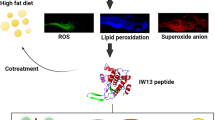
Abstract
Background
Pancreatic β-cells are susceptible to oxidative stress, leading to β-cell death and dysfunction due to enhanced ROS levels and type 2 diabetes. To inhibit the β-cells damages induced by the oxidative stress, the present study investigates the beneficial effect of various peptides (WL15, RF13, RW20, IW13 and MF18) of immune related proteins (cysteine and glycine-rich protein 2, histone acetyltransferase, vacuolar protein sorting associated protein 26B, serine threonine-protein kinase and CxxC zinc finger protein, respectively). Also, the molecular mechanism of WL15 from cysteine and glycine-rich protein 2 on β-cell regeneration was identified through PEPCK and insulin pathway.
Materials and methods
In this study, a total of five peptides including WL15, RF13, RW20, IW13, and MF18 were derived from immune-related proteins such as cysteine and glycine-rich protein 2, histone acetyltransferase, vacuolar protein sorting associated protein 26B, serine threonine-protein kinase and CxxC zinc finger protein, respectively. These protein sequences were obtained from an earlier constructed transcriptome database of a teleost Channa striatus. The identified peptides were evaluated for their antioxidant as well as antidiabetic activity. Based on the in silico analysis and in-vitro screening experiments, WL15 was predicted to have better antioxidant and antidiabetic activity among the five different peptides. Therefore, WL15 alone was further analyzed for apoptosis, antioxidant capacity, glucose metabolism, and gene expression performance, which was investigated on the alloxan (500 µM) induced zebrafish in vivo larval model.
Results
The results showed alloxan exposure to zebrafish larvae for a day, the ROS was generated in the β-cells. Interestingly, WL15 treatment showed a protective effect by reducing the toxicity of alloxan exposed zebrafish larvae by increasing their survival and heart rate. Moreover, WL15 reduced the intracellular ROS level and apoptosis in alloxan-induced larvae. The superoxide anion and lipid peroxidation levels are also reduced by improving the glutathione content after the WL15 treatment. Besides, WL15 treatment increased the proliferation rate of β-cells and decreased the glucose level. Further, the gene expression studies revealed that WL15 treatment normalized the PEPCK expression while upregulating the insulin expression in alloxan exposed larvae.
Conclusion
Overall, the findings indicate that WL15 of cysteine and glycine-rich protein 2 can act as a potential antioxidant for type 2 diabetes patients in respect of improving β-cell regeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The interplay between β-cell dysfunction and insulin resistance in the pathogenesis of diabetes is inherently complex [1]. When insulin-producing cells in pancreatic islets are unable to produce enough insulin to overcome peripheral insulin resistance, type 2 diabetes (T2D) develops, resulting in hyperglycemia. The β-cell dysfunction is linked to worsening glycemic control and treatment failure; it is critical to restoring β-cell functional mass in T2D management [2]. β-cell mass is reduced in T2D patients due to increased apoptosis, oxidative stress, necrosis, and autophagy. In T2D, reactive oxygen species (ROS) are formed due to elevated glucose levels caused by β-cell dysfunction [3]. Increased ROS production in β-cells due to mitochondrial overstimulation inhibits the electron transport chain, resulting in lower energy production, DNA damage and the formation of advanced glycation end products (AGEs) [4]. The pancreatic β-cells with low antioxidant capacity are vulnerable to high endogenous production of ROS, implying that oxidative stress plays a major role in damaging the β-cells [5]. ROS such as superoxide and hydrogen peroxide are primarily produced during mitochondrial oxidative phosphorylation. Oxidative phosphorylation is tightly linked to insulin secretion in β-cells, where more than 90% of the carbons in glucose are oxidized to CO2 when the substrate supply is available [6]. The glucokinase in the β-cells has rate-limiting enzymes for glucose metabolism, which have a much lower affinity for glucose than other hexokinases, allowing the β-cell to control the rate of glucose metabolism. As a result, the rate of mitochondrial oxidation in the β-cell is directly proportional to blood glucose levels. It is widely accepted that when blood glucose levels are chronically elevated in T2D, increased oxidative phosphorylation leads to increased ROS production in β-cells [7].
The pancreas of zebrafish and humans are nearly identical, so larvae are widely used to study pancreatic toxicity. Alloxan can cause oxidative stress in β-cells of zebrafish larvae and is known to develop pancreatic cell dysfunction [8, 9]. Evidence based research has shown that peptide with antidiabetic and antioxidant activity plays a major role in treating T2D and its associated oxidative stress complications [10]. Channa striatus is a popular food fish in Southeast Asia. The pharmacological properties of this fish may have been influenced by its high concentrations of amino acids and fatty acids. Glycine, lysine and arginine are the potent antioxidants present in this fish [11]. Antioxidant-rich foods have long been used to treat T2D by reducing oxidative stress and improving pancreatic cell proliferation and function. According to recent research, antioxidant molecules help to reduce hydrogen peroxide in β-cells to increase proliferation [12]. In this study, we aimed to screen the potential peptides identified from an earlier constructed transcriptome database (protein) of a teleost C. striatus and to evaluate the recovery effect of peptides on pancreatic β-cells toxicity through enhanced insulin expression and decreased transcriptional expression of phosphoenolpyruvate carboxykinase (PEPCK).
Materials and methods
In-silico studies.
