Abstract
Background
Icariin, the main pharmacological active flavonoid extracted from Epimedi herba, can regulate cellular processes in diverse diseases. The aim of this study was to explore the effects and mechanisms of icariin on proliferation and adipogenesis of bone marrow mesenchymal stem cells (BMSCs) in aplastic anemia (AA).
Methods and results
Bone marrow mesenchymal stem cells were isolated from posterior tibias and femurs of AA rats that were induced by benzene and cyclophosphamide and gavaged with icariin. The isolated BMSCs were characterized morphologically and immunologically by positive markers (CD29 and CD90) and negative markers (CD34 and CD45). CCK-8 assay was performed to examine the BMSCs proliferation. Cell apoptosis and cell cycle were detected by flow cytometry. Oil red O staining was carried out to evaluate the adipogenesis of BMSCs. The mRNA expression of PPARγ, C/EBP-α, and FABP4 was measured by qRT-PCR. The protein levels of p-p38/p38, p-JNK/JNK, p-ERK/ERK, PPARγ, C/EBP-α, and FABP4 were detected using Western blotting. Icariin promoted the proliferation of BMSCs from AA rats in a dose-dependent manner. The protein levels of p-p38/p38, p-JNK/JNK, and p-ERK/ERK were downregulated in BMSCs from AA rats after icariin treatment. Icariin inhibited the apoptosis and arrested cell cycle at G/S phase of BMSCs from AA rats. The adipogenesis of BMSCs from AA rats was also suppressed after icariin treatment. However, the effects of icariin on BMSCs were weakened by p38 agonist addition.
Conclusions
Icariin promoted the proliferation and inhibited the apoptosis and adipogenesis of BMSCs in AA by suppressing MAPK pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aplastic anemia (AA) is a rare and life-threatening bone marrow failure characterized by fatty bone marrow and poor hematopoietic function [1]. The decrease of hematopoietic function can lead to the genitourinary system disorder, intracranial hemorrhage, sepsis, etc., affecting normal life and work of patients [2]. Currently, the major therapeutic strategies for AA are hematopoietic stem cell transplantation (HSCT) and immunosuppressive therapy (IST) [3]. However, it is difficult to find a matched donor for HSCT, and some lymphocytotoxic agents used in IST may cause complications, such as infections, anaphylaxis fever, and subsequent malignant conditions [4,5,6]. Therefore, it is urgent to find promising drugs with low side-effects for AA treatment.
The changes of bone marrow hematopoietic microenvironment are closely associated with the pathogenesis of AA [7]. This microenvironment consists of diverse cells, including bone marrow mesenchymal stem cells (BMSCs) [8]. BMSCs act as a critical role in AA progression due to its multi-differentiation ability, which can differentiate to osteoblasts, chondrocytes, adipocytes, etc. [9]. Numerous studies have reported that BMSCs from AA patients appear proliferative defects and the enhanced adipocyte differentiation [10,11,12]. Luo et al. found that the proliferation capacity of BMSCs was reduced and adipocytes were increased in AA rats [11]. Li et al. also denoted that the adipogenic differentiation of BMSCs from children with AA was potentiated [12]. Therefore, finding effective drugs that can protect the proliferation and restrain the adipocyte differentiation of BMSCs in AA is of paramount importance.
Icariin is the main flavonoid constituent extracted from Epimedi herba [13]. It has been reported that icariin can affect the proliferation, apoptosis, and differentiation of BMSCs [14,15,16]. For instance, icariin can promote the proliferation of BMSCs from rats with fractures [14]. Icariin also exhibits the effect of inhibiting adipogenic differentiation of BMSCs [15]. However, the effects of icariin on the growth and differentiation of BMSC in AA remain unclear.
Mitogen-activated protein kinase (MAPK) pathway is an intracellular signaling pathway closely involved in the regulation of cell proliferation and differentiation [17]. Several previous studies indicate that MAPK pathway is related to the adipogenic differentiation of BMSCs [18,19,20]. Cho et al. demonstrated that BMP-2 induced the adipogenic differentiation of human BMSCs via upregulating MAPK pathway [18]. Zhao et al. indicated that MAPK pathway is activated during the adipogenic differentiation of BMSCs [19]. However, it is still illusive whether icariin can inhibit the adipogenesis of BMSCs in AA via affecting MAPK pathway.
In the present study, the effects of icariin on BMSCs isolated from AA rats were investigated by evaluating the proliferation, apoptosis, and adipogenesis of BMSCs. Moreover, the potential mechanism of icariin against adipogenesis of BMSCs in AA involving in MAPK pathway was uncovered. These findings provide a potential therapeutic drug for AA treatment and offer insights into the investigation of underlying mechanisms.
