Abstract
Background
Due to deficiencies in the expression of hormone receptors, such as PR, ER and HER2, it is challenging to treat triple-negative breast cancer, which does not respond to single targeted therapy. Ruxolitinib is a Janus kinase (JAK)1/JAK2 inhibitor. MK-2206 is an allosteric AKT inhibitor. Due to the limited activities of ruxolitinib and MK-2206 for monotherapy, the need for cotreatment with other drugs has emerged. This study is the first to examine the effects of ruxolitinib and MK-2206 cotreatment on apoptosis and JAK2/STAT5 and PI3K/AKT signaling in MDA-MB-231 breast cancer cells. Additionally, this work aimed to decrease the side effects of ruxolitinib and increase its anticancer effects with MK-2206 cotreatment.
Methods and results
Cell viability was reduced in a dose- and time-dependent manner after exposure to ruxolitinib, MK-2206 or both for 48 h, as shown by MTT assay. Ruxolitinib had a synergistic antiproliferative effect, as demonstrated by colony formation and wound healing assays. The effects of ruxolitinib, MK-2206 and their combination on apoptosis, as well as PI3K/AKT and JAK/STAT signaling, were examined by western blot analyses. Cotreatment with ruxolitinib and MK-2206 reduced proliferation with the dual inhibition of JAK2/STAT5 and PI3K/AKT signaling by decreasing PI3K, AKT, JAK2, STAT5, Caspase-9, Caspase-7, PARP, c-Myc, and Bcl-2 and increasing P53 and PTEN protein expression.
Conclusions
Our results revealed the roles of P53 and PTEN in the regulation of apoptosis and the PI3K/AKT and JAK2/STAT5 signaling pathways. The dual inhibition of JAK2/STAT5 and PI3K/AKT may reduce metastasis by decreasing tumor cell survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triple-negative breast cancer (TNBC) is known to involve a deficiency in the expression of hormone receptors, such as estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth Factor 2 receptor (HER2), or HER2 amplification [1, 2]. Therefore, performing TNBC therapy is difficult, and novel therapeutic approaches that target receptor tyrosine kinases (RTKs), PI3K/AKT/mTOR (PAM), RAS/MAPK, poly(ADP-ribose) polymerase (PARP), and JAK/STAT for TNBCs need to be developed [3,4,5,6,7,8]. Recent studies have shown that monotargeted therapies are not effective for TNBC because of intrinsic or acquired resistance.
A therapeutic strategy for TNBC is to inhibit the signaling pathways that have a role in tumor formation and metastatic progression [7]. Janus kinase (JAK) and its related signal transducer and activator of transcription (STAT) signaling play major roles in cancer progression; activation of JAK/STAT by cytokines and growth factors induces cell proliferation, survival, migration and differentiation [9,10,11]. STAT3 and STAT5 have a major role in tumor development, cell cycle regulation and apoptosis and have been found to be activated in breast, lung, prostate, and pancreatic cancers and other hematological malignancies [12,13,14]. Studies have shown that activation of JAK2/STAT5 causes resistance to PI3K/mTOR suppression in breast cancer. Ruxolitinib is an RTK inhibitor that suppresses JAK/STAT signaling targeting JAK1/JAK2 and was approved to treat patients with myelofibrosis and polycythemia vera [15].
PI3-kinase (PI3K) signaling has a major role in RTK signaling in breast cancer to modulate cell proliferation and has a crucial role in breast tumor formation [16, 17]. This pathway interacts with other signaling pathways to promote tumor formation and resistance to therapies [18]. Downstream of PI3K are protein kinase B (AKT) and mammalian target of rapamycin (mTOR). This signaling is modulated by multiple phosphatases that include inositol polyphosphate-4-phosphatase type II B (INPP4B) and phosphatase and tensin homolog (PTEN). MK-2206 is a potent allosteric AKT inhibitor that is currently the subject of clinical experiments, in which the cytotoxicity against different cancer types is being examined [19]. With the development of new drug targets and combined therapy, multiple oncogenic signaling pathways can be targeted, and drug resistance can be eliminated.
Combination therapy is a treatment type that uses a combination of multiple therapeutic agents in cancer treatment [20]. When used together with anticancer drugs, compared with the monotherapy approach, the combined therapy improves the efficacy of drugs by targeting key pathways synergistically or additively. Combination therapy possibly decreases drug resistance and provides some advantages, such as decreasing tumor growth, arresting mitotically active cells, and triggering apoptosis [21, 22]. Recently, studies have shown that JAK/STAT pathway activation is related to other signaling pathways, especially PI3K/AKT signaling [23]. Due to the advantages of combined therapy and the systemic side effects of ruxolitinib in the treatment of myelofibrosis, we treated both to decrease the cytotoxicity of ruxolitinib and to increase the anticancer effect of ruxolitinib with ruxolitinib and MK-2206 together in MDA-MB-231 cells, which are TNBC cells. In this study, we aimed to prevent drug resistance and reduce metastasis by especially affecting the survival of tumor-initiating cells using dual inhibition of JAK2/STAT5 and PI3K/AKT.
