Abstract
Renal ischemia-reperfusion (IR) injury triggers a cascade of signaling reactions involving an increase in Ca2 + charge and reactive oxygen species (ROS) levels resulting in necrosis, inflammation, apoptosis, and subsequently acute kidney injury (AKI).
Transient receptor potential (TRP) channels include an essential class of Ca2+ permeable cation channels, which are segregated into six main channels: the canonical channel (TRPC), the vanilloid-related channel (TRPV), the melastatin-related channel (TRPM), the ankyrin-related channel (TRPA), the mucolipin-related channel (TRPML) and polycystin-related channel (TRPP) or polycystic kidney disease protein (PKD2). TRP channels are involved in adjusting vascular tone, vascular permeability, cell volume, proliferation, secretion, angiogenesis and apoptosis.
TRPM channels include eight isoforms (TRPM1–TRPM8) and TRPM2 is the second member of this subfamily that has been expressed in various tissues and organs such as the brain, heart, kidney and lung. Renal TRPM2 channels have an important role in renal IR damage. So that TRPM2 deficient mice are resistant to renal IR injury. TRPM2 channels are triggered by several chemicals including hydrogen peroxide, Ca2+, and cyclic adenosine diphosphate (ADP) ribose (cADPR) that are generated during AKI caused by IR injury, as well as being implicated in cell death caused by oxidative stress, inflammation, and apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal ischemia-reperfusion (IR) injury, defined as the cessation and restoration of renal blood flow (RBF), is a major cause of acute kidney injury (AKI) [1, 2]. AKI is a worldwide health issue that is associated with a high rate of morbidity and mortality [3]. Renal IR injury is most commonly caused by transient or persistent renal hypoperfusion [4]. However, it may also be owing to temporary renal artery occlusion during suprarenal aortic aneurysm repair, nephrectomy, renal transplantation [5], cardiovascular surgery, hypovolemic, and septic shock [6, 7].
Interruption of RBF during the ischemic phase decreases medullary blood flow and reduces oxygen and glucose supply to tubular structures in this area, resulting in an imbalance in delivery, and demand [5]. Reduced O2 levels alter metabolism from aerobic to anaerobic, which cannot provide the requirement of aerobic tissues, resulting in fast depletion of intracellular ATP levels [8]. ATP depletion causes an increase in cytoplasm Ca2 + charge, hypoxanthine level inside the cells, and production of reactive oxygen species (ROS), which finally results in acidosis [9]. Apoptosis and necrosis are triggered by hypoxia, glucose deprivation, acidosis, and the formation of ROS [10]. In addition, reduced intracellular pH and ATP levels during the ischemia phase result in (1) lysosome membrane instability, followed by lysosome enzymes exudation and cell structure disturbance, and (2) inhibition of ionic pumps, especially the Na+/K+ ATPase [11].
Inhibition of Na+/K+ ATPase activity and Na+/H+ antiporter function, which attempts to correct the intracellular pH by pumping Na+ ions into the cell and protons out of the cell, results in increased intracellular Na+, water, and edema [12, 13]. Furthermore, because of ATP depletion, the cessation of Ca2+ pumping out of the cells and the suppression of Ca2+ reuptake into the endoplasmic reticulum raises intracellular Ca2+ levels. Furthermore, the cessation of Ca2+ pumping out of the cells and the suppression of Ca2+ reuptake into the endoplasmic reticulum due to ATP depletion raises intracellular Ca2+ levels [9].
The cytoplasmic Ca2+ overload activates Ca2+-dependent proteases like calpains, proteases, phospholipases, and caspases, which are inactive due to the acidic environment but can cause cell damage after pH normalization during reperfusion [9, 14]. Besides, Ca2+ overload leads to ROS production in mitochondria during ischemia, which is followed by the opening of the mitochondrial transition pore (mPTP) in reperfusion as a result of pH normalization, resulting in apoptosis and cell death [15]. Despite the fact that quick reperfusion recovers the oxygenation and substrates needed for aerobic ATP production and normalizes extracellular pH by washing out accumulated H+, it creates additional damage known as reperfusion damage [9, 16, 17]. Ca2+ overload causes activation of the calpains, ROS production, reduction of antioxidant capacity, mPTP opening, apoptosis, necrosis, endothelial dysfunction, alterations in outer medullary microcirculation, inflammation, and tubular injury [5, 18, 19].
TRPM2, the second TRPM channel subfamily member, enhances intracellular Ca2+, ROS generation, and oxidative stress, all of which are implicated in physiological and pathological processes [6, 20, 21]. TRPM2 has been found to play a deleterious role in IR injury in a variety of tissues, including the kidney, brain, and pancreas [22, 23]. Therefore, it is necessary to review the important pathophysiological mechanisms mediated by TRPM2 activation in renal IR injury. This review will focus on new results that provide light on the role of TRPM2 in IR-induced inflammation, oxidative stress, apoptosis, and AKI. Fig. 1 shows how TRPM2 channel opening activates oxidative stress, apoptosis, and inflammation signaling pathways in renal IR injury (Fig. 1).
Schematic diagram of activation of inflammatory cytokines and cells, production of ROS, oxidative stress, and apoptosis in renal IR injury and the role of TRPM2 channels activation in these pathways.
VCAM: Vascular cell adhesion molecule, ICAM: Intercellular Adhesion Molecule, ROS: Reactive oxygen species, NUDT9-H: Nudix-like domain homology domain, PARG: Poly (ADP-Ribose) Glycohydrolase, PARP: Poly(ADP-Ribose) Polymerase, ADPR: Adenosine diphosphate ribose, NFkB: Nuclear Factor kappa B, ERK: Extracellular signal-regulated kinases, AIF: apoptosis-inducing factor.
