 37(12):1471–1477. https://doi.org/10.1038/s41587-019-0310-0
Lenneman BR, Fernbach J, Loessner MJ, Lu TK, Kilcher S (2021) Enhancing phage therapy through synthetic biology and genome engineering. Curr Opin Biotechnol 68:151–159. https://doi.org/10.1016/j.copbio.2020.11.003
Wetzel KS et al (2021) CRISPY-BRED and CRISPY-BRIP: efficient bacteriophage engineering. Sci Rep 11(1):1–6. https://doi.org/10.1038/s41598-021-86112-6
Ramirez-Chamorro L, Boulanger P, Rossier O (2021) Strategies for bacteriophage T5 mutagenesis: expanding the Toolbox for Phage Genome Engineering. Front Microbiol 12. https://doi.org/10.3389/fmicb.2021.667332
Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F (2013) Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8(11):2281–2308. https://doi.org/10.1038/nprot.2013.143
States U et al (2022) “Molecular Epidemiology of Carbapenem-Resistant,” vol. 28, no. 6, https://doi.org/10.1089/mdr.2021.0352
Mu AR (2022) Analysis of Antibiotic Resistance and Biofilm-Forming capacity in tetracycline-resistant Bacteria from a Coastal lagoon. 28(6):654–659. https://doi.org/10.1089/mdr.2021.0255
Kang K, Imamovic L, Misiakou M, Bornakke M, Heshiki Y (2021) Expansion and persistence of antibiotic-specific resistance genes following antibiotic treatment. Gut Microbes 13(1):1–19. https://doi.org/10.1080/19490976.2021.1900995
Ndagi U, Falaki AA, Abdullahi M, Lawal M (2020) Antibiotic resistance: bioinformatics-based understanding as a functional strategy for drug. 18451–18468. https://doi.org/10.1039/d0ra01484b
Chen Y, Li W, Shi K, Fang Z, Yang Y, Zhang R (2023) “Isolation and characterization of a novel phage belonging to a new genus against Vibrio parahaemolyticus,” pp. 1–11,
Manohar P, Madurantakam Royam M, Loh B, Bozdogan B, Nachimuthu R, Leptihn S (2022) Synergistic Effects of phage-antibiotic combinations against Citrobacter amalonaticus. ACS Infect Dis 8(1):59–65. https://doi.org/10.1021/acsinfecdis.1c00117
Saini S, Shanbhag C, Saraogi I (2023) Chemical Approaches to tackle the Silent Pandemic of Antibiotic Resistance. no April. https://doi.org/10.51167/acm00051
Van Der Horst MA, Schuurmans JM, Smid MC (2011) De Novo Acquisition of Resistance to three antibiotics by Escherichia coli. 17(2). https://doi.org/10.1089/mdr.2010.0101
Ji X et al (2022) Molecular characteristics of extended-spectrum beta-lactamase-producing Escherichia coli strains. 28(6):750–757. https://doi.org/10.1089/mdr.2021.0298
Uba AI, Abdulazeez MA, Usman SS, Tabakoglu HO, Abubakar H (2015) “Phage Dısplay Technology As A Strong Alternatıve To Hybrıdoma Technology For Monoclonal Antıbody Productıon,”
Carroll D (2014) Genome engineering with targetable nucleases. Annu Rev Biochem 83:409–439. https://doi.org/10.1146/annurev-biochem-060713-035418
Blazanin M, Lam WT, Vasen E, Chan BK, Turner PE (2022) “Decay and damage of therapeutic phage OMKO1 by environmental stressors,” PLoS One, vol. 17, no. 2 February, pp. 1–13, https://doi.org/10.1371/journal.pone.0263887
Vera-Mansilla J, Sánchez P, Silva-Valenzuela CA, Molina-Quiroz RC (2022) Isolation and characterization of Novel Lytic Phages infecting Multidrug-Resistant Escherichia coli. Microbiol Spectr 10(1). https://doi.org/10.1128/spectrum.01678-21
Dong Y et al (2022) Characterization and genomic analysis of the First Podophage Infecting Shewanella, representing a novel viral cluster. Front Microbiol 13:1–13. https://doi.org/10.3389/fmicb.2022.853973
Majdani R, Ghahfarokhi ES (2022) Isolation and characterization of lytic bacteriophages against Pseudomonas aeruginosa isolates from human infections in the north-west of Iran. 14(2):203–213
Zelcbuch L et al (2021) Luminescent phage-based detection of klebsiella pneumoniae: from engineering to diagnostics. Pharmaceuticals 14(4). https://doi.org/10.3390/ph14040347
Pulkkinen EM, Hinkley TC, Nugen SR (2019) Utilizing in vitro DNA assembly to engineer a synthetic T7 nanoluc reporter phage for Escherichia coli detection. Integr Biol 11(3):63–68. https://doi.org/10.1093/intbio/zyz005
Braun P, Raab R, Bugert JJ, Braun S “Recombinant Reporter Phage rTUN1::,” 2023, https://doi.org/10.1021/acssensors.2c01822
Vogele K, Falgenhauer E, Von Schönberg S, Simmel FC, Pirzer T (2021) Small antisense DNA-Based gene silencing enables cell-free bacteriophage manipulation and genome replication. ACS Synth Biol 10(3):459–465. https://doi.org/10.1021/acssynbio.0c00402
