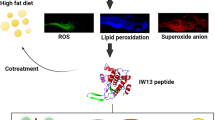
Abstract
This study reports the antioxidant property and molecular mechanism of a tryptophan-tagged peptide derived from a teleost fish Channa striatus of serine threonine-protein kinase (STPK). The peptide was tagged with tryptophan to enhance the antioxidant property of STPK and named as IW13. The antioxidant activity of IW13 peptide was investigated using in vitro methods such as DPPH, ABTS, superoxide anion radical scavenging and hydrogen peroxide scavenging assay. Furthermore, to investigate the toxicity and dose response of IW13 peptide on antioxidant defence in vitro, L6 myotubes were induced with generic oxidative stress due to exposure of hydrogen peroxide (H2O2). IW13 peptide exposure was found to be non-cytotoxic to L6 cells in the tested concentration (10, 20, 30, 40 and 50 μM). Also, the pre-treatment of IW13 peptide decreased the lipid peroxidation level and increased glutathione enzyme activity. IW13 peptide treatment upregulated the antioxidant enzyme genes: GPx (glutathione peroxidase), GST (glutathione S transferase) and GCS (glutamine cysteine synthase), in vitro in L6 myotubes and in vivo in zebrafish larvae against the H2O2-induced oxidative stress. The results demonstrated that IW13 renders protection against the H2O2-induced oxidative stress through a cellular antioxidant defence mechanism by upregulating the gene expression, thus enhancing the antioxidant activity in the cellular or organismal level. The findings exhibited that the tryptophan-tagged IW13 peptide from STPK of C. striatus could be a promising candidate for the treatment of oxidative stress–associated diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protein kinases are a family of enzymes that catalyze the phosphorylation of tyrosine, threonine and serine of target proteins, altering their activity and biological function. Among the protein kinases, serine threonine-protein kinase (STPK) has been involved in regulating various and diverse cellular functions such as differentiation, proliferation, tumorigenesis and antioxidant defence (Gopalakrishna and Jaken 2000). Even though STPK regulates the process of cellular antioxidant defence, STPK itself is a molecule that is susceptible to oxidative stress–induced modifications in their redox-sensitive regions, alteration of such regions hinder their activity and biological effects (Zarubin and Han 2005).
Tryptophan is a vital amino acid with an aromatic side chain and an indole ring. It has an important role, serving as a precursor for serotonin and melatonin biosynthesis and also comprises as one of the building blocks of protein. Besides this essential role as a biomolecule, the metabolites of tryptophan, such as indole and tryptamine, play a vital role in various biological processes within the body (Gostner et al. 2020). Indole compounds are potent hydroxyl radical scavengers, facilitated through the aromatic side chain present in them, which react freely with the uncoupled electrons (Lü et al. 2010). Two catabolic products known as kynurenine and N-formylkynurenine are formed from the tryptophan by the cleavage of its indole ring by a hydroxyl radical or single-electron attack. Further metabolic events yield two strong antioxidant molecules, 3-hydroxy kynurenine, 3-hydroxy anthranilic acid, niacin and serotonin from kynurenine and N-formyl-kynurenin (Perez-Gonzalez et al. 2014; Nayak et al. 2019; Gostner et al. 2020). Considering its antioxidant capabilities, tryptophan-fortified supplements are recommended in infant foods (Friedman 2018). Adequate intake of essential amino acid tryptophan has been vital for child growth and development (Nayak et al. 2019). When considering the physiological role of tryptophan, especially in fish, it accounts for an array of functional roles such as nutrition source and regulation of stress and immune response, and reported for maintaining antioxidant balance in the system (Moosmann and Behl 2000; Hoseini et al. 2019). In recent times, it has been reported that fishes use the regulation of oxidative defence mechanism in responding and coping to the environmental stresses such as low oxygen availability, temperature, salinity and drought (Birnie-Gauvin et al. 2017). Therefore, fish genome has been evolutionarily adapted to harness the effect of oxidative stress through key signaling molecules and genes involved in oxidant and antioxidant homeostasis to prevent oxidative stress–induced damage. Fish has been considered a promising source for bioactive compounds in recent years (Najafian and Babji 2012; Siauciunaite et al. 2019).
A practical approach towards bioactive peptide development with antioxidant property has important implications in therapeutic agents’ production. To achieve this goal, properties such as molecular weight, isoelectric point, the composition of amino acids, the concentration of hydrogen ions, hydrophobic characteristics and structural factors were taken into consideration for designing a peptide with antioxidant potential (Arockiaraj et al. 2012, 2013; Bhatt et al. 2014; Kumaresan et al. 2017; Tao et al. 2018).
In our previous study, we have developed a cationic peptide IE13 from serine threonine-protein kinase (STPK) of the teleost fish Channa striatus, which was further modified by replacing tryptophan in glutamic acid present in the C-terminal region at the thirteenth position as IW13 peptide. The IW13 peptide was tagged with tryptophan to enhance the antioxidant property of STPK. From our previous study (Prabha et al. 2020), the in vitro treatment of cationic peptide IW13 in human cervical carcinoma cells (HeLa), human lung cancer cells (A549) and breast cancer cells (MCF-7) found to exhibit anti-cancerous activity by bringing about cycle inhibition. When considering the known role of tryptophan for its antioxidant property and the involvement of serine/threonine in the process of antioxidant response (Mata-Cabana et al. 2012), therefore it has been hypothesized that the IW13 peptide may also exhibit antioxidant property. Hence, in this study, we have evaluated the antioxidant property, mechanism and toxicity of the tryptophan-tagged IW13 peptide by performing various antioxidant assays due to the pre-treatment of the peptide in vitro using L6 myotubes and in vivo with zebrafish larval model, after inducing generic oxidative stress due to H2O2 exposure. Furthermore, we have also studied the expression of antioxidant enzymes genes such as GPx (glutathione peroxidase), GST (glutathione S transferase) and GCS (glutamine cysteine synthase), and protein expression of active caspase 3 in zebrafish larvae to elucidate the molecular mechanism of IW13 peptide for findings its antioxidant ability.
Materials and method
Chemicals and reagents
L6 myoblast was procured from the National Centre for Cell Science (NCCS, Pune, India) (Passage number: 16). Dulbecco’s modified eagle’s medium (DMEM) (containing 4.5 g glucose, 4.0 mM L-Glutamine, 1 mM sodium pyruvate and 1.5 g/L of sodium bicarbonate) and antibiotic and antimycotic solutions were procured from Himedia, Mumbai, India. Fetal bovine serum (FBS) was procured from GIBCO Life Technologies, USA. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS 2, (2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid) were purchased from Sigma-Aldrich. DCFDA (2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)) fluorescent dye obtained from Merk-Millipore. Light Cycler 480 SYBR Green I RT-PCR Master mix was obtained from Roche. Caspase 3 antibody was purchased from R&D Systems, Minnesota, USA, and secondary antibody (anti-Rabbit) Dylight 488 was purchased from the Rockland antibodies & assays, Pennsylvania, USA. All other chemicals and reagents were of cell culture and molecular grade.
Determination of the in vitro antioxidant activity of IW13 peptide
The following in vitro experiments were performed to determine the antioxidant efficacy of the IW13 peptide: DPPH, ABTS, superoxide anion radical scavenging and hydroxyl radical scavenging activity.
DPPH radical scavenging activity assay
DPPH assay was performed as previously defined by Sarkar et al. (2020) with minor modifications. IW13 peptide was used in the concentrations ranging from 10, 20, 30, 40 and 50 μM, or Trolox has been combined with 0.5 mL of DPPH (25 μg/mL). Later, this mixture was incubated for about 30 min in a dark place; ethanol was used as a positive control. Absorption was measured at 517 nm using a UV-Vis spectrophotometer (UV1800, SHIMADZU, Kyoto, Japan). All experiments were conducted in triplicates. The percentage of DPPH radical scavenging activity was calculated using the following equation:
ABTS assay
ABTS assay was performed according to the protocol mentioned by Guru et al. (2021), with minor modifications. The peptide’s antioxidant activity was compared to Trolox, which served as a positive control in this experiment. The ABTS assay was carried out by dissolving the ABTS (7 mM) in freshly prepared potassium persulphate (2.45 mM) solution. Furthermore, the reaction mixture was diluted using 0.2 M PBS (pH 7.4) at 30 °C to an absorbance of 0.70 ± 0.02 at 734 nm. IW13 peptide was used in the concentrations ranging from 10, 20, 30, 40 and 50 μM, or Trolox was added to the above-mentioned reaction mixture and maintained at 30 °C for 60 min. A decrease in absorption was monitored using a UV-Vis spectrophotometer (UV1800, SHIMADZU, Kyoto, Japan) at a wavelength of 734 nm. All experiments were conducted in triplicates. Trolox’s antioxidant equivalent was calculated from the standard Trolox curve and expressed as Trolox equivalents (in mM).