Materials and methods
For this review, we gathered data from a variety of sources, including PubMed, Scopus, Web of Science (WOS), and EMBASE. The following keywords and or their equivalents were used for the search strategy; TRPM2, acute kidney injury, oxidative stress, inflammation, and renal ischemia-reperfusion.
General structural features of TRPM2
Transient receptor potential (TRP) channels with six transmembrane domains belong to a superfamily of monovalent and divalent cation permeable ion channels [24]. TRP channels are implicated in many biological processes, i.e., regulating vascular tone, permeability, proliferation, cell volume, secretion, apoptosis, angiogenesis, and cell death [25].
Based on differences in amino acid sequence similarities between the different gene products, this superfamily with 28 members is classified into six subgroups: canonical (C), vanilloid (V), melastatin (M), mucolipin (ML), polycystin (P), and, ankyrin (A) [26]. TRPM1 to TRPM8 are members of the TRPM subfamily. TRPM1 is the name of the first member to be characterized (M, melastatin) [27].
TRPM2 was first isolated from the human brain and named LTRPC-2 or TRPC7. This channel was later classified as TRPM2 [28]. The TRPM2 gene is found on chromosome 21q22.3 in humans. Its 6.5-kilobyte transcript codes a 1503-amino-acid protein with a molecular weight of 170 kDa. It covers 90 kb which contains 32 exons. The trpm2 mouse gene has 34 exons and produces a 1507-amino-acid protein and is highly similar to human TRPM2 gene [23, 24].
The TRPM2 channel is a protein with 1503 amino acids with six transmembrane fragments, namely S1 to S6, a loop domain between S5 and S6 that forms pores, and intracellular N and C termini [29, 30]. TRPM2 contains four homologous domains and a calmodulin (CaM)-binding IQ-like motif at its N terminus, which is needed for channel activation [31]. The TRP box, coiled-coil domain (CCD), and NUDT9 (Nudix-like domain or NUDT9 homology domain) are all found at the C terminus (NUDT9-H) [30, 31]. The most common endogenous ligand for TRPM2 is adenosine diphosphate ribose (ADPR), which binds to the NUDT9-H domain and opens the channel, allowing Ca2+ inflow. The enzymatic activity of NUDT9H catalyzes the conversion of ADPR to ribose-5-phosphate (R5P), with adenine monophosphate (AMP) acting as a negative regulator for ADPR gating TRPM2 [21, 30, 32]. TRPM2 has both ADPR hydrolase enzymatic and ion channel gating activity, and is referred to as a “coenzyme” because of its ability to convert ADPR into AMP and R5P [25, 29, 33]. Calmodulin (CaM) has been demonstrated to have a key role in TRPM2 channel facilitation and activation; it interacts directly with the IQ-like motif and modifies TRPM2’s Ca2+dependent activation, increasing intracellular Ca2+ levels [34].
TRPM2 has been found in a variety of organs (kidney, brain, heart, lung, liver, spleen, bone marrow, and pancreas) and cell types (neurons, hematopoietic, cardiomyocytes, endothelial cells, and pancreatic β -cells) [25, 26, 35, 36]. TRPM2 is almost expressed in the cortex and outer medulla of the kidney’s proximal tubular epithelial cells. TRPM2 receptors were also shown to be intracellular without a clear plasma membrane localization, whereas glomeruli, peritubular endothelial cells, and interstitial cells were spared [25]. In the ischemic AKI, the TRPM2 channel is activated by different molecules, including cyclic ADPR (cADPR), hydrogen peroxide, and Ca2+ [37] that are implicated in oxidative stress, cell death, apoptosis, and inflammation [36, 38].
Regulation of TRPM2
TRPM2 activators
Although ADPR is the most common TRPM2 activator, various ADPR analogs, including ADPR-2′-phosphate, ADPR2′-O-acetyl-, and 2′-deoxy-ADPR can activate the channel [6]. Furthermore, it has been shown that TRPM2 can be activated by several second messengers associated with adenine nucleotides that are metabolically related to ADPR, including cyclic ADPR (cADPR), nicotinamide adenine dinucleotide (NAD), and nicotinic acid adenine dinucleotide phosphate (NAADP) [6, 29]. Other molecules like reactive nitrogen species (NOS), ROS, and H2O2 are triggers of the TRPM2 channels and bind to them in pathological conditions, including oxidative stress, inflammation, and cell death [25, 39]. Besides, TRPM2 is activated by tumor amyloid β-peptide, necrosis factor α (TNF-α), and concanavalin A. All of these extracellular signals cause ADPR production. ADPR binds to the TRPM2 NUDT9-H domain on the C-terminus and activates TRPM2, permitting substantial penetration of monovalent or divalent cations such as K+, Na+, Zn2+, and Ca2 + [6, 24].TRPM2 is also regulated by intracellular Ca2+. Therefore, when ADPR interacts with TRPM2, Ca2+ is released [36]. TRPM2 is entirely activated by Ca2+; thus, the loss of outer or inner Ca2+ prevents ADPR from inducing TRPM2 currents. This impact might be due to the canal’s increased sensitivity to ADPR [40, 41]. Ca2+ also acts as a concentration-dependent gate for the TRPM2 channel. This can be due to conformational changes induced by CaM interaction with the TRPM2 IQ-like motif [6]. Ca2+-bound CaM enhances the interaction between CaM and the IQ-like motif, providing positive feedback for TRPM2 stimulation [36].