Li M, Shi D, Li Y, Xiao Y, Chen M, Chen L (2020) Recombination of T4-like phages and its activity against pathogenic Escherichia coli in Planktonic and Biofilm Forms. Virol Sin 35(5):651–661. https://doi.org/10.1007/s12250-020-00233-2
Amankwah S, Abdella K, Kassa T (2021) Bacterial biofilm destruction: a focused review on the recent use of phage-based strategies with other antibiofilm agents. Nanotechnol Sci Appl 14:161–177. https://doi.org/10.2147/NSA.S325594
Mohammadi M, Saffari M, Siadat SD, Hejazi SH (2023) Isolation, characterization, therapeutic potency, and genomic analysis of a novel bacteriophage vB _ KshKPC – M against carbapenemase – producing Klebsiella pneumoniae strains (CRKP) isolated from ventilator – associated pneumoniae (VAP) infection of COVID – 19 patients. Ann Clin Microbiol Antimicrob. https://doi.org/10.1186/s12941-023-00567-1
Nobrega FL et al (2018) Targeting mechanisms of tailed bacteriophages. Nat Rev Microbiol 16:760
Lammens EM, Nikel PI, Lavigne R (2020) Exploring the synthetic biology potential of bacteriophages for engineering non-model bacteria. Nat Commun 11(1):1–14. https://doi.org/10.1038/s41467-020-19124-x
Isaev A, Andriianov A, Znobishcheva E, Zorin E, Morozova N, Severinov K (2022) Editing of phage genomes—recombineering-assisted SpCas9 modification of Model Coliphages T7, T5, and T3. Mol Biol 56(6):801–815. https://doi.org/10.1134/S0026893322060073
Mahler M, Costa AR, van Beljouw SPB, Fineran PC, Brouns SJJ (2022) “Approaches for bacteriophage genome engineering,” Trends Biotechnol, vol. xx, no. xx, pp. 1–17, https://doi.org/10.1016/j.tibtech.2022.08.008
Shen J, Zhou J, Chen G-Q, Xiu Z-L (2018) Efficient Genome Engineering of a virulent Klebsiella. J Virol 92(17):1–20
Hoshiga F, Yoshizaki K, Takao N, Miyanaga K, Tanji Y (2019) Modification of T2 phage infectivity toward Escherichia coli O157:H7 via using CRISPR/Cas9. FEMS Microbiol Lett 366(4):1–7. https://doi.org/10.1093/femsle/fnz041
Salmonella M, Almoghrabi SZ, El-obeid T, Al-hadidi SH, Al H (2022) Retail chicken carcasses as a Reservoir. 28(7):824–831. https://doi.org/10.1089/mdr.2021.0414
Duong MM, Carmody CM, Ma Q, Peters JE, Nugen SR (2020) Optimization of T4 phage engineering via CRISPR/Cas9. Sci Rep 10(1):1–9. https://doi.org/10.1038/s41598-020-75426-6
Xia X (2023) “Optimizing protein production in therapeutic phages against a bacterial Pathogen, Mycobacterium abscessus,” no. 1, pp. 189–209,
Palma M (2023) “Aspects of phage-based vaccines for protein and Epitope Immunization,” pp. 1–23,
Maffei E et al (2021) Systematic exploration of Escherichia coli phage-host interactions with the BASEL phage collection. PLoS Biol 19(11):e3001424. https://doi.org/10.1371/journal.pbio.3001424
Bayat F, Didar TF, Hosseinidoust Z (2021) Emerging investigator series: bacteriophages as nano engineering tools for quality monitoring and pathogen detection in water and wastewater. Environ Sci Nano 8(2):367–389. https://doi.org/10.1039/d0en00962h
Airola C et al (2023)
Bondad-reantaso MG et al (2022) “Review of alternatives to antibiotic use in aquaculture,” no. pp. 1–31, 2023, https://doi.org/10.1111/raq.12786
Pan P et al (2023) Bacteriophage – based techniques for elucidating the function of zebrafish gut microbiota. 2039–2059. https://doi.org/10.1007/s00253-023-12439-x
Acknowledgements
No acknowledgements.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Sani Sharif Usman and Evangeline Christina conceived the idea; Sani Sharif Usman designed the review and gathered the information; Sani Sharif Usman, Abdullahi Ibrahim Uba and Evangeline Christina wrote the manuscript. The authors read and approved the final manuscript as well as agreed to authorship and submission of the manuscript for review.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Authors have declared that no competing interest exists.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Usman, S.S., Uba, A.I. & Christina, E. Bacteriophage genome engineering for phage therapy to combat bacterial antimicrobial resistance as an alternative to antibiotics. Mol Biol Rep 50, 7055–7067 (2023). https://doi.org/10.1007/s11033-023-08557-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08557-4