Abstract
Background
With an ageing population, the incidence of bone loss and obesity are increasing. Numerous studies emphasized the multidirectional differentiation ability of mesenchymal stem cells (MSCs), and reported betaine modulated the osteogenic differentiation and adipogenic differentiation of MSCs in vitro. We wondered how betaine affected the differentiation of hAD-MSCs and hUC-MSCs.
Methods and results
ALP staining and alizarin red S (ARS) staining were proved 10 mM betaine significantly increased the number of ALP-positive cells and plaque calcified extracellular matrices, accompanying by the up-regulation of OPN, Runx-2 and OCN. Oil red O staining demonstrated the number and size of lipid droplets were reduced, the expression of adipogenic master genes such as PPARγ, CEBPα and FASN were down-regulated simultaneously. For further investigating the mechanism of betaine on hAD-MSCs, RNA-seq was performed in none-differentiation medium. The Gene Ontology (GO) analysis showed fat cell differentiation and bone mineralization function terms were enriched, and KEGG showed PI3K-Akt signaling pathway, cytokine-cytokine receptor interaction and ECM-receptor interaction pathways were enriched in betaine treated hAD-MSCs, demonstrated betaine had a positive inducing effect on osteogenic of hAD-MSCs in the non-differentiation medium in vitro, which is opposite to the effect on adipogenic differentiation.
Conclusions
Our study demonstrated that betaine promoted osteogenic and compromised adipogenic differentiation of hUC-MSCs and hAD-MSCs upon low concentration administration. PI3K-Akt signaling pathway, cytokine-cytokine receptor interaction and ECM-receptor interaction were significantly enriched under betaine-treated. We showed hAD-MSCs were more sensitive to betaine stimulation and have a better differentiation ability than hUC-MSCs. Our results contributed to the exploration of betaine as an aiding agent for MSCs therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a widespread disease which the bone mass, strength and microarchitecture were impaired [1]. Obesity causes diabetes, coronary heart disease and osteoarthritis which increases the risk of death [2]. With an ageing population, the incidence of bone loss and obesity are increasing. However, there is no effective therapy to increase endogenous bone formation and reduce adipogenesis. Previous studies have shown that mesenchymal stem cells (MSCs) are multipotent cells that can be obtained from adipose tissue, peripheral blood, bone marrow, placenta, umbilical cord and Wharton’s jelly (WJ) [3,4,5] which are capable of osteogenic, chondrogenic, adipogenic and myogenic differentiation [6,7,8]. Adipose-derived mesenchymal stem cells are widely used because they are easy to isolate and have an abundant tissue source [9]. MSCs can cure some diseases mainly via secretion variety of cytokines, such as graft-versus-host disease (GVHD) [10], degenerative diseases, diabetes, osteoarthritis and liver fibrosis [11,12,13,14,15].
Small molecules have been used to explore the effects on the differentiation of MSCs. Betaine (N, N, N-trimethylglycine) is a kind of natural zwitterionic molecule and shows a wide distribution in many organisms. Betaine is a methyl donor and osmolyte that participates in the methionine cycle via betaine-homocysteine-methyltransferase (BHMT) [16]. Anthony J. Barak et al. [17] showed that betaine can remethylate homocysteine and remove S-adenosylhomocysteine (SAH), which may be used in the treatment of liver diseases. Betaine treatment prevents the decrease of osteogenic differentiation caused by alcohol-induced osteonecrosis of the femoral head (ONFH) [18]. Tabatabai et al. claimed osteogenesis differentiation medium with betaine increases the expression of Runx2 and OCN genes, but treatment with betaine alone did not (Tabatabai et al. 2021). Betaine promotes bone formation through signaling pathways such as cytosolic calcium influx, ERK activation and IGF-1 production in human osteoblasts [19]. Betaine promotes osteogenic differentiation of immortalized human dental pulp-derived cells by mediating intracellular calcium levels [20]. Yaelle Joselit et al. [21] found that betaine reduced fetal body fat in C57BL/6J mice and prevented liver triglyceride overaccumulation at late gestation. It has been shown that betaine can prevent increased hepatic lipid accumulation, improves glucose tolerance and insulin sensitivity [22]. However, the mechanism of betaine on adipogenic and osteogenic differentiation of AD-MSCs needs further study. Our study aims to study the effects of betaine on hAD-MSCs adipogenic and osteogenic differentiation and its potential molecular mechanism by applying transcriptome level analysis.