TRPM2 inhibitors
The first TRPM2 channel inhibitor discovered was adenosine monophosphate (AMP), which is formed by hydrolysis of ADPR. AMP competes with ADPR by binding to binding sites located on the NUDT9-H domain of TRPM2 ion channels [42]. As a TRPM2 antagonist, 8-Br-ADPR can compete with ADPR to prevent TRPM2 activation [37]. Furthermore, protons and a variety of divalent heavy metal cations like Cu2+, Hg2+, Pb2+, Fe2+, Se2+, and Zn2+ inhibit TRPM2 currents, all of these factors are targets of the TRPM2 channel pore region. Some structurally unrelated pharmacological agents that have been found to block the TRPM2 activity include clotrimazole, flufenamic acid (a member of nonsteroidal anti-inflammatory drugs), N-(p-amylcinnamoyl) anthranilic acid, and 2-Aminoethoxydiphenyl borate (2-APB) [6].
The role of TRPM2 channels in renal IR injury
TRPM2 and inflammation in renal IR injury
The inflammatory process triggered by renal IR damage induces cascades of proinflammatory cytokines (IL-1, IL-6, IL-10, and TNF-α) [43], chemokines (MCP, MIP-2, and IL-8) [44, 45], expression of various adhesion molecules (ICAM (Intercellular adhesion molecule), VCAM (Vascular cell adhesion molecule), P selectin, and E selectin by endothelial and parenchymal renal cells [46, 47]. The combination of cytokines, chemokines, and adhesion molecules recruit leukocytes and neutrophils infiltration into the ischemic kidney, resulting in improved leukocyte-endothelial interactions, the generation of additional ROS and cytokines, and eventually significant progression of kidney damage [48, 49].
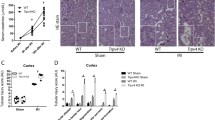
TRPM2 expression was found in tubular epithelial cells across the cortex and outer medulla using immunofluorescence. TRPM2 is also found in hematopoietic cells [31, 35]. Gao et al. have shown that mice Trpm2 knockout (Trpm2-KO) models are resistant to renal IR injury [31]. After IR injury, inflammatory cells like neutrophils entered the kidneys of Wild-Type (WT) mice, while this effect was less observed in Trpm2 KO animals. Chemokine production in monocytes is triggered by TRPM2-mediated Ca2+ influx, exacerbating neutrophil inflammatory properties [50, 51]. Because inflammation is an important mediator of IR injury, TRPM2 expression on hematopoietic cells could explain Trpm2-KO mice’s resistance to IR damage. Using bone marrow chimeric mice with Trpm2 KO in either hematopoietic or parenchymal cells, Gao et al. discovered that Trpm2 KO in parenchymal cells protected the kidney against IR injury, whereas Trpm2 KO in hematopoietic cells had no effect on ischemia-induced kidney damage. According to these data, TRPM2 in renal parenchymal cells appears to be a mediator in renal IR damage [31].
TRPM2-induced intracellular Ca2+ increase activates Ca2+-dependent signaling pathways such as the multi-subunit IB kinase (IKK) complex and extracellular signal-regulated kinase (ERK), which leads to nuclear factor B (NF-κB) activation [52, 53]. Studies identified the activation of NF-κB [54,55,56] and TRPM2 after renal IR damage [6, 31]. TRPM2 has been shown to modulate NF-κB signaling so that TRPM2 deficiency inhibited NF-κB signaling by blocking Jun N-terminal kinases (JNKs) signaling [57]. These findings imply that TRPM2 inhibiting and then preventing NF-κB activation can protect the kidney against inflammation.
Kurata et al. investigated the effects of TRPM2 on ischemic kidney in trpm2 KO mice by inducing bilateral IR at various ischemia periods (20, 25, and 30 min). A comparison of renal function in trpm2 KO and WT mice after 30 min of ischemia revealed a significant rise in plasma creatinine in KO mice, but not in 20 or 25 min of ischemia. In trpm2 KO, mRNA expression of kidney injury molecule 1 (Kim1) was considerably greater in 25 and 30 min of ischemia, and monocyte chemoattractant protein-1, (MCP-1 or Ccl2) was significantly higher in 20 and 25 min of ischemia. In contrast to the protective effect of TRPM2 deletion in the IR injury (28 min) described by Gao et al., trpm2 KO was deleterious in this investigation, as evidenced by a rise in plasma creatinine and higher mRNA expression levels of Kim1 and Ccl2 in several ischemia times. These disparities are most likely due to varied anesthetics and ischemia times in the two studies [58].
TRPM2 and oxidative stress in renal IR injury
When the balance between oxidants (free radicals) and antioxidants is disrupted, oxidative stress results, which, depending on the severity and duration, can cause tissue damage [59, 60]. Mammalian healthy cells produce a tiny amount of ROS when cellular metabolism is normal [61]. It is established that ROS moderate amounts affect many cellular signaling pathways, as well as proliferation, therefore playing a vital role in preserving cellular and tissue homeostasis [61].
It is known that several pathological conditions like IR injury, mitochondrial dysfunction, metabolism dysfunction, drug overdose, elevated Ca2+ levels, and aging increase the formation of ROS [62, 63]. Free radicals cause renal damage by DNA destruction, protein dysfunction, and lipid peroxidation ultimately leading to cell death [64].