Superoxide radical scavenging assay
The non-enzyme phenazine methosulphate nicotinamide adenine dinucleotide (PMS/NADH) reduces superoxide radicals to nitro blue tetrazolium (NBT) to purple colour formazan; this principle is exploited for determining the superoxide radical scavenging activity of IW13 peptide, and the assay was performed using a standard protocol (Sannasimuthu et al. 2018). Phosphate buffer (20 mM, pH 7.4), NBT (50 μM), NADH (73 μM) and PMS (15 μM), along with IW13 peptide (10, 20, 30, 40 and 50 μM), were mixed to a volume of 200 μL. The absorption of the chemical reaction was measured (UV-Vis spectrophotometer, UV1800, SHIMADZU, Kioto, Japan) against a blank, following 5-min incubation at room temperature to determine the amount of formazan formation by measuring the absorption at 562 nm. Superoxide anion radical scavenging activity has been calculated using the following equation and expressed in percentage.
Hydrogen peroxide scavenging assay
The H2O2 scavenging activity was estimated according to the protocol mentioned by Alam et al. (2013). In brief, 40 mM H2O2 solution was prepared in a 50 mM PBS with pH 7.4. Different concentrations (10 to 50 μM) of IW13 peptide were prepared using PBS, added to H2O2. The absorbance of the resulting chemical reaction was measured at 230 nm (UV-Vis spectrophotometer, UV1800, SHIMADZU, Kyoto, Japan). The absorbance was measured after 10 min against a blank solution without H2O2-containing PBS. Trolox was used as a positive control. The equation used for deriving the percentage of H2O2 scavenging activity is as follows:
In vitro study using L6 myotubes
Development of in vitro insulin-resistant model
L6 myoblast was maintained in DMEM with high glucose (25 mM/L) and 10% FBS and supplemented with the antibiotic-antimycotic solution with 10,000 U penicillin, 10 mg streptomycin and 25 μg amphotericin B per mL in 0.9% normal saline. Cells were maintained in a 5% CO2 environment at 37 °C (Anandharajan et al. 2006). In vitro insulin-resistant model was established by inducing the differentiation of L6 myoblast to myotubes by the method mentioned by Tang et al. (2017). For differentiation, the L6 cells were transferred to DMEM medium with 2% FBS for 4 days. The differentiation was confirmed by the extent of the multinucleation of cells. After differentiation, the cells were incubated for 24 h with DMEM containing high glucose (25 mM/L) (Sujatha et al. 2010; Kamaraj et al. 2017; Issac et al. 2020).
MTT-cytotoxicity assay
L6 cells’ viability was assessed on treatment with IW13 peptide using MTT assay (Issac et al. 2020). Approximately 3.6 × 105 myoblast cells/well were seeded in a 96-well plate and differentiate into myotubes. The subconfluent cells were treated with IW13 peptide with varying concentrations ranging from 10 μM, 20 μM, 30 μM, 40 μM and 50 μM or 0.01% Triton X-100 as a positive control and incubated for 24 h. After the incubation period, to each well, 5 mg/mL concentration of MTT solution was added and further incubated for 4 h. The formazan crystals which are formed during the reaction were dissolved in 100 μL of 0.01% dimethyl sulphoxide (DMSO), and the resulting absorbance was measured at 570 nm using an ELISA plate reader (Multiskan Go ELISA reader, Thermo Scientific, Finland). Percentage of cell viability was calculated using the following formula.
Estimation of SOD activity
SOD activity was estimated using the epinephrine method Hara and Fridovich (1972) with slight modifications. In a six-well plate, approximately 3 × 105 L6 myoblast cells were seeded and induced to differentiate into myotubes, following which IW13 peptide was treated (10 to 50 μM) and incubated for 24 h. After the incubation period, the treated cells were detached with 1 mL of lysis buffer, comprising HEPES 50 mM, NaCl 150 mM, EDTA 10 mM, Na4P2O7 10 mM, sodium orthovanate 1 mM, NaF 50 mM, aprotinin 10 μg/ml, leupeptin 10 μg/ml and Triton X-100 (1%). Cell lysates were homogenized using a Dounce homogenizer with 20 strokes, 0.5 cycles, 10 pulses, 2 min each and lag time of 1 min for each pulse. Later the lysate was centrifuged at 12000 rpm for 20 min at 4 °C (Kanaujia et al. 2010). Stock solutions containing 0.1 M carbonate buffer, 1.3 mM epinephrine and 0.6 mM EDTA were prepared. Cell lysates were diluted using 0.1 M carbonate buffer, and the prepared EDTA and epinephrine were added in equal proportion. This mixture was thoroughly mixed, and absorbance was read at 480 nm.
Estimation of CAT activity in L6 cell lysate
CAT activity was evaluated by the H2O2 decomposition method with a slight modification (Goth 1991). Around 3 × 105 L6 myoblast cells were seeded and maintained in culture conditions and induced to differentiate into myotubes. Furthermore, the cells were treated with IW13 peptide (10 to 50 μM) and incubated for 24 h. A stock solution of 65 μM H2O2 was prepared in 60 mM phosphate buffer (pH 7.4) containing 32.4 mM ammonium molybdate. The cell lysate was prepared as described earlier elsewhere. To the cell lysate, H2O2 was added and incubated at 37 °C for 60 s, then followed by the addition of ammonium molybdate to stop the enzymatic reaction with less time delay. The yellow complex formed by the reaction of ammonium molybdate and H2O2 is measured at 405 nm against the blank reagent using spectrophotometric measurement.
DCFDA intracellular fluorescent assay
The assay was done as described previously (Bernini et al. 2018; Sannasimuthu et al. 2019). Briefly, 3.6 × 105 myoblast (cells/well) were seeded in a 96-well plate and induced to differentiate into myotubes. The myotubes were incubated with 10 μM DCFDA and different concentrations (10 to 50 μM) of IW13 peptide for 30 min. The reaction mixture containing 20 μL of 30% H2O2 was used as the positive control for inducing generic oxidative stress. After a 1-h incubation with the DCFDA solution, the absorbance of the resulting chemical reaction was measured using a multimode microplate reader (Thermo Scientific) with excitation λ = 498 nm and emission λ = 530 nm and also the images were captured using a fluorescence microscope (Leica, Germany). Fluorescence signal from the DCFDA-stained L6 myotubes was quantified using Image J (V.1.49, NIH, USA) software.
In vivo study using zebrafish larvae model
Maintenance of the zebrafish and egg collection
Adult zebrafishes were purchased from a commercial dealer NSK aquarium, Kolathur, Tamilnadu, India. Fishes were kept under the following condition in a 3-L glass tank: 28.5 °C, with a 14/10-h light/dark cycle as described (Luzeena Raja et al. 2019). The fishes have been fed with live brine shrimp (Artemia salina) three times a day. After the acclimatization period (1 month) in lab conditions, the fishes were kept for breeding. For gaining embryos, four breeding groups were placed separately in a specific spawning tank with a male to female ratio of 2:1, equipped with a mesh at the bottom to prevent the eggs from being consumed by the adult fish. Spawning was induced at the onset of the light cycle. After 30 min, eggs were collected, rinsed with embryo medium and incubated in 12-well plates at 26 ± 1 °C until chemical treatment (Dambal et al. 2017); embryos were collected on a subsequent day after spawning, triggered by turning on the light. The collected embryos were observed under the microscope for differentiating the fertilized and unfertilized embryos; the embryos, which are fertilized and showing normal morphology, were utilized for the further experiments.
Embryo-toxicity assay
The embryos were collected from the breeding tank within 2 h of spawning, then incubated in 6-well plates (30 embryos/well) either with IW13 peptide dissolved in embryo medium or the embryo medium alone as the control. The following IW13 peptide exposure groups contained the following concentrations: 10 μM, 20 μM, 30 μM, 40 μM and 50 μM were maintained in a semi-static condition in which the fresh peptide solutions were replaced once in every 24 h until 96 hpf. The parameters such as mortality, heart rate and developmental malformation were recorded (Dambal et al. 2017). Embryos without any treatment were maintained as control, and H2O2 (1 mM) treatment served as a positive control for the induction of oxidative stress. All the experiments were performed in triplicate.