Materials and methods
Cell culture
Adipose-derived stem cells (hAD-MSCs) frozen at P5 were donated by The First Affiliated Hospital of the Chinese People’s Liberation Army General Hospital. Human umbilical cord derived mesenchymal stem cells (hUC-MSCs) were donated by Biotech & Biomedicine (Shenyang) Group. hAD-MSCs and hUC-MSCs were thawed at 37 °C in a water bath for 1–2 min and diluted with Dulbecco’s modified eagle medium (DMEM, Gbico, USA) supplemented with 10% fetal bovine serum (Excell, Australia) and 1% Penicillin (1000 Units/ml) /streptomycin (10,000 µg/ml) (Gibco, USA) dropwise to remove cryoprotectant agent. The samples were centrifuged at 1000 rpm for 5 min and the obtained pellet was suspended in Complete medium and cultured at 37 °C with 5% humidified CO2. hAD-MSCs of passage 5–11 (P5-P11) and the hUC-MSCs of passages 3–7 (P3-P7) were used.
Morphological characteristics and cytotoxicity test
The cytotoxic effects of betaine on hAD-MSCs and hUC-MSCs were determined using the Cell Counting Kit-8 (Dojindo, Japan). Briefly, hAD-MSCs (5000 cells/well, P11, n = 3) and hUC-MSCs (5000 cells/well, P7, n = 3) were plated in 96-well plates overnight and then supplemented with different concentrations of betaine (10 mM, 40 mM, 80 mM, 120 mM, Sigma-Aldrich, USA ) for 24 h, 48 h, 72 and 96 h, respectively. Betaine is dissolved in PBS ( TBD Science, China). Afterward, 10 µL of CCK-8 buffer was added to each well at 37 °C and incubated in dark for 3 h. The absorbance was measured at 450 nm wave length and the percentage cell number was calculated and normalized with the control.
Osteogenic differentiation
For the determination of osteoblast differentiation in vitro, hAD-MSCs at P12 were seeded in 12-well plates (1.0 × 105 cells/well, n = 4), hUC-MSCs at P8 were plated at 12-well plates (1.0 × 105 cells/well, n = 3). At 80% confluence, the medium were replaced with an osteogenic medium (α-MEM ,Gibco, USA) supplemented with 100 µg/ml L(+)-ascorbic acid (Sigma-Aldrich, USA), 5 mM β-Glycerophosphate disodium salt hydrate (Sigma-Aldrich, USA) and different concentrations of betaine (10 mM,80 mM).
ALP and alizarin red staining
BCIP/NBT Alkaline Phosphatase Color Development Kit (Beyotime, China) was used for ALP staining after 7 days for the early stage of osteogenic differentiation. Briefly, cells were fixed with 4% paraformaldehyde (Bioss Antibodies, China) for 20 min and washed by PBS for three times, followed by incubating with the ALP staining solution in a dark environment at room temperature for 15 min (hAD-MSCs) or 24 h (hUC-MSCs). Alizarin Red S (Solarbio, China) staining was performed after 21 days in the late stage of osteogenic differentiation. In a word, the cells were fixed with 4% paraformaldehyde for 15 min, then stained with 0.2% Alizarin Red S solution for 30 min. In the end, stained cells were washed and then observed. The staining area was quantified by ImageJ and normalized by control.
Adipogenic differentiation assays
hAD-MSCs (5 × 104 cells/well, n = 3) at P10 were seeded in 24-well plates. At 90% confluence, hAD-MSCs were cultivated in MesenCult™ Adipogenic Differentiation Kit (Human) (STEMCELL Technologies, Canada) with different concentrations betaine for 12 days and then Oil red O (Sangon Biotech, China) was used. The Oil red O working solution was prepared by mixing 0.5% oil red O reserve solution with ddH2O in a ratio of 3:2. For staining, briefly, cells were fixed with 4% paraformaldehyde for 10 min and washed by ddH2O, then treated with 60% isopropyl alcohol for 2 min, stained with Oil red O for 10 min, the cells were washed carefully and observed. The staining area was quantified by ImageJ and normalized by control.
