Abstract
Background:
Breast cancer metastatic programming involves an intricate process by which the tumor cell coevolves with the surrounding extracellular niche. The supporting cells from the local host stroma get transformed into cancer-associated stromal cells. This complex crosstalk leads to extracellular matrix remodeling, invasion, and eventually distant metastasis.
Methods:
In this review, we examine the protein-miRNA secretome that is crucial for this crosstalk. We also provide evidence from the literature for the pivotal role played by the various stromal cells like fibroblasts, adipocytes, and immune cells in promoting the process of EMT in breast cancer. Through in-silico analysis, we have also attempted to establish that stromal presence is integral to the process of EMT.
Results and Conclusion:
The in-silico analysis delineates the persuasive role of the stroma in mediating epithelial-to-mesenchymal transition. This review elucidates the importance of examining the role of the stromal niche that can yield promising diagnostic markers and pave avenues for formulating tailored anti-cancer therapy.
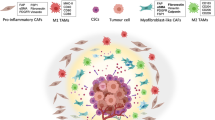
Graphical abstract
Process of EMT as driven by ‘stroma-hot’ tumors: The process of EMT is driven by the stromal cells. The stromal cells in the form of fibroblasts, adipocytes, endothelial cells, mesenchymal stromal cells and tissue associated macrophages secrete the miRNA-protein secretome that modulates the stromal niche and the tumor cells to be become ‘tumor associated’. This drives tumor progression and invasion. The ‘stromal-hot’ tumors eventually get the benefit of the surplus nurturing from the stroma that facilitates EMT leading to distant organ seeding and metastasis

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Death due to distant metastasis is a huge concern and barrier to effective treatment of breast cancer. The process of distant metastasis is a tightly regulated programmed process that gets initiated in the tumor–stromal niche when appropriate cues are exchanged. The cues help in the transformation of the polarized epithelial cells to attain plasticity to change into the hybrid epithelial–mesenchymal cells and then eventually the mesenchymal cells. This transition encompasses cellular and complex phenotypic changes that facilitate breach, migration, invasion, and colonization of tumor cells in distant favourable niches. This evolution is induced by an efficient dynamic and reciprocal crosstalk between the tumor and the surrounding stroma. The stroma in association with the tumor cells gets re-classified and refurbished as the tumor associated stroma. This extra tumoral matrix nurtures and supports tumor cells aiding and accelerating tumor progression. The cues that induce this transformation consist of secretome from the tumoral-stromal niche that is enriched for growth factors and chemokines. In addition to the proteins and chemokines, small regulatory RNA molecules like miRNAs also form a part of this blend of cues that effectively amend the tumoral cells and help them propel through the process of epithelial to mesenchymal plasticity. This review describes the process of epithelial to mesenchymal transition (EMT) that may be modulated by the tumoral extracellular matrix (ECM). The process by which the signalling from the stromal cells re-program the transcriptomic code of the tumoral cells is demonstrated using an in-silico analysis performed using breast tumor derived gene expression data of the The Cancer Genome Atlas (TCGA). The protein-miRNA secretome that is vital for the breast tumor–stromal crosstalk and the involvement of the various stromal cells in aiding clinical tumor progression of breast cancer is further reviewed.
The dynamic role of the stroma in modulating epithelial to mesenchymal transition
EMT is a controlled cellular program through which epithelial cells lose its features of apical–basal cellular polarity and attachment to basement membrane to transform into mesenchymal spindle like cells with features of invasive ability and stemness. This process was first identified by Greenburg and Hay in 1982 when they observed adult embryonic epithelial cells suspended in native collagen gels, get transformed into elongated mesenchymal like cells and eventually get detached and migrate [1]. Since its discovery, various studies have recognized its role in normal physiological processes like embryogenesis and wound healing and further exploited its potential in regenerative medicine for skin and heart muscle injuries. In normal cells, EMT is a well-known and vital developmental phenomenon that aids cells to differentiate into specific tissues. The disruption in homeostasis within a cancerous epithelial cell leads to EMT through genetic and epigenetic changes influenced by various factors. This transition is instrumental in aiding epithelial cells to get transformed into aggressive mesenchymal cells and thus is a key step for cancer cells to migrate and metastasize. The cells that undergo this transition are subjected to loss of epithelial cell characteristics such as cell–cell adhesion, apical polarity, and reduced expression of E-cadherin. Subsequent changes include gaining motility, stemness, increased N-cadherin & vimentin expression, loss of tight junction, cytoskeletal remodelling, and possible resistance to chemotherapy. These cellular changes complemented by the degradation of adherent junction leads the cells towards invasion. Metastasis then proceeds with migration of tumor cells from the site of initial formation to a secondary region involving a dynamic change in cellular phenotype and plasticity. Cells in the mesenchymal state then regain epithelial features and this process has been termed mesenchymal to epithelial transition (MET). This reversal of EMT is deemed imperative to establish a tumor in secondary sites during metastasis, a phenomenon termed as ‘reseeding’. This identifies that during EMT and MET, cells are in a transition state with flexibility to adapt and switch between either of the states based on the cues transmitted from the stromal environment to the tumors. This transition is a multifaceted process with distinct ‘hybrid’ or ‘intermediate’ phenotypes having both epithelial and mesenchymal features. This plasticity along the epithelial–mesenchymal axis featuring a hybrid E/M phase is termed epithelial–mesenchymal plasticity (EMP). Studies have shown these cells tend to be highly invasive with low proliferative abilities along with being chemo-resistant and possessing stemness attributes [2, 3].
It is now a well-accepted fact that the processes of tumor progression and distant metastasis are not tumor-cell autonomous. The ECM that surrounds the tumoral cells is tuned by the cues arising from the tumoral component. The structure of normal breasts comprises of an external layer of epithelial cells within which are present a collection of branched ducts with terminal ducts bulging into lobes. Within them are present several small ductules ending with structure called acinus, comprising of a lumen/cavity, lined by luminal epithelial cells, followed by myoepithelial cells attached to the basement membrane. Basement membrane clasps these structures to the ECM which is secreted by the cells of breast stroma. In addition, the stroma also modulates and controls epithelial cell polarity through signals from the basement membrane [4]. Breast tumors, however, fail to confine themselves within the defined structural organization of the breast tissue and are highly disorderly. This complex niche is now a heterogenous mixture of distinctive types of tumor cells in terms of plasticity, clonal evolution and genetic variations [5] along with stromal cells like fibroblasts, infiltrating endothelial cells, adipocytes and lymphocytes [6]. The tumor cells interact with the stromal cells, with a bi-directional signalling system to support each other and promote tumorigenesis. For instance, the stroma is signalled to produce abnormal levels of growth factors, cytokines like interleukin (IL-6) and other soluble factors along with increased angiogenesis to support tumor growth and its survival [4, 7]. The transformed stroma in turn forms an intricate nest harbouring and nurturing the tumor cells and fine-tuning the process of tumor progression [4]. Bulk of the breast matrix is composed of adipose tissue which is implicated in inducing EMT in various other cancer types too. These adipocytes in the tumor stroma induce expression of mesenchymal marker proteins triggering EMT alongside promoting proliferation, migration and invasion [8]. The cancer associated fibroblasts secrete several growth factors and chemokines and is driven by hypoxia; a phenomenon that occurs when oxygen supply becomes inadequate further increasing the formation of reactive oxygen species (ROS) [9]. Hypoxia in tumors is known to promote increased angiogenesis, desmoplasia, and inflammation. Hypoxia and the hypoxia driven genes are known to increase collagen production, deposition and matrix stiffness that leads to increased EMT. Mesenchymal stem cells in the stroma secrete factors which increase expression of EMT markers along with altering cell morphology and growth pattern [10]. Further cues influence the differentiation of M1 to M2 macrophages which are highly immunosuppressive and pro tumorigenic [7]. Breast stroma, along with the cellular components, also secretes non-cellular components [6]. This dynamic niche consisting of cellular and non-cellular entities transform into the cancer-associated specialised niche that propels tumor progression.