Peptide rank and toxicity prediction
PeptideRanker (http://distilldeep.ucd.ie/PeptideRanker/) is a server that uses a novel N-to-1 neural network to predict bioactive peptides. The Peptide Ranker predicts whether a peptide sequence is likely to be bioactive by giving scores ranging from 0 to 1. A peptide score of more than 0.75 indicates that a sequence has the potential to be bioactive [13]. ToxinPred was used to calculate the toxicity of predicted T-cell epitopes (http://crdd.osdd.net/raghava/toxinpred/). ToxinPred is a computer programme that predicts which peptides are toxic or non-toxic [14].
HPEPDOCK
HPEPDOCK (http://huanglab.phys.hust.edu.cn/hpepdock/) is a server that uses a hierarchical algorithm to investigate protein-peptide docking. The results obtained from the protein–ligand binding affinity are expressed in kcal/mol units of free energy. In the discovery studio software (Version 4.1), the interaction between the peptide and the receptor is visualized [15, 16].
In-vitro analysis.
α-amylase inhibition
To perform the α-amylase inhibition assay, the solution was prepared with 0.01 M CaCl2, and 0.5 M Tris–HCL (pH 6.9) added to 2 mg of starch. This mixture was kept in a boiling water bath for 5 min. The various concentrations (10 µM to 50 µM) of peptides (WL15, RF13, RW20, IW13, and MF18) were added to the reaction mixture and further added with pancreatic amylase dissolved in Tris–HCL buffer. The centrifugation was done to obtain the supernatant at 4000 rpm for 10 min. This resultant supernatant was measured at 595 nm in a spectrophotometer (UV 1800, SHIMADZU, Kyoto, Japan) [17].
Hydroxyl radical scavenging assay
To identify antioxidant activity, hydroxyl radical scavenging assay was performed [18]. The peptides with different concentrations were added to the reaction mixture containing EDTA (6 mM), ferrous sulphate (2 mM), PBS (0.2 mM), and phenanthroline (2 mM). Finally, 40 µL of 0.03% H2O2 was added to the reaction mixture and kept for 30 min incubation at 37 °C. Using a spectrophotometer, the absorbance was measured at 536 nm.
Experimental zebrafish larvae.
The wild-type zebrafish of AB strain were purchased from the NSK Aquarium at Kolathur, Tamil Nadu, India. To obtain embryos, a spawning tank was maintained with male and female fish in a 2:1 ratio, and a mesh was kept at the bottom of the tank to prevent fish from eating its eggs. At the start of the light cycle, spawning took place. After 2 h of spawning, the embryos are collected and incubated in a 12 well plate with an embryo medium at 28 °C [19, 20, 21]. The larvae were incubated with 500 µM alloxan for 1 day to induce oxidative stress in β-cells of larvae. For β-cells damage, the larvae were exposed to 500 µM alloxan for 3 days. The larvae were divided based on the treatment groups (n = 100 each). The control group, in which larvae were untreated; whereas, in alloxan group, the larvae were exposed to 500 μM alloxan; and the WL15 peptide treated group, the larvae were treated with different concentrations of WL15. All of the experiments were done per the Institute’s Animal Ethical Clearance guidelines (No. SAF/IAEC/211215/004).
Toxicity analysis in larvae
The protective effect of the WL15 peptide in alloxan-induced toxicity was investigated in 72 hpf zebrafish larvae [22, 23]. Briefly, different concentrations of the WL15 peptide were cotreated with alloxan and incubated for 24 h. The protective effect of WL15 was determined after the incubation period by calculating the mortality rate, heart rate, and percentage of deformities.
Measurement of alloxan mediated stress
The ROS, apoptosis, and lipid peroxidation level were measured using the 2′-7′-dichlorofluorescein diacetate (DCFDA), Diphenyl-1-pyrenylphosphine (DPPP) and acridine orange staining [24, 25, 26]. In brief, the larvae were exposed to 500 µM of alloxan for 1 day and cotreated with WL15 (50 µM). After treatment, the larvae were incubated for 1 h at room temperature in the dark with fluorescent stain (20 µg/mL). The larvae were washed and anesthetized with ice-cold PBS for visualization after incubation. The fluorescent microscope equipped with CoolSNAP-Pro colour digital camera (Olympus, Tokyo, Japan) was used to capture the larvae stained with fluorescent dye. The fluorescent intensity was measured using Image J software (Version 1.49, NIH, USA).
Quantification of superoxide anion and glutathione
The intracellular superoxide anion and glutathione depletion levels were measured using a modified version of a previously described protocol [27]. The larvae were incubated in the dark at 28.5 °C for 30 min with 20 µM of dihydroethidium (DHE) and naphthalene-2,3-dicarboxaldehyde (NDA). The larvae were washed in PBS buffer after incubation. Fluorescence microscopy was used to capture the images, and the intensity of fluorescent was measured using Image J software.
2NBDG uptake
The 2NBDG uptake assay in zebrafish larvae was performed as described previously [28]. The 72 hpf larvae were placed in a 6-well plate, exposed to 500 µM of alloxan, and cotreated with WL15 (50 µM) for 3 days. After treatment, the larvae were stained with 2NBDG and kept for 3 h of incubation. The stained larvae were washed with PBS buffer to remove the excess stain and anesthetized for visualization. A fluorescent microscope captured the image of 2NBDG uptake in β-cells of zebrafish larvae.