RNA-sequencing
hUC-MSC at P5 and hAD-MSCs at P12 were seeded in 60 mm plates and incubate cells in a 5% CO2 humidified incubator at 37 °C, the DMEM (Gbico, USA) supplemented with 10% fetal bovine serum (Excell, Australia), 1% Penicillin (1000 Units/ml) /streptomycin (10,000 µg/ml) (Gibco, USA) and different concentrations of betaine (0 mM,10 mM, 80 mM) were replaced when the cells approximately 80% confluent. Three parallel wells were set in each group for sampling. Cell pellets were collected after 72 h, and the RNA was extracted, RNA integrity number (RIN) > 7.0 was subjected to RNA sequencing (GenePlus Co., Ltd., China). All purified libraries were sequenced on DNBSEQ-T7RS of GenePlus Co., Ltd., which is located in Shenzhen, China, to acquire 150 bp paired-end sequence reads.
RNA-seq data analysis
RNA-seq data analysis procedures include raw reads processing, filtering, clean reads mapping and visualization. First, the quality of the raw data is checked with fastQC. Fastp filters out low-quality bases. Then cleaned sequence reads were mapping onto the human genome (GRCh38, Ensembel) using STAR. Gene expression levels were quantified using the FeatureCounts command. Principal Component Analysis was complete via Origin 2021 default parameters. The edge R package was used, which modelled the genes via negative binomial generalized log-linear model to identify significantly differentially expression genes (DEGs). Genes were considered differentially expressed between samples if they had an adjusted p value < 0.05 and |FC| >1.5. GO and KEGG enrichment analyses were performed using ClusterProfiler packages.
Quantitative real-time PCR (RT-qPCR)
The remaining total RNA of RNA-seq is reverse transcripted into cDNA using HiScript III RT SuperMix for qPCR (Vazyme Biotech, China) following the manufacturer’s protocol. RT-qPCR was performed using TB Green™ Premix Ex Taq™ II (Tli RNaseH Plus) (TaKaRa, Japan) according to the manufacturer’s instructions. The CFX96 Real-Time PCR Detection System (Bio-RAD, USA) was used for the experiment data generation. The mRNA levels of the target genes were normalized to the expression of GAPDH. The relative gene expression was quantified by the 2−ΔΔCt method.
ELISA
ADSCs (5 × 104 cells/well, P10, n = 2) and hUC-MSCs (5 × 104 cells/well, P7, n = 2) were seeded in 24-well plates, then the experimental groups were treated with different concentrations betaine (10mM, 80mM)for 72 h and the cell supernatant were collected. The cell supernatant of hUC-MSCs diluted 1:400 in diluent of samples and standards, cell supernatant of ADSCs diluted 1:50 in diluent of samples and standards. ELISA assay was carried out following the protocol of Human IL-6 ELISA kit (NEOBIOSCIENCE, China).
Statistical analysis
Statistical analysis was performed in Origin 2021, GraphPad Prism 8. All data are represented as the mean ± SD. Statistically significant differences were determined using One-way ANOVA or Two-way ANOVA. Statistical significance was considered when p < 0.05.
Results
High concentrations of betaine showed no toxicity to hAD-MSCs
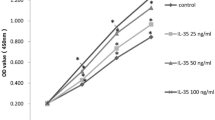
To test the potential toxicity of betaine on hAD-MSCs and hUC-MSCs, cells were maintained in a growth medium supplemented with 10, 40, 80, 120 mM betaine. The cell viability were assessed by CCK-8 assay. As shown in Fig. 1, low concentration (10 mM, 40 mM) betaine administration showed an elevated proliferation levels of hAD-MSCs at 96 h, and the high concentration (80 mM, 120 mM) betaine treatment showed an inhibitory effect on hAD-MSCs from 48 to 96 h. Surprisingly, 120 mM betaine still did not reach the sublethal concentration of hAD-MSCs and hUC-MSCs, and maintained the spindle-shaped and fibroblast-like morphology (Fig. 1a, b, Fig. S1a, b), indicating these cells were highly tolerant to betaine.
Proliferation of betaine-treated MSCs
a Morphological characteristics of hAD-MSCs treated with different concentrations of betaine (10, 40, 80, 120 mM) from 24 to 96 h, scale bar = 50 μm. b The cell proliferation (%) of betaine-treated hAD-MSCs were assessed by CCK-8 kit, n = 3. OD values were detected at a wavelength of 450 nm. *p < 0.05, **p < 0.01, ***p < 0.001.