SOD and CAT enzyme assays
At the end of the exposure studies (96 hpf), zebrafish larvae (n = 30) were collected and homogenized in a 100 mM Tris buffer (pH 7.8 at 4 °C) containing 150 mM KCL and 1 mM EDTA. The homogenate was centrifuged at 10000 rpm, 15 min at 4 °C. The resulting supernatant was used for total protein estimation, CAT, SOD and TBA assay. All the experiments were carried out in triplicates.
The protein content was measured against bovine serum albumin (BSA) as standard using the Bradford method (Bradford 1976). The absorbance was recorded at 595 nm. The SOD activity was measured by performing the standard assay mentioned by Han et al. (2016) with slight modification. The quantity of enzyme consumed to inhibit 50% of the nitroblue tetrazolium chloride photoreduction rate was considered equivalent to one unit of SOD activity (U). The lysate supernatant (50 μL) was mixed with 3 mL reaction buffer (containing 100 μM ethylenediaminetetraacetic acid, 50 mM pH 7.8 phosphate buffer, 750 μM nitroblue tetrazolium chloride, 130 mM methionine and 20 μM Riboflavin). The mixture was shaken and illuminated for 20 min with a 4000 lx fluorescent lamp. Immediately after illumination, the absorbance of the chemical mixture was measured at 560 nm. The activity was expressed as U/g protein.
CAT assay was performed according to the method mentioned by Techer et al. (2015) with 10 mM H2O2 as a substrate. The reduction in the absorption at 240 nm following H2O2 consumption was monitored and recorded for 2 min with a 15-s interval. One unit of CAT activity was defined micromoles of H2O2 decomposed per min at pH 7.0 and 25 °C.
Thiobarbituric acid reactive substances assay
Malondialdehyde (MDA) is a by-product of lipid peroxidation (LPO) reaction that results during oxidative stress. MDA content was measured using the standard TBA assay (Han et al. 2016). MDA is used as the final product of LPO as an indicator of the LPO level. Reaction mixture containing supernatant (50 μL) with, 0.37% SDS, 6.8% acetic acid (pH 3.5) and 1% TBA was incubated for 1 h in a boiling water bath at 80–90 °C. Furthermore, the mixture has been centrifuged for 15 min at 3000 rpm. The mixture was cooled at room temperature, and the absorbance was read at 532 nm spectrophotometrically. The MDA content is expressed as nM/mg protein.
Estimation of ROS levels in zebrafish larvae
The generation of ROS levels in zebrafish larvae was analyzed using the method described by Kang et al. (2013) with minor modifications. Intracellular ROS generation in zebrafish larvae was detected using an oxidation-sensitive fluorescent probe DCFDA. The embryos were treated with the following concentrations: 10 μM, 20 μM, 30 μM, 40 μM and 50 μM of IW13 peptide from 2 to 96 hpf; at the end of the exposure (96 hpf), the larvae were incubated with 1 mM of H2O2. After treatment with H2O2 for 1 h, the embryo medium was changed. The larvae were then treated with DCFDA solution (20 μg/mL) and incubated at 28.5 °C in the dark for 1 h. After the incubation, the embryos were rinsed with fresh embryonic medium and anesthetized before visualization. The representative larval fluorescent intensity from different exposure groups was captured using a fluorescence microscope equipped with a Cool SNAP-Pro colour digital camera (Olympus, Tokyo, Japan). Whole-body fluorescence was quantified using Image J (V.1.49, NIH, USA) software.
Whole-mount immunofluorescence
After the exposure study, 96 hpf zebrafish larvae were transferred to 1.5-mL tube and rinsed twice times with 1 mL of 1 x PBST (1x PBS, containing Tween-20). Paraformaldehyde (4%) was used for fixing the specimen by overnight incubation at 4 °C. Methanol was used for permeabilizing larvae. After permeabilization, 1 mL of PDT made up of 1 x PDST, 0.3% Triton X and 1% DMSO was added. After half an hour, PDT was discarded, and 500 μL blocking buffer was added. After the blocking procedure, 1 μL of rabbit anti-activated caspase 3 antibody (R&D Systems, Minnesota, USA) was used for incubation, then followed by the secondary antibody (anti-Rabbit, Dylight 488, Rockland antibodies & assays, Pennsylvania, USA) incubation step, which lasted for 8 h. Finally, the processed larvae were washed in 1 x PDT, and images were captured using a fluorescence microscope (Sorrells et al. 2013).
Real-time PCR
To study the influence of IW13 peptide on the antioxidant system, the expression of antioxidant enzyme genes were studied (Zhao et al. 2016). After the end of the exposure studies, the 96 hpf zebrafish larvae (n = 40) were homogenized, and total RNA isolation was performed (Lite et al. 2019; Raja et al. 2020). After total RNA isolation, cDNA synthesis was carried out, and quantitative real-time PCR was performed using the specific primers (Table 1) for the genes: GCS, GPx and GST; β-actin is considered the internal housekeeping gene (Lite et al. 2019). The fold expression was calculated using the 2–ΔΔCt method; the data was presented as the fold change normalized to the housekeeping gene (Livak and Schmittgen 2001).
Statistical analysis
All data were presented as mean ± standard deviation (SD) of three independent experiments. One-way ANOVA performed for the data set, followed by a Tukey multiple range test using GraphPad Prism 5.0; the statistical significance level was set at p < 0.05 and p < 0.001.
Results
Antioxidant activity of IW13 peptide in vitro assays
DPPH assay
To evaluate the antioxidant capacity of IW13 peptide, the DPPH assay was performed. The peptide IW13 showed enhanced antioxidant activity based on its concentrations used in the experiment. The IW13 peptide showed significant (p < 0.05) radical scavenging activity at 10 μM concentration (12.67%); in contrast, the higher concentration (50 μM) exhibited maximum radical scavenging activity (54.33%) when compared with the standard antioxidant Trolox (64.33%) (E-Suppl. Fig. 1A).
Effect of IW13 peptide on a SOD and b CAT activity in L6 myotubes. L6 myoblast were used as untreated control and 100 nM insulin was served as a positive control. The peptide treatment for 24 h in L6 myotubes showed dose-dependent increase in enzyme activity. Data were expressed as mean ± SD (n = 3). The asterisk denotes p < 0.05 as compared to the positive control
ABTS radical cation decolourization assay
This method utilizes a diode spectrophotometer to estimate the colour loss when an antioxidant is added with blue-green chromophore ABTS + (2,2-azino-bis (3-ethylbenzene-thiazolin-6-sulfonic acid). ABTS+ is decolourized and reduced into ABTS by antioxidants. Results showed that (E-Suppl. Fig. 1B) treatment with IW13 peptide at low concentration (10 μM) exhibited antioxidant activity (25.3%) in the ABTS assay. An increase in IW13 peptide concentration significantly (p < 0.05) enhanced the ABTS radical scavenging activity. The IW13 peptide was found to have an activity of 64.0% at 50 μM, while the positive control Trolox at 50 μM concentration showed activity of 74.6%.
SOD activity
The radical superoxide anion is a free radical which is toxic to different cellular reactions by acting as a potent oxidizing agent. Higher concentrations of peptide treatment significantly (p < 0.05) enhanced the superoxide radical scavenging activity of the IW13 peptide. The positive control (ascorbic acid) group exhibited a superoxide radical scavenging activity of 23.3% at 10 μM, whereas the IW13 peptide at 10 μM was recorded with an activity of 15.3%. In the higher IW13 peptide concentration of 50 μM, the activity was 54.0%, and the same concentration (50 μM) of ascorbic acid measured the activity of 64.3% (E-Suppl. Fig. 1C).
Hydrogen peroxide scavenging activity
To evaluate the effects of IW13 on hydroxyl trapping potential, a hydroxyl radical scavenging test was performed. The positive control Trolox at 50 μM concentration showed hydrogen peroxide scavenging activity of 77.3%, whereas the H2O2 scavenging activity of IW13 peptide was observed to be 61.0% in 50 μM treatment concentration (E-Suppl. Fig. 1D).
