Abstract
Introduction Rheumatoid arthritis (RA) is a common autoimmune disease across the globe that is chronic and systemic as well. The disease is linked with autoantibodies and is inflammatory, eventually targeting several molecules along with certain modified self-epitopes. The disease majorly affects the joints of an individual. Rheumatoid arthritis is manifested clinically by polyarthritis linked with the dysfunction of the joints. This chiefly affects the synovial joint lining and is linked with progressive dysfunction, premature death, along with socioeconomic implications. The macrophage activation, along with the activation of certain defense cells, results in a response to self-epitopes that helps in providing a better understanding of the disease pathogenesis.
Material and methodology For this review article, papers have been retrieved and reviewed from database including PubMed, Scopus and Web of science. Relevant papers were taken fulfilling the criteria for writing this review article.
Results This has resulted in the establishment of several new therapeutic techniques that serve as potential inhibitors of such cells. Researchers have gained an interest in understanding this disease to provide strategies for treatment in the last two decades. This also includes recognition followed by the treatment of the disease at its early stages. Various allopathic treatment approaches often have chronic and toxic teratogenic effects. However, to avoid this issue of toxicity followed by side effects, certain medicinal plants have been used in treating RA.
Conclusion Medicinal plants possess active phytoconstituents that entail antioxidants as well as anti-inflammatory properties, making them a helpful alternative to allopathic drugs that are often linked with highly toxic effects. This review paper entails a thorough discussion of the epidemiology, pathophysiology, diagnosis, and management of RA. The paper will also focus on the use of herbal plants in the treatment of the disease to avoid the side effects that generally occur in allopathic treatment.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is among the chronic diseases that result in joint inflammation [1]. The disease is autoimmune and is generally characterized by autoantibodies to IgG. Inappropriate treatment of the disease often results in accumulating damage in joints along with certain irreversible disabilities in the joints of the patient [1]. The disease is heterogeneous in nature that shows variable pathogenetic mechanisms as well as clinical presentation implicated between those who have a similar diagnosis or across the varied phases of the disease [2]. Undeniably, although autoantibodies are considered to be an important characteristic of the disease and are known as seropositive RA whereas some individuals are negative for such kinds of autoantibodies and they are known as seronegative RA [2]. The complexity of the disease has been known for years now and this involves certain environmental factors that elicit disease in the genetically susceptible individuals. The disease has affected over 1% of the population between 25 and 50 years of age [3].
The disease results in several types of deformities in the joints of the knees, feet, spine, and hips. This results in pain, inflammation, joint stiffness, reduced strength of muscles, and loss of mobility. All of these lead to hampering the activities of daily life [1]. Pro-inflammatory intermediaries play an important role in RA pathogenesis as they lead to the articular manifestation with the help of an expansion in inflammatory infiltration of certain cells like T cells, B lymphocytes, and macrophages, in addition to the bone erosion that results in autoantibody [4]. The production of pro-inflammatory mediators occurs due to several environmental as well as genetic factors as they activate the immune-like cells called monocytes, synovial fibroblasts, and macrophages taking part in the disease [5]. The activation of these immune-like cells causes the initiation of CD4 +T cells that are antigen-activated. Furthermore, the activation of CD4+T-cell results in the generation of cytokines like interleukin-1 and 6, along with TNF, which is considered the principal mediator of RA. These cytokines are responsible for the obstruction in the proteoglycans and collagen [5]. The production of collagenases and MMP proteolytic enzymes is primarily responsible for cartilage damage. In addition, certain protein molecules like cytokines and chemokines are responsible for tissue deterioration and synovitis. This happens by attracting the neutrophils, monocytes, cyclooxygenase (COX)-2 enzyme, and lymphocytes that trigger hyperplasia along with the development of pannus in synovial joints, leading to apoptosis in fibroblasts of the synovium [6].
This review paper entails a thorough discussion of the disease’s epidemiology, pathophysiology, diagnosis, and management. The diagnosis and management of RA will include the new developments that have happened in the diagnosis of the disease along with some novel treatment strategies. The paper will also focus on the use of herbal plants for treating RA to avoid the side effects that generally occur in allopathic treatment [7]. It is known that the conventional drugs that have been used in the treatment regimen for arthritis help in preventing the worsening of joints by directly targeting the inflammatory mediators. This eventually reduces inflammation and pain. However, the continuous usage of conventional drugs in slowing the growth of disease adversely affects the human body over time. Moreover, patients treated with herbal plant medications were seen to have minimal effect on rheumatoid arthritis. They usually affect the cardiovascular and gastrointestinal systems. Thus, several practitioners are trying to remediate the RA disease through herbal treatment in comparison to chemical drugs. Medicinal plants show fewer side effects along with being more compatible with prolonged consumption as compared to allopathic medications [8].
Classification
The criteria for classifying RA were proposed by the American Rheumatism Association for the first time in 1958. The criteria were revised again in the year 1987 by the American College of Rheumatology [9].
-
(1)
Hand joints arthritis: Swelling in wrist PIP or MCP for at least a period of 6 weeks.
-
(2)
Arthritis in three or more joints: Swelling in soft tissue or fluid was observed for at least a period of 6 weeks.
-
(3)
Morning stiffness: Morning stiffness is observed in and around the joints that often last for at least an hour before the maximal enhancement.
-
(4)
Symmetrical arthritis: Concurrent participation of the same joint areas on both the sides of the body {this means bilateral involvement of Prolactin-induced protein (PIPs), Monocyte chemoattractant protein-1(MCPs), or Microsomal Triglyceride Transfer Protein (MTPs)} for at least a period of 6 weeks.
-
(5)
Radiographic changes: Radiographs of RA posteroanterior hand and wrist show the attrition or unequivocal bony decalcification.
-
(6)
Rheumatoid factor: Identified by a method positive in less than 5% of normal controls.
-
(7)
Rheumatoid nodules: Bony prominences or in juxta-articular regions show subcutaneous nodules.
Epidemiology
Worldwide prevalence
Most of the epidemiological studies for RA have been done and they show that the prevalence of the disease is in the range of 0.5–1.0% among white individuals. Although vigorous epidemiological studies have been limited to other regions, some of the data has been pointed toward an analogous range. This looks as if it might be quite analogous to the estimated reports in Western countries [10]. On the other hand, the disease prevalence varies between ethnicities. A high incidence has been seen in Native American populations (approximately 5–6%). The adjusted ratios of disease prevalence were 0.45, 1.02, and 0.69 for females of Hispanic, African-American, or Asian descent, respectively, as compared with white females. Finally, the differences in terms of geography have been reported, though studies are narrow. For instance, in southern Europe, a lesser prevalence has been reported as compared with northern Europe [10].
Risk factors
Various risk factors, as summarized in Fig. 1, are involved in RA development; these risk factors include environmental factors, female sex, and genetics. Infectious agents, not getting enough vitamin D, bein exposed to silica, being overweight, smoking, and changes in the microbiota are all environmental risk factors [11].
Genetics
RA is a disease that has a strong genetic component. As per the studies, the phenotypic variance of RA is approximately 60%. This is concerned with the patients of RA who have been tested positive for Anti-citrullinated protein antibodies (ACPAs), while in the case of seronegative disease it is comparatively less. On the other hand, in the case of monozygotic twins, the disease concordance is around 12–15%. This means an understanding that the factors that are non-coding also play a significant role in propensity [12].
Class II HLA has a very strong association with RA. The shared epitope has been determined by a few alleles of HLA-antigen, i.e., the D-related locus (DR). FLS may also be important for synovial T-cell activation, and one possibility is that FLS stimulates T cells by antigen presentation on MHC class II HLA-DR molecules. They are considerably linked to the risk of the development of RA [13]. However, the other risk loci that possess weaker links have also been recognized, among which the majority are related to inflammatory as well as immune pathways. Studies have shown that, as per the pathway analysis and functional annotation, approximately 100 loci have been identified across the genome porting variants with RA susceptibility. Various proteins that are encoded by such genes could be potentially targeted by therapeutic agents [1]. The variants that have been associated with this disease are commonly mapped to the enhancer regions. This leads to the regulation of a few genes at secluded locations in a particular pattern. Therefore, genetically susceptible variants that map to diverse provinces of a chromosome might be responsible for regulating the same gene [14]. It becomes important to understand this complex regulation as it helps in defining which genes are significant in which cell types for the predilection to this disease [14].
Epigenetics
A lot of studies have revealed that genetic variants that are linked with this disease are augmented in epigenetic marks of active chromatin in CD4+T cells. Histone acetylation and DNA methylation play a significant role in the development of RA, along with the inclusion of epigenetics. In the case of monozygotic twins, DNA methylation at EXOSC1 varies among the unaffected and the affected twin. Most of the studies related to this disease regarding DNA methylation in the cases of unrelated individuals recognise a total of nine clusters with a discrepancy pattern in the HLA region regarding methylation [15]. While, in comparison with healthy controls, the studies suggested that risk variants’ genetic effects of HLA act under DNA methylation that has been altered [15]. DNA methylation helps in providing the means through which environmental factors can persuade some alterations in cellular activity [16]. For instance, in the case of individuals who smoke, the level of methylation seems to be higher in individuals with ACPA-positive RA than in those with ACPA-negative RA; this difference in the level of methylation has not been observed in non-smokers [16].