The antioxidant defense systems deteriorate after renal IR injury, and the activity of antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT) decreases, while the quantity of malondialdehyde (MDA), a lipid peroxidation product, rises [65]. Similar to oxidants, TRPM2 channels increase the cell membrane’s permeability to Ca2+, which promotes endothelial damage and ultimately, cell death [66, 67]. An increase in intracellular Ca2+ driven by Ca2+ entry through TRPM2 channels is part of the oxidant-induced breakdown of endothelial barrier function. Therefore, TRPM2 is considered a cellular redox sensor [68, 69]. As a result of these events (intracellular Ca2+ and oxidative stress), ADPR is generated and binds to the NUDT9-H domain, activating TRPM2 [70]. The production of ADPR takes place via two enzymes, including nuclear poly (ADPR) polymerases (PARPs) and poly (ADPR) glycohydrolases (PARGs) [71, 72]. PARP is triggered by oxidative stress-induced DNA damage and performs DNA repair tasks; whereas PARG hydrolyzes PARP chains to release free ADPR. Another avenue for ADPR formation in mitochondria is when oxidative stress results in the production of free ADPR [72, 73].
TRPM2 channel activation leads to a rise in Ca2+ entry and, as a result, a rise in Ca2+ adherence to calmodulin (CaM). CaM interaction with an IQ-like motif in the N-terminus of TRPM2 produces a positive response, activating TRPM2 channels and boosting the flow of Ca2+ through them. Increased intracellular Ca2+ activates Ca2+ dependent phospholipase A2, endonuclease, and proteases, ultimately leading to cell death in cells expressing TRPM2 channels [74,75,76]. While oxidative stress results in an excess of Ca2+, downregulating TRPM2-L, inhibiting TRPM2-S, or chelating Ca2+ might reduce TRPM2 activity and hence limit the rise in Ca2+ [28, 66, 77].
Furthermore, oxidative stress induces lipid peroxidation and the subsequent formation of highly electrophilic aldehydes such as 4-hydroxynonenal (4-HNE) that participate in renal IR injury [59, 60]. IR causes a significant rise in 4-HNE in WT mice kidneys, but Trpm2-KO mice have significantly lower 4-HNE levels [60]. The levels of H2O2, MDA, SOD, CAT and Glutathione (GSH) are increased in ischemic renal tissue while injection of 8 bromocyclic ADP ribose (8-BrcADPR) as an antagonist of cADPR, decreases the levels of all these enzymes[78]. Besides, renal IR injury increases TRPM2 expression while 8BrcADPR decreases it [37]. These findings show that cADPR is an excellent Ca2+ entry coreceptor via TRPM2 channels and it is possible that 8-Br‐cADPR altered oxidant and antioxidant enzymes levels (increase and decrease, respectively) by decreasing TRPM2 expression [37].
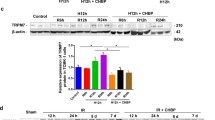
Tamm-Horsfall protein (THP) levels in AKI have been related to ROS production, and systemic oxidative stress. A HEK 293 recombinant cell line producing an inducible version of TRPM2 was used by La Favers et al. to demonstrate that THP suppressed TRPM2-mediated Ca2+ current and oxidative stress. The oxidative stress in vehicle-treated THP−/− animals was greater than in vehicle-treated THP+/+ animals. Inhibition of TRPM2 using 2-APB significantly reduced oxidative stress in THP+/+ and THP−/− mice exposed to IR injury. The disparity in oxidative stress found between THP+/+ and THP−/− animals was abolished after using 2-APB, showing that TRPM2 is a prime target for THP’s inhibitory impact on systemic oxidative stress [79].
TRPM2 and apoptosis in renal IR injury
In kidney cells of WT mice, IR damage increases apoptotic cell death by activating caspase-9 and caspase-3, reducing the expression of anti-apoptotic Bcl-xL and Bcl-2 proteins, which was reversed in Trpm2-KO animals exposed to IR [31]. This effect indicates that TRPM2 channels activate the apoptotic pathways after IR.
NADPH oxidase (NOX) is a transmembrane enzyme with several isoforms, including NOX oxidase 1 & 2, NOX1–5, NOX1 organizer, and NOX1 activator. The transfer of electrons from NADPH to O2 by NOX is the primary source of renal ROS in the cortex [80, 81].
IR damage stimulates NADPH oxidase and RAC1 (a key element of NAPDH oxidase activation) in the kidney of WT mice, while both of them decreased in Trpm2-KO animals [31]. The activated RAC1 interacts with TRPM2, which generates a possible positive feedback loop, resulting in TRPM2 membrane localization and greater oxidant injury [31, 37]. Treatment with NSC23766 as the RAC1 inhibitor before renal IR injury reduces NOX activation, PARP cleavage, and caspase-3 activity and increases the levels of anti-apoptotic proteins like Bcl-2 in mice subjected to IR injury [31].
ROS generated via activated NADPH oxidase causes DNA destruction and hence activates PARP, which functions as a modulator of cell death followed by IR injury [82]. The activated PARP increases the synthesis of PARP, which is converted into ADPR by PARG enzymes. ADPR stimulates Ca2+ influx through the TRPM2 channel and increases mitochondrial Ca2+ ion via NUDT9-H domain-TRPM2 C-terminal attachment [83]. The mitochondrial Ca2+ overload prompts the mitochondria’s swelling, destruction of the external membrane and the release of mitochondrial apoptosis factors into the cytosol, and induction of apoptosis [80, 84, 85]. PARP inhibitors restrict Ca2+ entrance through the TRPM2 channel by lowering ADPR synthesis, which is known to be the major activator of this channel [86].