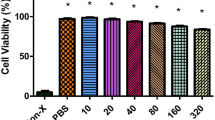
Cell viability
The cell viability was studied by performing an MTT assay in rat skeletal muscle cell line (L6 cells). Cell viability was determined after the 24-h treatment with IW13 peptide with various concentrations ranging from 10, 20, 30, 40 and 50 μM. Untreated cells were used as the control, whereas the cells treated with Triton X-100 (0.01%) were considered a positive control for assessing the cytotoxicity, which showed maximum cell lysis (17.5%). Moreover, IW13 peptide treatment (10 to 50 μM) in L6 cells observed with more than 80% cell viability, thus highlighting that IW13 peptide had low cytotoxicity effects in the treated concentrations (10 to 50 μM), since the viability percentages of cells were found to be similar to the control group (E-Suppl. Fig. 2).
SOD activity in L6 cells
SOD activity was observed to be significantly (p < 0.05) increased (0.43 units/10 μL ROS generation) in the 50 μM IW13 peptide treatment group, whereas insulin (100 nM), which was used as a positive control for this experiment, exhibited SOD activity of 0.55 units/10 μL (Fig. 1a). This data indicates the activation of the SOD by the IW13 peptide against the ROS generation.
CAT activity in L6 cells
CAT activity was observed to be significantly (p < 0.05) increased (0.40 units/10 μL ROS generation) in the 50 μM IW13 peptide treatment group, while the untreated control cells (L6 myoblast) showed an activity of 0.3 units/10 μL. Positive control (100 nM Insulin) exhibited higher CAT activity (0.49 units/10 μL) (Fig. 1b).
ROS levels in L6 myotube
The fluorescent intensity studied using the DCFDA stain directly proportional to the intracellular ROS levels. In the 10 μM IW13 peptide treatment, the concentration was observed to have a fluorescent intensity of 97.5%, whereas the 50 μM IW13 peptide exposure group measured a drop in their fluorescent intensity (48%) (Fig. 2). Since the fluorescence intensity is directly proportional to the intracellular ROS levels, it has been demonstrated that the treatment of IW13 peptide attenuated the intracellular ROS production induced by H2O2 exposure.
Measurement of intracellular ROS by DCFDA staining in L6 myotube. a Control, b H2O2 induced cells, c H2O2 + 10 μM IW13, d H2O2 + 20 μM IW13, e H2O2 + 30 μM IW13, f H2O2 + 40 μM IW13, g H2O2 + 50 μM IW13, h Percentage fluorescent intensity of experimental groups. Data represents the means ± SD of triplicates. *p < 0.05 as compared to control
In vivo exposure studies with zebrafish
Mortality and hatching rate
The mortality rate calculated during the exposure of IW13 peptide was from 2 to 96 hpf, and IW13 peptide concentrations were from 10 to 50 μM or 1 mM H2O2 served as a positive control. As shown in E-Suppl. Fig. 3, the mortality rate was over 67.5% for a 1 mM H2O2 treatment group. IW13 peptide treatment groups (30 and 40 μM concentrations) showed 2 and 11% mortality but not statistically significant compared to the control group.
The hatching rate was calculated at 48 hpf. Even the highest IW13 peptide exposure concentration (50 μM) did not alter the hatching rate. In comparison, the hatching rate was significantly (p < 0.05) reduced in H2O2 (1 mM)–treated group (E-Suppl. Fig. 4). This ascertains that the embryonic exposure to IW13 peptide in the experimental concentration found to exhibit no signs of developmental toxicity, which was evident in the developmental parameters such as mortality and hatching rate.
Heart rate
The heart rate of zebrafish embryos was calculated at 72 hpf to assess the cardio-toxicity of the IW13 peptide. Atrial and ventricular contractions were counted and recorded for 1 min in a microscope. The average heart rate is calculated measured per minute and presented in the graph. The results showed that the heart rate was found to be significantly (p < 0.01) reduced in H2O2 (1 mM) treated zebrafish larvae, whereas the IW13 peptide exposure groups (10–50 μM) did not show significant alteration in heart rate when compared to the embryos from the control (untreated) group (E-Suppl. Fig. 5).
Morphological malformations
Zebrafish embryos were treated with IW13 peptide and were found to have standard morphological architecture. However, 40 μM IW13 peptide concentration group larvae displayed bend spine deformity, and 50 μM IW13 peptide exposure group larvae were observed with yolk sac edema (E-Suppl. Fig. 6). In the positive control H2O2 (1 mM) group, larvae were observed with malformations such as the bent tail, yolk sac edema and bend spine. Thus, it is evident in the exposure of IW13 peptide above 40 μM induced, non-lethal deformities. A dose-specific outcome on the morphological parameters was observed in IW13 peptide concentration above 40 μM, showing the signs of sub-lethal effects (Fig. 3).
Representative photomicrographs of morphological malformation observed during the exposure period. Control and IW13 peptide treatment 10 μM, 20 μM and 30 μM did not show any malformation whereas 40 μM peptide concentration showed bend spine (BS) and 50 μM peptide concentration showed yolk sac edema (YE). The H2O2 treatment group observed with malformations such as YE and BS
Antioxidant enzyme activity in the zebrafish model
Results showed substantial increases in total SOD and CAT activity in 96 hpf zebrafish larva exposed to different concentrations of IW13 peptide when compared to control (E-Suppl. Fig. 7A and B). The IW13 peptide at 50 μM concentration shown to enhance (p < 0.05) the SOD (22 U/mg protein) and CAT (17 mmol/mg protein) activity. The SOD (6.6 U/mg protein) and CAT (5.3 mmol/mg protein) activity were significantly (p < 0.05) decreased in the H2O2 (1 mM) treated group.
Levels of lipid peroxidation
The 1 mM H2O2 treatment group showed significantly (p < 0.05) higher lipid peroxidation level (32 mmol/min/mg protein) when compared to the control group, which showed 8.3 mmol/min/mg protein (E-Suppl. Fig. 8). The IW13 peptide pre-treatment groups shown reduced lipid peroxidation. A maximum decrease (8.3 mmol/min/mg protein) in the lipid peroxidation level was observed at 50 μM IW13 peptide concentration compared with the untreated control group.
H2O2-induced ROS level in zebrafish larvae
DCFDA fluorescent staining detection was performed in 96 hpf zebrafish larvae treated with H2O2 to evaluate the effect of IW13 peptide for its in vivo ROS inhibition efficacy. In contrast to the group treated with H2O2 (1 mM), zebrafish larvae pre-treated with IW13 peptide significantly (p < 0.05) decreased the ROS levels, as shown in Fig. 4. ROS levels were increased up to 98% in zebrafish larvae treated with H2O2. However, a dose-dependent decrease in H2O2-induced ROS levels were observed in IW13 peptide pre-treated groups. The ROS level in 10 μM IW13 peptide exposure group was observed to be 83%; in contrast, the ROS level in the 50 μM peptide IW13 peptide exposure group dropped down to 38%. This shows that the ROS production in zebrafish larvae induced by the H2O2 can be effectively normalized by the IW13 peptide treatment. This further confirms the antioxidant activity of IW13 peptide, which showed a concentration-dependent reduction in intracellular ROS levels, even after the induction of generic oxidative stress by H2O2 treatment.
a Quantitative analysis of in vivo ROS generation in whole zebrafish larvae measured by image J. b Representative photomicrographs of 96 hpf zebrafish fish larvae stained with DCFDA. Experiments were performed in triplicate, and the data were expressed as mean ± SD. The asterisk represents the statistical significance at p < 0.05
Effect of IW13 peptide treatment on activated caspase 3 expression
The effect of IW13 peptide treatment on the expression of activated caspase 3 (Casp3 assay), produced from the apoptotic cells of zebrafish larvae, was studied by whole-mount immunofluorescence. The fluorescence intensity was increased in larvae treated with H2O2 (1 mM), signifying a higher population of apoptotic cells expressing activated caspase 3, while in the high peptide exposure (50 μM) group, the fluorescence intensity was reduced, similar to the untreated control group larvae (Fig. 5). The fluorescence intensity was 11.5% in 50 μM peptide exposure group, whereas in the H2O2 treatment group, larvae stained for activated caspase 3 was recorded with 19% fluorescent intensity.
a Quantitative graph of active caspase 3 expressions in 96 hpf zebrafish larvae calculated using Image J. b Representative fluorescence photomicrographs of zebrafish larvae. The untreated zebrafish larvae used as the control. Experiments are performed in triplicate and data are expressed as mean ± SD, *p < 0.05 indicates the fluorescence intensity was significantly higher than the control
Effect of IW13 peptide on the expression of antioxidant genes
The antioxidant enzymes such as GST, GPx and GCS were studied to understand the impact of IW13 peptide on the antioxidant enzyme genes’ transcriptional activity. The gene expression was as studied in 96 hpf zebrafish larvae pre-treated with IW13 peptide/H2O2. The 1 mM H2O2 treatment group showed a considerable downregulation in mRNA expression of genes: GST (0.5 fold), GPX (0.45 fold) and GCS (0.4 fold) respectively compared to the control group. IW13 pre-treated group showed significant (p < 0.05) upregulation in the expression of genes: GTS (1.9 fold), GPX (2.4 fold) and GCS (1.7 fold), respectively when compared to the control (untreated) group, demonstrating the ability of IW13 peptide to impact the expression of antioxidant genes: GST, GPX and GCS, thus rendering an enhanced antioxidant environment inside the cells helping to ameliorate ROS-induced effects even at an organismal level (Fig. 6).