Materials and methods
Drugs, chemical and antibodies
Ruxolitinib and MK-2206 were purchased from MedChemExpress (USA). Hoechst 33342 nuclear stain (ENZ-51031-0050) was purchased from Enzo Life Sciences. β-actin (CST-4970), PARP (CST-9532), Caspase-9 (CST-9508), Caspase-7 (CST-12827), and Caspase-3 (CST-9662) (1:1000 dilution) rabbit antibodies were purchased from Cell Signaling Technology (CST, Danvers, MA, USA). JAK2 (sc-390539), STAT5 (sc-74442), STAT3 (sc-8019), Caspase-8 (sc-56070), Bax (sc-20067), Bcl-2 (sc-7382), c-Myc (sc-40), PI3K (sc-1637), AKT1 (sc-5298), PTEN (sc-7974), and P53 (sc-126) (1:500 dilution) mouse antibodies were purchased from Santa Cruz Biotechnology (sc, Oregon, USA). HRP-conjugated anti-rabbit and anti-mouse secondary antibodies (1:5000 dilution) were purchased from CST.
Cell culture
MDA-MB-231 cells were cultured in DMEM (Gibco) containing 10% fetal calf serum (Gibco) and 100 U/100 mg/ml penicillin/streptomycin (Gibco) at 5% CO2 in humidified air at 37 °C (Memmert CO2 Incubator, INCO153med, Germany).
Viability assay
Cells were seeded at 1 × 104 density in 96-well plates and exposed to different concentrations of ruxolitinib (0–50 µM) and MK-2206 (0–7.5 µM) for 48 h. Then, 10 µl 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) dye (5 mg/ml in 1× PBS, Cayman Chemical, Michigan, USA) was added. After incubation at 37 °C for 4 h, the medium was removed, and then 200 µl of 10% SDS dissolved in 0.01M HCl was added. The absorbance of the suspensions was measured at 570 nm with a microplate reader (Multiskan™ FC Microplate Photometer, ThermoFisher Scientific, Multiskan FC China). The average of three independent experiment repetitions was obtained.
Analyzing the combination effects of ruxolitinib and MK-2206
CompuSyn software and the Chou–Talalay method were used to examine the combined effects of drug combination treatment and to determine the combination index (CI) value. CI value was calculated using the following equation:
(Dx)1 and (Dx)2 represent the doses for x% inhibition by ruxolitinib and MK-2206, respectively. Also, (D)1 and (D)2 represent their combination doses for x% inhibition. CI > 1 indicates antagonism, CI = 1 indicates additivite effect, and CI < 1 indicates synergism. Three independent experiments were performed to determine CI values [24].
Growth inhibition assay
Cells were seeded at 1 × 106 density in 6-well plates and exposed to ruxolitinib (22.5 µM), MK-2206 (7.5 µM) and their combination (18 µM ruxolitinib/5 µM MK-2206) at 24, 48, 72 and 96 h. The cells were stained with 50 µl trypan blue (0.4% w/v). The unstained cells were counted with a Neubauer hemocytometer under inverted microscopy.
Hoescht staining
Cells were seeded at 2.5 × 102 density in 6-well plates. The cells were treated with ruxolitinib, MK-2206, and their combination. The cells were washed twice with 1× assay buffer. Seventy microliters of Microscopy Dual Detection Reagent (including 1 µl of Hoechst 33342 and 2 µl of CYTO-ID® Green Detection Reagent in 1 ml of 1× assay buffer) was added to each sample. After incubation for 15 min at 37 °C, the cells were washed with 100 µl of 1× Assay Buffer and fixed with 4% formaldehyde for 20 min. The formaldehyde was removed by washing the cells three times with 1× assay buffer. The stained cells were analyzed by fluorescence microscopy.
Colony formation assay
Cells were seeded at 4 × 103 density in 6-well plates and incubated for 2 weeks. To remove the dead cells, 1× PBS solution was added to the cells. Colonies were fixed with acetic acid:methanol (1:3) for 20 min at room temperature and washed with distilled water. The cells were stained with crystal violet in methanol (0.5% w/v) for 15 min, and morphological images were taken under an inverted microscope.
Wound healing assay
A total of 5 × 105 cells/well on 6-well plates were seeded and incubated to adhere at 5% CO2 and 37 °C overnight. The cells were scraped with a 200 µl sterile pipette tip to create wounds. Wound widths were measured with an inverted microscope at 0, 24, 48, 72, and 96 h.
Protein extraction and immunoblotting
Cells were exposed to ruxolitinib, MK-2206 and their combination for 48 h. Every sample was washed, and 1× PBS was collected for 2 min at 13,200 rpm. Radioimmunoprecipitation assay (RIPA) solution (CST-9806) was added to lyse the cells for 5 min on ice. The lysed cells were shaken for 20 min at room temperature. The cells were centrifuged to remove cell debris for 15 min at 13,200 rpm, and the supernatant was collected. Total protein lysates (50 µg), which were measured by the Bradford protein assay, were loaded onto a 12% SDS‒PAGE gel and transferred to PVDF membranes (Merck Millipore) with a semidry transfer system (Hoefer). The membranes were blocked with 5% nonfat milk in 1× TBS-Tween 20 for 1 h at room temperature and incubated with primary and secondary antibodies at 4 °C overnight. The membranes were washed with 1× TBS for 10 min three times and were washed with 1× TBS-Tween 20 for 10 min one time. The protein expression of PARP, Caspase 9, β-actin, Caspase 8, Bax, Caspase 3, Bcl-2, Caspase 7, c-Myc, PI3K, AKT1, PTEN, P53, JAK2, STAT5 and STAT3 was analyzed with enhanced chemiluminescence (ECL, Thermo Fisher Scientific) in a Syngene G:Box Chemi XRQ system.