Glucose estimation in larvae
The treated zebrafish larvae from each group are taken to estimate the glucose level as per the test kit instructions (Robonik, India). The larvae were homogenized with 200 μL PBS buffer and centrifuged at 5000 g for 10 min. The supernatant obtained after the centrifugation process was used for analysis. The supernatant was added to the microplate reader to measure the glucose level at 510 nm.
Measurement of insulin and PEPCK gene expression
Using TRIzol Reagent method [29], the total RNA was isolated from untreated larvae, alloxan-exposed larvae, and WL15 treated larvae. The 1 µg of isolated RNA was converted into first-strand cDNA for real-time PCR using a cDNA reverse transcription kit. Amplification was performed in a cDNA mixture on a Light Cycler 96 Real-Time PCR machine using SYBER Green PCR Master Mix, with a final reaction volume of 20 µL. PCR was carried out using the primers listed in Table 3. The 2–ΔΔCt method was used to determine relative gene expression [30, 31].
Statistical analysis
GraphPad Prism was used to perform the statistical analysis (version 5.0). All experiments were done in triplicate, and statistical significance was determined by a p value of less than 0.05. The data were presented as a mean and standard deviation (SD).
Results
Insilico analysis.
The peptides including WL15 (1WHKNCFRCAKCGKSL15), RF13 (1RRGKGGRRVTMSF13), RW20 (1RPVKRKKGWPKGVKRGPPKW20), IW13 (1IKHFKKQRRLIPW13) and MF18 (1MRKKAVKVKHVKRREKKF18) derived from the protein sequeece of cysteine and glycine-rich protein 2 (CSRP2), histone acetyltransferase (HATs), vacuolar protein sorting associated protein 26B (VPS26B), serine threonine-protein kinase (STPK), and CxxC zinc finger protein (CxxC), respectively of an earlier constructed transcriptome dataset (protein sequence) of an teleost C. striatus were evaluated for toxicity using ToxinPred. Results showed that all the peptides were non-toxic (Table 1). PeptideRanker was used to screen these non-toxic peptides for the possibility of highly bioactive peptides. The WL15 has the highest PeptideRanker score of 0.93, indicating that this peptide was highly bioactive (Table 2). In contrast, MF18 has the lowest Peptide Ranker score of 0.23, with the least bioactive peptide possibility.
The HPEPDOCK tool was used to study the peptides (WL15, RF13, RW20, IW13, and MF18) interaction with the insulin receptor and glutathione (E-Suppl. Fig. 1). The binding score of the peptides with receptors is given in Table 2. The maximum binding affinity was noticed in the WL15 peptide; its insulin receptors are −218.16 kcal/mol units, and its glutathione is −190.74 kcal/mol units compared to other peptides Table 3.
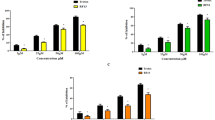
In-vitro α-amylase inhibition assay.
The α-amylase inhibition activity revealed that at a minimum concentration of 10 µM, WL15 inhibited 15%, RF13 inhibited 5%, RW20 inhibited 13%, IW13 inhibited 5%, and MF18 inhibited 2%. At a higher concentration of 50 µM, WL15 inhibited 62%, RF13 inhibited 30%, RW20 inhibited 51%, IW13 inhibited 23% and MF18 inhibited 15% compared to the control glimepiride (74%) (p > 0.05) at 50 µM. The WL15 peptide exhibited better antidiabetic activity than other peptides (E-Suppl. Fig. 2) considered for analysis.
In-vitro antioxidant activity.
The antioxidant activity of the WL15, RF13, RW20, IW13, and MF18 was preliminarily screened using hydroxyl radical scavenging activity. In this assay, at a minimum concentration of 5 µM, WL15 inhibited 12%, RF13 inhibited 4%, RW20 inhibited 7%, IW13 inhibited 5%, and MF18 inhibited 3%. At a higher concentration of 50 µM, WL15 inhibited 69%, RF13 inhibited 23%, RW20 inhibited 47%, IW13 inhibited 23% and MF18 inhibited 20% compared to the control Trolox (79%) (p > 0.05) at 50 µM. These results showed that the WL15 peptide had a better free radical scavenging activity compared to other peptides (E-Suppl. Fig. 3).
Protective effect of WL15 in zebrafish larvae.
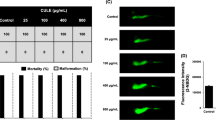
The mortality and heartbeat rate of alloxan induced zebrafish larvae was reduced when cotreated with different concentration (10 µM, 20 µM, 30 µM, 40 µM, and 50 µM) of WL15 (E-Suppl. Fig. 4A and 4B). The analysis revealed that cotreatment of the WL15 prevented the morphological abnormalities caused by the alloxan (500 µM) (E-Suppl. Fig. 5A and 5B). The larvae with no morphological abnormalities were found in the WL15 cotreatment at 40 µM and 50 µM.
Reduction of oxidative stress in β-cells.
The effect of WL15 on reducing ROS, apoptosis, and lipid peroxidation generated by alloxan in β-cell of zebrafish larvae was stained using DCFDA, acridine orange, and DPPP staining. The increase in fluorescent intensity was observed in alloxan exposed larvae, indicating enhanced ROS (82%), apoptosis (86%), and lipid peroxidation (73%) levels compared to untreated larvae (Figs. 1, 2, and 3). While the maximum decrease in ROS (30%), apoptosis (24%), and lipid peroxidation (34%) was observed in the 50 μM of WL15 treatment group. These results showed that the WL15 peptide was protective against oxidative stress in β-cells of zebrafish larvae.