Discussion
Free radicals are comprised of one or more unpaired electrons that are incredibly reactive towards the cellular molecules and result in adverse effects due to the disruption of the cellular process by accumulation of ROS (Mukwevho et al. 2014). Results from the current study demonstrate the antioxidant activity of IW13 peptide, which showed potent free radical scavenging activity in various in vitro as well as in vivo experiments performed in this study. DPPH radical scavenging activity of IW13 peptide at 50 μM showed an activity of 54%, while the positive control Trolox demonstrated a slightly higher percentage of activity (64%) at 50 μM concentration. The free radical scavenging rate gradually increased as the IW13 peptide concentration was increased from 10 to 50 μM concentration, thus highlighting the potential as well as the dose-dependent efficacy of the synthesized peptide. Similar to our results, Nayak and Buttar (2016) reported that L-tryptophan-isolated human milk showed extremely high radical scavenging capacity (7986 ± 468 μm Trolox equivalent (TE)/g). ABTS assay has been used for determining the antioxidant capability of the IW13 peptide. The results from the current study showed a maximum antioxidant activity for the IW13 peptide at 50 μM (64.0%). Similar to our results, in a study in which the synthesized antioxidant peptide: YFCLT (Tyr-Phe-Cys-Leu-Thr), derived from corn gluten hydrolysate, demonstrated with ABTS radical scavenging activity with an EC50 value of 37.63 μM (Wang et al. 2015). Superoxide anion radical is a well-known free radical. Even though not highly reactive, it can produce hydrogen peroxide and hydroxyl radicals by dismutation and other reactions in vivo. Superoxide anion radicals and their derivatives are toxic to cells by disrupting the DNA and cell membranes. Hence, in a healthy cellular environment, superoxide anion radicals need to be scavenged with indigenous antioxidants (Luo et al. 2013). The superoxide radical scavenging activity was studied to be enhanced by IW13 peptide treatment. The maximum superoxide radical scavenging activity was recorded at 50 μM (54%) IW13 peptide concentration. H2O2 is a reactive molecule that can damage the cells by producing hydroxyl radicals inside the cellular environment (Sasikumar 2015). In the current study, the antioxidant activity of Trolox has been estimated to be 77% (50 μM), whereas the IW13 peptide’s activity was estimated to be 61% at 50 μM concentration, thus highlighting its strong antioxidant efficacy of the IW13 peptide. Melatonin is an endogenous hormone derived from tryptophan, which has significantly increased the superoxide anion radical scavenging and H2O2 scavenging activity (Anwar et al. 2015). The high scavenging capacity of tryptophan-tagged IW13 peptide may be due to tryptophan, which consists of the aromatic side chain and the indole ring. Amino acids with aromatic side chains are known to react rapidly with hydroxyl radicals. Tryptophan is also readily converted to kynurenine and N-formylkynurenine through cleavage of indole ring by hydroxyl radical or one-electron oxidation. Thus, it can be conceived that the presence of tryptophan in our peptide could not only be directly involved in rendering antioxidant property but may also act as a source for the production of intermediate molecules such as melatonin, which in turn can act as a potent antioxidant biomolecule.
Extensive research has been conducted in aquaculture to optimize the tryptophan levels in commercial feeds for fishes. Sufficient levels of this amino acid have been reported to be essential for fish growth and reproduction; tryptophan showed to modulate fish behaviour, stress response and immune and antioxidant balance, thus accounting for the fish’s overall health. Tryptophan has been found to have the antioxidant activity on its own since it was highly reactive to free radicals such as hydroxyl radicals and renders antioxidant defence (Del Angel-Meza et al. 2011). This can be reflected results of the experimental studies conducted in animal’s models like rodents and fishes. Narin et al. (2010) reported that tryptophan was effective in protecting rabbits by reducing the generation of free radicals and lipid peroxidation resulting from hypoxic myocardial injury. Liu et al. (2015) in their experimental studies in ducks observed that the antioxidant levels were increased due to the tryptophan supplement. However, the antioxidant mechanisms activated by tryptophan were not clear and must be studied in detail to reveal the underlying molecular interaction.
Some of the oxidative products of tryptophan are also known to act as potent antioxidants such as 3-hydroxykynurenine, 3-hydroxy anthranilic acid and melatonin (Nayak and Buttar 2016). Tsopmo et al. (2009) studied the enzymatic hydrolysates of human milk peptides for their antioxidant activity, and have found tryptophan-containing fragment to have the highest oxygen radical absorbance capacity value as compared to the human milk peptides without tryptophan.
The impaired secretion of insulin and glucose in peripheral tissues reported oxidative stress in diabetic animal models (Zatalia and Sanusi 2013). Hyperglycemia has been shown to prevent diabetic complications by inhibiting ROS generation or its neutralization (Otero et al. 2005; Vincent et al. 2007; Issac et al. 2017, 2018). Evans et al. (2003) also proposed the role of oxidative stress to initiate insulin resistance through the modification of intracellular signaling pathways. Several clinical trials have proved that increased insulin sensitivity in insulin-resistant individuals is facilitated by antioxidants, such as vitamin E and C, or glutathione (Jia et al. 2009). The present study was carried out to test that the addition of tryptophan to the peptide (IW13) could make the tryptophan-tagged IW13 peptide to be more effective in reducing the oxidative stress. Insulin is an effective inhibitor of ROS; we were interested in studying the effects of the antioxidant treatment conditions by keeping the 100 nM insulin treatment as a comparative reference (positive control) for SOD and CAT assays. Our 24-h treatment with IW13 peptide in L6 myotubes proved significantly to enhance the antioxidant defence against the H2O2-induced generic oxidative stress. Similar to our current study results, Sannasimuthu et al. (2018) reported the scavenging activity of a peptide derived from Spirulina, even at a lower concentration of 12.5 μM, but did not show a dose-dependent efficacy. However, in our case, the tryptophan-tagged IW13 peptide showed enhanced activity with higher dose concentration.
In this study, zebrafish embryos/larvae were used as a vertebrate model to study the effects of IW13 peptide during development phases and determine its toxicity. Zebrafishes have been extensively used in eco-toxicological experiments and many toxicity assessment studies to examine the influence of the toxicant (Huang et al. 2018). The parameters such as mortality, heartbeat rate and developmental malformation were used as the endpoints for evaluating the developmental toxicity of IW13 peptide during the embryo-larval stages of zebrafish. The heart is one of the organs that form first in the zebrafish embryo and function throughout development. Our study results showed that exposure to IW13 peptide had not shown lethal to zebrafish larvae even at a higher concentration (50 μM). However, 40 and 50 μM developed non-lethal malformation, which are the signs for impact at the sub-lethal dose. Overall, IW13 exposure assessment in zebrafish larvae reveals that IW13 exhibits less toxicity or teratogenic impact even during the early developmental window, which is the most susceptible period for a toxicant effect.
As with many other species, fish can compete for ROS with antioxidant enzymes, such as SOD and CAT, which can minimize and restore harmful effects of ROS and turn superoxide anion O2− to H2O2 and then to H2O and O2 (Parlak 2018). Antioxidant enzyme’s SOD and CAT in the zebrafish larvae decreased after exposure to H2O2 (1 mM). However, IW13 peptide treatment significantly reduced the activity of the antioxidant enzyme in a dose-dependent manner. Measurement of MDA is a key parameter for the determination of oxidative stress levels. The lipid peroxidation levels were decreased significantly in IW13 peptide pre-treated zebrafish larvae. This displays that IW13 peptide can ease oxidative damage to zebrafish larvae.