Betaine promoted osteogenesis differentiation in hAD- and hUC-MSCs
To determine the effect of betaine on osteogenic differentiation of hAD-MSCs and hUC-MSCs, ALP and Alizarin red S (ARS) staining were evaluated. 10 mM betaine significantly increased the number of ALP-positive cells for 7 days differentiation (Fig. 2a, d; Fig. S2a, c) and exhibited a significant increase in plaque calcified extracellular matrices after 21 days osteogenic induction compared to control (Fig. 2b, d; Fig. S2b, c), whereas 80 mM betaine had an inhibitory effect on osteogenesis, indicating that long-term treatment with high concentration of betaine inhibit the proliferation of hAD-MSCs and hUC-MSCs, which was consistent with CCK-8 assay (Fig. 1a, b, Fig. S1a, b). As shown in Fig. 2e, the expression levels of OPN, Runx-2, and OCN in 10 mM betaine-treated group were significantly up-regulated compared with B_0 mM.
Effect of betaine on hAD-MSCs osteogenic and adipogenic differentiation
a ALP staining was performed after 7 days (scale bar = 500 μm) and b ARS staining was performed after 3 weeks (scale bar = 100 μm), c Oil red O staining was performed after 12 days (scale bar = 100 μm). d The staining area were quantified by ImageJ, n = 4. e The mRNA expression levels of adipogenic and osteogenic markers, n = 3. Data were presented as the mean ± SD, *p < 0.05, **p < 0.01.
Betaine inhibited adipogenic differentiation of hAD-MSCs
To evaluate the effect of betaine on the adipogenic potential of hAD-MSCs, oil red O staining was performed. The reduction of lipid droplets number and size were accompanied by a significant decreased in the expression of adipogenic master genes such as PPARγ, CEBPα, FASN, SCD1 and FABP4, indicating adipogenic differentiation of hAD-MSCs was significantly suppressed by betaine (Fig. 2c-e). Due to the weak adipogenic potential of hUC-MSCs, it is difficult to observe the inhibition of 80 mM betaine-treated (data not shown), which is consistent with the compromise adipogenic differentiation potential of the MSCs derived from umbilical cord [23]. Previous studies have revealed that osteogenic and adipogenic differentiation of MSCs may be mutually exclusive [24, 25], in consistent with the ALP, ARS and oil red O staining of 10 mM betaine treatment, however, osteogenic and adipogenic of hAD-MSCs were down-regulated both in 80 mM betaine-treated group (Fig. 2a-d ) suggesting that betaine may be not only have an inhibiting effect but also be harmful for the cells upon high concentration. Therefore, 10 mM should be the optimal concentration of betaine for hAD-MSCs and hUC-MSCs.
Transcriptome analysis showed potential mechanisms of betaine on hAD-MSCs differentiation
To investigate the mechanism of betaine involved in the differentiation of hAD-MSCs and hUC-MSCs, RNA-seq was performed in none-differentiation medium with betaine. Since the proliferation and differentiation experiments showed hAD-MSCs were more sensitive to betaine stimulation and have a better differentiation ability than hUC-MSCs (Figs. 1 and 2, Fig. S1-2), we focused on the effect of betaine on hAD-MSCs for further studies.
Principal component analysis (PCA) showed the separation of the control group from the betaine treated group, the first three PCs (PC1, PC2 and PC3) explained 78.4% of the variation (Fig. 3a). There were 314 significantly differentially expression genes (DEGs) shared in 10 mM and 80 mM betaine-treated groups, 74 genes were up-regulated and 240 genes were down-regulated (Fig. 3f). PPARGC1A, IGF1, SCD were down-regulated by 2.13, 1.56, and 1.59 folds in 10 mM betaine-treated group respectively (Fig. 3b, Table. S1), FASN, IGF1, PDE3B, SCD, SOCS3 were down-regulated by 1.78, 2.89, 3.22, 2.93 and 1.5 folds in 80 mM betaine-treated group (Fig. 3c, Table. S1), these genes were proved up-regulated during the adipogenic differentiation of MSCs in previous study [26]. 80 mM betaine treatment showed a 1.53 folds down-regulation of NOX4, Shigetada Furukawa et al. [27] demonstrated that NOX4 induces adipogenesis in hAD-MSCs. We observed that BMP6 and BMPR1B were down-regulated in both betaine-treated groups, BMPR1B is a proved regulator of bone homeostasis [28]. Several important cytokines involved in ECM breakdown and inflammation such as MMP3, TGFB3 and CCL7 were down-regulated (Table. S1) [29]. RT-qPCR and ELISA were applied to examine the expression levels of the 8 candidate genes of hAD-MSCs which were in accordance with RNA-seq (Fig. 3d, e).