Statistical analysis
Statistical analysis for every experiment was performed by one-way analysis of variance (ANOVA) using GraphPad Prism 9.0.0. Standard deviation (SD) values indicate three independent experiments with at least three replicates, used for determining error bars in the graphs. S. D values the intensities of the protein bands obtained by western blot analysis were measured by using the ImageJ program. Each band of proteins was measured three times to use the mean value of three measurements. For the calculation of relative expression levels, each value was divided by the value of β-actin. P values were considered statistically significant as * P < 0.05; ** P < 0.01; ***P < 0.001; **** P < 0.0001.
Results
The combination of ruxolitinib with MK-2206 reduced cell viability
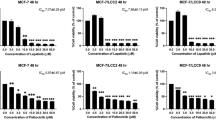
To clarify the cytotoxic effect of ruxolitinib and MK-2206 on cells, an MTT assay was used. The cells were exposed to different concentrations of ruxolitinib (0–40 µM) [25, 26], MK-2206 (0–10 µM) [27,28,29,30,31] and their combination at different ratios (1:1, 1:1.5, 1:1.75, 1:4) for 48 h. No changes in cell viability were observed when cells were exposed to ruxolitinib, MK-2206, and their combinations for 24 h. When cells were exposed to ruxolitinib, MK-2206, and their combinations for 72 h, toxic effects on cell viability were observed. Therefore, the MTT test was performed for 48 h, The cell viability decreased with only ruxolitinib and only MK-2206 treatment in a dose-dependent manner (Fig. 1a, b). The most appropriate dose concentrations of ruxolitinib, MK-2206, and their combination were determined to be 22.5 µM, 7.5 µM, and 18 µM + 5 µM, by measuring the reduction of cell viability by 51%, 58%, and 50%, respectively (Fig. 1a, b, c) When the cells were treated with ruxolitinib, MK-2206 and their combination, cell proliferation was reduced in a time-dependent manner. The cytotoxic effect was reduced with the combined treatment (Fig. 1d). Ruxolitinib was synergized with MK-2206 (CI 0.95).
Effect of ruxolitinib, MK-2206, and their combination on MDA-MB-231 cell viability. Cell viability was measured by MTT assay after ruxolitinib (a), MK-2206 (b), and their combination (c) treatment. The trypan blue assay was used to determine the time-dependent effect of ruxolitinib, MK-2206, and their combination on cell viability (d). ± SD values indicate three independent experiments with at least three replicates. Statistical differences were analyzed using one-way ANOVA and two-way ANOVA. *, **, *** and **** indicate P < 0.05, P < 0.01, P < 0.001, and P < 0.0001, respectively
The combination of ruxolitinib with MK-2206 inhibits metastasis
To understand the metastatic effect of ruxolitinib and MK-2206 on cells, a colony formation assay was used. No changes in cell proliferation were observed for the cells in the untreated control group treated with 0.1% DMSO. While there was a time-dependent decrease in cell proliferation for the cells treated with ruxolitinib compared to the untreated control group, no significant reduction in cell proliferation was detected in the cells exposed to MK-2206. Exposure to cotreated ruxolitinib and MK-2206 cells significantly reduced colony formation compared to that of the control group, DMSO-treated cells and the single drug-treated cells (Fig. 2a).
Determination of the metastatic effect of ruxolitinib and MK-2206 on MDA-MB-231 cells (a). Determination of the effects of ruxolitinib and MK-2206 on lateral cell movement in MDA-MB-231 breast cancer cells (b). ± SD values indicate three independent experiments with at least three replicates. Every group was compared with the untreated group. Statistical differences were analyzed using two-way ANOVA. *, **, *** and **** indicate P < 0.05, P < 0.01, P < 0.001, and P < 0.0001, respectively
The effects of ruxolitinib and MK-2206 on lateral cell movement in MDA-MB-231 cells were examined by wound healing analysis (Fig. 2b). It was found that the width of the wound opened in the untreated control and DMSO-treated groups of MDA-MB-231 cells gradually closed over time. It was found that the width of the wound opened in MDA-MB-231 cells exposed to ruxolitinib and MK-2206 and together could not close in time. The results showed that combined treatment with ruxolitinib and MK-2206 increased the closure rate of wound widths that were opened in the cells compared to that of a single application of drugs (Fig. 2b).
The combination of ruxolitinib with MK-2206 induces apoptosis
The effect of ruxolitinib, MK-2206, and their combination on apoptosis was examined by western blot analyses (Fig. 3a). The cells were exposed to 22.5 µM ruxolitinib, 7.5 µM MK-2206 and 18 µM ruxolitinib + 5 µM MK-2206 for 48 h. As shown in Fig. 3a, the expression of PARP, Caspase-9, Caspase-3, and Caspase-7 decreased in the cells treated with the combination of ruxolitinib + MK-2206 for 48 h. No significant change was observed in the expression of Caspase-8 in the cells treated with ruxolitinib, MK-2206, and ruxolitinib + MK-2206. As shown in Fig. 3b, proapoptotic Bax expression increased in the cells treated with ruxolitinib and MK-2206, while antiapoptotic Bcl-2 expression was reduced. Additionally, c-Myc expression was decreased in the cells treated with both ruxolitinib and MK-2206 (Fig. 3b).