The protein-microRNA secretome as key facilitators of tumor cell transformation and plasticity: the stromal link
During tumor progression, the tumor tempers the stromal cells to construct a favourable niche in the presence of secreted proteins, growth factors and pro-migratory cytokines such as epidermal growth factor (EGF), hepatocyte growth factor (HGF), transforming growth factor (TGF-β), fibroblast growth factor (FGF), insulin-like growth factor (IGF) and signalling molecules like Hedgehog and Wnt. These proteins enhance the expression of transcription factors such as Snail, ZEB1, SIP1 etc., through associated pathways [9, 11]. Stimulation of Wnt, TGF-β and notch signalling pathways also lead to repression of E-cadherin, and activation of Snail, Slug, ZEB1 and ZEB2 which further induce EMT. The triggered EMT would lead to further changes in the niche including that of activation of MMPs that help in degradation of the stroma to aid the migration of the invading tumor cell. In addition to secreted proteins, miRNAs also play an important and essential role in regulating gene expression and eventually the protein map for tuning different biological pathways. They are small, endogenous, non-coding RNA molecules, comprising of about 20–22 nucleotides and mediate gene silencing and cleavage of mRNA transcripts at post-transcriptional level. miRNAs are transcribed by RNA polymerase II and on transcription, are released as un-processed pri-miRNA, longer than its usual length, with 5′ cap and 3′-poly A tails, along with several introns. This is sliced by a complex of Drosha and DGCR8 into pre-miRNA which is then transported out of the nucleus, into the cytoplasm, and gets further cleaved by Dicer and associates with guide miRNA to be loaded onto the RNA Inducing Silencing Complex (RISC). miRNA associated with the RISC complex, can base pair with complementary region in the target mRNA and bring about silencing of the target gene or degradation of the mRNA [12]. Thus, miRNAs play a central role in controlling various cellular functions and are key epigenetic regulators. In the tumor micro-environment, several miRNAs are released in exosomes or vesicles from both the tumor cells as well as the stromal elements. They regulate each other by modulating protein expression and this creates a tumor promoting environment.
To further analyse the integral role of the stroma in shaping the tumor and aiding in tumor progression, we performed an in-silico analysis by using the breast tumors of the TCGA cohort. The TCGA data was accessed from the TCGA Research Network: https://www.cancer.gov/tcga (accessed on 15 November 2020). The TCGA gene expression data was used to infer the stromal scores to predict level of infiltrating stromal cells in the tumors along with the cumulative ESTIMATE score using the ESTIMATE algorithm. The ESTIMATE (Estimation of Stromal and Immune cells in Malignant Tumour tissues using Expression data) is a tool that uses gene expression data for predicting tumor purity, and the presence of infiltrating stromal/immune cells in tumor tissues [13]. Tumors were stratified based on high and low stromal content derived from the ESTIMATE score. A list of genes (n = 40) and miRNAs (n = 67) implicated in EMT were identified from literature and their expression profile was analysed in these tumor groups within each subtype of breast cancer- the ER+, HER2+ and the triple negative (TNBC). Significant differentially expressed miRNAs and genes (p < 0.05) in three subtypes were then further analysed for their expression pattern (Figs. 1 and 2; Table 1). From the results it is very clear that majority of the genes implicated in driving EMT are highly expressed in the stromal high tumors across all the subtypes of breast cancer. The same trend can be seen with the miRNAs as well. There are 2 clusters of miRNAs; one cluster up regulated and the other down regulated in the high and low stromal tumors respectively. This may be attributed to the functionality of the miRNAs as they are known to be tumor-suppressing or promoting. The result indicates that the presence of stroma is crucial to activate genes and pathways that are important for the process for EMT, and this seems to be tuned down in ‘stroma-cold’ tumors. This intrigued us to delineate the specific role of the stromal cells in the process of EMT and the protein-miRNA secretome that aids in the dynamic tumor stroma crosstalk, which is discussed in detail below.
The part played by cancer associated fibroblasts (CAFs)
Fibroblast cells present in ECM are functionally responsible for maintaining the ECM configuration and have an important role to play in the process of wound healing. CAFs are the most abundant type of stromal component associated with a growing tumor, partakes in nurturing its growth, proliferation and promoting migration [6]. Originally, CAFs are activated from quiescent fibroblasts, acquire spindle shaped morphology with high expression of the smooth muscle actin fibers rendering it contractile property along with altered gene expression profiles [7, 41]. The activation signals are produced from the tumor mass in the form of growth factors like TGF-β, FGF, inflammatory factors like PDGF, ROS, tumor induced hypoxia etc. These growth factors could promote “mesenchymal–mesenchymal” transitions of the resident fibroblasts to convert them into CAFs or sometimes they could also be sourced from bone marrow derived mesenchymal-stem cells and recruited at the tumor site [6, 7]. The other precursors of CAFs include bone marrow fibrocytes, mesenchymal stem cells, adipocytes, pericytes, and smooth muscle cells [42, 43]. Intracellular and soluble normal fibroblasts are lost during initial stages mostly but are transformed and stabilized into CAFs during the course of EMT [44]. These CAFs then mediate cell–cell interactions, secretion of paracrine factors, cytokines, and chemokines to alter the integrity of the surrounding epithelial cell mass. They facilitate ECM structure remodelling allowing immunosuppression, metastasis and stemness [42, 43]. Interestingly, it is also observed that CAFs, like cancer cells, undergo Warburg effect producing lactate and ATP energy to fuel tumor cells [45]. Besides, CAFs have also been observed to synthesize ECM components and secrete them in the stroma, thereby re-defining the stromal organization [6]. It was also reported that upon co-culturing MCF-7 cells with fibroblast cells, CAFs diminish apoptosis and promotes hyperplasia induced by Estrogen receptor (ER) by modulating the levels of ER and its transactivation [46]. During single/collective cell migration, CAFs produce ECM degrading proteases like MMP2, MMP3 and MMP9 creating micro tracks through the dense stroma facilitating migration [47, 48]. Moreover, CAFs modulate immune response by secreting pro-inflammatory cytokines like IL-1,6,8, SDF-1, NFkβ and TNF-α as well as chemotactic protein 1 (MCP1/CCL2) enriching the stroma with lymphocytes and eventually transforming them into tumor promoting lymphocytes [7, 49]. CAFs inhibit the T-cell infiltration and recruit other immunosuppressive components to the tumor microenvironment (TME) rendering it immunologically cold. It was also reported that CSF1 produced by CAF neutralize the anti-tumor immunity, recruiting TAMs [43].
Further, activated CAFs secrete high levels of growth factors that includes TGF-β, HGF, IGF, nerve growth factor (NGF), EGF, and FGF2 [7, 50]. By co-culturing the breast tumor stroma derived fibroblasts with cancer cell lines, it was showed that CAFs in co-culture, secreted TGF-β, which induced the TGF-β/SMAD signalling pathway and promoted EMT of the breast cancer cell lines [51]. Multiple other mechanisms of EMT induction by CAFs have also been reported namely induction through oxidative stress and senescent fibroblasts via senescence activated secretory pathway (SASP) [52]. Another mechanism reported is the reciprocal regulation of EMT by TGF-β and FGF secreted from stromal cells that drives transitioning of endothelial cells to myofibroblasts. TGF-β induces the formation of myofibroblast in the tissue microenvironment whereas FGF2 suppresses TGF-β by regulation of ELK1 transcription factor [53].
Various studies have also demonstrated the regulatory roles of miRNAs secreted by CAFs on tumor progression. Induced expression of miR-21 in CAFs contributed to invasive potential of pancreatic cancer, through up regulation of MMP-3,9, PDGF and CCL-7 [54]. High miR-143 expression in CAFs induced collagen type III expression via TGF-β/SMAD signalling [55]. In-vitro studies have also showed that CAFs derived exosomes with miR-181d-5p, targets CDX2 and thereby induced proliferation and repressed apoptosis in breast cancer cell lines [56]. High miR-7 expression in CAFs decreased RAS-association domain family 2 and enhanced migration in in-vitro studies [57]. High miR-9 levels in CAFs associated with TNBC was reported to induce EMT through E-cadherin loss [58]. Exosomal SNHG3 secreted by CAFs acts as sponge for miR-330-5p inhibiting oxidative phosphorylation, increasing glycolysis, carboxylation and hence promoting tumor proliferation in breast cancer [59]. The increase in the levels of fibronectin by miR-200 in the CAFs promoted ECM remodelling, activating the invasion and metastasis of breast cancer cells in vitro and in vivo [60].