Intracellular ROS generation can be detected using the DCFDA, an oxidation-sensitive dye. DCFDA does not exhibit fluorescence without ROS; however, it produces fluorescence when coming in contact with the ROS (Lee et al. 2013). In the present study, the untreated control group, which is not treated with IW13 peptide, showed very minimal fluorescence signal. In contrast, the positive control larvae exposed H2O2, observed with an intense fluorescent signal highlighting the increased ROS levels. However, the groups pre-treated with IW13 peptide were found to attenuate the increased ROS levels. Earlier studies have shown that high ROS levels can cause a wide range of biochemical and physiological lesions due to oxidative stress, and also reported to affect the metabolic processes contributing to cell death and inflammation (Finkel and Holbrook 2000). Since the IW13 peptide showed optimum antioxidant activity at a concentration of 50 μM. Therefore, we are interested in studying the activated caspase 3 expression in 50 μM IW13 peptide exposure.
Apoptosis is known to be a process of programmed cell death, particularly in response to environmental toxicants in multicellular organisms. Caspase 3 has been described as one of the key molecules involved in the execution of apoptosis (Shi et al. 2011). Results from our study showed that in increased caspase 3 expression in zebrafish larvae treated with H2O2 (1 mM), when compared to control, cell death was induced by H2O2 exposure in zebrafish larvae; however, the strength of the fluorescent signal was reduced by the pre-treatment with IW13 peptide, which indicates that the H2O2 exposure leads to increased oxidative stress in the cellular environment further contributing to caspase 3–dependent apoptotic response. The current study findings demonstrated that treatment of IW13 peptide could effectively ameliorate the caspase 3–dependent apoptosis induced by reducing the free radical levels inside the cellular environment.
We also examined the effects of IW13 peptides on antioxidant enzyme genes such as GPx, GST and GCS. GSH-related enzymes such as GST, GPx and GCS were the key players in the intracellular antioxidant defence system (Li et al. 2015). This study showed H2O2 exposure to downregulate the expression of genes: GST, GPx and GCS in zebrafish larvae, whereas the IW13 peptide pre-treatment groups showed upregulation in the expression of antioxidant genes GST, GPx and GCS.
Conclusion
The result of this study indicated that the tryptophan-tagged IW13 peptide substantially reduced the ROS levels concomitant with the enhanced antioxidant enzyme activity levels both in vitro and in vivo. The administration of IW13 peptide decreased the lipid peroxidation level both in vitro and in vivo, and also increased the activity of glutathione enzyme. The expression of antioxidant enzyme genes: GST, GPx and GCS was found to be upregulated in the experimental groups pre-treated with the IW13 peptide. IW13 peptide was found to be non-cytotoxic and did not exhibit toxic effect on the zebrafish larvae model, in parallel to enhance antioxidant enzyme activity in the in vitro and in vivo experiments. Therefore, it can be conceived that IW13 peptide tagged with tryptophan can be a promising candidate for treating both diabetes and its related complications since disruption of the antioxidant balance in peripheral tissues are reported with insulin resistance and aberrant insulin signaling. Hence, this peptide’s therapeutic potential must be further explored for the treatment of oxidative stress–associated diseases.
Data availability
The required data have been provided in the article as table and figures in the main article as well as in the E-supplement document.
Abbreviations
- A549 cells:
-
Human lung cancer cells
- ABTS:
-
2, 2′-Azino-bis-3-ethylbenzothiazoline-6-sulfonic acid
- BSA:
-
Bovine serum albumin
- CAT:
-
Catalase
- DCFDA:
-
Dichlorofluorescin diacetate
- DMEM:
-
Dulbecco’s modified eagle’s medium
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- FBS:
-
Fetal bovine serum
- GPx:
-
Glutathione peroxidase
- GST:
-
Glutathione S transferase
- GCS:
-
Glutamyl cysteine synthetase
- HeLa cells :
-
Human cervical carcinoma cells
- LPO:
-
Lipid peroxidation
- MCF cells:
-
Breast cancer cell line
- MDA:
-
Malondialdehyde
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NBT:
-
Nitro blue tetrazolium
- NCCS:
-
National Centre for Cell Science
- PMS/NADH:
-
Phenazine methosulphate nicotinamide adenine dinucleotide
- SOD:
-
Superoxide dismutase
- STPK:
-
Serine threonine-protein kinase
References
Alam MN, Bristi NJ, Rafiquzzaman M (2013) Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J 21:143–152. https://doi.org/10.1016/j.jsps.2012.05.002
Anandharajan R, Jaiganesh S, Shankernarayanan NP, Viswakarma RA, Balakrishnan A (2006) In vitro glucose uptake activity of Aegles marmelos and Syzygium cumini by activation of Glut-4, PI3 kinase and PPARγ in L6 myotubes. Phytomedicine 13:434–441. https://doi.org/10.1016/j.phymed.2005.03.008
Anwar MJ, Muhammad BY, Bader AA, Abdulghani M, Mahmood D, Haider M (2015) An insight into the scientific background and future perspectives for the potential uses of melatonin. Egypt J Basic Appl Sci 2:139–152. https://doi.org/10.1016/j.ejbas.2015.05.003
Arockiaraj J, Easwvaran S, Vanaraja P, Singh A, Othman RY, Bhassu S (2012) Molecular cloning, characterization and gene expression of an antioxidant enzyme catalase (MrCat) from Macrobrachium rosenbergii. Fish Shellfish Immunol 32:670–682. https://doi.org/10.1016/j.fsi.2012.01.013
Arockiaraj J, Gnanam AJ, Muthukrishnan D, Thirumalai MK, Pasupuleti M, Milton J, Kasi M (2013) Macrobrachium rosenbergii cathepsin L: molecular characterization and gene expression in response to viral and bacterial infections. Microbiol Res 168:569–579. https://doi.org/10.1016/j.micres.2013.04.007
Bernini R, Barontini M, Cis V, Carastro I, Tofani D, Chiodo R, Lupattelli P, Incerpi S (2018) Synthesis and evaluation of the antioxidant activity of lipophilic phenethyl trifluoroacetate esters by in vitro ABTS, DPPH and in cell-culture DCF assays. Molecules 23:1–14. https://doi.org/10.3390/molecules23010208
Bhatt P, Kumaresan V, Palanisamy R, Chaurasia MK, Gnanam AJ, Pasupuleti M, Arockiaraj J (2014) Immunological role of C4 CC chemokine-1 from snakehead murrel Channa striatus. Mol Immunol 57:292–301. https://doi.org/10.1016/j.molimm.2013.10.012
Birnie-Gauvin K, Costantini D, Cooke SJ, Willmore WG (2017) A comparative and evolutionary approach to oxidative stress in fish: a review. Fish Fish 18:928–942. https://doi.org/10.1111/faf.12215
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Choi CY, An KW, An MI (2008) Molecular characterization and mRNA expression of glutathione peroxidase and glutathione S-transferase during osmotic stress in olive flounder (Paralichthys olivaceus). Comp Biochem Physiol A Mol Integr Physiol 149:330–337. https://doi.org/10.1016/j.cbpa.2008.01.013
Dambal VY, Selvan KP, Lite C, Barathi S, Santosh W (2017) Developmental toxicity and induction of vitellogenin in embryo-larval stages of zebrafish (Danio rerio) exposed to methyl Paraben. Ecotoxicol Environ Saf 141:113–118. https://doi.org/10.1016/j.ecoenv.2017.02.048
Del Angel-Meza AR, Dávalos-Marín AJ, Ontiveros-Martinez LL et al (2011) Protective effects of tryptophan on neuro-inflammation in rats after administering lipopolysaccharide. Biomed Pharmacother 65:215–219. https://doi.org/10.1016/j.biopha.2011.02.008
El Mouatassim S, Guérin P, Ménézo Y (1999) Expression of genes encoding antioxidant enzymes in human and mouse oocytes during the final stages of maturation. Mol Hum Reprod 5:720–725. https://doi.org/10.1093/molehr/5.8.720