Effects of ruxolitinib, MK-2206, and ruxolitinib + MK-2206 on apoptosis. The effects of ruxolitinib, MK-2206, and ruxolitinib + MK-2206 on apoptosis (a) and Bcl-2 family members (b) were investigated using β-actin as a loading control by western blot. Nuclear condensation and DNA fragmentation were observed in the cells exposed to ruxolitinib, MK-2206, and ruxolitinib + MK-2206 by Hoechst 33,342 staining (c). The error bars represent the mean ± SD from three independent experiments. *, **, *** and **** indicate P < 0.05, P < 0.01, P < 0.001, and P < 0.0001, respectively
The cells were stained with Hoechst 33342 nuclear stain to observe morphologic changes, such as nuclear condensation and DNA fragmentation, in the apoptotic cells (Fig. 3c). While DNA fragmentation was not detected in the untreated control cells or DMSO-treated cells, DNA fragmentation increased in the cells exposed to only MK-2206 and ruxolitinib + MK-2206. According to our findings, cotreatment with ruxolitinib and MK-2206 in MDA-MB-231 cells can trigger apoptosis by decreasing the expression of Caspase-9 and Caspase-7, playing a role in the intrinsic pathway.
The combination of ruxolitinib with MK-2206 inhibits PI3K/AKT and JAK2/STAT5 signaling
The effects of ruxolitinib, MK-2206, and their combination on PI3K/AKT and signaling were observed by western blot analyses (Fig. 4a, b). Only the MK-2206 combined with ruxolitinib treatment stimulated PTEN and P53 upregulation. PI3K expression decreased after only ruxolitinib and ruxolitinib + MK-2206 treatment. However, AKT expression decreased with the combined treatment (Fig. 4a). While JAK2 was upregulated after single doses of ruxolitinib and MK-2206, JAK2 was downregulated after combined treatment with ruxolitinib and MK-2206. Although STAT5 expression increased with only ruxolitinib treatment, STAT5 decreased with only MK-2206 treatment. However, STAT5 expression completely disappeared after cotreatment with ruxolitinib and MK-2206. STAT3 expression increased at the same rate in the MDA-MB-231 cells treated with ruxolitinib or MK-2206 and cotreated with ruxolitinib and MK-2206. According to these results, cotreatment with ruxolitinib and MK-2206 may inhibit PI3K/AKT and JAK2/STAT5 signaling in cells by increasing P53 and PTEN expression and decreasing PI3K, AKT, JAK2 and STAT5 expression.
Effects of ruxolitinib, MK-2206, and ruxolitinib + MK-2206 on the PI3K/AKT signaling pathway (a) and JAK/STAT signaling (b). β-Actin was used as a loading control. Error bars represent the mean ± SD from three independent experiments. *, **, *** and **** indicate P < 0.05, P < 0.01, P < 0.001, and P < 0.0001, respectively
Discussion
TNBC exhibits aggressive features that are characterized by poor prognosis and, consequently, a high risk of metastasis. Due to deficiencies in PR, ER, and HER2, TNBC therapy becomes difficult to perform [3]. Single-targeted therapy is not effective for TNBC because of genetic heterogeneity and acquired resistance. Combined targeted therapy approaches aimed at inhibiting oncogenic signaling networks have been investigated to achieve efficient therapy of the intractable TNBC subtype [4, 32]. Therefore, the development of targeted anticancer drugs that target signaling pathways and regulate tumor formation and metastatic progression is necessary.
The ruxolitinib drug has been approved drug for treating cancer patients who have a risk of myelofibrosis and patients who have an insufficient response to hydroxyurea or are intolerance of hydroxyurea [15]. Because of the toxicities of ruxolitinib, it is used together with other drugs [15, 25, 33, 34]. In this study, we aimed both to decrease the side effects of ruxolitinib and increase the anticancer effects of ruxolitinib using MK-2206 together in MDA-MB-231 cells. In this work, we first studied the effects of ruxolitinib in combination with MK-2206 in MDA-MB-231 cells and then evaluated the cytotoxic and growth inhibitory effects of ruxolitinib, MK-2206, and ruxolitinib + MK-2206 in MDA-MB-231 cells. Schneider et al. [35] investigated the antiproliferative effect of ruxolitinib and calcitriol, both individualy and in combination, on MCF-7, SKBR3, and MDA-MB-468 using the BrdU assay. They demonstrated that ruxolitinib reduces cell proliferation in MCF-7, SKBR3, and MDA-MB-468 cells and determined that the IC50 values of the ruxolitinib are 30.42 µM in MCF-7, 13.94 µM in SKBR3, and 10.87 µM in MDAMB-468 cells [35]. Savaee et al. [36] showed that 10 µM MK-2206 reduced the cell viability of PC-3 cells. As shown in our findings, the viability of cells treated with ruxolitinib, MK-2206, and their combination was reduced in a dose- and time-dependent manner. The determined dose concentrations of ruxolitinib, MK-2206, and their combination were found to be 22,5 μM, 5 μM, and 18 μM + 5 μM for 48 h and decreased cell viability to 51, 58, and 50%, respectively.