Role of adipocytes and cancer associated adipocytes
Breast tissue is predominantly composed of adipose tissue, which mainly helps in storage of lipid molecules and provides support, along with other components. Tumor cells are commonly found to metastasize to adipose rich regions to derive maximum energy. The fact that adipose tissue in humans increase with obesity directly correlates with the high incidence of cancer associated with this condition [61]. This indicates that there is a very close association of tumor cells with adipocytes which affect tumor–stromal interaction to achieve EMT. Epidemiological studies in breast cancer show the involvement of obesity with increased number of adipocytes as a risk factor promoting disease progression [62]. Adipocytes are the lipid storing cells and comprises of lipid derived hormones, leptin, adiponectin (APN), collagen VI which are collectively termed as adipokines. These employ endocrine signalling to regulate obesity, metabolism along with inflammation and tumor progression in tumor stroma environment [6, 63]. Adipocytes get activated by the stimuli through tumor cells and secreted proteins to form cancer associated adipocytes (CAAs) which are intermediate cells with the expression of FSP1 (Ferroptosis Suppressor Protein 1) [64]. The characteristic feature of CAAs are their differential shrunken phenotype brought about by Wnt/β-catenin pathway stimulated by paracrine signalling from cancer cells with subsequent loss of lipid content, decreased expression of adipocyte markers aP2 and FABP4 with high expression of proteases and MMP11 as shown by co-culture studies of normal adipocytes with cancer cells [63]. These CAAs also express fibroblasts markers like S100A4/FSP-1 to gain migratory capacity and move towards the tumor core to interact with them and these are termed adipocyte derived fibroblasts (ADF) [65].
The lipolysis of CAAs is known to directly fuel the tumor growth. CAAs also secret pro-inflammatory cytokines like IL-6 and IL-1β [8, 63] which render the associated tumors invasive and aggressive abilities. Besides, they also secrete CCL5 which aids in invasion and motility. CCL5 secreted from peritumoral adipocytes have also been shown to promote metastasis [5, 66]. Another member of IL6 family cytokines, Oncostatin-M (OSM) is also secreted by CAAs and is known to induce stemness [67]. Further, IL-6 secretion by CAAs has been shown to induce EMT in breast cancer, through STAT3 signalling [6]. The increased level of IL-6 is also associated with invasion, angiogenesis, and metastasis by activation and over expression of JAK/STAT, TNF-α, and induction of EMT signalling pathways [61]. Moreover, in-vitro co-culture studies of adipocytes with breast cancer cell lines like MCF-7 and MDA-MB-468 showed increased expression of MMP9 associated with migration and invasion [63]. Recent in-vitro co-culture studies also presented the EMT inducing potential of adipocytes through increased expression of EMT associated transcription factors like Twist 1 and Snail, vimentin, MMP9, decrease in cell adhesion protein E-cadherin and metastasis suppressor gene Kiss1 [68]. Another interesting study reported that adipocyte high tumors were enriched for metastasis and inflammation related gene signature while the adipocytes low tumors were associated with advanced grades and high proliferation [61]. In-vitro co-culture studies have also demonstrated that increased expression of mmu-miR-5112 by adipocytes targeted CPEB1 gene which is a negative regulator of IL-6, thereby promoting IL-6 secretion [69]. Pre-adipocytes secreted exosomes with miR-140 have been shown to modulate stemness and migration of breast cancer cells by targeting SOX9 signalling in breast cancer [70].
Endothelial cells and their contribution towards EMT
Endothelial cells are the inner-most, single layer of cells that line the vasculature [71, 72]. They are also an integral part of tumor-associated vasculature and are termed tumor-derived endothelial cells (TECs). The tumor associated endothelial cells are of various forms like Circulatory Endothelial Cells (CEC), Endothelial Progenitor Cells (EPC), Circulating Endothelial Progenitor Cells (CEPC), Aneuploid TECs and the more recently identified exocytic procoagulant Endothelial Micro Particles [71, 72]. CECs, as the name suggests are found in circulation. EPCs originate from the bone marrow and express surface markers like CD133, CD34, VEGFR2 [72]. CEPCs are like EPCs but are present in circulation and have lost CD133. Aneuploid TECs are endothelial cells with aneuploidy of chromosomes. Such genetic abnormality is generated from “cancerization of stromal endothelial cells” and “endothelialization of carcinoma cells” in the hypoxic tumor microenvironment. In either of the cases, trans-differentiation and heterotypic cell fusion constitute the primary pathways leading these processes [71]. Endothelial Micro Particles are a distinct class of TECs that are formed as vesicles, post activation, and comprise of endothelial cell specific surface markers and cytoplasmic status [72]. Studies have also shown that endothelial cells in bone marrow receive signals in the form of TGF-β from tumor stroma. Consequently, these endothelial cells undergo a transition into fibroblasts like mesenchymal cells via endothelial to mesenchymal transition with downregulation of CD31 and increased CAF markers FSP1 and α-SMA [73, 74]. CAFs secret chemokines like CXCL12 and this also helps in recruiting EPCs to tumor site [75]. The tumor and stromal cells are induced by the hypoxia derived factor HIF-1α and signalled to secrete angiogenesis growth factor VEGF. VEGF recruits the activated TECs, at the tumor site, to bring about “angiogenic sprouting” to improve and extend blood vessels throughout the tumor mass [76]. This supplies the tumor core with oxygen, nutrients availability [77], promotes growth and survival of the tumor mass and aids in transmission of the various paracrine signalling factors. EPCs and vascular endothelial cells via the expression of various integrins like α1β1, α2β1, and α5β1 have been shown to be involved in angiogenesis and are supported by pericytes via TGF-β and PDGFB-PDGFR-β signalling [76]. Pericytes are among diverse cell types found in breast tissue, which are responsible for lining the outer surface of blood vessels by providing structural support [78, 79]. VEGF-C along with other pro-angiogenic factors like PDGF, FGF-2, and EGF then promote the growth of lymphatic endothelial cells. In response, these cells secret high levels of pro-chemotactic chemokine ligand CCL21 which promotes migration of the tumor cells towards the newly formed lymph vessels leading to metastasis [76]. It is also reported that NF-kappa signalling stimulates these cancer associated endothelial cells to produce TNF-α and CXCL1/2 which further signals myeloid cells to produce S100 A8/9, resulting in increased survival and chemo-resistance of breast cancer cells [80]. Using in-vitro experimentation, it was shown that supernatant from cultured endothelial cells consists of paracrine signalling factors like basal membrane and ECM proteins nidogen-1, biglycan, cyr61, hspg2 that activates the pro-migratory STAT3 signalling pathway and induces invasion and cell migration in breast cancer cells [81]. Endothelial cells have also been featured as EMT inducers via PAI-1 and CCL5 signalling [5]. It has also been shown that prolonged TGF-β signalling from the tumor stroma promotes proliferation of endothelial cells preventing trans-endothelial migration (TEM) of cells into vessels while a brief exposure promotes TEM through down regulation of Twist, CSF1 and LHX2/PDGFβ signalling [82, 83]. Additionally, miRNAs are also known to play a role in endothelial cell-tumor crosstalk. It is reported that miR-7 affects the interaction between endothelial cells and tumor cells and was found to negatively correlate with survival in breast cancer patients. miR-7 expressed in the breast cancer cells also inhibited proliferation, migration, and invasion of endothelial cells [84]. ALK1 gene is also known to be critical for the process of angiogenesis and is expressed in endothelial cells. ALK1 gene is targeted by miR-199b-5p which is generally repressed in breast cancer cells. The process of vasculature genesis is tightly controlled by endothelial cell-tumor crosstalk where miR-199b-5p targets ALK1 gene [85].