Smoking
Tobacco use has long been associated with an increased risk of the disease. The link between the consumption of tobacco and the disease is strongest or even constrained to ACPA-positive disease in those with at least one copy of the shared epitope [10]. The relationship between smoking and the shared epitope increases the risk by 20-fold or somewhat even more than that as compared with non-smokers (non-smokers-no shared epitope). The current status of smoking is linked with increased pro-inflammatory cytokines as well as increased disease activity. Changes to epigenetics can also change the higher risk that comes with smoking [17].
Sex
Usually, females are twice to thrice more prone to RA than males. The cumulative life span of risk regarding the development of adult-onset disease is estimated at 3.6% for females and 1.7% for males [10]. The frequency of the disease in females is higher, which is accredited to the corresponding possessions of estrogen on the individual’s immune system [1, 10]. However, the function of hormonal factors in disease development remains controversial. In females, nulliparity has been seen as one of the reasons that increases the risk of this disease, while pregnancy is also linked with the remission of the disease. Although disease flickers are common in the postpartum period. In females, the disease is mostly symptomatic around the time of menopause or middle age. Males get sick later than females because they have much higher levels of ACPAs and are more likely to have RA [1].
Microbiota
Periodontal diseases are also linked with an augmented risk of disease development. Although RA and periodontal disease are quite different in clinical aspects, their mechanisms bear certain similarities, along with inflammatory bone erosions as well as chronic inflammation [6]. Interestingly, the link between periodontal disease and RA is somewhere arbitrated by the oral microbiota [18]. For instance, Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis are linked with the risk of rheumatoid arthritis. Aside from periodontal microbiota, gut microbiota has been shown to play an important role in the increased risk of RA. The diversity of gut microbiota has been seen to be lower in those who have RA as compared with the normal population. Rare taxa, such as Actinobacteria, are more abundant in RA patients, while abundant taxa diversity is reduced [19]. Along with these, the levels of Prevotellacopriin intestine have been seen to spot early disease. This is because this bacterium has been found very commonly in those patients that are untreated with new-onset RA rather than in those who do not have RA or in those with established disease [19]. The current study shows that the peptides of another two new autoantigens that possess certain sequence homology with Prevotella peptides along with some other species of gut bacteria have been isolated from the HLA-DR molecules of those individuals that have been suffering from RA. This finding shows a strong linkage between autoimmunity, the environment, and disease.
Dust inhalation
Exposure to silica has been identified as one of the environmental risk factors for RA. A study revealed that firefighters, along with other emergency responders who had been exposed to dust at the site of the World Trade Center collapse in the year 2001 in New York, the United States, had a high risk of systemic autoimmune diseases including RA. Glass fibres, silica, pulverised cement, asbestos, and other materials, among which certain elements are considered to be the risk factors for RA. Industrial exposure to textile dust is related to the augmented risk of RA development in the Malaysian female population [20].
Others
Mycoplasma organisms, Campylobacter, Porphyromonasgingivalis, Salmonella, and Proteus mirabilis are some of the microbes that have been found in the environment and are associated with RA. In addition to this, viruses like the Rubella virus and Epstein–Barr virus (EBV) are the causes of RA. Furthermore, structural and functional changes in the intestinal microbiome have been linked to disease, with patients having less diversity in the gut microbiome than healthy people [21]. Certain factors are implicated in RA and are also known as modifiable lifestyle factors. For instance, obesity is one of the factors that is constantly as well as autonomously linked with a reserved rise in the risk of successive rheumatoid arthritis, with a peculiar ratio of around 1.45 in those individuals that have a body mass index (BMI) of more than or equal to 30 kg per m2 as compared with individuals with less than 25 kg per m2 of BMI. A self-effacing connection has been discovered between long-term consumption of alcohol and a reduced risk of RA. When x-symptomology of posttraumatic stress disorder shown by women is elevated, the risk of developing RA also increases [21].
Mortality
Cardiovascular disease has been reported as a common cause of premature death in those who are suffering from RA. Individuals with this disease show increased rates of prevalence when it comes to cardiovascular risk factors; rates of hypertension, hyperlipidemia, diabetes mellitus, and obesity, and the rates are reported to be 9.9%, 6.0%, 18.6%, and 4.4%, respectively. Both genetic as well as serological factors play a significant role in the identification of people with RA who are generally at a higher risk of cardiovascular disease. A study has reported that females with RA show a high risk of total mortality (95% CI 1.25–1.57; HR 1.40) as compared with individuals that do not suffer from RA or respiratory disease [22].
Pathogenesis
Rheumatoid arthritis has been understood as a systemic disease along with certain immunological events that happen on mucosal surfaces outside the joint along with primary lymphoid tissues, whereas the central player is the synovium [4]. The synovium helps in serving two main functions in homeostasis. It helps in the production of lubricants that facilitate the surfaces of the cartilage to be operated in a low-friction environment, along with presenting important nutrients to the cartilage, which generally is deficient in its supply of blood [10]. The healthy synovium can be understood as a delicate structure that consists of macrophage-like synoviocytes, an intimal lining that is composed of FLS, and a sub lining. This sub lining is made up of blood vessels, adipocytes, scattered immune cells, and fibroblasts [4]. The intimal lining does not count as a barrier in a conventional way because the lining is short of a basement membrane as well as tight junctions. So, it leaks a lot and proteins and cells can move through it pretty easily into the synovial fluid [10].
There are two main pathogenetic changes that are evident in the synovium in the case of individuals with RA. The first main pathogenetic change involves the expansion of the intimal lining due to an increase in as well as activation of both synoviocyte types. These are understood as one of the most prominent sources of proteases and cytokines. The second pathogenetic change linked with RA is adaptive immune cell infiltration into the subliming of synovium [23] .
The cardinal sign of this disease is the cartilage and bone damage that occurs because of the synovial incursion into neighbouring articular structures [1]. The pathways that are involved in joint damage are heterogeneous. This comprises distinctive mechanisms between those who are ACPA negative and those who are ACPA positive, along with those who encompass some other autoantibodies. Neutrophils, mast cells, and macrophages altogether contribute to damaging the joints by releasing MMPs and cytokines. The study showed that joint disorder happens because of certain mechanical factors that also involve unpretentious inflammatory involvement. Erosions of bone also occur because of the activation and maturation of osteoclasts. This maturation and activation occur due to the receptor activator of the nuclear factor-κB ligand, which is generated by T cells. These osteoclasts potentially damage the mineralized bone matrix by constructing proteases [24].
The pro-inflammatory as well as tissue-damaging cellular activities that occur in synovitis are generally integrated by cytokine networks [1]. The main function that is played by cytokines when it comes to the pathogenesis of the disease is significantly established for TNF. They take part in the activation of leukocytes and production of MMP, followed by angiogenesis, along with promoting pain [4].
Cells, inflammation, and rheumatoid arthritis
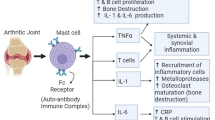
The main indication of synovitis is engendering pain as well as swelling in the accretion of inflammation mediators in the joint synovium that leads to the erosion of bone and cartilage. The cell variants have been diagnosed to be activated where there is inflammation. The lymphocytes, along with macrophages from blood circulation, start migrating into the synovium, followed by macrophage maturation that results in an increasing macrophage quantity in the synovium. This promotes the recruitment of inflammatory cells [4].
The hyperplastic synovial lining occurs due to the other migrated macrophages as well as synoviocytes that are quite similar to the fibroblasts. APC interaction with T cells occurs that leads to activation of T cell signalling followed by macrophage activation in the synovium. This macrophage activation releases the inflammatory mediators such as IL-1, IL-6, and TNF-alpha that cause the lymphocytes’ migration along with recruiting the PMN to enter into the joint space via hyperplastic synovial lining [4, 5].
The proteases such as MMP-1 and MMP-3 are released by PMN further and cartilage is destroyed by reactive oxygen species (ROS). Cartilage destruction is mediated by the release of additional proteases that are matrix metalloproteinase-1 and 3. In addition to this, osteoclast activation occurs that results in destroying the bone in rheumatoid arthritis. This is done by releasing RANKL [4] Fig. 2.
NF-kB signaling in RA
NF-kB plays a significant role in rheumatoid arthritis by taking part in inflammation. Consequently, the level of NF-kB is reported to be high in patients with arthritis. NF-kB signalling activation occurs due to various immune cells and the release of diverse stimuli of the RANKL receptor. The immune cells that wander at the synovium membrane and cause cartilage damage are T cells, B cells, macrophages, and synovial fibroblast. Thus, NF-kB is responsible for the mediation of high expression of chemokines (MMP-1 & 2, IL-8, MCP-1 & 2, iNOS, COX-1 & 2) and cytokines (TNF-alpha, IL-6, IL-1beta, IL-18). The NF-kB-inducing kinase is activated due to the B-cell-activating factor and CD40 as they are reported to be highly expressed in individuals with arthritis [25].