TRPM2 channels are oxidative stress sensors, which means that oxidative stress either directly stimulates TRPM2 channels or increases cADPR levels [86]. The stimulation of TRPM2 channels by oxidative stress, as well as a rise in Ca2+ levels within the cell, indicated that it was linked to cell damage [38]. The TRPM2 blockade via ACA results in a decreased Ca2+ level within cells and consequently reduced cell death in H2O2 toxicity-exposed cells [68].
Intravenous injection of 2-APB as a TRPM2 inhibitor before ischemia protect the kidney against IR injury. This protective effect was shown by the reduction of apoptotic cells [87]. Cell apoptosis caused by nuclease, Ca2+-dependent phospholipase, and protease activation is recognized as one of the processes generating renal IR damage. Increased intracellular Ca2+ causes a rise in mitochondrial Ca2+ levels through the mitochondrial Ca2+ channels, and high mitochondrial Ca2+ load triggers irreversible processes that lead to cell apoptosis. 2-APB most likely protects the kidneys by reducing intracellular Ca2+ accumulation, which causes cell apoptosis following ischemia [87].
TRPM2 is activated by H2O2 under pathological circumstances like inflammation, oxidative stress and, cell death [88]. H2O2 also activates the TRPM2 channel directly and indirectly through NAD+-dependent ADPR production processes such as PARP activation, particularly PARP-1 and PARG enzymes in the nucleus [38]. H2O2 causes apoptosis by a variety of ways, including overexpression of Fas/Fas ligand, which activates the cell death pathway [89]. It also triggers the death of mitochondrial cells by modulating the mPTP [90]. H2O2 causes a rise in free Ca2+ inside cells, which leads to an increase in mitochondrial matrix Ca2+, which opens the mPTP [91]. When active, the mPTP decouples oxidative phosphorylation from ATP generation and promotes cytochrome c release into the cytosol. Cytochrome c attaches to Apaf-1(apoptotic peptidase activating factor 1) to create the apoptosome. After then, Caspase9, Caspase3, and Caspase7 are activated, leading to cell death [70].
TRPM2 and tissue damage in renal IR injury
Gao et al. investigated the function of the TRPM2 ion channel in kidney damage due to IR injury. Bilateral IR injury (28 min ischemia and 24 min reperfusion) caused histological damage to the kidney, comprising cast formation, an increase in the levels of blood urea nitrogen (BUN) and creatinine (Cr), and epithelial cell membrane damage in WT animal models while these effects were improved positively in TRPM2-KO animals [31]. TRPM2 deletion decreased renal dysfunction significantly, as demonstrated by lower serum Cr, BUN, and kidney injury molecule 1 (KIM-1) and lipocalin-2 (NGAL, a marker of kidney injury) levels [31, 57, 78]. Treatment with NSC23766 (RAC1 inhibitor), 8-Br‐cADPR, and 2-APB before ischemia, decreases ischemic damage in WT mice, while there was no additional amelioration in renal function in TRPM2-KO mice [78].
Conclusion
From this review it can be concluded that TRPM2 ion channels through various mechanisms such as ROS production, oxidative stress, Ca2+ overload, apoptosis, and inflammation are involved in AKI induced by IR injury. There is a crosstalk between the TRPM2 and its role in oxidative stress, inflammation, apoptosis, and inflammatory cells activation through Ca2+ overload.
The pharmacological inhibition of TRPM2 protects the kidney against IR damage. Therefore, the study of TRPM2 ion channels using their antagonists, agonists, modulators, as well as genetic deletion of TRPM2 and the use of animal models with manipulated TRPM2 channels can provide promising ideas for a better understanding of TRPM2 function under particular renal physiological and pathophysiological conditions and possibly a strategy for treating or even preventing the harmful effects of AKI caused by IR injury.
References
Karimi F, Nematbakhsh M (2021) Mas Receptor Blockade Promotes Renal Vascular Response to Ang II after Partial Kidney Ischemia/Reperfusion in a Two-Kidney-One-Clip Hypertensive Rats Model. International Journal of Nephrology. ;2021
Gholampour F, Karimifard F, Owji S (2015) Berberine improves liver injury following renal ischemia reperfusion in rats. Iran J Sci Technol 39(A1):17
Liu A, Yang B (2019) Roles of TRPM7 in renal ischemia-reperfusion injury. Curr Protein Pept Sci 20(8):777–788
Patschan D, Patschan S, Müller G (2012) Inflammation and microvasculopathy in renal ischemia reperfusion injury. Journal of transplantation. ;2012
De Rosa S, Antonelli M, Ronco C (2017) Hypothermia and kidney: a focus on ischaemia–reperfusion injury. Nephrol Dialysis Transplantation 32(2):241–247
Zhan K-y, Yu P-l, Liu C-h, Luo J-h, Yang W (2016) Detrimental or beneficial: the role of TRPM2 in ischemia/reperfusion injury. Acta Pharmacol Sin 37(1):4–12
Legrand M, Mik EG, Johannes T, Payen D, Ince C (2008) Renal hypoxia and dysoxia after reperfusion of the ischemic kidney. Mol Med 14(7):502–516