The part played by mesenchymal stromal cells (MSCs)
Mesenchymal stromal cells (MSCs) also known as mesenchymal stem cells are multi-potent, heterogenous mixture of spindle shaped adherent cells with self-renewal and stem like properties [86]. They originate primarily from bone marrow and commonly reside in peri-vascular niches of most of the human tissues [77, 87]. These cells differentiate majorly into tri-lineages-osteoblasts, chondrocytes and adipocytes [88]. The heterogeneity of these cells arises from clonal convergence and sub-clones generated based on the environment they reside in [87]. Studies have showed that MSCs are able to migrate and incorporate into tumor, differentiate, or remain primitive, thereby contributing to the stroma and support or inhibit tumor progression. Tumors undergoing necrosis and the surrounding injured tissues release cytokines and chemokines like CXCL1,2,4 which binds to CXCR4 receptors present on MSCs either in local tissue regions or in circulation and recruit it to the tumor site [89, 90]. The MSCs extravasate and engraft into tumor mass and if they get activated into tumor promoting MSCs, they secret IL-6, Wnt5a, BMP and Gremlin-1 and create a niche similar to that of cancer stem cells (CSCs), with Gremlin-1; promoting CSC self-renewal and producing a stem cell pool. Tumors will then secrete more chemo-attractants like TGF-β and SDF-1a, recruiting more MSCs into this niche [91, 92].The MSCs from the tumor mass is essentially different from the naïve MSCs present in normal tissues. This is due to reprogramming/activation of MSCs by the tumor microenvironment. Waterman and colleagues also identified polarization and fate determination of MSCs by Toll receptor signalling. MSCs express Toll receptors 1–5 and gets polarized into anti-tumor MSC1 or pro-tumor MSC2, based on the type of inducing signals that activate the receptors [93, 94]. Owing to its self-renewal capacities, MSCs are also able to support tissue repair and regenerative activities. Besides, they secret chemokines and other factors signalling the neighbouring cells and regulating their survival, maturation, and apoptosis [77]. Further, MSCs secrets chemokines like IL-8, growth factors like VEGF, TGF-β and other metabolites into the TME to support tumor vascularity [87, 95]. TGF-β promotes endothelial differentiation and angiogenesis and other growth factors like EGF activates PIK3/Akt survival pathway [91]. They also recruit mast cells to increase vascular permeability [89]. MSCs also directly interact with cancer cells via gap junction protein connexions or formation of F-actin tunnelling tubes and promote tumor growth [87]. MSCs then favour tumor metastasis by promoting motility via chemokine CCL5, which interacts with CCR5 receptor on tumors and helps in metastasis. CCL5 secretion is in turn promoted by tumor released osteopontin (OPN). They also create metastatic niche at secondary sites and promote tumor initiation via IL-6 secretion and JAK-STAT pathway activation [93, 96]. The immune-modulation via secretion of macrophage inflammatory protein-2, TGF-β1, and the pro-inflammatory cytokines, along with reducing the cytotoxicity of NK cells and T cells, protects the tumors from immune responses [91, 97]. It is also reported that MSCs can undergo differentiation into CAFs, through expression of CAFs specific markers α-SMA, FSP etc. on stimulation by TGF-β and TGF-β/SMAD signalling. This has been experimentally demonstrated in an in-vitro setting using breast cancer cell lines [93, 98]. MSCs also secrete exosomes comprising of miRNAs, growth factors, bioactive lipids and growth factors essential to support tumor progression [89]. In-vitro co-culture studies on breast cancer cell lines with MSCs have shown EMT induction on breast cancer cell lines through increased expression of N-cadherin, Vimentin, Twist and Snail and down regulation of E-cadherin, via TNFα and IFNγ stimulation and TGF-β signalling [93]. There exist very few reports on the miRNA regulation of these cells. MSCs secrete miR-16 and miR-92a and is associated with anti-angiogenic response. miR-16 down regulates VEGF while miR-92a down regulates HGF secretion, both of which supresses angiogenesis [99, 100]. In-vitro studies have also shown that exosomal secreted miR-23b from bone marrow MSCs induce cycle arrest and suppress migration and invasion in breast cancer stem cells [101].
Immune cells and their vital role in EMT progression
Macrophages are phagocytic cells of the immune system derived from myeloid progenitor cells and recruited at the site of injury and foreign invasions. These cells secret pro-inflammatory cytokines IL-1 and TNFα and prime the host immune response [6, 7]. The myeloid progenitor cells differentiate into immature monocytes in the bone marrow and are released into circulation. On receiving chemokine signals like CCL2, CCL5, CCL7, CXCL8 and CXCL12 secreted from the stromal cells, these monocytes are recruited at the TME [102]. Here, based on the different environmental cues, they are polarized to either M1 or M2 macrophages based on differential surface marker expression. The M1 are “classically” activated macrophages signalled by LPS or IFN-c and are tumor inhibiting in nature. The TME however signals the monocytes towards an “alternatively” activated M2 polarization state. These M2 macrophages are pro-tumorigenic, produce anti-inflammatory cytokines, promote angiogenesis, and wound healing [6, 7]. The close interaction of immune cells with tumor cells impacts each other either to promote or suppress tumor progression. TME also constitutes of other immune suppressive cells like NK cells, Tregs, and MDSC in high levels [103]. The process of EMT generally makes the TME invasive, and immunosuppressive. During EMT or MET the genes in tumor cells undergo alterations to develop neoantigens which increases immunogenicity. The cytotoxic CD8+T cell that normally engages in lysing the tumor cell refrains from the same during EMT due to change in antigen susceptibility which leads to immune escape [104]. The mesenchymal like cells express Twist 1, RUNX which regulate FOXP3 expression and activate Treg cells. The activation of MMPs in TME increases immune infiltration and these immune cells interact with tumor cells to create a pro-tumorigenic environment. The increased secretion of pro-inflammatory cytokines are also found to suppress EMT by increasing the immune cell recruitment as shown in previous studies [105].
Tumor associated macrophages (TAMs) secret CCL18 which binds to breast cancer specific receptor PITPNM3 and this induces a downstream signalling that leads to increased expression of integrin clusters on tumor cell surface which can interact with the stromal ECM and promote adhesion [106]. At a later stage, these TAMs, like CAFs also secrets ECM remodelling serine proteases, MMP 2, 3, 7, 9, cathepsins and lysosomal enzymes which disrupt these integrin cancer cell surface-ECM interactions and favor tumor migration and invasion [7]. Further, TAMs have been shown to induce EMT in various types of cancers through different mechanisms dependent on the environmental cues. While TLR4 signalling in pancreatic cancer drives EMT, in breast cancer it is instigated by increased CCL18 secretion [6, 107]. Eventually, the signalling converges into events of reduced expression of epithelial markers like E-cadherin and acquisition of mesenchymal characters alongside activation of ZEB1 [87]. Moreover, NF-kβ mediated signalling and stabilization of other transcription factors like Snail is also brought about by TAMs [7]. Recently, in vitro co-culture studies on breast cancer cell lines with monocyte cell line demonstrated that the M1 polarized macrophages inhibited the outgrowth of the aggressive mesenchymal cell line MDA-MB-231 with re-expression of E-cadherin and acquisition of quiescent morphology, while the M2 polarized macrophages led the dormant, epithelial MCF-7 more proliferative with loss of E-cadherin and acquisition of spindle morphology [108]. These findings suggest that in order to maintain the plasticity along the epithelial-mesenchymal axis, the TME signalling could possibly polarize monocytes to both M1 and M2 phenotypes to bring about the required balance between the two phenotypes, in the process also promoting distant metastasis and drug resistance brought about by EMP.