Materials and methods
Experimental animals and treatments
Animal experiments were carried out in compliance with the guidelines of the Animal Ethics Committee of Zhejiang Chinese Medical University Laboratory Animal Research Center. Clean-grade Sprague–Dawley (SD) rats (n = 54, 6 weeks old, weighing 200–250 g) were obtained from the Animal Centre of the Academy of Military Medical Sciences (Beijing, China) and maintained in a temperature-controlled room (24 ± 1 ℃) with a 12 h/12 h light/dark schedule. The AA rat model was established by subcutaneously injecting 1 mL/kg benzene per two days for one week and then intraperitoneally injecting 25 mg/kg cyclophosphamide per two days for three times for two weeks. AA model rats were gavaged with a 1:1 mix of normal saline and dimethyl sulfoxide (DMSO) (the solvent of icariin), different doses of icariin (0.1, 0.5, 1, 5, and 10 μM), and/or 10 μM icariin plus p38 agonist (P79350; Invitrogen, Carlsbad, CA, USA). SD rats in control group were treated with physiological saline. After treatment for three weeks, all rats were sacrificed by 3% 150 mg/kg pentobarbital sodium and disinfected with 75% ethanol for isolating BMSCs.
Isolation and characterization of BMSCs
BMSCs were isolated from the femurs and tibias of SD rats. Briefly, femurs and tibias were removed from SD rats and washed with phosphate-buffered saline (PBS) three times in a sterile environment. BMSCs were flushed from posterior tibias and femurs with PBS and then cultured in Roswell Park Memorial Institute (RPMI) medium containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 1% l-glutamine. Third generation well-grown cells were harvested and digested with 0.25% trypsin. After centrifuging for 5 min at 4 ℃ and 1000 rpm, cells were resuspended and identified under a light microscope (Olympus, Japan).
BMSCs isolated from AA rats were analyzed for the presence of MSCs specific markers (CD29, CD34, CD45, and CD90) by flow cytometry. In brief, BMSCs were washed with PBS and blocked with 2% bovine serum albumin (BSA). Then, cells were incubated with primary antibodies against CD29, CD34, CD45, and CD90 for 2 h, followed by the secondary antibody (Alexa fluor 546). Finally, BMSCs were analyzed using a flow cytometer (BD Biosciences, NJ, USA).
Cell proliferation assay
BMSCs suspension was inoculated into a 96-well plate (1 × 103 cells/well) and cultured for 24, 48, and 72 h at 37 ℃. Then, 10 μL CCK-8 solution (Beyotime, China) was added to the wells to incubating for 2 h. Subsequently, the absorbances were measured at 450 nm using a Varioskan™ LUX multimode microplate reader (Thermo scientific, CA, USA).
Flow cytometry
BMSCs cells treated with icariin for 24 h were resuspended in 100 µL binding buffer to 1 × 106 cells/mL. The apoptosis of BMSCs was detected using an Annexin V/propidium iodide (PI) apoptosis detection kit (KeyGen Biotech, China). Cells were stained with 10 µL fluorescein isothiocyanate (FITC)-Annexin V and 5 µL PI under darkness. For cell cycle detection, cells were fixed in 70% ethanol for 12 h at 4 °C. Then, fixed cells were incubated with 10 µg/mL PI and 100 µg/mL RNase for 30 min at 37 °C in the dark. Finally, apoptosis and cell cycle condition were detected using a flow cytometer with CellQuest software (BD Biosciences, NJ, USA).
Adipocyte differentiation and oil red O staining
BMSCs were seeded in six-well plates (4 × 104 cells/well) and cultured in adipogenic-induction medium (high sugar Dulbecco’s Modified Eagle Medium (DMEM) supplemented with dexamethasone 10-8 M, B-glycerin, and ascorbic acid). After 20 days of adipocyte differentiation, cells were fixed with 4% paraformaldehyde and then stained with oil red O (Cyagen Biosciences, China). Finally, the lipid droplet formation was observed under a microscope (Olympus, Japan).
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from BMSCs using TRIzol reagent (Invitrogen, CA, USA). cDNA was synthesized using a PrimeScript RT reagent kit (Tiangen, China). qRT-PCR was performed using the SYBR@ Green reagent (Roche, IN, USA) in a Roche LightCycler® 96 System. The reaction program of qRT-PCR was 95 ℃ for 2 min, and 40 cycles of 95 ℃ for 20 s, 56 ℃ for 20 s, and 72 ℃ for 20 s. The primers were presented in Table 1. The relative mRNA expression was calculated using the 2−∆∆Ct method. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control.