A Representative photomicrographs of oxidative stressed β-cells in zebrafish larvae (n = 10/group) stained by DCFDA fluorescent probe. B Quantitative analysis of in-vivo ROS generation in β-cells of zebrafish larvae. The fluorescence intensity was quantified using ImageJ. The untreated and glimepiride (10 μM) larvae were used as control and positive control in the experiment, respectively. The β-cells are marked inside the white box. Experiments were performed in triplicate, and the data were expressed as mean ± SD. * represents the statistical significance at p < 0.05
A Representative photomicrographs of apoptotic β-cells in zebrafish larvae (n = 10/group) stained by acridine orange fluorescent probe. B Quantitative analysis of in-vivo apoptosis in β-cells of zebrafish larvae. The fluorescence intensity was quantified using ImageJ. The untreated and glimepiride (10 μM) larvae were used as control and positive control in the experiment, respectively. The β-cells are marked inside the white box. Experiments were performed in triplicate, and the data were expressed as mean ± SD. * represents the statistical significance at p < 0.05
A Representative photomicrographs of lipid peroxidation in β-cells of zebrafish larvae (n = 10/group) stained by DPPP fluorescent probe. B Quantitative analysis of in-vivo lipid peroxidation in β-cells of zebrafish larvae. The fluorescence intensity was quantified using ImageJ. The untreated and glimepiride (10 μM) larvae were used as control and positive control in the experiment, respectively. The β-cells are marked inside the white box. Experiments were performed in triplicate, and the data were expressed as mean ± SD. * represents the statistical significance at p < 0.05
Effect of WL15 on superoxide anion and glutathione depletion.
The DHE and NDA staining were used to examine the effect of WL15 on reducing superoxide anion and glutathione depletion in β-cells of zebrafish larvae. In alloxan-exposed larvae, the fluorescent intensity of superoxide anion (77%) was higher, while a decrease in glutathione level (9%) was observed when compared to untreated larvae (Fig. 4 and 5). These superoxide anion levels (36%) in the β-cells of zebrafish are reduced when WL15 peptides are cotreated with alloxan due to an increase in glutathione content (39%).
A Representative photomicrographs of glutathione level in β-cells of zebrafish larvae (n = 10/group) stained by NDA fluorescent probe. B Quantitative analysis of glutathione in β-cells of zebrafish larvae. The fluorescence intensity was quantified using ImageJ. The untreated and glimepiride (10 μM) larvae were used as control and positive control in the experiment, respectively. The β-cells are marked inside the white box. Experiments were performed in triplicate, and the data were expressed as mean ± SD. * represents the statistical significance at p < 0.05
A Representative photomicrographs of superoxide anion in β-cells of zebrafish larvae (n = 10/group) stained by DHE fluorescent probe. B Quantitative analysis of superoxide anion in β-cells of zebrafish larvae. The fluorescence intensity was quantified using ImageJ. The untreated and glimepiride (10 μM) larvae were used as control and positive control in the experiment, respectively. The β-cells are marked inside the white box. Experiments were performed in triplicate, and the data were expressed as mean ± SD. * represents the statistical significance at p < 0.05
Effect of WL15 on alloxan induced β-cells damages.
To investigate the protective effect of alloxan induced β-cells damages, the larvae were treated with 500 µM of alloxan for 3 days. The alloxan exposed zebrafish larvae showed a decrease in fluorescent intensity, indicating a significant (p < 0.05) decrease in the size of pancreatic β-cells in larvae (Fig. 6). However, cotreatment of WL15 (50 µM) increased the fluorescent intensity indicating the protective effect against β-cell damages and an increase in the size of β-cells in larvae.
Glucose estimation.
The glucose levels were estimated in the zebrafish larvae using a glucose estimation kit. High glucose levels were observed in the 500 μM alloxan treatment group (160 mg/dL) compared to the control group (84 mg/dL). A significant (p < 0.05) reduction in the glucose level was observed in WL15 cotreated group (E-Suppl. Fig. 6). WL15 at 50 μM was able to reverse the condition (113 mg/dL), similar to the glimepiride treatment (101 mg/dL). These results suggest that WL15 could attenuate post-prandial hyperglycemia conditions.
Effect of WL15 on insulin and PEPCK expression.
The insulin expression in alloxan exposed larvae was investigated to check whether the WL15 can enhance the insulin mRNA levels. The alloxan (500 µM) exposed larvae with β-cell damage showed a decrease in insulin expression level (0.35 fold), while when the WL15 peptide at 50 µM was cotreated, the insulin level was significantly (p < 0.05) upregulated (1.8 fold) (E-Suppl. Fig. 7A). Further the PEPCK expression was investigated, which transcriptionally regulated by insulin and act as a rate limiting step in gluconeogenesis. Overexpression of PEPCK causes excessive glucose production in the liver in patients with T2D. As a result inhibiting PEPCK has been proven to be a therapeutic target for lowering blood sugar. These findings prompted us to use real-time PCR to examine changes in PEPCK gene expression in response to WL15 treatment. When compared to the control group, alloxan increased PEPCK mRNA expression (2.2 fold) (E-Suppl. Fig. 7B). However, 50 µM of WL15 cotreatment inhibited PEPCK mRNA expression (1.6 fold), respectively. This is comparable to the inhibitory efficacy of 10 µM of glimepiride on PEPCK expression (1.4 fold). From the result, we can assume that the inhibitory effect of WL15 on PEPCK expression is due to insulin upregulation. When taken together, WL15 may increase insulin production while decreasing PEPCK expression, resulting in normal glucose metabolism.