RANKL-RANK in RA
The main issues that are reported in arthritis include bone loss and erosion. It is well articulated by juxta-articular osteoporosis, which means loss of bone mass. In the case of osteoporosis, cells (osteoclasts) are the effectors that are related to the disease pathogenesis that results in osteoporosis. In RA, osteoclasts’ activation has been found to contribute to bone destruction [26]. while synoviocytes liberate the receptor activator for RANKL (NF-kB ligand). This activated NF-kB ligand results in the RANK activation of the osteoclast cell. The osteoclast (multinucleated or matured) displays the receptor of the RANK receptor on the membrane of the cell membrane [26]. The NF-kB ligand gets attached to the mature osteoclast, leading to the intracellular signalling pathways’ activation; this osteoclast, which is activated, encourages the TRAF protein, resulting in Src kinase protein activation. This leads to PI3K/mTOR/Akt pathway activation. This activation has taken place by both canonical as well as non-canonical pathways that help in mediating the inflammatory mediators that cause apoptosis, which results in osteoporosis.
Diagnosis
The diagnosis of RA is made easier by its clinical symptoms. Additional diagnostic symptoms include radiographic abnormalities and a positive rheumatoid factor. X-rays, joint aspiration, antinuclear antibodies, CRP, and rheumatoid factor are all used in the investigation of RA [27].
Pre-clinical RA
Rantapaa-Dahlqvist and coworkers showed in 2003 that high RF of different isotypes and CCP were present in preserved blood samples from a Swedish sample set of 83 patients with RA and controls, even years before the diagnosis of RA [28]. Soon later, Nielen et al. [29] used serum samples from the Dutch Sanquin biobank of blood donor samples, which included 79 patients with RA and controls, to show comparable results. Studies supporting this found comparable results over the following years, but with slightly different biomarkers (including various tests for autoantibodies) and in different cohorts, such as the United States Military, North American nurses, and a Norwegian sample set. C-reactive protein, many cytokines and chemokines, and gene expression in inflammatory pathways have all been shown to be higher before a diagnosis of RA [30], though the amount of increase varies from study to study.
Several recent prospective studies of early RA development have supplemented the two early notable prospective studies of preclinical RA, but the majority of the early looks at preclinical RA were through studies of unintentionally collected samples from subjects prior to the development of RA. Among these is the Dutch “arthralgia” cohort, which includes people who report pain and stiffness in 44 joints but who test negative for IA at baseline based on the examination of two doctors. These people may test positive for RF and/or CCP2. In order to determine how frequently and when people develop arthritis, it is necessary to monitor them prospectively. Early work reported that rates of development of RA in subjects with RF and CCP2 positive at baseline were approximately 40% over a median of 28 months of follow up, with incident rates of 50% in less than 2 years in patients who had baseline CCP levels above the 75th percentile [31]. This was just one of many findings generated from this cohort.
Additional biomarker studies in preclinical RA
IgG, IgA, and IgM isotypes of CCP2 were present prior to a diagnosis of RA, with IgG being most commonly present prior to a diagnosis of RA (35%), followed by IgA (24%), and then IgM (12%). Furthermore, using multiplex technology to identify ACPAs to a wide variety of antigens, Brink, Rantapaa-Dahlqvist and colleagues identified that immune responses to citrullinated proteins appear to be restricted to a small number of targets early in RA and expand towards the time of diagnosis of RA [32]. Moreover, while there was not a specific early antigen targeted across all subjects, autoantibodies to citrullinated fibrinogen and vimentin were early and did not seem to rise dramatically over time, while antibodies to other citrullinated antigens, including enolase and filaggrin, appeared later and rose prior to diagnosis; finally, antibodies to antigens such as citrullinated collagen appeared only immediately prior to a diagnosis of RA, but increased in levels after the onset of clinically apparent arthritis. Similar results have been demonstrated in studies using the Dutch Sanquin biobank and the Dutch ‘arthralgia’ cohort, and the United States Department of Defense Serum Repository (DoDSR). Also, Suwannalai and his team showed that ACPA avidity seems to go up over time during preclinical RA until IA symptoms show up [33].
Medical history
It is important to assess both personal and family medical histories. The effects of medicine include the reduction of symptoms like pain, stiffness, discomfort, and trouble moving. RA status can also be determined by the distribution and number of afflicted joints. For example, the same joint on both sides typically becomes affected by the condition. Even so, the condition might just affect one joint. RA can affect both small and large joints, but it typically starts in small joints like the hands and feet [34].
Physical examination
All joints should be examined by the physician to check for warmth, swelling, discomfort, and restricted or painful motions. During a regular physical exam, rheumatoid nodules and a low-grade fever may be found to be other signs [35].
Biochemical characteristics
The results of testing for CRP, adenosine deaminase, renal function, uric acid, and liver function should be assessed. Complete blood counts of people with RA often show that they have anaemia (lower haemoglobin or red blood cells) and thrombocytopenia [36].
Rheumatic factor
Rheumatic factor is typically found in 80% of RA patients. Nevertheless, it may be found in people who have been given a diagnosis for another condition, such as infectious hepatitis, sarcoidosis, TB, parasitic infection, infectious mononucleosis, syphilis, Sjogren’s syndrome, or systemic lupus erythematosus [37]. Subjects having a family history of the condition are more likely to have the rheumatic factor found in them. Therefore, thorough knowledge of patients’ medical status and history is necessary for appropriate interpretation of the rheumatic component. An aggressive stage of the disease is indicated by a high titer of the rheumatic factor. The rheumatic factor test result that is normal or negative is 14 IU/ml. Rheumatic factor levels above 14 IU/ml are considered abnormally high or positive. Autoimmune conditions like RA are linked to an increased rheumatic factor. In order to diagnose RA from the standpoint of persistent polyarthritis, high titer IgM RF is utilized. It used to be the sole serologic indicator for RA diagnosis [38].
Classification criteria
The diagnosis of RA requires taking into account a number of criteria. These symptoms include morning stiffness in the joints, symmetrical arthritis, arthritis of the hand joints, and alterations in radiography. A patient’s desire for reassurance, explanation, and knowledge increases significantly after receiving a diagnosis of RA [39]. Inflammation, the rate of advancement, and the degree of structural alterations can all be determined through a thorough evaluation. The patient’s lifestyle as a whole, including their free time, job, and domestic responsibilities, should be assessed. In rheumatoid arthritis (RA), engaging in physical activity does not increase joint disease. Patients should begin with beginner activities to preserve muscular strength and joint mobility due to the possibility of developing growing joint rigidity and abnormalities. Patients with RA have decreased shoulder mobility and reduced knee flexion [40].
Prevention and management
The approaches that help prevent the disease are chiefly aimed at those individuals that have been dealing with undifferentiated arthritis [1]. Preventive strategies include both pharmacological and risk factor modification strategies. Individuals who participate in oral health and/or smoking cessation programmes are less likely to develop RA. Along with this, increasing the number of fish you eat and losing weight have been thought of as ways to change risk factors that could help prevent the disease [10].
Pharmacological treatment of RA
TNF-blockers (etanercept, infliximab, adalimumab, and certolizumab), IL-1 antagonist tocilizumab, B-cell depleting rituximab, T-cell co-stimulator abatacept, IL-6 antagonist tocilizumab, and IL-1 receptor antagonist are medications for RA (anakinra). The side effects of tocilizumab include headaches, nasopharyngitis, upper respiratory tract infections, and gastrointestinal perforations. Agents that target proximal effects of the immune response and IL-17 are two novel biological medicines currently in research. Future contributions from novel conventional drugs with DMARD-like effects are also possible. The results of clinical investigations with the kinase inhibitors SYK and JAK were positive, and more research is being done on additional targets [41].
Treatment for RA typically entails a four-pronged approach that is based on the principles of planned management. Reducing pain and inflammation in RA is the goal of NSAIDs and steroidal anti-inflammatory medications (corticosteroids). Disease-modifying antirheumatic medications (DMARDs) and biological medicines slow the spread of damage and delay the onset of the illness. Drugs used to treat arthritis might interfere with conception and pregnancy; since RA affects more women than males, special care must be taken when devising management methods for women of reproductive years. RA can be treated with cyclosporine, azathioprine, minocycline, myochrysine, gold, sulfasalazine, hydroxychloroquine, leflunomide, and methotrexate, among other drugs [42].