Myeloid-derived suppressor cells are immature, deregulated, and dysfunctional myeloid cell precursors seen in spleens, peripheral blood and in and around the tumor. They are activated by tumor cells and then produce prostaglandins to promote their own proliferation. These cells primarily deplete the concentration of essential amino acids such as arginine and lysine which are needed by other immune cells to develop and function and further produce ROS under hypoxic conditions. They also promote the M2 macrophage phenotype by secreting TGF-β, suppress the functioning of NK cells, T lymphocytes and Dendritic cells [105, 109]. MDSCs are also found to upregulate the expression of PD1, PDL1, LAG3, CTLA4, and TIM3 [109].
TAMs also release exosomes containing several miRNAs and among them high miR-21 expression in monocytes caused M2 polarization and EMT induction through Snail expression [110]. Further, cytokine IL-1Ra induced high expression of miR-100 in TAMs and promoted cancer stemness via Hedgehog signalling pathway in in-vitro models [111]. miR-146a and miR-222 were associated with induction and recruitment of M2 macrophages, via CXCR4 targeting in a breast cancer mouse model [112]. High expression of miR-519a-3p showed resistance to apoptosis in breast cancer cells and was shown to be associated with poor survival. It suppresses the target genes coding for different caspases making them insensitive to apoptotic signalling cascade. It also down regulates the expression of NKG2D and prevents killing of tumor cells by NK cells [113]. miR-200c, is a master regulator in controlling immune suppression, and when overexpressed in tumor cells was demonstrated to make them more sensitive to immune therapy [104]. This points towards the fact that tumor–stromal interactions may be amenable to targeted treatment strategies like immune therapy that may be used in combination with conventional therapies.
Discussion and future perspectives
It is now a well-accepted fact that tumor progression that leads to metastasis is a process that is multistep and multidimensional and is not a tumor cell self-directed process. The stroma plays a critical role by providing a sheltered nest that harbours and nurtures the tumor cells. They not only provide the required nutrients but also provide signalling cues that bestow invasive properties and immune evasive ability. To enable transition of tumor and stromal cells through this process of EMT and to render the plasticity required for this, proteins and epigenetic machinery in the form of miRNAs form the tools. They help in dispatching the cues between tumor and stroma and in sculpting the trail toward distant metastasis. The expression of proteins and miRNAs crucial for the process of EMT is exceedingly distinctive in the presence and absence of stroma. This is a clear illustration of the fact that stroma is instrumental in driving tumor progression. In the review we have presented evidence from literature about the integral role of stroma for the above-mentioned dynamic crosstalk. This is exceptionally valuable comprehension and useful in designing therapeutic strategies for anticancer treatment.
The focus should shift towards designing more molecules that target both the stroma and the tumor cells concurrently. Some of them are already in various phases of clinical trials and have shown promising results [114]. Though our understanding of the stromal role in tumor progression has deepened over the last decade, several questions remain unexplored and challenges to overcome. Bulk of the data obtained for the stromal contribution towards EMT arises from pre-clinical model systems and results obtained from pre-clinical research may be highly inconsistent due to the variety of pre-clinical models used like xenografts, spheroids etc. The implication of these biomarkers derived in cancer therapeutics should be evaluated further through the scope of larger research and cohort-based studies. The dual role of some of the stromal cells in being anti-tumorigenic and pro-tumorigenic add a layer of complexity and makes it challenging to discern the stromal role in tumor progression further. More research is necessary to understand this dual role of stromal cells and the stromal secretome in metastatic progression and therapeutic resistance. Anticancer research and thought-provoking leads on the might of the stromal niche are indeed promising and pave way to a new era of personalised medicine.
References
Greenburg G, Hay ED (1982) Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol 95(1):333–339. https://doi.org/10.1083/jcb.95.1.333
Georgakopoulos-Soares I, Chartoumpekis DV, Kyriazopoulou V, Zaravinos A (2020) EMT factors and metabolic pathways in cancer. Front Oncol. https://doi.org/10.3389/fonc.2020.00499
Jolly MK, Boareto M, Huang B, Jia D, Lu M, Ben-Jacob E et al (2015) Implications of the hybrid epithelial/mesenchymal phenotype in metastasis. Front Oncol 5:155. https://doi.org/10.3389/fonc.2015.00155
Kim JB, Stein R, O’Hare MJ (2005) Tumour–stromal interactions in breast cancer: the role of stroma in tumourigenesis. Tumour Biol 26(4):173–185. https://doi.org/10.1159/000086950
Kvokackova B, Remsik J, Jolly MK, Soucek K (2021) Phenotypic heterogeneity of triple-negative breast cancer mediated by epithelial-mesenchymal plasticity. Cancers. https://doi.org/10.3390/cancers13092188
Poltavets V, Kochetkova M, Pitson SM, Samuel MS (2018) The role of the extracellular matrix and its molecular and cellular regulators in cancer cell plasticity. Front Oncol 8:431. https://doi.org/10.3389/fonc.2018.00431
Taddei ML, Giannoni E, Comito G, Chiarugi P (2013) Microenvironment and tumor cell plasticity: an easy way out. Cancer Lett 341(1):80–96. https://doi.org/10.1016/j.canlet.2013.01.042
Lee Y, Jung WH, Koo JS (2015) Adipocytes can induce epithelial-mesenchymal transition in breast cancer cells. Breast Cancer Res Treat 153(2):323–335. https://doi.org/10.1007/s10549-015-3550-9
De Francesco EM, Maggiolini M, Musti AM (2018) Crosstalk between notch, HIF-1alpha and GPER in breast cancer EMT. Int J Mol Sci. https://doi.org/10.3390/ijms19072011
Martin FT, Dwyer RM, Kelly J, Khan S, Murphy JM, Curran C et al (2010) Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res Treat 124(2):317–326. https://doi.org/10.1007/s10549-010-0734-1
Pupo M, Pisano A, Abonante S, Maggiolini M, Musti AM (2014) GPER activates notch signaling in breast cancer cells and cancer-associated fibroblasts (CAFs). Int J Biochem Cell Biol 46:56–67. https://doi.org/10.1016/j.biocel.2013.11.011
Vasudevan S, Tong Y, Steitz JA (2007) Switching from repression to activation: microRNAs can up-regulate translation. Science 318(5858):1931–1934. https://doi.org/10.1126/science.1149460
Yoshihara K, Shahmoradgoli M, Martínez E, Vegesna R, Kim H, Torres-Garcia W et al (2013) Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun 4:2612. https://doi.org/10.1038/ncomms3612
Lin Z, Hu Y, Lin R, Ye H (2020) The effect of overexpression of the HOXD10 gene on the malignant proliferation, invasion, and tumor formation of pancreatic cancer cell PANC-1. Gland Surg 9(2):385–391. https://doi.org/10.21037/gs.2020.03.28
Tomaskovic-Crook E, Thompson EW, Thiery JP (2009) Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res 11(6):213. https://doi.org/10.1186/bcr2416
Liu KW, Hu B, Cheng SY (2011) Platelet-derived growth factor signaling in human malignancies. Chin J Cancer 30(9):581–584. https://doi.org/10.5732/cjc.011.10300