Evans JL, Goldfine ID, Maddux BAGG (2003) Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes 52:1–8. https://doi.org/10.2337/diabetes.52.1.1
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247. https://doi.org/10.1038/35041687
Friedman M (2018) Analysis, nutrition, and health benefits of tryptophan. Int J Tryptophan Res 11:1–12. https://doi.org/10.1177/1178646918802282
Gopalakrishna R, Jaken S (2000) Protein kinase C signaling and oxidative stress. Free Radic Biol Med 28:1349–1361. https://doi.org/10.1016/S0891-5849(00)00221-5
Gostner JM, Geisler S, Stonig M, Mair L, Sperner-Unterweger B, Fuchs D (2020) Tryptophan metabolism and related pathways in psychoneuroimmunology: the impact of nutrition and lifestyle. Neuropsychobiology 79:89–99. https://doi.org/10.1159/000496293
Goth L (1991) A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta 196:143–151. https://doi.org/10.1016/0009-8981(91)90067-M
Guru A, Lite C, Freddy AJ, Issac PK, Pasupuleti M, Saraswathi NT, Arasu MV, al-Dhabi NA, Arshad A, Arockiaraj J (2021) Intracellular ROS scavenging and antioxidant regulation of WL15 from cysteine and glycine-rich protein 2 demonstrated in zebrafish in vivo model. Dev Comp Immunol 114:103863. https://doi.org/10.1016/j.dci.2020.103863
Han Y, Liu T, Wang J, Wang J, Zhang C, Zhu L (2016) Genotoxicity and oxidative stress induced by the fungicide azoxystrobin in zebrafish (Danio rerio) livers. Pestic Biochem Physiol 133:13–19. https://doi.org/10.1016/j.pestbp.2016.03.011
Hara PM, Fridovich I (1972) The role of superoxide anion in the epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Hoseini SM, Pérez-Jiménez A, Costas B, Azeredo R, Gesto M (2019) Physiological roles of tryptophan in teleosts: current knowledge and perspectives for future studies. Rev Aquac 11:3–24. https://doi.org/10.1111/raq.12223
Huang D, Li H, He Q, Yuan W, Chen Z, Yang H (2018) Developmental toxicity of diethylnitrosamine in zebrafish embryos/juveniles related to excessive oxidative stress. Water Air Soil Pollut 229:1–11. https://doi.org/10.1007/s11270-018-3739-8
Issac PK, Ishan M, Sujatha S, Alwin D (2017) Antihyperglycemic and antihyperlipidemic activity of Jatropha gossypifolia methanolic extract in streptozotocin-nicotinamide induced diabetic rats. Asian J Pharm Clin Res 10:326–330. https://doi.org/10.22159/ajpcr.2017.v10i11.20985
Issac PK, Arun J, Sri Snehaa C, Sujatha S (2018) Tannins of Jatropha gossypifolia exert anti-hyperlipidemic effect. Eur Biomed Pharm Sci 5:607–614
Issac PK, Guru A, Chandrakumar SS, Lite C, Saraswathi NT, Arasu MV, al-Dhabi NA, Arshad A, Arockiaraj J (2020) Molecular process of glucose uptake and glycogen storage due to hamamelitannin via insulin signalling cascade in glucose metabolism. Mol Biol Rep 47:6727–6740. https://doi.org/10.1007/s11033-020-05728-5
Jia J, Zhang X, Hu YS, Wu Y, Wang QZ, Li NN, Guo QC, Dong XC (2009) Evaluation of in vivo antioxidant activities of Ganoderma lucidum polysaccharides in STZ-diabetic rats. Food Chem 115:32–36. https://doi.org/10.1016/j.foodchem.2008.11.043
Kamaraj N, Rajaguru PY, Issac PK, Sundaresan S (2017) Fabrication, characterization, in vitro drug release and glucose uptake activity of 14-deoxy, 11, 12-didehydroandrographolide loaded polycaprolactone nanoparticles. Asian J Pharm Sci 12:353–362. https://doi.org/10.1016/j.ajps.2017.02.003
Kanaujia A, Duggar R, Pannakal ST, Yadav SS, Katiyar CK, Bansal V, Anand S, Sujatha S, Lakshmi BS (2010) Insulinomimetic activity of two new gallotannins from the fruits of Capparis moonii. Bioorg Med Chem 18:3940–3945. https://doi.org/10.1016/j.bmc.2010.04.032
Kang MC, Cha SH, Wijesinghe WAJP, Kang SM, Lee SH, Kim EA, Song CB, Jeon YJ (2013) Protective effect of marine algae phlorotannins against AAPH-induced oxidative stress in zebrafish embryo. Food Chem 138:950–955. https://doi.org/10.1016/j.foodchem.2012.11.005
Kumaresan V, Nizam F, Ravichandran G, Viswanathan K, Palanisamy R, Bhatt P, Arasu MV, al-Dhabi NA, Mala K, Arockiaraj J (2017) Transcriptome changes of blue-green algae, Arthrospira sp. in response to sulfate stress. Algal Res 23:96–103. https://doi.org/10.1016/j.algal.2017.01.012
Lee SH, Ko CI, Jee Y, Jeong Y, Kim M, Kim JS, Jeon YJ (2013) Anti-inflammatory effect of fucoidan extracted from Ecklonia cava in zebrafish model. Carbohydr Polym 92:84–89. https://doi.org/10.1016/j.carbpol.2012.09.066
Li S, Chen J, Xie P, Guo X, Fan H, Yu D, Zeng C, Chen L (2015) The role of glutathione detoxification pathway in MCLR-induced hepatotoxicity in SD rats. Environ Toxicol 30:1470–1480. https://doi.org/10.1002/tox.22017
Lite C, Ahmed SSSJ, Santosh W, Seetharaman B (2019) Prenatal exposure to bisphenol-A altered miRNA-224 and protein expression of aromatase in ovarian granulosa cells concomitant with elevated serum estradiol levels in F 1 adult offspring. J Biochem Mol Toxicol 33:e22317. https://doi.org/10.1002/jbt.22317
Liu Y, Yuan JM, Zhang LS, Zhang YR, Cai SM, Yu JH, Xia ZF (2015) Effects of tryptophan supplementation on growth performance, antioxidative activity, and meat quality of ducks under high stocking density. Poult Sci 94:1894–1901. https://doi.org/10.3382/ps/pev155
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Lü JM, Lin PH, Yao Q, Chen C (2010) Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med 14:840–860. https://doi.org/10.1111/j.1582-4934.2009.00897.x
Luo HY, Wang B, Li ZR, Chi CF, Zhang QH, He GY (2013) Preparation and evaluation of antioxidant peptide from papain hydrolysate of Sphyrna lewini muscle protein. LWT Food Sci Technol 51:281–288. https://doi.org/10.1016/j.lwt.2012.10.008
Luzeena Raja G, Divya Subhashree K, Lite C, Santosh W, Barathi S (2019) Transient exposure of methylparaben to zebrafish (Danio rerio) embryos altered cortisol level, acetylcholinesterase activity and induced anxiety-like behaviour. Gen Comp Endocrinol 279:53–59. https://doi.org/10.1016/j.ygcen.2018.11.001
Mata-Cabana A, García-Domínguez M, Florencio FJ, Lindahl M (2012) Thiol-based redox modulation of a cyanobacterial eukaryotic-type serine/threonine kinase required for oxidative stress tolerance. Antioxid Redox Signal 17:521–532. https://doi.org/10.1089/ars.2011.4483
Moosmann B, Behl C (2000) Cytoprotective antioxidant function of tyrosine and tryptophan residues in transmembrane proteins. Eur J Biochem 267:5687–5692. https://doi.org/10.1046/j.1432-1327.2000.01658.x
Mukwevho E, Ferreira Z, Ayeleso A (2014) Potential role of sulfur-containing antioxidant systems in highly oxidative environments. Molecules 19:19376–19389. https://doi.org/10.3390/molecules191219376
Najafian L, Babji AS (2012) A review of fish-derived antioxidant and antimicrobial peptides: their production, assessment, and applications. Peptides 33:178–185. https://doi.org/10.1016/j.peptides.2011.11.013
Narin F, Narin N, Başarslan F et al (2010) The effect of L-tryptophan on the heart in rabbits via chronic hypoxia. Turkish J Med Sci 40:257–263. https://doi.org/10.3906/sag-0901-3
Nayak BN, Buttar HS (2016) Evaluation of the antioxidant properties of tryptophan and its metabolites in in vitro assay. J Complement Integr Med 13:129–136. https://doi.org/10.1515/jcim-2015-0051
Nayak BN, Singh RB, Buttar HS (2019) Role of tryptophan in health and disease: systematic review of the anti-oxidant, anti-inflammation, and nutritional aspects of tryptophan and its metabolites. World Heart J 11:161–178
Otero P, Bonet B, Herrera E, Rabano A (2005) Development of atherosclerosis in the diabetic BALB/c mice: prevention with Vitamin E administration. Atherosclerosis 182:259–265. https://doi.org/10.1016/j.atherosclerosis.2005.02.024