Infliximab and methotrexate are frequent treatments for RA. Infliximab and methotrexate together are more effective than methotrexate alone in preventing bone and cartilage degeneration. Biologics and disease-modifying antirheumatic medications (DMARDs) are powerful immunosuppressants that raise the danger of developing life-threatening infections and, likely, several types of cancer. The elderly has the highest incidence of illnesses and cancers. The patient should get a blood test to rule out infectious disorders such as tuberculosis, hepatitis, and others that are not readily apparent to the naked eye before beginning treatment with any biologic or DMARD. Certain disease-modifying antirheumatic drugs (DMARDs) are associated with an increased risk of liver disease [43].
Primary biological agents for RA are ineffective because they trigger the body’s production of biosimilars. The first biosimilar of infliximab (IFX), called CT-P13, has been approved by the European Medicines Agency. The Phase III clinical trials comparing CT-P13 or PLANETRA to the original IFX indicated that both were equally effective, with a similar safety profile and pharmacokinetics. Chemicals are the source of nonbiologic DMARDs. Most are taken orally once or twice weekly, while others may be given as injections. Inhibiting the immune system with DMARDs slows the development of RA. Most doctors use methotrexate because it is the DMARD that works the fastest and best [44].
A subset of DMARDs includes biologics. It’s possible that biologic DMARDs, as opposed to synthetic ones, would work faster and provide a more tailored response to inflammation than would be possible if the immune system were to be suppressed. It is possible that they are being utilised now to treat patients who are resistant to standard DMARDs. These drugs don’t dampen the immune system overall like some other RA treatments do since they target specific points in the inflammatory process. Even when other medications have poor efficacy, a biologic can delay, adapt, or mitigate the illness for many people with RA. Cyclophosphamide greatly reduces B lymphocyte function and causes cytotoxic effects on T cells. Cyclophosphamide treatment has been linked to decreased immunoglobulin secretion. Many organs and tissues, including the heart and kidneys, are harmed by this medicine because of its cytotoxic effects. It is teratogenic; thus, it shouldn’t be used during pregnancy or breastfeeding [45].
Neovascularization is a critical part of the progression and maintenance of RA. For the synovium and its pannus, angiogenesis is crucial because it ensures a steady supply of inflammatory cells and blood-borne nutrients. There was an uptick in integrin v3 expression in the pannus’s blood vessels. The intra-articular administration of integrin v3 antagonists has been demonstrated in an animal model [46].
After treatment with an intra-articular cyclic peptide (integrin v3 antagonist), joint inflammation, synovial infiltration, and pannus development are all decreased, and vascular apoptosis is triggered. In RA patients, this medication is also useful for avoiding erosion-related complications. Randomized clinical trials are required to prove its safety and effectiveness for RA treatment. Immunosuppression may be lifesaving for those with advanced RA. When it comes to immune suppression, the risk of spontaneous hematoma ablation is by far the most important aspect. It has been shown through research that many tissues need to be clarified, including: the best person to transplant stem cells (ii), the risk of death, especially in weak patients and elderly subjects (iii), the recurrence rate over a considerable follow-up (iv), and the successful management with methotrexate a week after hematopoietic stem-cell transplantation [47].
Biological DMARDs alone or in combination with methotrexate
As a DMARD for RA, methotrexate is used alone. Methotrexate decreases tissue inflammation and prevents cartilage and tissue deterioration and disintegration. To improve the immunological response, it is given along with biological DMARDs. The folic acid antagonist methotrexate may contribute to associated illnesses. Folic acid should therefore be given to methotrexate-using patients [48].
Rituximab
A B-cell surface-attaching antibody called rituximab lowers B-cell numbers in the blood for at least 6 months. If anti-TNF therapy was unsuccessful, it was approved to treat RA when paired with methotrexate because of its clinical effectiveness, which is comparable to that of anti-TNF therapy. Rituximab (MabThera) and methotrexate are typically used to treat neoplastic conditions, including follicular lymphoma and active, severe RA that is resistant to conventional anti-rheumatic medications. Rituximab is given by slow intravenous infusion [49]. B lymphocytes have a significant role in the aetiology of RA. Inflammatory cytokines, including TNF, are secreted by synovial B cells, which secrete a significant number of inflammatory cytokines, including TNF, in RA. B cells may also function as antigen-presenting cells, which have the major histocompatibility complex and the foreign antigen complex on their surface. The T cell receptor on T cells may then be used to recognise this complex. Patients with RA who are stressed out can use anti-anxiety medications [50].
Nonsteroidal anti-inflammatory drugs
Since NSAIDs have both analgesic and anti-inflammatory properties, they are often recommended to treat localised inflammation. Only RA patients can benefit from using NSAIDs, as they cannot stop erosions or slow the course of the disease [51].
Analgesics
Aspirin, ibuprofen, naproxen, and paracetamol are NSAIDs that are used to treat RA. These medications block cyclo-oxygenase, which prevents prostaglandin production throughout the inflammatory process. When used in high doses, all NSAIDs have anti-inflammatory properties and are beneficial. The primary drawbacks of NSAIDs as a class are associated with gastrointestinal, nephrotoxic, and cardiovascular side effects. Also, it’s hard to know how a person who takes a certain NSAID will react [52].
Corticosteroids
Corticosteroids are used to reduce discomfort and slow the spread of the disease, which has a remarkable positive impact on RA patients. By blocking the enzyme phospholipase A2, they display outstanding anti-inflammatory properties by stopping the production of arachidonic acid from phospholipid in the cell membrane. However, corticosteroids’ side effects can include cataracts, diabetes mellitus, weight gain, osteoporosis, and hypertension. In some patients with severe RA, corticosteroids are injected into the joints [53].
Immunosuppressive medications
Since many years ago, using immunosuppressive medications to treat RA has been advised. Immunosuppressive drugs like chlorambucil, cyclosporine A, cyclophosphamide, azathioprine, and methotrexate have been given to people with active RA [54].
Hemopoietic stem-cell transplantation (HSCT)
Although hemopoietic stem cell transplantation (HSCT) is efficient, further clinical research is required to determine how effective it is on a wide scale [55].
Vaccination
Despite DMARD selection, immunisation for human papillomavirus, hepatitis A and B, influenza, and pneumococcus is recommended when indicated. The live vaccine is not recommended in subjects receiving DMARDS, but it may be recommended in patients who are not receiving DMARD medication. Vaccines can be used at any time during treatment, but if they are given before DMARD therapy, the immune response may be less [56].
Janus kinase (JAK) inhibitors
JAK inhibitors are a new subclass of DMARDs that block the JAK, or Janus kinase, which is important in the body’s immunological response. These are medications that doctors administer to patients who have refractory joint injuries to biologics and DMARDs. Tofacitinib is an orally given JAK kinase inhibitor. Tofacitinib is a targeted medicine that specifically inhibits enzymes involved in the inflammatory process of the joints [57].
TNF blockers
There are now five TNF blockers available for the treatment of RA [58]. Adalimumab, infliximab, and etanercept are classified as first-generation drugs, while golimumab and certolizumab are second-generation medications. The five TNF inhibitors can all be used to treat RA.
Surgery
If the condition does not respond to medical treatment, surgery is advised. Some of the surgeries that are used to treat RA are synovectomy, arthroplasty or reconstructive surgery, and tendon realignment [59].
Synovectomy
The inflammatory synovium is removed during this surgery. It is feasible on knees, elbows, wrists, fingers, hips, and so forth. An orthopaedic therapist will help the patient understand how their joints will move after surgery. Recovery is based on the surgical technique used and the location of the incision. When medication fails to reduce pain, synovectomy is advised. It may also be necessary if the pain continues after 6–12 months of medication, such as with DMARDs. Although synovectomy can reduce symptoms, it cannot treat RA [60].
Repair of tendons
Tendon rupture or loosening is brought on by joint inflammation and injury near joints. Surgery is used to repair the tendons that surround the joints [61].
Replaces joints
When joints sustain serious deterioration, they are replaced. Inadequate use of medical therapy to treat clinical symptoms Joint replacement makes the outlook better, as only 4–13% of major joint replacements need to be redone within 10 years. This is especially true for knee and hip joints [62].
Total replacement of joints
Damaged joints are removed with this treatment, and a metal and plastic prosthesis is implanted. The replacement of a joint is indicated when there is significant joint injury and inadequate medical intervention to control symptoms. Only 4–13% of significant joint replacements occur over the long term, which is beneficial. requiring another intervention within 10 years. The most common knee and hip replacements are common [63].
Complications associated with pharmacotherapy in RA
The conventional treatment approach uses pharmacotherapy. Due to various advancements in allopathic medicine, they have been found useful in reducing the disease progression of the disease as they help in providing symptomatic relief along with improving the quality of life that has been impaired. However, using synthetic drugs for a prolonged time has resulted in various toxic side effects for the patient. Certolizumab pegol is an FDA-approved drug that has been used as an immunosuppressant in RA. However; the drug shows toxic effects by escalating the risk of urinary tract infections and respiratory infections. Similarly, there are some other drugs that show adverse effects on prolonged usage, such as respiratory infections and tuberculosis infection in individuals with RA, along with severe teratogenic effects [64] Table 1.