Discussion
The teleost C. striatus had a higher amount of protein and essential amino acids, as determined by the proximate composition and amino acid content; when the right enzyme and protein are combined, a high yield of bioactive peptides is obtained [32]. In our previous studies [19, 20, 33, 34, 35], we reported that the proteins namely CSRP2, VPS26B, HATs, STPK, and CxxC in which the derived peptides including WL15, RF13, RW20, IW13, and MF18 possessed to have antioxidant, antidiabetic and antimicrobial properties. In this observation, we initially screened these peptides for their better understanding of antioxidant and antidiabetic nature, especially on evaluating them on the β-cell damaged zebrafish in-vivo larval model. Using PeptideRanker and ToxinPred server, the toxicity and bioavailability of the peptides are investigated. All the five peptides of WL15, RF13, RW20, IW13, and MF18 are predicted to be non-toxic. While the WL15 peptide has better bioavailability compared to other peptides.
The antioxidant and antidiabetic activity of these peptides was predicted with molecular docking studies. The Protein Data Bank was used to obtain the protein structures of insulin receptors (PDB ID: 1IR3) and glutathione (PDB ID: 2I3Y). The peptides WL15, RF13, RW20, IW13, and MF18 are docked with insulin receptors and glutathione using HPEPDOCK webserver. In comparison to other peptides, WL15 had a higher affinity for binding to the insulin receptor and glutathione. Further, the in-vitro hydroxyl radical scavenging assay and α-amylase assay were performed to identify the peptides with better antioxidant and antidiabetic activity. The highly reactive species is the hydroxyl radical, which causes severe damage to nearby biomolecules. The presence of hydrogen donating ability in the compounds has a better ability to scavenge hydroxyl radicals [36]. On the other hand, if the α-amylase enzyme is inhibited, starch is degraded into oligosaccharides and monosaccharides before being absorbed. As a result, glucose absorption is reduced, which lowers the post-prandial blood glucose levels [17]. In both hydroxyl radical scavenging and amylase inhibition assay, WL15 showed better activity compared to other peptides (RF13, RW20, IW13, and MF18). In addition, recent studies have identified antioxidant and antidiabetic properties of WL15 in both in-vitro and in-vivo models. The hydrophobic (leucine and alanine) and aromatic (phenylalanine and histidine) amino acids are present in the WL15 peptide, which has strong antioxidant properties by transferring electrons to free radicals. Leucine and glycine are amino acids that are present in the WL15 peptide and have been shown to slow the progression of diabetes [10, 19]. However, no research has been conducted on the effect of WL15 peptide on normalizing glucose metabolism and reducing pancreatic toxicity, so the protective effect of WL15 peptide was investigated in the alloxan induced β-cell damaged zebrafish larvae model.
After being exposed to 500 µM alloxan for 1 day, the oxidative stress in β-cells of zebrafish larvae was developed. Alloxan also reduces the survival and heartbeat rate of larvae with morphological abnormalities such as bent spine and bent tail. Mortality and heart rate have become sensitive indicators for evaluating the protective effect of WL15 due to these variations. The cotreatment of WL15 improved the heartbeat rate and survival rate of 500 µM alloxan exposed zebrafish larvae, while larvae are protected from abnormalities formation. Since WL15 at 50 µM showed the highest activity range with no toxicity, this optimum concentration was taken for further experiments. Pancreatic cells are susceptible to oxidative stress due to their low antioxidant enzyme level of expression [37]. In diabetic animals, antioxidant defense systems in the β-cells are significantly disrupted in addition to increased free radical production [38]. Alloxan is specifically used for experimental diabetes conditions because it is selectively toxic to pancreatic β-cells and directly affects islet cell permeability by regulating ROS and lipid peroxidation production [39]. The amino acid composition of the WL15 are majorly leucine and glycine. By acting as a source of energy and regulating cell metabolism, leucine and glycine are known to encourage insulin release from pancreatic β-cells. Additionally, leucine in peptides controls the expression of vital metabolic genes in β-cells to prevent islet dysfunction and diabetes [40]. According to the results (Figs. 5, 6), oxidative stress increased in the β-cells of the zebrafish larvae after exposure to alloxan. The WL15 at 50 µM successfully reduced ROS and apoptosis, and the level of lipid peroxidation in the β-cells portion of the larvae was also significantly reduced. High levels of superoxide cause cellular dysfunction and apoptotic cell death, and they significantly increase in the organ during glutathione depletion [41]. Using DHE and NDA staining, it was discovered that alloxan significantly increased superoxide anion and depleted glutathione level in the β-cells region; however, the WL15 treatment group showed a gradual decrease in superoxide anion and normalized the glutathione level. It indicates that the WL15 peptide inhibits ROS formation in the pancreatic β-cells.