Puchalapalli M, Mu L, Edwards C, Kaplan-Singer B, Eni P, Belani K et al (2019) The Laminin-α1 chain-derived peptide, AG73, binds to syndecans on MDA-231 breast cancer cells and alters filopodium formation. Anal Cell Pathol 2019:9192516. https://doi.org/10.1155/2019/9192516
Jezierska A, Motyl T (2009) Matrix metalloproteinase-2 involvement in breast cancer progression: a mini-review. Med Sci Monit 15(2):Ra32–40
Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW et al (1999) The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell 98(2):137–146. https://doi.org/10.1016/s0092-8674(00)81009-0
Huang H (2018) Matrix metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: recent advances. Sensors. https://doi.org/10.3390/s18103249
Zeng R, Huang J, Zhong MZ, Li L, Yang G, Liu L et al (2016) Multiple roles of WNT5A in breast cancer. Med Sci Monit 22:5058–5067. https://doi.org/10.12659/msm.902022
Guo D, Huang J, Gong J (2012) Bone morphogenetic protein 4 (BMP4) is required for migration and invasion of breast cancer. Mol Cell Biochem 363(1–2):179–190. https://doi.org/10.1007/s11010-011-1170-1
Carbognin L, Miglietta F, Paris I, Dieci MV (2019) Prognostic and predictive implications of PTEN in breast cancer: unfulfilled promises but intriguing perspectives. Cancers. https://doi.org/10.3390/cancers11091401
Elias D, Ditzel HJ (2015) Fyn is an important molecule in cancer pathogenesis and drug resistance. Pharmacol Res 100:250–254. https://doi.org/10.1016/j.phrs.2015.08.010
Kai K, Iwamoto T, Zhang D, Shen L, Takahashi Y, Rao A et al (2018) CSF-1/CSF-1R axis is associated with epithelial/mesenchymal hybrid phenotype in epithelial-like inflammatory breast cancer. Sci Rep 8(1):9427. https://doi.org/10.1038/s41598-018-27409-x
Teng Y, Loveless R, Benson EM, Sun L, Shull AY, Shay C (2021) SHOX2 cooperates with STAT3 to promote breast cancer metastasis through the transcriptional activation of WASF3. J Exp Clin Cancer Res 40(1):274. https://doi.org/10.1186/s13046-021-02083-6
Jiang LH, Zhang HD, Tang JH (2018) MiR-30a: a novel biomarker and potential therapeutic target for cancer. J Oncol 2018:5167829. https://doi.org/10.1155/2018/5167829
Xiong H, Zhao W, Wang J, Seifer BJ, Ye C, Chen Y et al (2017) Oncogenic mechanisms of Lin28 in breast cancer: new functions and therapeutic opportunities. Oncotarget 8(15):25721–25735. https://doi.org/10.18632/oncotarget.14891
Hou B, Ishinaga H, Midorikawa K, Nakamura S, Hiraku Y, Oikawa S et al (2018) Let-7c inhibits migration and epithelial–mesenchymal transition in head and neck squamous cell carcinoma by targeting IGF1R and HMGA2. Oncotarget 9(10):8927–8940. https://doi.org/10.18632/oncotarget.23826
Wang K, Peng GG, Tan YL, He SZ, Luo CF (2021) MiR-99a inhibits proliferation of oral squamous cell carcinoma by targeting mTOR pathway. Shanghai Kou Qiang Yi Xue 30(1):44–49
Sheedy P, Medarova Z (2018) The fundamental role of miR-10b in metastatic cancer. Am J Cancer Res 8(9):1674–1688
Yang FR, Li HJ, Li TT, Zhao YF, Liu ZK, Li XR (2019) Prognostic value of microRNA-15a in human cancers: a meta-analysis and bioinformatics. Biomed Res Int 2019:2063823. https://doi.org/10.1155/2019/2063823
Kim HK, Park JD, Choi SH, Shin DJ, Hwang S, Jung HY et al (2020) Functional link between miR-200a and ELK3 regulates the metastatic nature of breast cancer. Cancers. https://doi.org/10.3390/cancers12051225
Piperigkou Z, Franchi M, Riethmüller C, Götte M, Karamanos NK (2020) miR-200b restrains EMT and aggressiveness and regulates matrix composition depending on ER status and signaling in mammary cancer. Matrix Biol Plus 6–7:100024. https://doi.org/10.1016/j.mbplus.2020.100024
Song C, Liu LZ, Pei XQ, Liu X, Yang L, Ye F et al (2015) miR-200c inhibits breast cancer proliferation by targeting KRAS. Oncotarget 6(33):34968–34978. https://doi.org/10.18632/oncotarget.5198
Zeng Y, Gao T, Huang W, Yang Y, Qiu R, Hou Y et al (2019) MicroRNA-455-3p mediates GATA3 tumor suppression in mammary epithelial cells by inhibiting TGF-β signaling. J Biol Chem 294(43):15808–15825. https://doi.org/10.1074/jbc.RA119.010800
Xu WX, Liu Z, Deng F, Wang DD, Li XW, Tian T et al (2019) MiR-145: a potential biomarker of cancer migration and invasion. Am J Transl Res 11(11):6739–6753
Yang X, Feng KX, Li H, Wang L, Xia H (2020) MicroRNA-199a inhibits cell proliferation, migration, and invasion and activates AKT/mTOR signaling pathway by targeting B7-H3 in cervical cancer. Technol Cancer Res Treat 19:1533033820942245. https://doi.org/10.1177/1533033820942245
Ye T, Liang Y, Zhang D, Zhang X (2020) MicroRNA-16-1-3p represses breast tumor growth and metastasis by inhibiting PGK1-Mediated warburg effect. Front Cell Dev Biol 8:615154. https://doi.org/10.3389/fcell.2020.615154
Huang X, Xu X, Ke H, Pan X, Ai J, Xie R et al (2022) MicroRNA-16-5p suppresses cell proliferation and angiogenesis in colorectal cancer by negatively regulating forkhead box K1 to block the PI3K/Akt/mTOR pathway. Eur J Histochem. https://doi.org/10.4081/ejh.2022.3333
Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C (2001) Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell 12(9):2730–2741. https://doi.org/10.1091/mbc.12.9.2730
Hurtado P, Martinez-Pena I, Pineiro R (2020) Dangerous liaisons: circulating tumor cells (CTCs) and cancer-associated fibroblasts (CAFs). Cancers. https://doi.org/10.3390/cancers12102861
Jenkins L, Jungwirth U, Avgustinova A, Iravani M, Mills A, Haider S et al (2022) Cancer-associated fibroblasts suppress CD8+T-cell infiltration and confer resistance to immune-checkpoint blockade. Cancer Res 82(16):2904–2917. https://doi.org/10.1158/0008-5472.CAN-21-4141
Libring S, Shinde A, Chanda MK, Nuru M, George H, Saleh AM et al (2020) The dynamic relationship of breast cancer cells and fibroblasts in fibronectin accumulation at primary and metastatic tumor sites. Cancers. https://doi.org/10.3390/cancers12051270
Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG et al (2009) The reverse warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 8(23):3984–4001. https://doi.org/10.4161/cc.8.23.10238
Morgan MM, Livingston MK, Warrick JW, Stanek EM, Alarid ET, Beebe DJ et al (2018) Mammary fibroblasts reduce apoptosis and speed estrogen-induced hyperplasia in an organotypic MCF7-derived duct model. Sci Rep 8(1):7139. https://doi.org/10.1038/s41598-018-25461-1
Sinha D, Saha P, Samanta A, Bishayee A (2020) Emerging concepts of hybrid epithelial-to-mesenchymal transition in cancer progression. Biomolecules. https://doi.org/10.3390/biom10111561
Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C et al (2007) Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol 9(8):893–904. https://doi.org/10.1038/ncb1616
Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R et al (2005) Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121(3):335–348. https://doi.org/10.1016/j.cell.2005.02.034
Prindull G, Zipori D (2004) Environmental guidance of normal and tumor cell plasticity: epithelial mesenchymal transitions as a paradigm. Blood 103(8):2892–2899. https://doi.org/10.1182/blood-2003-08-2807
Yu Y, Xiao CH, Tan LD, Wang QS, Li XQ, Feng YM (2014) Cancer-associated fibroblasts induce epithelial–mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br J Cancer 110(3):724–732. https://doi.org/10.1038/bjc.2013.768