During pregnancy too, treating RA with anti-rheumatoid drugs such as cyclophosphamide, azathioprine, and methotrexate has been a challenging approach as they lead to various life-threatening teratogenic side effects, including congenital abnormalities, issues with foetal growth, and a high risk of abortion. Therefore, recent therapies have been shifted towards complementary and alternative herbal medicines that show minimal side effects [65]. Such herbal medicines are made from various commonly used herbal medicinal plants. The advantage of using herbal plants includes the presence of active components like terpenes, phenols, sesquiterpene lactones, flavonoids, and carotenoids, along with their antioxidant and anti-inflammatory properties. These properties make them an effective treatment approach for RA treatment [66].
Advantages of using herbal plants in RA treatment
Although patients frequently receive the above-mentioned mainstream medications, they have adverse side effects. Additionally, some of these medications are rather costly. A growing number of patients are turning to natural remedies as a result of these restrictions, with over 36% of adults in the USA adopting complementary and alternative (CAM) therapies [72]. Numerous disorders, including cancer, infectious diseases, and autoimmunity, have been thoroughly researched in relation to natural goods. However, there are a number of grounds for scepticism from both the general public and professional communities, including challenges in evaluating the efficacy of these items and a lack of knowledge regarding their mechanism of action [73]. As the National Institutes of Health (NIH)/National Centre for Complementary and Integrative Health (NCCIH), USA, has also emphasised, identifying the mechanism of action of natural products is of utmost importance. Here is a summary of some of the most important research that backs the use of common natural items to treat arthritis Fig. 3.
Through a variety of mechanisms, including the inhibition of effector molecules (such as pro-inflammatory cytokines and chemokines), the induction of anti-inflammatory mediators (such as IL-4 and IL-10), the regulation of the Th17/Treg balance, and the modulation of osteo-immune cross-talk, natural products can control arthritic inflammation [74]. These outcomes are the result of bioactive substances found in plant-derived or other natural products controlling inflammatory molecular mediators like MAPK (mitogen-activated protein kinase), NF-kB (nuclear factor kappa-light-chain-enhancer of activated B cells), and STAT3 (signal transducer and activator of transcription 3). Natural products can also influence the Th17/Treg balance by regulating the relative levels of transcription factors such as STAT3, IRF-4 (interferon regulatory factor 4), RORt (RAR-related orphan receptor gamma), and Foxp3 (forkhead box P3), as well as important cytokines such as IL-1, IL-6, and TGF- (transforming growth factor-). Similar to this, natural products can affect not only the T cell response but also the osteo-immune crosstalk and bone health by acting via certain cytokines (for example, IL-17) and other mediators like RANKL [75]. In this regard, natural items with the aforementioned qualities could be used alone or in combination with other common anti-arthritic medications to treat RA. Such combination medicines must be thoroughly examined for their compatibility and efficacy, though, in order to rule out any unanticipated medication interactions that might alter the effectiveness and adverse effects of these therapeutic agents.
Given below is a list (Table 2) of various plants that includes active constituents and characteristics they exhibit. The assessment of these herbal plants has been done via various evidence-based data that demonstrate the chemical constituent and the mechanism of some of the herbal plants that are useful in repressing the molecular signaling pathways and/or inflammatory mediators that are involved in RA.
Clinical usage of herbal plants in herbal formulations
Herbal plants are often utilized as a complimentary therapy alongside conventional medications in the treatment of arthritis. Various plants, including Withania somnifera, Cannabis, Emblica officinalis, Terminalia bellerica, Boswellia serrata,Terminalia chebula, Curcuma longa, Alpinia calcarata, Aconitum heterophyllym, Tinospora cordifolia (Guduchi), Cissampelos pariera, Picrorhiza kur and Cassia fifistula (Amaltas). Table 3 lists the many plants that contain active ingredients that are responsible for anti-inflammatory and antioxidant activity. The preclinical and clinical studies that evaluated these herbal formulations provided evidence-based data demonstrating the mechanism of such herbal plants and the active constituents of herbal plants responsible for suppressing the inflammatory mediators or the molecular signaling pathways involved in arthritis.
Nonpharmacological approach for RA
There are many risk levels of RA, and managing RA at each level helps both to prevent and to treat clinical cases of RA. At the first primary level, the twins and blood relatives of RA patients are the suspects, and they will be watched closely to lower the risk of pathogenesis symptoms. Risk factors need to be reduced at the secondary level, while patients’ prevention at the tertiary level will concentrate on damage-limiting strategies. The negative effects of RA should be kept to a minimum at the last stage of development to prevent relapse cases [91].
Patients with RA have extreme anxiety and depression, which worsens the situation. Poly unsaturated fatty acids (PUFAs) have been linked to a decrease in brain illnesses like anxiety and depression. Omega-3 PUFAs have been shown in numerous trials to lessen the feelings of anxiety in patients compared to controls [92]. As a result, RA sufferers can manage their condition using these nonpharmacological methods.
Future prospects
Future use of medicinal plants Due to the estimated 500,000 plants that exist worldwide, the majority of which have not yet been investigated in medical practice, and the possibility that present and future research on medical activities will result in efficient disease treatment, medicinal plants have a bright future. The use of medicinal plants has a long history, but using the entire plant or raw materials for treatment or experimentation has a number of disadvantages, including changes in the plant’s compounds in various climates, the concurrent development of synergistic compounds that result in the unexpected changes in bioactivity, or adverse effects of antagonists and changes or loss of bioactivity due to variability and accumulation, storage, and preparation of raw material. Easy analysis of the treatment results and identification of dangerous doses to regulate the effectiveness of the therapeutic formulation [93]. Beginning with the isolation, purification, and identification of plant components, chemistry included the creation of herbal remedies. Depending on the complexity of the chemicals, it could take weeks, months, or even years to identify the structures of active compounds from extracts when developing drugs from biological molecules derived from plant sources. Modern precision instruments like high-performance liquid chromatography (HPLC/MS), liquid chromatography mass spectrometry (LC/MS), magnetic field, and nuclear magnetic resonance (NMR) have significantly increased the rate of bioassay-guided fractionation. NMR is a recent major breakthrough for the categorization of compounds that are extremely limited in quantity in their organisms of origin [94]. Despite decades of successful research into creating therapeutic plants, several obstacles still stand in the way of further progress. Research has been done on the herbal product’s quality. Standardisation of raw materials is a crucial concern for the plant business. During development, processing, and collection, herbaceous plants are easily susceptible to infection. The two main issues with herbal medications are contamination and heavy metal pollution. To produce herbal pharmaceuticals, it is therefore vital to increase the number and quality of bioactive chemicals while also making an attempt to discover more new herbal drugs. The quality and safety of plant-derived medicines should be thoroughly and accurately studied due to the expanding use of natural substances worldwide, and traditional and millennial beliefs about these issues cannot be trusted. Therefore, scientific and enlightening studies are essential to obtain reliable information for the use of medicinal plants in health care. On the other hand, one issue with medicinal plants is the extinction of plant species as a result of unethical usage of natural resources. According to the International Union for Conservation of Nature, there are between 50,000 and 80,000 species of flowering plants that are utilised for therapeutic reasons. Among these figures, around 15,000 species are at risk of going extinct due to habitat loss and heavy harvesting, and 20% of their wildlife resources are declining as a result of excessive plant consumption and expanding human populations [95]. As a result, it is important to take into account the environmental code of ethics that protects biodiversity when using natural resources to find new natural remedies. GAPs (good agricultural practises) are intended to control production, guarantee quality, and allow the standardisation of herbal medications [96]. GAP is a method that uses top-notch, secure, and uncontaminated (raw pharmaceuticals) herbal remedies to deal with a variety of issues. Environmental ecology, production sites, germplasm, cultivation, collection, and quality features of pesticide detection, macroscopic or microscopic validation, chemical identification of active chemicals, and metal element checking are just a few of the extensive items covered by GAP [97]. The GAP is seriously promoted and implemented in many nations. For instance, GAP has encouraged the growing of conventional medicinal plants in China in regions where these plants are currently traditionally grown [98]. Despite the fact that many people today utilise herbal remedies as part of their basic healthcare, there are still numerous questions concerning the effectiveness and safety of employing plants. The use of herbal medicines in conjunction with conventional treatment has the potential to improve healthcare, but there are still many significant obstacles that must be overcome. One of the major issues in this discipline is the absence of accurate translation and interpretation of publications and research findings on plants by experts worldwide. In reality, researchers and practitioners should receive training in the utilisation of plant chemicals in both traditional and modern medicine in order to realise the effective integration of plants into a medical system. Additionally, the empirical justifications for plant use in conventional medicine should be transformed into arguments that are supported by data. Finally, it is important to provide answers to a number of queries regarding the standardisation of herbal medications and natural products as well as safety, precise dosage, treatment length, side effects, acute and chronic toxicities, and safety. If these problems are fixed, using plants as medicine will be a safe and cost-effective choice.