Further, their effect on protecting the β-cells from damage and dysfunction was investigated in zebrafish larvae. To induce β-cells damages or dysfunction, the larvae are exposed to 500 µM alloxan for 72 h. The 2NBDG treatment on zebrafish larvae allows for complete staining of β-cells [42]. In comparison to the control group, the size and fluorescent intensity of β-cells in the alloxan-exposed group decreased. The size of pancreatic beta cells was reduced due to damage, but damages were significantly (p < 0.05) reduced, and pancreatic regeneration was observed after cotreatment with the WL15 peptide. Insulin is produced and released by β-cells in the pancreas in response to blood glucose levels. β-cells in people with T2D have to work harder to produce enough insulin to keep blood sugar levels under control. This condition may also lead to β-cell dysfunction [1]. WL15 peptide at 50 µM was able to control the high glucose level equally compared to the positive control glimepiride (10 µM) in the larvae. Some patients with T2D may experience hyperglycemia as a result of insulin inability to suppress the expression of PEPCK, a rate-limiting enzyme in gluconeogenesis. PEPCK overexpression has been previously reported to reduce insulin signaling and insulin sensitivity in transgenic mice [43]. In this study, the mRNA expression of insulin levels was downregulated, and PEPCK was upregulated in the alloxan-treated group, but this was reversed by the cotreatment of WL15, suggesting that WL15 may induce insulin secretion, thus reducing gluconeogenesis by blocking the expression of PEPCK.
In conclusion, we showed that WL15 could normalize glucose metabolism by selectively inhibiting PEPCK expression and increasing insulin levels. WL15 was also found to be protective against ROS-induced pancreatic toxicity. Thus, chronic WL15 peptide consumption may aid in treating hyperglycemia and preventing diabetic complications. The use of a larvae model limits this study and more research is needed to determine clinical relevance to humans.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Code availability
Not applicable.
Change history
06 April 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11033-023-08365-w
Abbreviations
- AGEs:
-
Advanced glycation end products
- PEPCK:
-
Phosphoenolpyruvate carboxykinase
- DHE:
-
Dihydroethidium
- NDA:
-
NDA, naphthalene-2,3-dicarboxal-dehyde
- T2D:
-
Type 2 diabetes
- ROS:
-
Reactive oxygen species
- DCFDA:
-
2'-7'-Dichlorofluorescein diacetate
- DPPP:
-
Diphenyl-1-pyrenylphosphine
- SD:
-
Standard deviation
- EDTA:
-
Ethylenediaminetetraacetic acid
- 2-NBDG:
-
2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-D-glucose
- DCFDA:
-
2',7'-Dichlorodihydrofluorescein diacetate
- CSRP2:
-
Cysteine and glycine-rich protein 2
- HATs:
-
Histone acetyltransferase
- VPS26B:
-
Vacuolar protein sorting associated protein 26B
- STPK:
-
Serine threonine-protein kinase
References
Cerf ME (2013) Beta cell dysfunction and insulin resistance. Front Endocrinol (Lausanne) 4:1–12. https://doi.org/10.3389/fendo.2013.00037
Saisho Y (2015) β-cell dysfunction: its critical role in prevention and management of type 2 diabetes. World J Diabetes 6:109. https://doi.org/10.4239/wjd.v6.i1.109
Gurgul-Convey E, Mehmeti I, Plötz T et al (2016) Sensitivity profile of the human EndoC-βH1 beta cell line to proinflammatory cytokines. Diabetologia 59:2125–2133. https://doi.org/10.1007/s00125-016-4060-y
Drews G, Krippeit-Drews P, Duïfer M (2010) Oxidative stress and beta-cell dysfunction. Pflugers Arch Eur J Physiol 460:703–718. https://doi.org/10.1007/s00424-010-0862-9
Eguchi N, Vaziri ND, Dafoe DC, Ichii H (2021) The role of oxidative stress in pancreatic β cell dysfunction in diabetes. Int J Mol Sci 22:1–18. https://doi.org/10.3390/ijms22041509
Stancill JS, Broniowska KA, Oleson BJ et al (2019) Pancreatic -cells detoxify H2O2 through the peroxiredoxin/thioredoxin antioxidant system. J Biol Chem 294:4843–4853. https://doi.org/10.1074/jbc.RA118.006219
Matschinsky FM (1996) A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes 45:223–241. https://doi.org/10.2337/diab.45.2.223
Moss LG, Caplan TV, Moss JB (2013) Imaging beta cell regeneration and interactions with islet vasculature in transparent adult zebrafish. Zebrafish 10:249–257. https://doi.org/10.1089/zeb.2012.0813
Zang L, Maddison LA, Chen W (2018) Zebrafish as a model for obesity and diabetes. Front Cell Dev Biol 6:1–13. https://doi.org/10.3389/fcell.2018.00091
Guru A, Issac PK, Saraswathi NT et al (2021) Deteriorating insulin resistance due to WL15 peptide from cysteine and glycine-rich protein 2 in high glucose-induced rat skeletal muscle L6 cells. Cell Biol Int 45:1698–1709. https://doi.org/10.1002/cbin.11608
Mohd Shafri MA, Abdul Manan MJ (2012) Therapeutic potential of the haruan (Channa striatus): from food to medicinal uses. Malays J Nutr 18:125–136