Parlak V (2018) Evaluation of apoptosis, oxidative stress responses, AChE activity and body malformations in zebrafish (Danio rerio) embryos exposed to deltamethrin. Chemosphere 207:397–403. https://doi.org/10.1016/j.chemosphere.2018.05.112
Perez-Gonzalez A, Muñoz-Rugeles L, Alvarez-Idaboy JR (2014) Tryptophan: antioxidant or target of oxidative stress? A quantum chemistry elucidation. RSC Adv 4:56128–56131. https://doi.org/10.1039/c4ra11635f
Prabha N, Sannasimuthu A, Kumaresan V, Elumalai P, Arockiaraj J (2020) Intensifying the anticancer potential of cationic peptide derived from serine threonine protein kinase of Teleost by tagging with Oligo Tryptophan. Int J Pept Res Ther 26:75–83. https://doi.org/10.1007/s10989-019-09817-3
Raja GL, Lite C, Subhashree KD et al (2020) Prenatal bisphenol-A exposure altered exploratory and anxiety-like behaviour and induced non-monotonic, sex-specific changes in the cortical expression of CYP19A1, BDNF and intracellular signaling proteins in F1 rats. Food Chem Toxicol 142:111442. https://doi.org/10.1016/j.fct.2020.111442
Sannasimuthu A, Kumaresan V, Pasupuleti M, Paray BA, al-Sadoon MK, Arockiaraj J (2018) Radical scavenging property of a novel peptide derived from C-terminal SOD domain of superoxide dismutase enzyme in Arthrospira platensis. Algal Res 35:519–529. https://doi.org/10.1016/j.algal.2018.09.028
Sannasimuthu A, Kumaresan V, Anilkumar S, Pasupuleti M, Ganesh MR, Mala K, Paray BA, al-Sadoon MK, Albeshr MF, Arockiaraj J (2019) Design and characterization of a novel Arthrospira platensis glutathione oxido-reductase-derived antioxidant peptide GM15 and its potent anti-cancer activity via caspase-9 mediated apoptosis in oral cancer cells. Free Radic Biol Med 135:198–209. https://doi.org/10.1016/j.freeradbiomed.2019.03.006
Sarkar P, Stefi RV, Pasupuleti M, Paray BA, al-Sadoon MK, Arockiaraj J (2020) Antioxidant molecular mechanism of adenosyl homocysteinase from cyanobacteria and its wound healing process in fibroblast cells. Mol Biol Rep 47:1821–1834. https://doi.org/10.1007/s11033-020-05276-y
Sasikumar V (2015) Evaluation of free radical scavenging activity of various leaf extracts from Kedrostis foetidissima (Jacq.) Cogn. Biochem Anal Biochem 04:42–16. https://doi.org/10.1016/j.fshw.2015.02.001
Shi X, Gu A, Ji G, Li Y, di J, Jin J, Hu F, Long Y, Xia Y, Lu C, Song L, Wang S, Wang X (2011) Developmental toxicity of cypermethrin in embryo-larval stages of zebrafish. Chemosphere 85:1010–1016. https://doi.org/10.1016/j.chemosphere.2011.07.024
Siauciunaite R, Foulkes NS, Calabrò V, Vallone D (2019) Evolution shapes the gene expression response to Oxidative Stress. Int J Mol Sci 20:3040. https://doi.org/10.3390/ijms20123040
Sorrells S, Toruno C, Stewart RA, Jette C (2013) Analysis of apoptosis in zebrafish embryos by whole-mount immunofluorescence to detect activated caspase 3. J Vis Exp:1–8. https://doi.org/10.3791/51060
Sujatha S, Anand S, Sangeetha KN, Shilpa K, Lakshmi J, Balakrishnan A, Lakshmi BS (2010) Biological evaluation of (3β)-STIGMAST-5-EN-3-OL as potent anti-diabetic agent in regulating glucose transport using in vitro model. Int J Diabetes Mellit 2:101–109. https://doi.org/10.1016/j.ijdm.2009.12.013
Tang D, Bin CQ, Xin XL, Aisa HA (2017) Anti-diabetic effect of three new norditerpenoid alkaloids in vitro and potential mechanism via PI3K/Akt signaling pathway. Biomed Pharmacother 87:145–152. https://doi.org/10.1016/j.biopha.2016.12.058
Tao J, Zhao YQ, Chi CF, Wang B (2018) Bioactive peptides from cartilage protein hydrolysate of spotless smoothhound and their antioxidant activity in vitro. Mar Drugs 16:1–18. https://doi.org/10.3390/md16040100
Techer D, Milla S, Fontaine P, Viot S, Thomas M (2015) Acute toxicity and sublethal effects of gallic and pelargonic acids on the zebrafish Danio rerio. Environ Sci Pollut Res 22:5020–5029. https://doi.org/10.1007/s11356-015-4098-2
Tsopmo A, Diehl-Jones BW, Aluko RE, Kitts DD, Elisia I, Friel JK (2009) Tryptophan released from mother’s milk has antioxidant properties. Pediatr Res 66:614–618. https://doi.org/10.1203/PDR.0b013e3181be9e7e
Vincent AM, Russell JW, Sullivan KA, Backus C, Hayes JM, McLean LL, Feldman EL (2007) SOD2 protects neurons from injury in cell culture and animal models of diabetic neuropathy. Exp Neurol 208:216–227. https://doi.org/10.1016/j.expneurol.2007.07.017
Wang L, Ding L, Wang Y, Zhang Y, Liu J (2015) Isolation and characterisation of in vitro and cellular free radical scavenging peptides from corn peptide fractions. Molecules 20:3221–3237. https://doi.org/10.3390/molecules20023221
Zarubin T, Han J (2005) Activation and signaling of the p38 MAP kinase pathway. Cell Res 15:11–18. https://doi.org/10.1038/sj.cr.7290257
Zatalia SR, Sanusi H (2013) The role of antioxidants in the pathophysiology, complications, and management of diabetes mellitus. Acta Med Indones 45:141–147
Zhao X, Ren X, Zhu R, Luo Z, Ren B (2016) Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria-mediated apoptosis in zebrafish embryos. Aquat Toxicol 180:56–70. https://doi.org/10.1016/j.aquatox.2016.09.013
Acknowledgements
The authors acknowledge Mrs. N. Prabha, Asst. Professor, Department of Microbiology, SRM Arts and Science College, Kattankulathur 603 203, Chennai, Tamil Nadu, India, for providing the peptide to carry out this study.The authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSP-2020/111), King Saud University, Riyadh, Saudi Arabia. Universiti Putra Malaysia contribution to the research is made possible through research grant LRGS/1/2019/UPM/1.
Funding
The authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSP-2020/111), King Saud University, Riyadh, Saudi Arabia. Universiti Putra Malaysia contribution to the research is made possible through research grant LRGS/1/2019/UPM/1.
Author information
Authors and Affiliations
Contributions
Conceived, designed the experiments and wrote the article: Praveen Kumar Issac, Christy Lite, Ajay Guru, Manikandan Velayutham and Jesu Arockiaraj; performed the experiments: Praveen Kumar Issac, Christy Lite, Ajay Guru and Manikandan Velayutham; analyzed the data and wrote the article: Praveen Kumar Issac, Christy Lite, Ajay Guru, Manikandan Velayutham, Giva Kuppusamy, Saraswathi N.T., Ebtesam M. Al Olayan, Abeer S. Aloufi, Mohamed A. Elokaby, Preetham Elumalai, Aziz Arshad and Jesu Arockiaraj; contributed reagents/materials/analysis and tools and wrote the article: Giva Kuppusamy, Saraswathi N.T., Ebtesam M. Al Olayan, Abeer S. Aloufi, Mohamed A. Elokaby, Preetham Elumalai, Aziz Arshad and Jesu Arockiaraj; supervised the research: Jesu Arockiaraj; All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Declaration and informed consent
This research does not involve any human objects; however, we have performed assays using zebrafishes. The fishes were collected, handled, experimented and sampled as per the Institute animal ethical handling procedure and guidelines. All authors are aware of the details of their research work that are presented in the current manuscript and gave their consent to publication.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 2897 kb).
Rights and permissions
About this article
Cite this article
Issac, P.K., Lite, C., Guru, A. et al. Tryptophan-tagged peptide from serine threonine-protein kinase of Channa striatus improves antioxidant defence in L6 myotubes and attenuates caspase 3–dependent apoptotic response in zebrafish larvae. Fish Physiol Biochem 47, 293–311 (2021). https://doi.org/10.1007/s10695-020-00912-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-020-00912-7