Conclusion
As discussed in this paper, our research into the epidemiology, pathogenesis, genetics, diagnosis, management, and combining herbal plant formulations in therapies for treating RA has reached a state which has turned from an extremely immobilising disease for which no effectual medication existed that could potentially control the disease well. Despite the fact that various allopathic drugs have shown a success rate in providing relief from symptoms as well as aiding in the control of disease progression, these drugs/allopathic medications have been linked to a number of noxious side effects. The review article is concerned with the emerging use of several herbal medicinal plants that have some active chemical constituents that have been found to be helpful in exhibiting potent therapy for RA. In order to maximise therapeutic success, nonpharmacological strategies should be used in conjunction with pharmaceutical therapies.
Abbreviations
- ACPA:
-
Anti-citrullinated protein antibodies
- AKT:
-
Ak strain transforming
- ALT:
-
Alanine transaminase
- APC:
-
Antigen-presenting cell
- BMI:
-
Body mass index
- CCP:
-
Anti-cyclic citrullinated peptide-2
- CD4:
-
Clusters of differentiation 4
- CMV:
-
Cytomegalovirus
- COX:
-
Cyclooxygenase
- CRP:
-
c-reactive protein
- DMARDs:
-
Disease-modifying antirheumatic drugs
- DNA:
-
Deoxyribonucleic acid
- DR:
-
D-related locus
- EBV:
-
Epstein–Barr virus
- ERK:
-
Extracellular signal-regulated kinase
- ESR:
-
Erythrocyte sedimentation rate
- EXOSC1:
-
Exosome component 1
- FDA:
-
Food and Drug Administration
- FLS:
-
Fibroblast-like synoviocytes
- GM-CSF:
-
Granulocyte macrophage colony-stimulating factor
- HIV:
-
Human immunodeficiency virus
- HLA:
-
Human leukocyte antigens
- IFNs:
-
Interferons
- Ig:
-
immunoglobulin
- IL:
-
Interleukin
- iNOS:
-
Inducible nitric oxide synthase
- JNK:
-
c-Jun N-terminal kinase
- LOX:
-
Lysyl oxidase
- LTB4:
-
Leukotriene B4
- MAP Kinase:
-
Mitogen activated protein kinases
- MCP-1:
-
Monocyte chemoattractant protein-1
- MMPs:
-
Matrix metalloproteinases
- mRNA:
-
Messenger ribonucleic acid
- mTOR:
-
Mammalian target of rapamycin
- MTP:
-
Microsomal triglyceride transfer protein
- NF-kB:
-
Nuclear factor-Κb
- NO:
-
Nitric oxide
- NSAID:
-
Non-steroidal anti-inflammatory drug
- PDGF:
-
Platelet-derived growth factor
- PGE2:
-
Prostaglandin E2
- PI3Ks:
-
Phosphoinositide 3-kinases
- PIP:
-
Prolactin-induced protein
- RA:
-
Rheumatoid arthritis
- RANK:
-
Receptor activator of NF-κB
- RANKL:
-
Receptor activator of nuclear factor kappa-B ligand
- RF:
-
Rheumatoid factor
- ROS:
-
Reactive oxygen species
- STAT4:
-
Signal transducer and activator of transcription 4
- Th1:
-
T helper type 1
- Th2:
-
T helper type 2
- TNF:
-
Tumor necrosis factor
- TRAF:
-
Tumor necrosis factor receptor–associated factor
- WBC:
-
White blood cell
References
Guo Q, Wang Y, Xu D et al (2018) Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res 6:1–14
Araki Y, Mimura T (2016) The mechanisms underlying chronic inflammation in rheumatoid arthritis from the perspective of the epigenetic landscape. J Immunol Res 2016:1–10. https://doi.org/10.1155/2016/6290682
van Delft MAM, Huizinga TWJ (2020) An overview of autoantibodies in rheumatoid arthritis. J Autoimmun 110:102392. https://doi.org/10.1016/J.JAUT.2019.102392
Yap HY, Tee SZY, Wong MMT et al (2018) Pathogenic role of immune cells in rheumatoid arthritis: implications in clinical treatment and biomarker development. Cells 7:161. https://doi.org/10.3390/CELLS7100161
Edilova MI, Akram A, Abdul-Sater AA (2021) Innate immunity drives pathogenesis of rheumatoid arthritis. Biomed J 44:172–182. https://doi.org/10.1016/J.BJ.2020.06.010
de Molon RS, Rossa C, Thurlings RM et al (2019) Linkage of periodontitis and rheumatoid arthritis: current evidence and potential biological interactions. Int J Mol Sci 20:4541. https://doi.org/10.3390/IJMS20184541
Sofowora A, Ogunbodede E, Onayade A (2013) The role and place of medicinal plants in the strategies for disease prevention. Afr J Tradit Complement Altern Med 10:210–229. https://doi.org/10.4314/AJTCAM.V10I5.2
Singh S, Singh TG, Mahajan K, Dhiman S (2020) Medicinal plants used against various inflammatory biomarkers for the management of rheumatoid arthritis. J Pharm Pharmacol 72:1306–1327. https://doi.org/10.1111/JPHP.13326
Anand U, Jacobo-Herrera N, Altemimi A, Lakhssassi N (2019) A comprehensive review on medicinal plants as antimicrobial therapeutics: potential avenues of biocompatible drug discovery. Metabolites 9:258. https://doi.org/10.3390/METABO9110258
Smolen JS, Aletaha D, Barton A et al (2018) Rheumatoid arthritis. Nat Rev Dis Primers 4:18001. https://doi.org/10.1038/NRDP.2018.1
Pradeepkiran JA (2019) Insights of rheumatoid arthritis risk factors and associations. J Transl Autoimmun 2:100012. https://doi.org/10.1016/J.JTAUTO.2019.100012
Svendsen AJ, Gervin K, Lyle R et al (2016) Differentially methylated DNA regions in monozygotic twin pairs discordant for rheumatoid arthritis: an epigenome-wide study. Front Immunol 7:510. https://doi.org/10.3389/fimmu.2016.00510
van Drongelen V, Holoshitz J (2017) Human leukocyte antigen-disease associations in rheumatoid arthritis. Rheum Dis Clin North Am 43:363–376. https://doi.org/10.1016/J.RDC.2017.04.003
Lim J, Kim K (2019) Genetic variants differentially associated with rheumatoid arthritis and systemic lupus erythematosus reveal the disease-specific biology. Sci Rep 9:1–7. https://doi.org/10.1038/s41598-019-39132-2
Cribbs A, Feldmann M, Oppermann U (2015) Towards an understanding of the role of DNA methylation in rheumatoid arthritis: therapeutic and diagnostic implications. Ther Adv Musculoskelet Dis 7:206–219. https://doi.org/10.1177/1759720X15598307
Ciechomska M, Roszkowski L, Maslinski W (2019) DNA methylation as a future therapeutic and diagnostic target in rheumatoid arthritis. Cells 8:953. https://doi.org/10.3390/CELLS8090953
Sokolove J, Wagner CA, Lahey LJ et al (2016) Increased inflammation and disease activity among current cigarette smokers with rheumatoid arthritis: a cross-sectional analysis of US veterans. Rheumatology 55:1969–1977. https://doi.org/10.1093/RHEUMATOLOGY/KEW285
Koziel J, Mydel P, Potempa J (2014) The link between periodontal disease and rheumatoid arthritis: an updated review. Curr Rheumatol Rep 16:408–408. https://doi.org/10.1007/S11926-014-0408-9
Jeong Y, Kim JW, You HJ et al (2019) Gut microbial composition and function are altered in patients with early rheumatoid arthritis. J Clin Med 8:693. https://doi.org/10.3390/JCM8050693
Novella-Navarro M, Plasencia-Rodríguez C, Nuño L, Balsa A (2021) Risk factors for developing rheumatoid arthritis in patients with undifferentiated arthritis and inflammatory arthralgia. Front Med 8:668898. https://doi.org/10.3389/fmed.2021.668898
Chen J, Wright K, Davis JM et al (2016) An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med 8:43. https://doi.org/10.1186/S13073-016-0299-7
Jagpal A, Navarro-Millán I (2018) Cardiovascular co-morbidity in patients with rheumatoid arthritis: a narrative review of risk factors, cardiovascular risk assessment and treatment. BMC Rheumatol 2:10. https://doi.org/10.1186/S41927-018-0014-Y
Bustamante MF, Garcia-Carbonell R, Whisenant KD, Guma M (2017) Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res Ther 19:110. https://doi.org/10.1186/S13075-017-1303-3
Kim JM, Lin C, Stavre Z et al (2020) Osteoblast-osteoclast communication and bone homeostasis. Cells 9:2073. https://doi.org/10.3390/CELLS9092073.