To better understand the overall changes induced by betaine, Biological Process (BP) of Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis were applied. The most enriched BP terms is extracellular matrix organization shared in administration groups. Ossification, fat cell differentiation and bone mineralization were also enriched (Fig. 4a, c). KEGG showed PI3K-Akt and MAPK signaling pathway were the most significantly enrichment signaling pathway in 10 mM betaine treatment. Cytokine-cytokine receptor interaction, ECM-receptor interaction, TGF-beta signaling pathway and cholesterol metabolism were also enriched in administration groups (Fig. 4b, d). PI3K-Akt signaling pathway has been shown to play an important role in adipogenic and osteogenic differentiation previously [30,31,32]. Takashi Hoshiba et al. [24] demonstrated extracellular matrix (ECM) played an important role in controlling the balance between osteogenic and adipogenic differentiation of MSCs. In conclusion, these results indicated that betaine regulated adipogenic and osteogenic differentiation of hAD-MSCs through PI3K-Akt signaling pathway, cytokine-cytokine receptor interaction and ECM-receptor interaction in non-differentiated medium in vitro.
Effect of betaine on gene expression of hAD-MSCs
a PCA analysis of different concentrations betaine treatment groups. b, c Volcano plot of DEGs in B_10 mM vs. B_0 mM and B_80 mM vs. B_0 mM. Purple and cyan indicate |FC| > 1.5 and p.adj < 0.05. d qPCR analysis of genes expression levels in hAD-MSCs treated with different concentration betaine. e Determination of IL6 expression in hAD-MSCs with and without betaine-treated by ELISA. f Overlap between B_10 mM vs. B_0mM differentially expressed genes (adj. p value < 0.05 and log2 FC > 1.5) and B_80 mM vs. B_0mM differentially expressed genes.
Enrichment analysis of DEGs in hAD-MSCs.
a, c Biological Process of GO terms using DEGs of B_10 mM vs. B_0 mM, B_80 mM vs. B_0 mM. The size of the circles is proportional to the number of genes in that category, reflecting differential expression, while color indicates p.adj. b, d KEGG analysis associated with the DEGs. Vertical coordinate represents pathway name, and horizontal coordinate represents GeneRatio. The size and color of points respectively represent the number of differentially expressed genes in the pathway and p.adj.
Discussion
In this study, we demonstrated betaine promoted hUC-MSCs and hAD-MSCs proliferation upon low concentration treatment, and 10 mM betaine inhibited adipogenesis and promoted osteogenesis, especially for hAD-MSCs. At the transcriptional level, the complex interactions between PI3K-Akt signaling pathway, cytokine-cytokine receptor interaction and ECM-receptor interaction were involved in the regulation of hAD-MSCs differentiation by betaine. Takashi Hoshiba et al. [24] proved that ECM plays an important role in balancing the osteogenesis and adipogenesis of MSCs. Recently, data had shown that adipose-derived stem cells differentiation could be determined by signal transducing events from the interactions of cells with the extracellular matrix (ECM) and the ECM bound growth factors [33]. Weihua Yu et al. [32] demonstrated that the PI3K/Akt pathway plays an important role in adipogenesis of human mesenchymal stem cells. We found that NOX4, PPARGC1A, PDE3B, SOCS3 showed the decreased expression upon betaine treatment of hAD-MSCs, previous studies showed overexpression of NADPH oxidase 4 (NOX4) promoted ROS generation, which then enhanced lipogenesis, intracellular lipid accumulation [34]. PPARGC1A was a master regulator of adipogenesis and its down-regulation associated with the reduced adipogenesis [35]. PDE3B regulated insulin-induced glucose uptake, GLUT-4 translocation [36], its phosphorylation by Akt accelerates the degradation of cAMP, resulting in reduced lipogenesis [37]. SOCS3 binds to JAK kinase and the cytokine receptor to inhibit STAT3 activation, resulting in downregulation of Runx2 and ALP expression in MSCs [38][39], these data were consistent with that betaine inhibited hAD-MSCs adipogenic differentiation.