Tavalli et al. [34] demonstrated that ruxolitinib has a synergistic effect with lapatinib to eliminate SUM149 mammary tumor cells in transitory drug exposure to lapatinib and ruxolitinib colony formation assays. Schneider et al. [35] demonstrated that the combination of Ruxolitinib and Calcitriol synergistically inhibits the proliferation of SKBR3 and MDA-MB-468 cells using the BrdU assay (SKBR3: CI 0.759–0.836, MDA-MB-468: CI 0.676–0.787). Khan et al. [37] illustrated that peripheral blood CD34+ cells from primary myelofibrosis (PMF) patients that were treated with MK-2206 inhibited colony formation in a dose-dependent manner. Additionally, the researchers showed that MK-2206 had a synergistic effect with ruxolitinib in subduing the growth of JAK2V617F-mutant SET2 cells (CI < 1) [37]. Sim et al.[38] showed that 5 µM MK2206 decreased cell migration by 30% in HeLa cells. Li et al. [19] demonstrated that MK-2206 has a synergistic effect with WZB117 in MDA-MB-231cells (CI 0.21). Similar to these results, we found that ruxolitinib synergizes with MK-2206, inhibiting the formation of colonies in MDA-MB-231 cells (CI 0.95) (Fig. 2a). We determined that the combination of ruxolitinib and MK-2206 inhibits cell migration (Fig. 2b). According to our results, ruxolitinib has a synergistic antiproliferative effect with MK-2206 in MDA-MB-231 cells.
Apoptosis is organized by a chain of events [39]. Studies have shown that ruxolitinib and MK-2206, when used together with other drugs, inhibit the growth of cancer cells synergistically by triggering apoptosis, but there are no studies on the combination of these two drugs with each other [25, 40, 41]. Pro- and anti-apoptotic members of the Bcl-2 family act as regulators of apoptosis [25, 39]. Due to the activation of anti-apoptotic proteins that belong to the Bcl-2 family, which contains Bcl-2, Mcl-1, Bcl-w and Bcl-XL, the resistance of several cancer cells to chemotherapy has increased [42]. In many malignancies, such as aggressive TNBCs, the MYC oncogene is overexpressed [42]. Li et al. [43] demonstrated that ruxolitinib increased the expression of cleaved caspase 3, 8, 9, and PARP in LS411N and SW620 colorectal cancer cells at 48 h. Lim et al. [25] presented that the synergistic effects of ruxolitinib and calcitriol were associated with reduced protein levels of JAK2, phosphorylated JAK2, c-Myc, Bcl-2 and Bcl-XL, and with increased levels of caspase-3 and Bad proteins in MCF7-HER18 breast cancer cells. Chen et al. [44] determined that the combination of MK-2206 with MLN4924 synergistically induces apoptosis in SK-BR3 and MDA-MB-231 cells. They demonstrated that cotreatment MK-2206 and MLN4924 reflect a much higher percentage of Annexin V + population in FACS analysis, induce apoptotic DNA fragmentation, and increased cleavage of PARP and Caspase 3 in SK-BR3 and MDA-MB231 cells. Also, they showed that MK-2206 significantly blocked AKT activation induced by MLN4924 treatment, and demonstrated that MK-2206 synergistically enhances the induction of apoptosis induced by MLN4924. After cotreatment with ruxolitinib and MK2206, pro-apoptotic Bax expression was increased significantly, while Caspase-9, Caspase-7, PARP, c-Myc, and Bcl-2 expression were decreased (Fig. 3a, b). Nuclear condensation and DNA fragmentation are the main characteristics of apoptotic cells [45]. Exposing the cells to ruxolitinib + MK-2206 increased DNA fragmentation (Fig. 3c). According to our findings, the combination of ruxolitinib and MK-2206 in MDA-MB-231 cells induced the intrinsic pathway of apoptosis by decreasing the expression of Caspase-9 and Caspase-7 proteins, and compared to single treatments, the combination was more effective in triggering apoptosis.
PI3K/AKT signaling is usually activated in breast cancers and has an important role in tumorigenesis, apoptosis, and autophagy [46]. PTEN functions as a tumor suppressor and negative regulator of this pathway. In many cancers, the PTEN-inactivated form genetically concludes with the upregulation of PI3K signals. Additionally, mutations in P85, which is a regulatory subunit of PI3K, have been identified in tumors in human. The activities of P53 and c-Myc, which are transcriptional regulators, are affected by the PI3K-AKT pathway. These proteins have all been linked to oncogenic transformation, but their exact roles during PI3K-mediated oncogenesis are still unknown. AKT phosphorylates and suppresses P53 activity; however, the activity of c-Myc is increased by AKT. The effects of AKT and PI3K on transcriptional regulators also play a major role in tumor progression. Recently, studies have shown that stimulating the phosphorylation of proapoptotic proteins and Caspase-9 by AKT triggers the inactivation of these proteins [47]. Karagianni et al. [48] showed that ruxolitinib significantly reduced p-AKT levels at 24 h, and p-STAT3 levels at 48 h post-treatment in MyLa Cutaneous T-cell lymphomas (CTCL) cells. Sim et al. [38] demonstrated that 5 µM MK2206 reduced phospho-AKT (Ser473-Thr308), and not changed total AKT for 24 h in HeLa cells. Savaee et al. [36] illustrated that combined treatment with MK-2206 and salinomycin increased apoptosis of the cancer cells by reducing total AKT expression. Hirai et al. [40] indicated that MK-2206 synergistically suppressed AKT pathway in cotreatment with cytotoxic drugs such as antimetabolites, topoisomerase inhibitors, DNA crosslinkers, and anti-microtubule agents in ovarian A2780 and lung NCI-H460 tumor cells. According to our findings, cotreatment with ruxolitinib and MK-2206 inhibits PI3K/AKT signaling in MDA-MB-231 cells by increasing P53 and PTEN expression and decreasing PI3K and AKT expression (Fig. 4a).