Fang Q, Zhou C, Nandakumar KS (2020) Molecular and cellular pathways contributing to joint damage in rheumatoid arthritis. Mediators Inflamm 2020:3830212. https://doi.org/10.1155/2020/3830212
Steffen U, Schett G, Bozec A (2019) How autoantibodies regulate osteoclast induced bone loss in rheumatoid arthritis. Front Immunol 10:1483. https://doi.org/10.3389/FIMMU.2019.01483
Breedveld FC (2002) Current and future management approaches for rheumatoid arthritis. Arthritis Res Ther 4(2):1–6. https://doi.org/10.1186/AR548
Rantapää-Dahlqvist S, De Jong BAW, Berglin E et al (2003) Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 48:2741–2749. https://doi.org/10.1002/ART.11223
Nielen MMJ, Van Schaardenburg D, Reesink HW et al (2004) Specific autoantibodies precede the symptoms of rheumatoid arthritis a study of serial measurements in blood donors. Arthritis Rheum 50:380–386. https://doi.org/10.1002/art.20018
Jørgensen KT, Wiik A, Pedersen M et al (2008) Cytokines, autoantibodies and viral antibodies in premorbid and postdiagnostic sera from patients with rheumatoid arthritis: case–control study nested in a cohort of norwegian blood donors. Ann Rheum Dis 67:860–866. https://doi.org/10.1136/ARD.2007.073825
Silman AJ, Hennessy E, Ollier B (1992) Incidence of rheumatoid arthritis in a genetically predisposed population. Rheumatology 31:365–368. https://doi.org/10.1093/RHEUMATOLOGY/31.6.365
Bos WH, Wolbink GJ, Boers M et al (2010) Arthritis development in patients with arthralgia is strongly associated with anti-citrullinated protein antibody status: a prospective cohort study. Ann Rheum Dis 69:490–494. https://doi.org/10.1136/ARD.2008.105759
Sokolove J, Bromberg R, Deane KD et al (2012) Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS ONE 7:e35296. https://doi.org/10.1371/JOURNAL.PONE.0035296
Suwannalai P, Van De Stadt LA, Radner H et al (2012) Avidity maturation of anti-citrullinated protein antibodies in rheumatoid arthritis. Arthritis Rheum 64:1323–1328. https://doi.org/10.1002/ART.33489
Gvozdenović E, Dirven L, Van Den Broek M et al (2014) Intra articular injection with corticosteroids in patients with recent onset rheumatoid arthritis: subanalyses from the BeST study. Clin Rheumatol 33:263–267. https://doi.org/10.1007/S10067-013-2465-2
Sandler RD, Dunkley L (2018) Osteoarthritis and the inflammatory arthritides. Surgery (United Kingdom) 36:21–26. https://doi.org/10.1016/j.mpsur.2017.10.004
Wilson A, Yu HT, Goodnough LT, Nissenson AR (2004) Prevalence and outcomes of anemia in rheumatoid arthritis: a systematic review of the literature. Am J Med 116:50–57. https://doi.org/10.1016/j.amjmed.2003.12.012
Ingegnoli F, Castelli R, Gualtierotti R (2013) Rheumatoid factors: clinical applications. Dis Markers 35:727–734. https://doi.org/10.1155/2013/726598
Lerkvaleekul B, Jaovisidha S, Sungkarat W et al (2017) The comparisons between thermography and ultrasonography with physical examination for wrist joint assessment in juvenile idiopathic arthritis. Physiol Meas 38:691–700. https://doi.org/10.1088/1361-6579/AA63D8
Laurent L, Anquetil F, Clavel C et al (2015) IgM rheumatoid factor amplifies the inflammatory response of macrophages induced by the rheumatoid arthritis-specific immune complexes containing anticitrullinated protein antibodies. Ann Rheum Dis 74:1425–1431. https://doi.org/10.1136/ANNRHEUMDIS-2013-204543
Muehleman C, Green J, Williams JM et al (2002) The effect of bone remodeling inhibition by zoledronic acid in an animal model of cartilage matrix damage. Osteoarthr Cartil 10:226–233. https://doi.org/10.1053/joca.2001.0506
Grigor C, Capell H, Stirling A et al (2004) Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 364:263–269. https://doi.org/10.1016/S0140-6736(04)16676-2
St.Clair EW, Van Der Heijde DMFM, Smolen JS et al (2004) Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum 50:3432–3443. https://doi.org/10.1002/art.20568
Yoo DH, Prodanovic N, Jaworski J et al (2017) Efficacy and safety of CT-P13 (biosimilar infliximab) in patients with rheumatoid arthritis: comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study. Ann Rheum Dis 76:355–363. https://doi.org/10.1136/ANNRHEUMDIS-2015-208786
Roubille C, Richer V, Starnino T et al (2015) The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 74:480–489. https://doi.org/10.1136/ANNRHEUMDIS-2014-206624
Rathbun AM, Harrold LR, Reed GW (2016) A prospective evaluation of the effects of prevalent depressive symptoms on disease activity in rheumatoid arthritis patients treated with biologic response modifiers. Clin Ther 38:1759–1772. https://doi.org/10.1016/J.CLINTHERA.2016.06.007
Storgard CM, Stupack DG, Jonczyk A et al (1999) Decreased angiogenesis and arthritic disease in rabbits treated with an αvβ3 antagonist. J Clin Invest 103:47–54. https://doi.org/10.1172/JCI3756
O’Dell JR, Haire CE, Erikson N et al (1996) Treatment of rheumatoid arthritis with methotrexate alone, sulfasalazine and hydroxychloroquine, or a combination of all three medications. N Engl J Med 334:1287–1291. https://doi.org/10.1056/NEJM199605163342002
Shea B, Swinden MV, Tanjong Ghogomu E et al (2013) Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev 2013:CD000951. https://doi.org/10.1002/14651858.CD000951.pub2
Smolen JS, Cohen SB, Tony HP et al (2017) A randomised, double-blind trial to demonstrate bioequivalence of GP2013 and reference rituximab combined with methotrexate in patients with active rheumatoid arthritis. Ann Rheum Dis 76:1598–1602. https://doi.org/10.1136/ANNRHEUMDIS-2017-211281
Jones G, Ding C (2010) Tocilizumab: a review of its safety and efficacy in rheumatoid arthritis. Clin Med Insights Arthritis Musculoskelet Disord 3:81–89. https://doi.org/10.4137/CMAMD.S4864
Bermas BL (2014) Non-steroidal anti inflammatory drugs, glucocorticoids and disease modifying anti-rheumatic drugs for the management of rheumatoid arthritis before and during pregnancy. Curr Opin Rheumatol 26:334–340. https://doi.org/10.1097/BOR.0000000000000054
Wen H-Y, Chiang C-C, Chen R-Y et al (2023) Immunosensing for early detection of rheumatoid arthritis biomarkers: anti-cyclic citrullinated peptide antibodies based on tilted-fiber bragg grating biosensor. Bioengineering 10:261. https://doi.org/10.3390/bioengineering10020261
Hasegawa T, Kaneko Y, Izumi K, Takeuchi T (2017) Efficacy of denosumab combined with bDMARDs on radiographic progression in rheumatoid arthritis. Joint Bone Spine 84:379–380. https://doi.org/10.1016/J.JBSPIN.2016.05.010
Felson DT, Anderson JJ, Meenan RF (1990) The comparative efficacy and toxicity of second-line drugs in rheumatoid arthritis results of two metaanalyses. Arthritis Rheum 33:1449–1461. https://doi.org/10.1002/ART.1780331001
Di Giuseppe D, Wallin A, Bottai M et al (2014) Long-term intake of dietary long-chain n-3 polyunsaturated fatty acids and risk of rheumatoid arthritis: a prospective cohort study of women. Ann Rheum Dis 73:1949–1953. https://doi.org/10.1136/ANNRHEUMDIS-2013-203338
Blanco FJ, Möricke R, Dokoupilova E et al (2017) Secukinumab in active rheumatoid arthritis: a phase III randomized, double-blind, active comparator: and placebo-controlled study. Arthritis Rheumatol 69:1144–1153. https://doi.org/10.1002/ART.40070
Fleischmann R, Cutolo M, Genovese MC et al (2012) Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum 64:617–629. https://doi.org/10.1002/art.33383
Pannell WC, Savin DD, Scott TP et al (2015) Trends in the surgical treatment of lumbar spine disease in the United States. Spine J 15:1719–1727. https://doi.org/10.1016/J.SPINEE.2013.10.014
Momohara S, Inoue E, Ikari K et al (2014) Recent trends in orthopedic surgery aiming to improve quality of life for those with rheumatoid arthritis: data from a large observational cohort. J Rheumatol 41:862–866. https://doi.org/10.3899/JRHEUM.131018
Kanbe K, Chiba J, Inoue Y et al (2015) Analysis of clinical factors related to the efficacy of shoulder arthroscopic synovectomy plus capsular release in patients with rheumatoid arthritis. Eur J Orthop Surg Traumatol 25:451–455. https://doi.org/10.1007/S00590-014-1570-5