Yapca OE, Borekci B, Suleyman H (2013) Ischemia-reperfusion damage. Eurasian J Med 45(2):126
Kalogeris T, Baines CP, Krenz M, Korthuis RJ (2012) Cell biology of ischemia/reperfusion injury. Int Rev cell Mol biology 298:229–317
Li C, Jackson RM (2002) Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiology-Cell Physiol 282(2):C227–C41
Sugiyama S, Hanaki Y, Ogawa T, Hieda N, Taki K, Ozawa T (1988) The effects of SUN 1165, a novel sodium channel blocker, on ischemia-induced mitochondrial dysfunction and leakage of lysosomal enzymes in canine hearts. Biochem Biophys Res Commun 157(2):433–439
Salvadori M, Rosso G, Bertoni E (2015) Update on ischemia-reperfusion injury in kidney transplantation: Pathogenesis and treatment. World J transplantation 5(2):52
Roberts BN, Christini DJ (2011) NHE inhibition does not improve Na + or Ca2 + overload during reperfusion: using modeling to illuminate the mechanisms underlying a therapeutic failure. PLoS Comput Biol 7(10):e1002241
Inserte J, Hernando V, Garcia-Dorado D (2012) Contribution of calpains to myocardial ischaemia/reperfusion injury. Cardiovascular Res 96(1):23–31
Chatauret N, Badet L, Barrou B, Hauet T (2014) Ischemia-reperfusion: From cell biology to acute kidney injury. Progrès en urologie 24:S4–S12
Granger DN, Kvietys PR (2015) Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol 6:524–551
Lemasters J, Bond J, Chacon E, Harper I, Kaplan S, Ohata H et al (1996) The pH paradox in ischemia-reperfusion injury to cardiac myocytes. Myocardial ischemia: mechanisms, reperfusion, protection. :99–114
Heyman SN, Rosenberger C, Rosen S (2010) Experimental ischemia–reperfusion: biases and myths—the proximal vs. distal hypoxic tubular injury debate revisited. Kidney Int 77(1):9–16
Schrier RW, Arnold PE, Van Putten VJ, Burke TJ (1987) Cellular calcium in ischemic acute renal failure: role of calcium entry blockers. Kidney Int 32(3):313–321
Takahashi N, Kozai D, Kobayashi R, Ebert M, Mori Y (2011) Roles of TRPM2 in oxidative stress. Cell Calcium 50(3):279–287
Yu P, Xue X, Zhang J, Hu X, Wu Y, Jiang L-H et al (2017) Identification of the ADPR binding pocket in the NUDT9 homology domain of TRPM2. J Gen Physiol 149(2):219–235
Hiroi T, Wajima T, Negoro T, Ishii M, Nakano Y, Kiuchi Y et al (2013) Neutrophil TRPM2 channels are implicated in the exacerbation of myocardial ischaemia/reperfusion injury. Cardiovascular Res 97(2):271–281
Uchida K, Tominaga M (2011) TRPM2 modulates insulin secretion in pancreatic β-cells. Islets 3(4):209–211
Miller BA, Cheung JY (2016) TRPM2 protects against tissue damage following oxidative stress and ischaemia–reperfusion. J Physiol 594(15):4181–4191
Marko L, Mannaa M, Haschler T, Krämer S, Gollasch M (2017) Renoprotection: focus on TRPV 1, TRPV 4, TRPC 6 and TRPM 2. Acta Physiol 219(3):591–614
Guinamard R, Paulais M, Lourdel S, Teulon J (2012) A calcium-permeable non-selective cation channel in the thick ascending limb apical membrane of the mouse kidney. Biochim et Biophys Acta (BBA)-Biomembranes 1818(5):1135–1141
Huang Y, Fliegert R, Guse AH, Lü W, Du J (2020) A structural overview of the ion channels of the TRPM family. Cell Calcium 85:102111
Zhang W, Hirschler-Laszkiewicz I, Tong Q, Conrad K, Sun S-C, Penn L et al (2006) TRPM2 is an ion channel that modulates hematopoietic cell death through activation of caspases and PARP cleavage. Am J Physiology-Cell Physiol 290(4):C1146–C59
Rosenbaum T (2015) Activators of TRPM2: Getting it right. J Gen Physiol 145(6):485–487
Zhang Z, Tóth B, Szollosi A, Chen J, Csanády L (2018) Structure of a TRPM2 channel in complex with Ca2 + explains unique gating regulation. Elife 7:e36409
Gao G, Wang W, Tadagavadi RK, Briley NE, Love MI, Miller BA et al (2014) TRPM2 mediates ischemic kidney injury and oxidant stress through RAC1. J Clin Investig 124(11):4989–5001
Wang L, Fu T-M, Zhou Y, Xia S, Greka A, Wu H (2018) Structures and gating mechanism of human TRPM2. Science 362:6421
Abumaria N, Li W, Clarkson AN (2019) Role of the chanzyme TRPM7 in the nervous system in health and disease.Cellular and Molecular Life Sciences. :1–10
Tong Q, Zhang W, Conrad K, Mostoller K, Cheung JY, Peterson BZ et al (2006) Regulation of the transient receptor potential channel TRPM2 by the Ca2 + sensor calmodulin. J Biol Chem 281(14):9076–9085
Turlova E, Feng Z-p, Sun H-s (2018) The role of TRPM2 channels in neurons, glial cells and the blood-brain barrier in cerebral ischemia and hypoxia. Acta Pharmacol Sin 39(5):713–721
Cheung JY, Miller BA (2017) Transient Receptor Potential–Melastatin Channel Family Member 2: Friend or Foe. Trans Am Clin Climatol Assoc 128:308
Eraslan E, Tanyeli A, Polat E, Polat E (2019) 8-Br‐cADPR, a TRPM2 ion channel antagonist, inhibits renal ischemia–reperfusion injury. J Cell Physiol 234(4):4572–4581
Malko P, Jiang L-H (2020) TRPM2 channel-mediated cell death: An important mechanism linking oxidative stress-inducing pathological factors to associated pathological conditions.Redox Biology. :101755