For hUC-MSCs, we observed that several cytokines such as CXCL3, CXCL5, CXCL8, IL6, CCL20, IL24, IL36B, MMP3 were up-regulated (Fig. S3b-d), just as obtained in GO enrichment analysis that cellular chemotaxis and cell differential were enriched (Fig. S3f, h). KEGG enrichment analysis showed that DEGs were significantly enriched in cytokine-cytokine receptor interactions (Fig. S3g, i), suggesting the potential mechanism of betaine regulation of hUC-MSCs differentiation.
We observed BMPR1B were down-regulated upon low and high concentration of betaine treatment [28], whereas Emmanuel Biver et al. [40] proved the expression of BMPR1B increased significantly during early osteoblastic differentiation, and the expression of IL6 in hUC-MSCs and hAD-MSCs showed opposite trend in betaine administration (Fig. 3d, e, Fig. S3d, e), indicating the complex connection between gene regulation network and phenotype outcome.
In conclusion, data presented in this study demonstrated that betaine promoted osteogenic and compromised adipogenic differentiation of hUC-derived and hAD-derived mesenchymal stem cells upon low concentration administration. These effects were mediated by PI3K-Akt signaling pathway, cytokine-cytokine receptor interaction and ECM-receptor interaction. Here, we showed hAD-MSCs were more sensitive to betaine stimulation and have a better differentiation ability than hUC-MSCs. To our knowledge, this study would be the first investigation that betaine had a positive inducing effect on the osteogenic differentiation of hAD-MSCs in the non-differentiation medium in vitro, which is opposite to the effect on adipogenic differentiation. Our study provided theoretical basis for the application of betaine in the treatment of osteoporosis and diseases caused by obesity.
Data Availability
The datasets used and analyzed during the current study are available from the https://www.ncbi.nlm.nih.gov/sra/PRJNA849741, https://www.ncbi.nlm.nih.gov/sra/PRJNA885273.
Change history
12 September 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11033-023-08779-6
References
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377(9773):1276–1287. https://doi.org/10.1016/S0140-6736(10)62349-5
Kopelman PG (2000) Obesity as a medical problem. Nature 404(6778):635–643. https://doi.org/10.1038/35007508
Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R (2000) Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: implications for their use in cell therapy. Exp Hematol 28(6):707–715. https://doi.org/10.1016/s0301-472x(00)00160-0
Hass R, Kasper C, Böhm S, Jacobs R (2011) Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 9:12. https://doi.org/10.1186/1478-811X-9-12
Zhang C, Han X, Liu J, Chen L, Lei Y, Chen K et al (2022) Single-cell transcriptomic analysis reveals the Cellular heterogeneity of mesenchymal stem cells. https://doi.org/10.1016/j.gpb.2022.01.005. Genomics Proteomics Bioinformatics
Almalki SG, Agrawal DK (2016) Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation 92(1–2):41–51. https://doi.org/10.1016/j.diff.2016.02.005
Liechty KW, Mackenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R et al (2000) Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med 6(11):1282–1286. https://doi.org/10.1038/81395
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147. https://doi.org/10.1126/science.284.5411.143
Schäffler A, Büchler C (2007) Concise review: adipose tissue-derived stromal cells–basic and clinical implications for novel cell-based therapies. Stem Cells 25(4):818–827. https://doi.org/10.1634/stemcells.2006-0589
Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M et al (2004) Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363(9419):1439–1441. https://doi.org/10.1016/S0140-6736(04)16104-7
Jaber H, Issa K, Eid A, Saleh FA (2021) The therapeutic effects of adipose-derived mesenchymal stem cells on obesity and its associated diseases in diet-induced obese mice. Sci Rep 11(1):6291. https://doi.org/10.1038/s41598-021-85917-9