Bavik C, Coleman I, Dean JP, Knudsen B, Plymate S, Nelson PS (2006) The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res 66(2):794–802. https://doi.org/10.1158/0008-5472.Can-05-1716
Akatsu Y, Takahashi N, Yoshimatsu Y, Kimuro S, Muramatsu T, Katsura A et al (2019) Fibroblast growth factor signals regulate transforming growth factor-beta-induced endothelial-to-myofibroblast transition of tumor endothelial cells via Elk1. Mol Oncol 13(8):1706–1724. https://doi.org/10.1002/1878-0261.12504
Zhang L, Yao J, Li W, Zhang C (2018) Micro-RNA-21 regulates cancer-associated fibroblast-mediated drug resistance in pancreatic cancer. Oncol Res 26(6):827–835. https://doi.org/10.3727/096504017X14934840662335
Naito Y, Sakamoto N, Oue N, Yashiro M, Sentani K, Yanagihara K et al (2014) MicroRNA-143 regulates collagen type III expression in stromal fibroblasts of scirrhous type gastric cancer. Cancer Sci 105(2):228–235. https://doi.org/10.1111/cas.12329
Wang H, Wei H, Wang J, Li L, Chen A, Li Z (2020) MicroRNA-181d-5p-containing exosomes derived from CAFs promote EMT by regulating CDX2/HOXA5 in breast cancer. Mol Ther Nucleic Acids 19:654–667. https://doi.org/10.1016/j.omtn.2019.11.024
Shen Z, Qin X, Yan M, Li R, Chen G, Zhang J et al (2017) Cancer-associated fibroblasts promote cancer cell growth through a mir-7-RASSF2-PAR-4 axis in the tumor microenvironment. Oncotarget 8(1):1290–1303. https://doi.org/10.18632/oncotarget.13609
Baroni S, Romero-Cordoba S, Plantamura I, Dugo M, D’Ippolito E, Cataldo A et al (2016) Exosome-mediated delivery of miR-9 induces cancer-associated fibroblast-like properties in human breast fibroblasts. Cell Death Dis 7(7):e2312. https://doi.org/10.1038/cddis.2016.224
Li Y, Zhao Z, Liu W, Li X (2020) SNHG3 functions as miRNA sponge to promote breast Cancer cells growth through the metabolic reprogramming. Appl Biochem Biotechnol 191(3):1084–1099. https://doi.org/10.1007/s12010-020-03244-7
Tang X, Hou Y, Yang G, Wang X, Tang S, Du YE et al (2016) Stromal miR-200s contribute to breast cancer cell invasion through CAF activation and ECM remodeling. Cell Death Differ 23(1):132–145. https://doi.org/10.1038/cdd.2015.78
Tokumaru Y, Oshi M, Katsuta E, Yan L, Huang JL, Nagahashi M et al (2020) Intratumoral adipocyte-high breast cancer enrich for metastatic and inflammation-related pathways but associated with less cancer cell proliferation. Int J Mol Sci. https://doi.org/10.3390/ijms21165744
Proebstle TM, Huber R, Sterry W (1996) Detection of early micrometastases in subcutaneous fat of primary malignant melanoma patients by identification of tyrosinase-mRNA. Eur J Cancer 32A(10):1664–1667. https://doi.org/10.1016/0959-8049(95)00534-x
Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B et al (2011) Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res 71(7):2455–2465. https://doi.org/10.1158/0008-5472.Can-10-3323
Rybinska I, Mangano N, Tagliabue E, Triulzi T (2021) Cancer-associated adipocytes in breast cancer: causes and consequences. Int J Mol Sci. https://doi.org/10.3390/ijms22073775
Bochet L, Lehuédé C, Dauvillier S, Wang YY, Dirat B, Laurent V et al (2013) Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res 73(18):5657–5668. https://doi.org/10.1158/0008-5472.Can-13-0530
Pinilla S, Alt E, Abdul Khalek FJ, Jotzu C, Muehlberg F, Beckmann C et al (2009) Tissue resident stem cells produce CCL5 under the influence of cancer cells and thereby promote breast cancer cell invasion. Cancer Lett 284(1):80–85. https://doi.org/10.1016/j.canlet.2009.04.013
Junk DJ, Bryson BL, Smigiel JM, Parameswaran N, Bartel CA, Jackson MW (2017) Oncostatin M promotes cancer cell plasticity through cooperative STAT3-SMAD3 signaling. Oncogene 36(28):4001–4013. https://doi.org/10.1038/onc.2017.33
Kushiro K, Chu RA, Verma A, Núñez NP (2012) Adipocytes promote B16BL6 melanoma cell invasion and the epithelial-to-mesenchymal transition. Cancer Microenviron 5(1):73–82. https://doi.org/10.1007/s12307-011-0087-2
Lee J, Hong BS, Ryu HS, Lee HB, Lee M, Park IA et al (2017) Transition into inflammatory cancer-associated adipocytes in breast cancer microenvironment requires microRNA regulatory mechanism. PLoS ONE 12(3):e0174126. https://doi.org/10.1371/journal.pone.0174126
Gernapudi R, Yao Y, Zhang Y, Wolfson B, Roy S, Duru N et al (2015) Targeting exosomes from preadipocytes inhibits preadipocyte to cancer stem cell signaling in early-stage breast cancer. Breast Cancer Res Treat 150(3):685–695. https://doi.org/10.1007/s10549-015-3326-2
Lin PP (2020) Aneuploid circulating tumor-derived endothelial cell (CTEC): a novel versatile player in tumor neovascularization and cancer metastasis. Cells. https://doi.org/10.3390/cells9061539
Astekar M, Metgud R, Sharma A, Soni A (2013) Hidden keys in stroma: unlocking the tumor progression. J Oral Maxillofac Pathol 17(1):82–88. https://doi.org/10.4103/0973-029X.110742
Bussard KM, Mutkus L, Stumpf K, Gomez-Manzano C, Marini FC (2016) Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res 18(1):84. https://doi.org/10.1186/s13058-016-0740-2
Sigurdsson V, Hilmarsdottir B, Sigmundsdottir H, Fridriksdottir AJ, Ringner M, Villadsen R et al (2011) Endothelial induced EMT in breast epithelial cells with stem cell properties. PLoS ONE 6(9):e23833. https://doi.org/10.1371/journal.pone.0023833
Mao Y, Keller ET, Garfield DH, Shen K, Wang J (2013) Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev 32(1–2):303–315. https://doi.org/10.1007/s10555-012-9415-3
Spaw M, Anant S, Thomas SM (2017) Stromal contributions to the carcinogenic process. Mol Carcinog 56(4):1199–1213. https://doi.org/10.1002/mc.22583
Eiro N, Gonzalez LO, Fraile M, Cid S, Schneider J, Vizoso FJ (2019) Breast cancer tumor stroma: cellular components, phenotypic heterogeneity, intercellular communication, prognostic implications and therapeutic opportunities. Cancers. https://doi.org/10.3390/cancers11050664
Kim J (2019) Pericytes in breast cancer. Adv Exp Med Biol 1147:93–107. https://doi.org/10.1007/978-3-030-16908-4_3
Cooke VG, LeBleu VS, Keskin D, Khan Z, O’Connell JT, Teng Y et al (2012) Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell 21(1):66–81. https://doi.org/10.1016/j.ccr.2011.11.024
Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG et al (2012) A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 150(1):165–178. https://doi.org/10.1016/j.cell.2012.04.042
Ferraro DA, Patella F, Zanivan S, Donato C, Aceto N, Giannotta M et al (2019) Endothelial cell-derived nidogen-1 inhibits migration of SK-BR-3 breast cancer cells. BMC Cancer 19(1):312. https://doi.org/10.1186/s12885-019-5521-8
Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E (2009) Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol 11(11):1287–1296. https://doi.org/10.1038/ncb1973
Tsuji T, Ibaragi S, Hu GF (2009) Epithelial-mesenchymal transition and cell cooperativity in metastasis. Cancer Res 69(18):7135–7139. https://doi.org/10.1158/0008-5472.can-09-1618
Cui YX, Bradbury R, Flamini V, Wu B, Jordan N, Jiang WG (2017) MicroRNA-7 suppresses the homing and migration potential of human endothelial cells to highly metastatic human breast cancer cells. Br J Cancer 117(1):89–101. https://doi.org/10.1038/bjc.2017.156
Lin X, Qiu W, Xiao Y, Ma J, Xu F, Zhang K et al (2019) MiR-199b-5p suppresses tumor angiogenesis mediated by vascular endothelial cells in breast cancer by targeting ALK1. Front Genet 10:1397. https://doi.org/10.3389/fgene.2019.01397
Keating A (2006) Mesenchymal stromal cells. Curr Opin Hematol 13(6):419–425. https://doi.org/10.1097/01.moh.0000245697.54887.6f