JAK2 has an important role in regulating the apoptosis and proliferation of cancer cells by activating different signaling pathways, such as the STAT5/STAT3 and PI3K/AKT signaling pathways [33]. STAT5 and STAT3 are STAT family members and regulate the expression of genes that have roles in angiogenesis, survival and cell growth [7]. Therefore, resistance to therapy and recurrence may occur. Pro- and antiapoptotic proteins, such as Bak/Bax and Bcl-2, are also related to JAK/STAT signaling. The PI3K/AKT and JAK/STAT pathways control the expression of proteins that regulate mitochondrial apoptosis. Girardot et al. [49] showed that P53 interacts with tyrosine-phosphorylated and unphosphorylated (U) STAT5 by coimmunoprecipitation. Moreover, they showed that WT P53 might suppress STAT5 transcriptional activity in myeloid neoplasms. Recent studies have focused on JAK2/STAT3 signaling in breast cancer models. Britschgi et al. [9] determined that dual inhibition of PI3K/mTOR and JAK2/STAT5, which were treated with a combination of BEZ235 and NVP-BSK805 in RAS-mutated MDA-MB-231 LM2 and PTEN-deficient MDA-MB-468 breast cancer cells and in the mouse breast cancer cell Line 4T1, caused the activation of Bim and inhibition of Mcl-1. Additionally, they showed that inhibiting JAK2 prevents the resistance to PI3K/mTOR inhibition and that the combination therapy of PI3K/mTOR and JAK2 negatively affects tumor growth, cancer cell number and metastasis [7, 9]. Li et al. [43] demonstrated that ruxolitinib decreased the expression of JAK1, JAK2, and STAT1 in LS411N and SW620 colorectal cancer cells. Wilson et al. [31] determined that Ruxolitinib effectively inhibited JAK/STAT signaling in SNU182 and SNU423 cells with a reduction pSTAT1 and pSTAT3. According to our study, the combined treatment of ruxolitinib and MK-2206 can similarly inhibit the JAK/STAT signaling pathway in MDA-MB-231 cells by decreasing JAK2 expression and STAT5 expression. In addition, our study showed for the first time that the increased expression of P53 and PTEN tumor suppressor genes at the protein level after combined treatment with ruxolitinib and MK-2206 may be associated with the inhibition of STAT5 expression in breast cancer cells (Fig. 4b).
Conclusions
In conclusion, the JAK2 inhibitor ruxolitinib has a synergistic antiproliferative effect with the AKT inhibitor MK-2206. Additionally, compared to single use of drugs, the combined treatment with ruxolitinib and MK-2206 is more effective. The increased expression of P53 and PTEN tumor suppressor genes at the protein level with combined treatment with ruxolitinib and MK-2206 may have an important role in triggering apoptosis in MDA-MB-231 cells by dual inhibition of JAK2/STAT5 and PI3K/AKT signaling. The dual inhibition of JAK2/STAT5 and PI3K/AKT may reduce metastasis by decreasing the survival of tumor cells.
References
Yang Y, Zhou H, Liu W et al (2018) Ganoderic acid a exerts antitumor activity against MDA-MB-231 human breast cancer cells by inhibiting the Janus kinase 2/signal transducer and activator of transcription 3 signaling pathway. Oncol Lett 16:6515–6521. https://doi.org/10.3892/ol.2018.9475
Franklin DA, James JL, Axelrod ML, Balko JM (2020) MEK inhibition activates STAT signaling to increase breast cancer immunogenicity via MHC-I expression. Cancer Drug Resist 3:603–612. https://doi.org/10.20517/cdr.2019.109
Khan Mohammad A, Jain Vineet K, JA Md. Rizwanullah, KJ (2019) PI3K/AKT/mTOR pathway inhibitors in triple-negative breast cancer: a review on drug discovery and future challenges. Drug Discov Today 24:2181–2191. https://doi.org/10.1016/j.drudis.2019.09.001
He J, McLaughlin RP, van der Noord V et al (2019) Multi-targeted kinase inhibition alleviates mTOR inhibitor resistance in triple-negative breast cancer. Breast Cancer Res Treat 178:263–274. https://doi.org/10.1007/s10549-019-05380-z
Jhan JR, Andrechek ER (2017) Triple-negative breast cancer and the potential for targeted therapy. Pharmacogenomics 18:1595–1609. https://doi.org/10.2217/pgs-2017-0117
Bianchini G, Balko JM, Mayer IA et al (2016) Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 13:674–690. https://doi.org/10.1038/nrclinonc.2016.66
Yeh JE, Toniolo PA, Frank DA (2013) JAK2-STAT5 signaling. JAK-STAT 2:e24635. https://doi.org/10.4161/jkst.24635
Mumin NH, Drobnitzky N, Patel A et al (2019) Overcoming acquired resistance to HSP90 inhibition by targeting JAK-STAT signalling in triple-negative breast cancer. BMC Cancer 19:102. https://doi.org/10.1186/s12885-019-5295-z
Britschgi A, Andraos R, Brinkhaus H et al (2012) JAK2/STAT5 inhibition circumvents resistance to PI3K/mTOR blockade: a rationale for cotargeting these pathways in metastatic breast cancer. Cancer Cell 22:796–811. https://doi.org/10.1016/j.ccr.2012.10.023