Li L, Hui H, Jia X, Zhang J, Liu Y, Xu Q et al (2016) Infusion with human bone marrow-derived mesenchymal stem cells improves β-cell function in patients and non-obese mice with severe diabetes. Sci Rep 6:37894. https://doi.org/10.1038/srep37894
Matas J, Orrego M, Amenabar D, Infante C, Tapia-Limonchi R, Cadiz MI et al (2019) Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: repeated MSC Dosing is Superior to a single MSC dose and to Hyaluronic Acid in a controlled Randomized Phase I/II Trial. Stem Cells Transl Med 8(3):215–224. https://doi.org/10.1002/sctm.18-0053
Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C et al (2018) Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol 14(8):493–507. https://doi.org/10.1038/s41581-018-0023-5
Takeuchi S, Tsuchiya A, Iwasawa T, Nojiri S, Watanabe T, Ogawa M et al (2021) Small extracellular vesicles derived from interferon-γ pre-conditioned mesenchymal stromal cells effectively treat liver fibrosis. NPJ Regen Med 6(1):19. https://doi.org/10.1038/s41536-021-00132-4
Kharbanda KK, Rogers DN, Mailliard ME, Siford GL, Barak AJ, Beckenhauer HC et al (2005) Role of elevated S-adenosylhomocysteine in rat hepatocyte apoptosis: protection by betaine. Biochem Pharmacol 70(12):1883–1890. https://doi.org/10.1016/j.bcp.2005.09.021
Barak AJ, Beckenhauer HC, Mailliard ME, Kharbanda KK, Tuma DJ (2003) Betaine lowers elevated s-adenosylhomocysteine levels in hepatocytes from ethanol-fed rats. J Nutr 133(9):2845–2848. https://doi.org/10.1093/jn/133.9.2845
Yang Q, Yin W, Chen Y, Zhu D, Yin J, Zhang C et al (2019) Betaine alleviates alcohol-induced osteonecrosis of the femoral head via mTOR signaling pathway regulation. Biomed Pharmacother 120:109486. https://doi.org/10.1016/j.biopha.2019.109486
Villa I, Senesi P, Montesano A, Ferraretto A, Vacante F, Spinello A et al (2017) Betaine promotes cell differentiation of human osteoblasts in primary culture. J Transl Med 15(1):132. https://doi.org/10.1186/s12967-017-1233-5
Kornsuthisopon C, Nantanapiboon D, Rochanavibhata S, Nowwarote N, Namangkalakul W, Osathanon T (2022) Betaine promotes osteogenic differentiation in immortalized human dental pulp-derived cells. BDJ Open 8(1):31. https://doi.org/10.1038/s41405-022-00123-7
Joselit Y, Nanobashvili K, Jack-Roberts C, Greenwald E, Malysheva OV, Caudill MA et al (2018) Maternal betaine supplementation affects fetal growth and lipid metabolism of high-fat fed mice in a temporal-specific manner. Nutr Diabetes 8(1):41. https://doi.org/10.1038/s41387-018-0035-z
Szkudelska K, Szkudelski T (2022) The anti-diabetic potential of betaine. Mechanisms of action in rodent models of type 2 diabetes. Biomed Pharmacother 150:112946. https://doi.org/10.1016/j.biopha.2022.112946
Chi Y, Han ZB, Xu FY, Wang YW, Feng XM, Chen F et al (2014) Adipogenic potentials of mesenchymal stem cells from human bone marrow, umbilical cord and adipose tissue are different. Zhongguo Shi Yan Xue Ye Xue Za Zhi 22(3):588–594. https://doi.org/10.7534/j.issn.1009-2137.2014.03.003
Hoshiba T, Kawazoe N, Chen G (2012) The balance of osteogenic and adipogenic differentiation in human mesenchymal stem cells by matrices that mimic stepwise tissue development. Biomaterials 33(7):2025–2031. https://doi.org/10.1016/j.biomaterials.2011.11.061
Kang Q, Song WX, Luo Q, Tang N, Luo J, Luo X et al (2009) A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev 18(4):545–559. https://doi.org/10.1089/scd.2008.0130
Yi X, Wu P, Liu J, Gong Y, Xu X, Li W (2019) Identification of the potential key genes for adipogenesis from human mesenchymal stem cells by RNA-Seq. J Cell Physiol 234(11):20217–20227. https://doi.org/10.1002/jcp.28621
Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y et al (2004) Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114(12):1752–1761. https://doi.org/10.1172/JCI21625
Chen D, Ji X, Harris MA, Feng JQ, Karsenty G, Celeste AJ et al (1998) Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol 142(1):295–305. https://doi.org/10.1083/jcb.142.1.295
Page-Mccaw A, Ewald AJ, Werb Z (2007) Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 8(3):221–233. https://doi.org/10.1038/nrm2125