Hass R, von der Ohe J, Ungefroren H (2020) Impact of the tumor microenvironment on tumor heterogeneity and consequences for cancer cell plasticity and stemness. Cancers. https://doi.org/10.3390/cancers12123716
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147. https://doi.org/10.1126/science.284.5411.143
Cammarota F, Laukkanen MO (2016) Mesenchymal stem/stromal cells in stromal evolution and cancer progression. Stem Cells Int 2016:4824573. https://doi.org/10.1155/2016/4824573
Alsayed Y, Ngo H, Runnels J, Leleu X, Singha UK, Pitsillides CM et al (2007) Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood 109(7):2708–2717. https://doi.org/10.1182/blood-2006-07-035857
Torsvik A, Bjerkvig R (2013) Mesenchymal stem cell signaling in cancer progression. Cancer Treat Rev 39(2):180–188. https://doi.org/10.1016/j.ctrv.2012.03.005
Sneddon JB, Zhen HH, Montgomery K, van de Rijn M, Tward AD, West R et al (2006) Bone morphogenetic protein antagonist gremlin 1 is widely expressed by cancer-associated stromal cells and can promote tumor cell proliferation. Proc Natl Acad Sci U S A 103(40):14842–14847. https://doi.org/10.1073/pnas.0606857103
Ridge SM, Sullivan FJ, Glynn SA (2017) Mesenchymal stem cells: key players in cancer progression. Mol Cancer 16(1):31. https://doi.org/10.1186/s12943-017-0597-8
Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM (2010) A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS ONE 5(4):e10088. https://doi.org/10.1371/journal.pone.0010088
Li W, Zhang X, Wu F, Zhou Y, Bao Z, Li H et al (2019) Gastric cancer-derived mesenchymal stromal cells trigger M2 macrophage polarization that promotes metastasis and EMT in gastric cancer. Cell Death Dis 10(12):918. https://doi.org/10.1038/s41419-019-2131-y
Hsu HS, Lin JH, Hsu TW, Su K, Wang CW, Yang KY et al (2012) Mesenchymal stem cells enhance lung cancer initiation through activation of IL-6/JAK2/STAT3 pathway. Lung Cancer 75(2):167–177. https://doi.org/10.1016/j.lungcan.2011.07.001
Keating A (2012) Mesenchymal stromal cells: new directions. Cell Stem Cell 10(6):709–716. https://doi.org/10.1016/j.stem.2012.05.015
Shangguan L, Ti X, Krause U, Hai B, Zhao Y, Yang Z et al (2012) Inhibition of TGF-β/Smad signaling by BAMBI blocks differentiation of human mesenchymal stem cells to carcinoma-associated fibroblasts and abolishes their protumor effects. Stem Cells 30(12):2810–2819. https://doi.org/10.1002/stem.1251
Lee JK, Park SR, Jung BK, Jeon YK, Lee YS, Kim MK et al (2013) Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS ONE 8(12):e84256. https://doi.org/10.1371/journal.pone.0084256
Kalinina N, Klink G, Glukhanyuk E, Lopatina T, Efimenko A, Akopyan Z et al (2015) miR-92a regulates angiogenic activity of adipose-derived mesenchymal stromal cells. Exp Cell Res 339(1):61–66. https://doi.org/10.1016/j.yexcr.2015.10.007
Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU et al (2014) Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal 7(332):ra63. https://doi.org/10.1126/scisignal.2005231
Mantovani A, Allavena P, Sozzani S, Vecchi A, Locati M, Sica A (2004) Chemokines in the recruitment and shaping of the leukocyte infiltrate of tumors. Semin Cancer Biol 14(3):155–160. https://doi.org/10.1016/j.semcancer.2003.10.001
Romeo E, Caserta CA, Rumio C, Marcucci F (2019) The vicious cross-talk between tumor cells with an EMT phenotype and cells of the immune system. Cells. https://doi.org/10.3390/cells8050460
Camp FA, Brunetti TM, Williams MM, Christenson JL, Sreekanth V, Costello JC et al (2022) Antigens expressed by breast cancer cells undergoing EMT stimulate cytotoxic CD8(+) T cell immunity. Cancers. https://doi.org/10.3390/cancers14184397
Segovia-Mendoza M, Morales-Montor J (2019) Immune tumor microenvironment in breast cancer and the participation of estrogen and its receptors in cancer physiopathology. Front Immunol 10:348. https://doi.org/10.3389/fimmu.2019.00348
Chen J, Yao Y, Gong C, Yu F, Su S, Chen J et al (2011) CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell 19(4):541–555. https://doi.org/10.1016/j.ccr.2011.02.006
Liu CY, Xu JY, Shi XY, Huang W, Ruan TY, Xie P et al (2013) M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Invest 93(7):844–854. https://doi.org/10.1038/labinvest.2013.69
Yang M, Ma B, Shao H, Clark AM, Wells A (2016) Macrophage phenotypic subtypes diametrically regulate epithelial–mesenchymal plasticity in breast cancer cells. BMC Cancer 16:419. https://doi.org/10.1186/s12885-016-2411-1
Zhu H, Gu Y, Xue Y, Yuan M, Cao X, Liu Q (2017) CXCR2(+) MDSCs promote breast cancer progression by inducing EMT and activated T cell exhaustion. Oncotarget 8(70):114554–114567. https://doi.org/10.18632/oncotarget.23020
Hsieh CH, Tai SK, Yang MH (2018) Snail-overexpressing cancer cells promote M2-like polarization of tumor-associated macrophages by delivering MiR-21-abundant exosomes. Neoplasia 20(8):775–788. https://doi.org/10.1016/j.neo.2018.06.004
Wang W, Liu Y, Guo J, He H, Mi X, Chen C et al (2018) miR-100 maintains phenotype of tumor-associated macrophages by targeting mTOR to promote tumor metastasis via Stat5a/IL-1ra pathway in mouse breast cancer. Oncogenesis 7(12):97. https://doi.org/10.1038/s41389-018-0106-y
Li Y, Zhao L, Shi B, Ma S, Xu Z, Ge Y et al (2015) Functions of miR-146a and miR-222 in tumor-associated macrophages in breast cancer. Sci Rep 5:18648. https://doi.org/10.1038/srep18648
Breunig C, Pahl J, Kublbeck M, Miller M, Antonelli D, Erdem N et al (2017) MicroRNA-519a-3p mediates apoptosis resistance in breast cancer cells and their escape from recognition by natural killer cells. Cell Death Dis 8(8):e2973. https://doi.org/10.1038/cddis.2017.364
Xu M, Zhang T, Xia R, Wei Y, Wei X (2022) Targeting the tumor stroma for cancer therapy. Mol Cancer 21(1):208. https://doi.org/10.1186/s12943-022-01670-1
Acknowledgements
We thank the Department of Health Research, Ministry of Health & Family Welfare, India, for the Young Scientist fellowship [File no. R.12014/16/2019-HR/E office: 3205182] to M.G.N till July 2022 and SKAN Research Trust for Program Scientist fellowship thereafter. We thank University Grants Commission (UGC), India for JRF fellowship to A.D.M (221610111590) and C.M.N (221610066058).
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
AD: Conceptualization, Data curation, Interpretation of data, Writing—Original draft preparation. CM: Conceptualization, Data curation, Interpretation of data, Writing—Original draft preparation. JSP: Reviewing and Editing. MGN: Conceptualization, Data curation, Interpretation of data, Writing—Original draft preparation, Writing—Review & Editing. All authors have read and approved the final manuscript. AD and CM have contributed equally to this work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Ethical approval has been obtained from Institutional Ethics Review Board [IERB] at St. John’s Medical College and Hospital (No. 267/2022) for analysis of secondary data/public datasets from TCGA.
Additional information
Publisher’s Note
Springer nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mavatkar, A.D., Naidu, C.M., Prabhu, J.S. et al. The dynamic tumor–stromal crosstalk: implications of ‘stromal-hot’ tumors in the process of epithelial–mesenchymal transition in breast cancer. Mol Biol Rep 50, 5379–5393 (2023). https://doi.org/10.1007/s11033-023-08422-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08422-4