Wang J, Wang H (2017) Oxidative stress in pancreatic beta cell regeneration. Oxid Med Cell Longev. https://doi.org/10.1155/2017/1930261
Yu Z, Wu S, Zhao W et al (2018) Identification and the molecular mechanism of a novel myosin-derived ACE inhibitory peptide. Food Funct 9:364–370. https://doi.org/10.1039/c7fo01558e
Pearman NA, Ronander E, Smith AM, Morris GA (2020) The identification and characterisation of novel bioactive peptides derived from porcine liver. Curr Res Food Sci 3:314–321. https://doi.org/10.1016/j.crfs.2020.11.002
Cakır B, Okuyan B, Sener G, Tunali-Akbay T (2021) Investigation of beta-lactoglobulin derived bioactive peptides against SARS-CoV-2 (COVID-19): in silico analysis. Eur J Pharmacol 891:173781. https://doi.org/10.1016/j.ejphar.2020.173781
Velayutham M, Guru A, Gatasheh MK et al (2022) Molecular docking of SA11, RF13 and DI14 peptides from vacuolar protein sorting associated protein 26B against cancer proteins and in vitro investigation of its anticancer potency in Hep-2 cells. Int J Pept Res Ther. https://doi.org/10.1007/s10989-022-10395-0
Sangeetha R, Vedasree N (2012) In vitro α -amylase inhibitory activity of the leaves of thespesia populnea. ISRN Pharmacol 2012:1–4. https://doi.org/10.5402/2012/515634
Manjunathan T, Guru A, Arokiaraj J, Gopinath P (2021) 6-gingerol and semisynthetic 6-gingerdione counteract oxidative stress induced by ROS in zebrafish. Chem Biodivers. https://doi.org/10.1002/cbdv.202100650
Guru A, Lite C, Freddy AJ et al (2021) Intracellular ROS scavenging and antioxidant regulation of WL15 from cysteine and glycine-rich protein 2 demonstrated in zebrafish in vivo model. Dev Comp Immunol 114:103863. https://doi.org/10.1016/j.dci.2020.103863
Issac PK, Lite C, Guru A et al (2021) Tryptophan-tagged peptide from serine threonine-protein kinase of Channa striatus improves antioxidant defence in L6 myotubes and attenuates caspase 3–dependent apoptotic response in zebrafish larvae. Fish Physiol Biochem 47:293–311. https://doi.org/10.1007/s10695-020-00912-7
Sudhakaran G, Prathap P, Guru A et al (2022) Anti-inflammatory role demonstrated both in vitro and in vivo models using non-steroidal tetranortriterpenoid, Nimbin (N1) and its analogues (N2 and N3) that alleviate the domestication of alternative medicine. Cell Biol Int 24:327–332. https://doi.org/10.1002/cbin.11769
Velayutham M, Ojha B, Issac PK et al (2021) NV14 from serine O-acetyltransferase of cyanobacteria influences the antioxidant enzymes in vitro cells, gene expression against H2O2 and other responses in vivo zebrafish larval model. Cell Biol Int 45:2331–2346. https://doi.org/10.1002/cbin.11680
Sarkar P, Guru A, Raju SV et al (2021) GP13, an Arthrospira platensis cysteine desulfurase-derived peptide, suppresses oxidative stress and reduces apoptosis in human leucocytes and zebrafish (Danio rerio) embryo via attenuated caspase-3 expression. J King Saud Univ - Sci 33:101665. https://doi.org/10.1016/j.jksus.2021.101665
Sudhakaran G, Prathap P, Guru A et al (2022) Reverse pharmacology of Nimbin-N2 attenuates alcoholic liver injury and promotes the hepatoprotective dual role of improving lipid metabolism and downregulating the levels of inflammatory cytokines in zebrafish larval model. Mol Cell Biochem. https://doi.org/10.1007/s11010-022-04448-7
Haridevamuthu B, Manjunathan T, Guru A (2022) Amelioration of acrylamide induced neurotoxicity by benzo [b ] thiophene analogs via glutathione redox dynamics in zebrafish larvae. Brain Res 1788:147941. https://doi.org/10.1016/j.brainres.2022.147941
Lite C, Guru A, Juliet MJ, Arockiaraj J (2022) Embryonic exposure to butylparaben and propylparaben induced developmental toxicity and triggered anxiety-like neurobehavioral response associated with oxidative stress and apoptosis in the head of zebrafish larvae. Environ Toxicol. https://doi.org/10.1002/tox.23545
Li Y, Li X, Chu Q et al (2020) Russula alutacea Fr. polysaccharide ameliorates inflammation in both RAW264.7 and zebrafish (Danio rerio) larvae. Int J Biol Macromol 145:740–749. https://doi.org/10.1016/j.ijbiomac.2019.12.218
Lee J, Jung DW, Kim WH et al (2013) Development of a highly visual, simple, and rapid test for the discovery of novel insulin mimetics in living vertebrates. ACS Chem Biol 8:1803–1814. https://doi.org/10.1021/cb4000162
Velayutham M, Guru A, Arasu MV et al (2021) GR15 peptide of S-adenosylmethionine synthase (SAMe) from Arthrospira platensis demonstrated antioxidant mechanism against H2O2 induced oxidative stress in in-vitro MDCK cells and in vivo zebrafish larvae model. J Biotechnol 342:79–91. https://doi.org/10.1016/j.jbiotec.2021.10.010
Guru A, Sudhakaran G, Velayutham M et al (2022) Daidzein normalized gentamicin-induced nephrotoxicity and associated pro-inflammatory cytokines in MDCK and zebrafish: possible mechanism of nephroprotection. Comp Biochem Physiol Part C Toxicol Pharmacol 258:109364. https://doi.org/10.1016/j.cbpc.2022.109364