Chen J, Crawford R, Chen C, Xiao Y (2013) The key regulatory roles of the PI3K/Akt signaling pathway in the functionalities of mesenchymal stem cells and applications in tissue regeneration. Tissue Eng Part B Rev 19(6):516–528. https://doi.org/10.1089/ten.TEB.2012.0672
Osyczka AM, Leboy PS (2005) Bone morphogenetic protein regulation of early osteoblast genes in human marrow stromal cells is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase signaling. Endocrinology 146(8):3428–3437. https://doi.org/10.1210/en.2005-0303
Yu W, Chen Z, Zhang J, Zhang L, Ke H, Huang L et al (2008) Critical role of phosphoinositide 3-kinase cascade in adipogenesis of human mesenchymal stem cells. Mol Cell Biochem 310(1–2):11–18. https://doi.org/10.1007/s11010-007-9661-9
Shaik S, Martin EC, Hayes DJ, Gimble JM, Devireddy RV (2019) Transcriptomic profiling of adipose derived stem cells undergoing osteogenesis by RNA-Seq. Sci Rep 9(1):11800. https://doi.org/10.1038/s41598-019-48089-1
Higuchi M, Dusting GJ, Peshavariya H, Jiang F, Hsiao ST, Chan EC et al (2013) Differentiation of human adipose-derived stem cells into fat involves reactive oxygen species and forkhead box O1 mediated upregulation of antioxidant enzymes. Stem Cells Dev 22(6):878–888. https://doi.org/10.1089/scd.2012.0306
Alshammari GM, Balakrishnan A (2019) Pumpkin (Cucurbita ficifolia Bouché) extract attenuate the adipogenesis in human mesenchymal stem cells by controlling adipogenic gene expression. Saudi J Biol Sci 26(4):744–751. https://doi.org/10.1016/j.sjbs.2018.10.002
Zmuda-Trzebiatowska E, Oknianska A, Manganiello V, Degerman E (2006) Role of PDE3B in insulin-induced glucose uptake, GLUT-4 translocation and lipogenesis in primary rat adipocytes. Cell Signal 18(3):382–390. https://doi.org/10.1016/j.cellsig.2005.05.007
Dipilato LM, Ahmad F, Harms M, Seale P, Manganiello V, Birnbaum MJ (2015) The role of PDE3B phosphorylation in the inhibition of lipolysis by insulin. Mol Cell Biol 35(16):2752–2760. https://doi.org/10.1128/MCB.00422-15
Carow B, Rottenberg ME (2014) SOCS3, a Major Regulator of infection and inflammation. Front Immunol 5:58. https://doi.org/10.3389/fimmu.2014.00058
Nicolaidou V, Wong MM, Redpath AN, Ersek A, Baban DF, Williams LM et al (2012) Monocytes induce STAT3 activation in human mesenchymal stem cells to promote osteoblast formation. PLoS ONE 7(7):e39871. https://doi.org/10.1371/journal.pone.0039871
Biver E, Soubrier AS, Thouverey C, Cortet B, Broux O, Caverzasio J et al (2012) Fibroblast growth factor 2 inhibits up-regulation of bone morphogenic proteins and their receptors during osteoblastic differentiation of human mesenchymal stem cells. Biochem Biophys Res Commun 427(4):737–742. https://doi.org/10.1016/j.bbrc.2012.09.129
Funding
This work was supported by Liaoning Revitalization Talents Program (XLYC2002027), Programs from the Department of Education of Liaoning Province (LQ2020022). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
of competing interest.
Number of patent application: 202211292063 .X. The authors have no relevant financial or non-financial interests to disclose.
Author contributions Statement
Yue Jing and Jian Zhou designed and finished this study; Yue Jing analyze and visualize the data; Fenghua Guo validate the data; Lin Yu, Xiaomeng Ren and Xiushan Yin revised the paper. All authors reviewed and approved the final manuscript.
This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors Yue Jing and Jian Zhou contributed equally to this work.
The original online version of this article was revised: The missing equally contribution statement “The authors Yue Jing and Jian Zhou contributed equally to this work” is included.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jing, Y., Zhou, J., Guo, F. et al. Betaine regulates adipogenic and osteogenic differentiation of hAD-MSCs. Mol Biol Rep 50, 5081–5089 (2023). https://doi.org/10.1007/s11033-023-08404-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-023-08404-6