Furth PA (2014) STAT signaling in different breast cancer sub-types. Mol Cell Endocrinol 382:612–615. https://doi.org/10.1016/j.mce.2013.03.023
Halim CE, Deng S, Ong MS, Yap CT (2020) Involvement of STAT5 in oncogenesis. Biomedicines 8:316. https://doi.org/10.3390/biomedicines8090316
Owen KL, Brockwell NK, Parker BS (2019) JAK-STAT signaling: a double-edged sword of immune regulation and cancer progression. Cancers 11:2002. https://doi.org/10.3390/cancers11122002
Orlova A, Wagner C, de Araujo ED et al (2019) Direct targeting options for STAT3 and STAT5 in cancer. Cancers 11:1930. https://doi.org/10.3390/cancers11121930
Jang H, Ko H, Song K, Kim Y (2019) A sesquiterpenoid from farfarae flos induces apoptosis of MDA-MB-231 human breast cancer cells through inhibition of JAK–STAT3 signaling. Biomolecules 9:278. https://doi.org/10.3390/biom9070278
Stover DG, Gil Del Alcazar CR, Brock J et al (2018) Phase II study of ruxolitinib, a selective JAK1/2 inhibitor, in patients with metastatic triple-negative breast cancer. NPJ Breast Cancer 4:10. https://doi.org/10.1038/s41523-018-0060-z
Pascual J, Turner NC (2019) Targeting the PI3-kinase pathway in triple-negative breast cancer. Ann Oncol 30:1051–1060. https://doi.org/10.1093/annonc/mdz133
Stemke-Hale K, Gonzalez-Angulo AM, Lluch A et al (2008) An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res 68:6084–6091. https://doi.org/10.1158/0008-5472.CAN-07-6854
LoRusso PM (2016) Inhibition of the PI3K/AKT/mTOR pathway in solid tumors. J Clin Oncol 34:3803–3815. https://doi.org/10.1200/JCO.2014.59.0018
Li Y-L, Weng H-C, Hsu J-L et al (2019) The combination of MK-2206 and WZB117 exerts a synergistic cytotoxic effect against breast cancer cells. Front Pharmacol 10:1311. https://doi.org/10.3389/fphar.2019.01311
Eroglu O, Celik EG, Kaya H et al (2019) Investigation of methylation profiles of TP53, caspase 9, caspase 8, caspase 3 genes treated with DNA methyl transferase inhibitor (DNMTi) zebularine (ZEB) and caffeic acid phenethyl ester (CAPE) on MCF-7 and MDA-MB-231 breast cancer cell lines. J Cancer Ther 10:69–85. https://doi.org/10.4236/jct.2018.101006
Eroglu O, Kaya H, Celik EG et al (2019) Triple effect of doxorubicin, 5-fluorouracil, propranolol on cell survival on MCF-7 breast cancer cell line. J Biosci Med 7:74–85. https://doi.org/10.4236/jbm.2019.72007
Mokhtari RB, Homayouni TS, Baluch N et al (2017) Combination therapy in combating cancer. Oncotarget 8:38022–38043. https://doi.org/10.18632/oncotarget.16723
Choong ML, Pecquet C, Pendharkar V et al (2013) Combination treatment for myeloproliferative neoplasms using JAK and pan-class I PI3K inhibitors. J Cell Mol Med 17:1397–1409. https://doi.org/10.1111/jcmm.12156
Chou TC (2010) Drug combination studies and their synergy quantification using the chou-talalay method. Cancer Res 70:440–446. https://doi.org/10.1158/0008-5472.CAN-09-1947
Lim ST, Jeon YW, Gwak H et al (2018) Synergistic anticancer effects of ruxolitinib and calcitriol in estrogen receptor-positive, human epidermal growth factor receptor 2-positive breast cancer cells. Mol Med Rep 17:5581–5588. https://doi.org/10.3892/mmr.2018.8580
Civallero M, Cosenza M, Pozzi S, Sacchi S (2017) Ruxolitinib combined with vorinostat suppresses tumor growth and alters metabolic phenotype in hematological diseases. Oncotarget 8:103797–103814. https://doi.org/10.18632/oncotarget.21951
Tao K, Yin Y, Shen Q et al (2016) Akt inhibitor MK-2206 enhances the effect of cisplatin in gastric cancer cells. Biomed Reports 4:365–368. https://doi.org/10.3892/br.2016.594
Ma BBY, Lui VWY, Hui CWC et al (2013) Preclinical evaluation of the AKT inhibitor MK-2206 in nasopharyngeal carcinoma cell lines. Invest New Drugs 31:567–575. https://doi.org/10.1007/s10637-012-9896-5
Simioni C, Neri LM, Tabellini G et al (2012) Cytotoxic activity of the novel Akt inhibitor, MK-2206, in T-cell acute lymphoblastic leukemia. Leukemia 26:2336–2342. https://doi.org/10.1038/leu.2012.136
Jiao P, Zhou YS, Yang JX et al (2013) MK-2206 induces cell cycle arrest and apoptosis in HepG2 cells and sensitizes TRAIL-mediated cell death. Mol Cell Biochem 382:217–224. https://doi.org/10.1007/s11010-013-1737-0
Wilson JM, Kunnimalaiyaan S, Gamblin TC, Kunnimalaiyaan M (2014) MK2206 inhibits hepatocellular carcinoma cellular proliferation via induction of apoptosis and cell cycle arrest. J Surg Res 191:280–285. https://doi.org/10.1016/j.jss.2014.05.083
You KS, Yi YW, Cho J, Seong Y-S (2021) Dual inhibition of AKT and MEK pathways potentiates the anti-cancer effect of gefitinib in triple-negative breast cancer cells. Cancers 13:1205. https://doi.org/10.3390/cancers13061205
Ishida S, Akiyama H, Umezawa Y et al (2018) Mechanisms for mTORC1 activation and synergistic induction of apoptosis by ruxolitinib and BH3 mimetics or autophagy inhibitors in JAK2-V617F-expressing leukemic cells including newly established PVTL-2. Oncotarget 9:26834–26851. https://doi.org/10.18632/oncotarget.25515