Haridevamuthu B, Manjunathan T, Guru A, Saravana R (2022) Hydroxyl containing benzo[b]thiophene analogs mitigates the acrylamide induced oxidative stress in the zebrafish larvae by stabilizing the glutathione redox cycle. Life Sci. https://doi.org/10.1016/j.lfs.2022.120507
Ghassem M, Arihara K, Babji AS et al (2011) Purification and identification of ACE inhibitory peptides from Haruan (Channa striatus) myofibrillar protein hydrolysate using HPLC-ESI-TOF MS/MS. Food Chem 129:1770–1777. https://doi.org/10.1016/j.foodchem.2011.06.051
Guru A, Velayutham M, Arockiaraj J (2022) Lipid-lowering and antioxidant activity of RF13 peptide from vacuolar protein sorting-associated protein 26B (VPS26B) by modulating lipid metabolism and oxidative stress in HFD induced obesity in zebrafish larvae. Int J Pept Res Ther 28:74. https://doi.org/10.1007/s10989-022-10376-3
Prabha N, Guru A, Harikrishnan R et al (2022) Neuroprotective and antioxidant capability of RW20 peptide from histone acetyltransferases caused by oxidative stress-induced neurotoxicity in in vivo zebrafish larval model. J King Saud Univ - Sci 100:101861. https://doi.org/10.1016/j.jksus.2022.101861
Nagaram P, Pasupuleti M, Arockiaraj J (2020) CxxC zinc finger protein derived peptide, MF18 functions against biofilm formation. Protein J 39:337–349. https://doi.org/10.1007/s10930-020-09904-1
Pavithra K, Vadivukkarasi S (2015) Evaluation of free radical scavenging activity of various extracts of leaves from Kedrostis foetidissima (Jacq.) Cogn. Food Sci Hum Wellness 4:42–46. https://doi.org/10.1016/j.fshw.2015.02.001
Lenzen S, Drinkgern J, Tiedge M (1996) Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 20:463–466. https://doi.org/10.1016/0891-5849(96)02051-5
Evans JL, Goldfine ID, Maddux BA, Grodsky GM (2002) Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev 23:599–622. https://doi.org/10.1210/er.2001-0039
Ramkumar KM, Lee AS, Krishnamurthi K et al (2009) Gymnema montanum H. protects against alloxan-induced oxidative stress and apoptosis in pancreatic β-cells. Cell Physiol Biochem 24:429–440. https://doi.org/10.1159/000257480
Vahdatpour T, Nokhodchi A, Zakeri-Milani P et al (2019) Leucine–glycine and carnosine dipeptides prevent diabetes induced by multiple low-doses of streptozotocin in an experimental model of adult mice. J Diabetes Investig 10:1177–1188. https://doi.org/10.1111/jdi.13018
Moriya S, Yokoyama H, Fukuda M et al (2000) Glutathione depletion enhances the formation of superoxide anion released into hepatic sinusoids after lipopolysaccharide challenge. Alcohol Clin Exp Res 24:59–63. https://doi.org/10.1111/j.1530-0277.2000.tb00014.x
Nam YH, Hong BN, Rodriguez I et al (2015) Synergistic potentials of coffee on injured pancreatic islets and insulin action via KATP channel blocking in zebrafish. J Agric Food Chem 63:5612–5621. https://doi.org/10.1021/acs.jafc.5b00027
Kwon SJ, Hwang SJ, Jung Y et al (2017) A synthetic Nitraria alkaloid, isonitramine protects pancreatic β-cell and attenuates post-prandial hyperglycemia. Metabolism 70:107–115. https://doi.org/10.1016/j.metabol.2017.02.002
Acknowledgements
The authors extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2021/191), King Saud University, Riyadh, Saudi Arabia.
Funding
Researchers Supporting Project Number (RSP-2021/191), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
AG and JA contributed to the concept and design of the study; AG and GS performed the experiments; MHA. BOA. AJ and JA contributed significantly to resources, data analysis, manuscript preparation and perform the analysis with constructive discussions; JA supervised and checked the manuscript; All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This research does not involve any human objects; however, we have performed few assays using zebrafish embryo and larvae. The fish were handled and experimented carefully as per the Institute Animal Handling Procedure and Ethical Approval and Clearence (No. SAF/IAEC/211215/004).
Consent to participate
All the authors listed in the manuscript have approved the manuscript.
Consent for publication
The data provided in the manuscript is approved by all authors for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The affiliation of the author "Annie Juliet" is corrected as "Foundation for Aquaculture Innovations and Technology Transfer (FAITT), Thoraipakkam, Chennai 600 097, Tamil Nadu, India.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guru, A., Sudhakaran, G., Almutairi, M.H. et al. β-cells regeneration by WL15 of cysteine and glycine-rich protein 2 which reduces alloxan induced β-cell dysfunction and oxidative stress through phosphoenolpyruvate carboxykinase and insulin pathway in zebrafish in-vivo larval model. Mol Biol Rep 49, 11867–11879 (2022). https://doi.org/10.1007/s11033-022-07882-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07882-4