Nazıroğlu M (2007) New molecular mechanisms on the activation of TRPM2 channels by oxidative stress and ADP-ribose. Neurochem Res 32(11):1990–2001
McHugh D, Flemming R, Xu S-Z, Perraud A-L, Beech DJ (2003) Critical intracellular Ca2 + dependence of transient receptor potential melastatin 2 (TRPM2) cation channel activation. J Biol Chem 278(13):11002–11006
Starkus J, Beck A, Fleig A, Penner R (2007) Regulation of TRPM2 by extra-and intracellular calcium. J Gen Physiol 130(4):427–440
Kolisek M, Beck A, Fleig A, Penner R (2005) Cyclic ADP-ribose and hydrogen peroxide synergize with ADP-ribose in the activation of TRPM2 channels. Mol Cell 18(1):61–69
Ramesh G, Reeves WB (2004) Inflammatory cytokines in acute renal failure. Kidney Int 66:S56–S61
Furuichi K, Wada T, Kaneko S, Murphy PM (2008) Roles of chemokines in renal ischemia/reperfusion injury. Front Biosci 13:4021–4028
Stroo I, Stokman G, Teske GJ, Raven A, Butter LM, Florquin S et al (2010) Chemokine expression in renal ischemia/reperfusion injury is most profound during the reparative phase. Int Immunol 22(6):433–442
Rabb H, O’Meara YM, Maderna P, Coleman P, Brady HR (1997) Leukocytes, cell adhesion molecules and ischemic acute renal failure. Kidney Int 51(5):1463–1468
Kher A, Meldrum KK, Wang M, Tsai BM, Pitcher JM, Meldrum DR (2005) Cellular and molecular mechanisms of sex differences in renal ischemia–reperfusion injury. Cardiovascular Res 67(4):594–603
Thurman JM (2007) Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol 123(1):7–13
Malek M, Nematbakhsh M (2015) Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Ren injury Prev 4(2):20
Robledo-Avila FH, Ruiz-Rosado JdD, Brockman KL, Partida-Sánchez S (2020) The TRPM2 Ion channel regulates inflammatory functions of neutrophils during listeria monocytogenes infection. Front Immunol 11:97
Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y et al (2008) TRPM2-mediated Ca 2 + influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med 14(7):738–747
Liu T, Zhang L, Joo D, Sun S-C (2017) NF-κB signaling in inflammation. Signal Transduct Target therapy 2(1):1–9
Faouzi M, Penner R (2014) Trpm2. Mammalian Transient Receptor Potential (TRP) Cation Channels. :403–26
Sung FL, Zhu TY, Au-Yeung KK, Siow YL, Karmin O (2002) Enhanced MCP-1 expression during ischemia/reperfusion injury is mediated by oxidative stress and NF-κB. Kidney Int 62(4):1160–1170
Cao CC, Ding XQ, Ou ZL, Liu CF, Li P, Wang L et al (2004) In vivo transfection of NF-κB decoy oligodeoxynucleotides attenuate renal ischemia/reperfusion injury in rats. Kidney Int 65(3):834–845
Kenan Kinaci M, Erkasap N, Kucuk A, Koken T, Tosun M (2012) Effects of quercetin on apoptosis, NF-κB and NOS gene expression in renal ischemia/reperfusion injury. Experimental and Therapeutic Medicine 3(2):249–254
Wang Y, Chen L, Wang K, Da Y, Zhou M, Yan H et al (2019) Suppression of TRPM2 reduces renal fibrosis and inflammation through blocking TGF-β1-regulated JNK activation. Biomed Pharmacother 120:109556
Kurata Y, Tanaka T, Cernecka H, Eitner F, Nangaku M (2022) TRPM2 Plays a Minor Role in AKI and Kidney Fibrosis. Kidney360 3(1):153
Langley B, Ratan RR (2004) Oxidative stress-induced death in the nervous system: Cell cycle dependent or independent? J Neurosci Res 77(5):621–629
Chandra J, Samali A, Orrenius S (2000) Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med 29(3–4):323–333
Bardaweel SK, Gul M, Alzweiri M, Ishaqat A, ALSalamat HA, Bashatwah RM (2018) Reactive oxygen species: The dual role in physiological and pathological conditions of the human body. Eurasian J Med 50(3):193
Dias V, Junn E, Mouradian MM (2013) The role of oxidative stress in Parkinson’s disease. J Parkinson’s disease 3(4):461–491
Makris D, Mertens PR, Dounousi E, Giamouzis G, Nseir S (2018) Oxidative Stress in the Critically Ill Patients. Pathophysiology and Potential Interventions. Hindawi
Kehrer JP (1993) Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol 23(1):21–48
Cakir M, Polat A, Tekin S, Vardi N, Taslidere E, Rumeysa Duran Z et al (2015) The effect of dexmedetomidine against oxidative and tubular damage induced by renal ischemia reperfusion in rats. Ren Fail 37(4):704–708
Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T et al (2002) LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell 9(1):163–173
Fonfria E, Marshall IC, Benham CD, Boyfield I, Brown JD, Hill K et al (2004) TRPM2 channel opening in response to oxidative stress is dependent on activation of poly (ADP-ribose) polymerase. Br J Pharmacol 143(1):186–192
Hack CT, Buck T, Bagnjuk K, Eubler K, Kunz L, Mayr D et al (2019) A Role for H2O2 and TRPM2 in the Induction of Cell Death: Studies in KGN Cells. Antioxidants 8(11):518
Di A, Mehta D, Malik AB (2016) ROS-activated calcium signaling mechanisms regulating endothelial barrier function. Cell Calcium 60(3):163–171
Miller BA, Zhang W (2011) TRP channels as mediators of oxidative stress.Transient Receptor Potential Channels. :531–44