Eversden L, Maggs F, Nightingale P, Jobanputra P (2007) A pragmatic randomised controlled trial of hydrotherapy and land exercises on overall well being and quality of life in rheumatoid arthritis. BMC Musculoskelet Disord 8:23. https://doi.org/10.1186/1471-2474-8-23
Gehrmann R, Harten R, Renard RL et al (2016) Biomechanical evaluation of patellar tendon repair techniques: comparison of double krackow stitch with and without cerclage augmentation. Orthop Rheumatol 5:00163. https://doi.org/10.15406/MOJOR.2016.05.00163
Köhler BM, Günther J, Kaudewitz D, Lorenz HM (2019) Current therapeutic options in the treatment of rheumatoid arthritis. J Clin Med 8:938. https://doi.org/10.3390/JCM8070938
Choudhary M, Kumar V, Malhotra H, Singh S (2015) Medicinal plants with potential anti-arthritic activity. J Intercult Ethnopharmacol 4:147. https://doi.org/10.5455/JICE.20150313021918
Feng Y, Zhang R, Zhao Z et al (2023) Efficacy and safety of electroacupuncture combined with medication for rheumatoid arthritis: a systematic review and meta-analysis. Heliyon 9:e14014. https://doi.org/10.1016/j.heliyon.2023.e14014
Farzaei MH, Farzaei F, Abdollahi M et al (2016) A mechanistic review on medicinal plants used for rheumatoid arthritis in traditional persian medicine. J Pharm Pharmacol 68:1233–1248. https://doi.org/10.1111/jphp.12606
Nimesh S (2018) Herbal drug is better than allopathic drug in the treatment of rheumatoid arthritis. Int J Pharmacogn 5(9):539–545. https://doi.org/10.13040/IJPSR.0975-8232.IJP.5
Chiu HY, Huang HL, Li CH et al (2015) Increased risk of chronic kidney disease in rheumatoid arthritis associated with cardiovascular complications: a national population-based cohort study. PLoS ONE 10:1–13. https://doi.org/10.1371/journal.pone.0136508
Bhupinder K, Reena G, Gupta M (2017) Natural products in treatment of rheumatoid arthritis. Int J Green Pharm 11:356–363
Teles KA, Medeiros-Souza P, Lima FAC et al (2017) Cyclophosphamide administration routine in autoimmune rheumatic diseases: a review. Rev Brasi de Reumatol (English Edition) 57:596–604. https://doi.org/10.1016/j.rbre.2016.09.008
Grainger R, Walker J (2014) Rheumatologists’ opinions towards complementary and alternative medicine: a systematic review. Clin Rheumatol 33:3–9. https://doi.org/10.1007/s10067-013-2379-z
Ekor M (2014) The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol 4:1–10. https://doi.org/10.3389/FPHAR.2013.00177
Venkatesha SH, Dudics S, Astry B, Moudgil KD (2016) Control of autoimmune inflammation by celastrol, a natural triterpenoid. Pathog Dis 74:1–12. https://doi.org/10.1093/FEMSPD/FTW059
Che CT, Wong MS, Lam CWK, McPhee DJ (2016) Natural products from chinese medicines with potential benefits to bone health. Molecules 21:239. https://doi.org/10.3390/MOLECULES21030239
Kaur A, Nain P, Nain J (2012) Herbal plants used in treatment of rheumatoid arthritis: a review. Int J Pharm Pharm Sci 4:44–57
Arya V, Kumar Gupta V, Kaur R, Pharm M (2011) A review on plants having anti-arthritic potential morphological and microscopic studies of aerial parts of ceylon leadwort view project Vikrant Arya. Int J Pharm Sci Rev Res 7:239
Goel A, Kulshrestha S (2021) Review on anti-rheumatoid arthritis potential of medicinal plants. Int J Curr Res Rev 13:16–32. https://doi.org/10.31782/IJCRR.2021.13303
Shashank D, Rajendra S, Mistry A (2018) An overview of phytoconstituents and pharmacological activities of Celastrus paniculatus Willd. J Pharm Res 16:307–313
Li M, He J, Jiang LL et al (2013) The anti-arthritic effects of Aconitum vilmorinianum, a folk herbal medicine in Southwestern China. J Ethnopharmacol 147:122–127. https://doi.org/10.1016/j.jep.2013.02.018
Gokhale AB, Damre AS, Kulkarni KR, Saraf MN (2002) Preliminary evaluation of anti-inflammatory and anti-arthritic activity of S. lappa, A. speciosa and A. aspera. Phytomedicine 9:433–437. https://doi.org/10.1078/09447110260571689
Pharm IJ, Res P, Bk M et al (2012) Hepatoprotective potency of Achyranthes aspera: an in-vivo study. Int J Pharm Phytopharmacol Res 1:387–390
Singh B, Bani S, Gupta DK et al (2003) Anti-inflammatory activity of ‘TAF’ an active fraction from the plant Barleria prionitis Linn. J Ethnopharmacol 85:187–193. https://doi.org/10.1016/S0378-8741(02)00358-6
Nazir N, Koul S, Qurishi MA et al (2007) Immunomodulatory effect of bergenin and norbergenin against adjuvant-induced arthritis—a flow cytometric study. J Ethnopharmacol 112:401–405. https://doi.org/10.1016/j.jep.2007.02.023
Fan AY, Lao L, Zhang RX et al (2005) Effects of an acetone extract of Boswellia carterii Birdw. (Burseraceae) gum resin on adjuvant-induced arthritis in Lewis rats. J Ethnopharmacol 101:104–109. https://doi.org/10.1016/j.jep.2005.03.033
Wu SQ, Otero M, Unger FM et al (2011) Anti-inflammatory activity of an ethanolic Caesalpinia sappan extract in human chondrocytes and macrophages. J Ethnopharmacol 138:364–372. https://doi.org/10.1016/j.jep.2011.09.011
Kumar VL, Roy S (2007) Calotropis procera latex extract affords protection against inflammation and oxidative stress in Freund’s complete adjuvant-induced monoarthritis in rats. Mediat Inflamm 2007:047523. https://doi.org/10.1155/2007/47523
Jeyadevi R, Sivasudha T, Rameshkumar A, Kumar LD (2013) Anti-arthritic activity of the indian leafy vegetable Cardiospermum halicacabum in Wistar rats and UPLC-QTOF- MS/MS identification of the putative active phenolic components. Inflamm Res 62:115–126. https://doi.org/10.1007/s00011-012-0558-z
Mukhopadhyay MK, Nath D (2011) Phytochemical screening and toxicity study of Saraca asoca bark methanolic extract. Int J Phytomed 3:498–505
Agarwal T, Singh R, Shukla AD et al (2012) Comparative analysis of antibacterial activity of four piper betel varieties. Pelagia Res Libr 3:698–705
Radu AF, Bungau SG (2021) Management of rheumatoid arthritis: an overview. Cells 10:2857. https://doi.org/10.3390/CELLS10112857
Zhou L, Xiong JY, Chai YQ et al (2022) Possible antidepressant mechanisms of omega-3 polyunsaturated fatty acids acting on the central nervous system. Front Psychiatry 13:1991. https://doi.org/10.3389/FPSYT.2022.933704/BIBTEX
Kowalska K (2011) Natural compounds involved in adipose tissue mass control in in vitro studies. Postepy higieny i medycyny doswiadczalnej (Online) 65:515–523. https://doi.org/10.5604/17322693.955499
Schroeder FC, Gronquist M (2006) Extending the scope of NMR spectroscopy with microcoil probes. Angew Chem Int Ed 45:7122–7131. https://doi.org/10.1002/ANIE.200601789
Ross IA (2005) Medicinal plants of the world. Med Plants World 3:1–623. https://doi.org/10.1007/978-1-59259-887-8/COVER
Chan K, Shaw D, Simmonds MSJ et al (2012) Good practice in reviewing and publishing studies on herbal medicine, with special emphasis on traditional chinese medicine and chinese materia medica. J Ethnopharmacol 140:469–475. https://doi.org/10.1016/J.JEP.2012.01.038
Makunga NP, Philander LE, Smith M (2008) Current perspectives on an emerging formal natural products sector in South Africa. J Ethnopharmacol 119:365–375. https://doi.org/10.1016/J.JEP.2008.07.020
Jianzhang M, Ke R, Kun C (2013) Research and practice on biodiversity in situ conservation in China: pro-gress and prospect. Biodivers Sci 20:551–558. https://doi.org/10.3724/SP.J.1003.2012.08118
Acknowledgements
Authors are grateful to the GLA University, management for providing support.
Funding
None.
Author information
Authors and Affiliations
Contributions
AS: Original draft preparation, AG: Supervision and Editing.
Corresponding author
Ethics declarations
Conflict of interest
All authors declared that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, A., Goel, A. Pathogenesis of rheumatoid arthritis and its treatment with anti-inflammatory natural products. Mol Biol Rep 50, 4687–4706 (2023). https://doi.org/10.1007/s11033-023-08406-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08406-4