Tavallai M, Booth L, Roberts JL et al (2016) Rationally repurposing ruxolitinib (Jakafi®) as a solid tumor therapeutic. Front Oncol. https://doi.org/10.3389/fonc.2016.00142
Schneider J, Jeon YW, Suh YJ, Lim ST (2022) Effects of ruxolitinib and calcitriol combination treatment on various molecular subtypes of breast cancer. Int J Mol Sci 23:2535. https://doi.org/10.3390/ijms23052535
Savaee M, Bakhshi A, Yaghoubi F et al (2022) Evaluating the effects of separate and concomitant use of MK-2206 and salinomycin on prostate cancer cell line. Rep Biochem Mol Biol 11:157–165. https://doi.org/10.52547/rbmb.11.1.157
Khan I, Huang Z, Wen Q et al (2013) AKT is a therapeutic target in myeloproliferative neoplasms. Leukemia 27:1882–1890. https://doi.org/10.1038/leu.2013.167
Sim HJ, Song MS, Lee SY (2021) Kv3 channels contribute to cancer cell migration via vimentin regulation. Biochem Biophys Res Commun 551:140–147. https://doi.org/10.1016/j.bbrc.2021.03.019
Çakir HK, Eroglu O (2021) In vitro anti-proliferative effect of capecitabine (Xeloda) combined with mocetinostat (MGCD0103) in 4T1 breast cancer cell line by immunoblotting. Iran J Basic Med Sci 9:1515–1522. https://doi.org/10.22038/IJBMS.2021.58393.12971
Hirai H, Sootome H, Nakatsuru Y et al (2010) MK-2206, an allosteric akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther 9:1956–1967. https://doi.org/10.1158/1535-7163.MCT-09-1012
Cheng Y, Zhang Y, Zhang L et al (2012) MK-2206, a novel allosteric inhibitor of Akt, synergizes with gefitinib against malignant glioma via modulating both autophagy and apoptosis. Mol Cancer Ther 11:154–164. https://doi.org/10.1158/1535-7163.MCT-11-0606
Kim JW, Gautam J, Kim JE et al (2019) Inhibition of tumor growth and angiogenesis of tamoxifen-resistant breast cancer cells by ruxolitinib, a selective JAK2 inhibitor. Oncol Lett 17:3981–3989. https://doi.org/10.3892/ol.2019.10059
Li X, Wang Z, Zhang S et al (2021) Ruxolitinib induces apoptosis of human colorectal cancer cells by downregulating the JAK1/2-STAT1-Mcl-1 axis. Oncol Lett 21:1–14. https://doi.org/10.3892/ol.2021.12613
Chen X, Cui D, Bi Y et al (2018) AKT inhibitor MK-2206 sensitizes breast cancer cells to MLN4924, a first-in-class NEDD8-activating enzyme (NAE) inhibitor. Cell Cycle 17:2069–2079. https://doi.org/10.1080/15384101.2018.1515550
Xu X, Rajamanicham V, Xu S et al (2019) Schisandrin A inhibits triple negative breast cancer cells by regulating Wnt/ER stress signaling pathway. Biomed Pharmacother 115:108922. https://doi.org/10.1016/j.biopha.2019.108922
Vagia E, Mahalingam D, Cristofanilli M (2020) The landscape of targeted therapies in TNBC. Cancers 12:916. https://doi.org/10.3390/cancers12040916
Bader AG, Kang S, Zhao L, Vogt PK (2005) Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer 5:921–929. https://doi.org/10.1038/nrc1753
Karagianni F, Piperi C, Mpakou V et al (2021) Ruxolitinib with resminostat exert synergistic antitumor effects in cutaneous T-cell lymphoma. PLoS ONE 16:1–14. https://doi.org/10.1371/journal.pone.0248298
Girardot M, Pecquet C, Chachoua I et al (2015) Persistent STAT5 activation in myeloid neoplasms recruits p53 into gene regulation. Oncogene 34:1323–1332. https://doi.org/10.1038/onc.2014.60
Acknowledgements
The results shown in this paper were part of the PhD thesis of Esin Guvenir Celik. The authors gratefully acknowledge the support given under the Research Projects Department of Bilecik Şeyh Edebali University. MDA-MB-231 cells were kindly donated by Professor Hatice Mehtap Kutlu for our doctorate research project.
Funding
This work was financially supported by the Research Projects Department of Bilecik Şeyh Edebali University [Project No. 2018-02.BŞEÜ.01-01].
Author information
Authors and Affiliations
Contributions
EGC and OE contributed to the study conception and design. Material preparation, data collection and analysis were performed by EGC. The draft of the manuscript was written by EGC and OE, and EGC and OE approved the version to be published. EGC and OE read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or nonfinancial interests to disclose. The authors of this paper state that they have no conflicts of interest.
Ethical approval
The research performed in article used a commercially available cell line, and we declare that it does not include any studies with human participants or animals by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guvenir Celik, E., Eroglu, O. Combined treatment with ruxolitinib and MK-2206 inhibits the JAK2/STAT5 and PI3K/AKT pathways via apoptosis in MDA-MB-231 breast cancer cell line. Mol Biol Rep 50, 319–329 (2023). https://doi.org/10.1007/s11033-022-08034-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-08034-4