Catara G, Grimaldi G, Schembri L, Spano D, Turacchio G, Monte ML et al (2017) PARP1-produced poly-ADP-ribose causes the PARP12 translocation to stress granules and impairment of Golgi complex functions. Sci Rep 7(1):1–17
Kashio M, Tominaga M (2017) Redox-Sensitive Trp Channels: Trpa1 and Trpm2. Redox: Principles and Advanced Applications. :203
Jiang L-H, Li X, Mortadza SAS, Lovatt M, Yang W (2018) The TRPM2 channel nexus from oxidative damage to Alzheimer’s pathologies: An emerging novel intervention target for age-related dementia. Ageing Res Rev 47:67–79
Kosieradzki M, Rowiński W (eds) (2008) Ischemia/reperfusion injury in kidney transplantation: mechanisms and prevention. Transplantation proceedings: Elsevier
Orrenius S, Zhivotovsky B, Nicotera P (2003) Regulation of cell death: the calcium–apoptosis link. Nat Rev Mol Cell Biol 4(7):552–565
Wehage E, Eisfeld I Jr, Jüngling E, Zitt C, Lückhoff A (2002) Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide: a splice variant reveals a mode of activation independent of ADP-ribose. J Biol Chem 277(26):23150–23156
Zhang W, Chu X, Tong Q, Cheung JY, Conrad K, Masker K et al (2003) A novel TRPM2 isoform inhibits calcium influx and susceptibility to cell death. J Biol Chem 278(18):16222–16229
Kar F, Hacioglu C, Senturk H, Donmez DB, Kanbak G (2020) The role of oxidative stress, renal inflammation, and apoptosis in post ischemic reperfusion injury of kidney tissue: the protective effect of dose-dependent boric acid administration. Biol Trace Elem Res 195(1):150–158
LaFavers KA, Macedo E, Garimella PS, Lima C, Khan S, Myslinski J et al (2019) Circulating uromodulin inhibits systemic oxidative stress by inactivating the TRPM2 channel. Sci Transl Med 11(512):eaaw3639
Schriewer JM, Peek CB, Bass J, Schumacker PT (2013) ROS-Mediated PARP activity undermines mitochondrial function after permeability transition pore opening during myocardial ischemia–reperfusion. J Am Heart Association 2(2):e000159
Chen S, Meng X-F, Zhang C (2013) Role of NADPH oxidase-mediated reactive oxygen species in podocyte injury. BioMed research international. 2013
Morales J, Li L, Fattah FJ, Dong Y, Bey EA, Patel M et al (2014) Review of poly (ADP-ribose) polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases.Critical Reviews™ in Eukaryotic Gene Expression. 24(1)
Buelow B, Song Y, Scharenberg AM (2008) The Poly (ADP-ribose) polymerase PARP-1 is required for oxidative stress-induced TRPM2 activation in lymphocytes. J Biol Chem 283(36):24571–24583
Zhang F, Xie R, Munoz FM, Lau SS, Monks TJ (2014) PARP-1 hyperactivation and reciprocal elevations in intracellular Ca2 + during ROS-induced nonapoptotic cell death. Toxicol Sci 140(1):118–134
Giorgi C, Baldassari F, Bononi A, Bonora M, De Marchi E, Marchi S et al (2012) Mitochondrial Ca2 + and apoptosis. Cell Calcium 52(1):36–43
Miller BA (2004) Inhibition of TRPM2 function by PARP inhibitors protects cells from oxidative stress-induced death. Br J Pharmacol 143(5):515–516
Yildar M, Aksit H, Korkut O, Ozyigit MO, Sunay B, Seyrek K (2014) Protective effect of 2-aminoethyl diphenylborinate on acute ischemia–reperfusion injury in the rat kidney. J Surg Res 187(2):683–689
Kashio M, Tominaga M (2017) The TRPM2 channel: a thermo-sensitive metabolic sensor. Channels 11(5):426–433
Facchinetti F, Furegato S, Terrazzino S, Leon A (2002) H2O2 induces upregulation of Fas and Fas ligand expression in NGF-differentiated PC12 cells: Modulation by cAMP. J Neurosci Res 69(2):178–188
Takeyama N, Miki S, Hirakawa A, Tanaka T (2002) Role of the mitochondrial permeability transition and cytochrome C release in hydrogen peroxide-induced apoptosis. Exp Cell Res 274(1):16–24
Li L, Sha Z, Wang Y, Yang D, Li J, Duan Z et al (2019) Pre–treatment with a combination of Shenmai and Danshen injection protects cardiomyocytes against hypoxia/reoxygenation–and H2O2–induced injury by inhibiting mitochondrial permeability transition pore opening. Experimental and therapeutic medicine 17(6):4643–4652
Acknowledgements
The author (s) received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Compliance with ethical standards
This manuscript does not contain any human or animal studies by the authors. This manuscript is an overview of research on the subject of Pathological Mechanisms Induced by TRPM2 Ion Channels Activation in Renal Ischemia-Reperfusion Injury” and the author (s) received no financial support for the research, authorship, and/or publication of this manuscript. This manuscript does not contain any studies with human participants performed by any of the authors. This manuscript does not contain any studies with animals performed by any of the authors. This manuscript does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khanahmad, H., Mirbod, S.M., karimi, F. et al. Pathological Mechanisms Induced by TRPM2 Ion Channels Activation in Renal Ischemia-Reperfusion Injury. Mol Biol Rep 49, 11071–11079 (2022). https://doi.org/10.1007/s11033-022-07836-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07836-w