Western blotting
Total protein of BMSCs was extracted by lysing in ice-cold lysis buffer with protease inhibitor cocktail (Sigma, CA, USA) for 30 min. The protein lysates were separated through SDS-PAGE and then transferred to polyvinylidene fluoride membranes (Millipore, MA, USA). After incubating with blocking buffer, membranes were incubated with primary antibodies, including anti-p38, p-p38, JNK, p-JNK, ERK, p-ERK, PPARγ, C/EBP-α, FABP4, and GAPDH (1:500, CST, MA, USA), at 4 ℃ overnight. Followed by that, membranes were incubated with the horseradish peroxidase-conjugated secondary antibody (1:1000, CST) for 1 h. Protein images were visualized using an enhanced chemiluminescence detection kit (Solarbio, China) and captured using the ChemiDoc Imaging System (Bio-Rad, CA, USA). GAPDH was used as the loading control.
Statistical analysis
Each experiment was performed in triplicate. All data were presented as the mean ± standard deviation. Statistical analyses were performed using SPSS 27.0 (IBM, NY, USA). Statistically significant differences were calculated using one-way analysis of variance followed by Tukey’s test. P < 0.05 was considered statistically significant.
Results
Morphological characterization of BMSCs in AA model
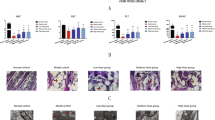
BMSCs were isolated from the tibia and femur of rats to identify the morphological characteristics of AA and normal tissues. BMSCs presented shorter spindle-shaped morphology and arranged irregularly than normal cells. Moreover, the vacuoles were increased in BMSCs from AA tissues compared with that from control tissues (Fig. 1A). Further, the isolated BMSCs from AA rats showed the positive expression of MSC biomarkers CD29 and CD90, and the negative expression of CD34 and CD45 (Fig. 1B).
Icariin promotes the proliferation of BMSCs in AA
To investigate the effect of icariin on BMSCs in AA, the proliferation of BMSCs were evaluated. As shown in Fig. 2, the proliferation ability of BMSCs from AA model was significantly declined after cultured for 48 h and 72 h compared with that from control (P < 0.001). Icariin treatment rescued the decreased proliferation of BMSCs from AA model in a dose-dependent manner. In particular, when treated with 10 μM icariin, the proliferation of BMSCs was significantly increased (P < 0.01) (Fig. 2). Therefore, 10 μM icariin was used to treat BMSCs in the subsequent experiments.
Icariin dose-dependently promotes the BMSCs proliferation in AA The proliferation of BMSCs from AA rats was measured by CCK-8 assay. AA rats (n = 6 per group) were treated with different doses of icariin (0.1, 0.5, 1, 5, 10 µm) for 24, 48, and 72 h. ***P < 0.001 vs. the control; #P < 0.05, ##P < 0.01, and ###P < 0.001 vs. the AA model; ∆∆P < 0.01 and ∆∆∆P < 0.001 vs. the 0.1 µm icariin treatment
Icariin inhibits the MAPK signaling pathway in BMSCs from AA model
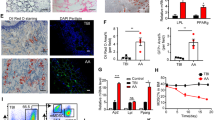
It has been reported that MAPK signaling pathway is involved in regulating BMSCs proliferation and differentiation [21]. p38, JNK, and ERK are three major protein kinases of MAPK pathway [22]. Our results showed that the protein expression of phosphor (p)-p38/p38, p-JNK/JNK, and p-ERK/ERK in BMSCs from AA model were significantly up-regulated compared with that from control (P < 0.001). Icariin administration down-regulated the expression of p-p38/p38, p-JNK/JNK, and p-ERK/ERK in BMSCs from AA model, whereas p38 agonist weakened the effect of icariin (Fig. 3).
Icariin inhibits the MAPK pathway in BMSCs of AA The relative protein levels of p-p38/p38, p-JNK/JNK, and p-ERK/ERK were determined by Western blotting. AA rats (n = 6 per group) were treated with 10 µm icariin and/or p38 agonist. ***P < 0.001 vs. the control; ##P < 0.01 and ###P < 0.001 vs. the AA model; ∆P < 0.05 and ∆∆P < 0.01 vs. the icariin treatment
Icariin suppresses the apoptosis of BMSCs from AA model via inhibiting MAPK signaling pathway
To further confirm the promotive effect of icariin on BMSCs proliferation in AA, the apoptosis of BMSCs was determined. Flow cytometry showed that the apoptosis of BMSCs was significantly increased in the AA model compared to that in the control (P < 0.001). Icariin treatment dramatically decreased the apoptosis of BMSCs in the AA model (P < 0.001) (Fig. 4A). In addition, the cell cycle phase ratio was also evaluated. BMSCs from AA model presented an increased ratio of G/S phase in comparison to the control (P < 0.001). After icariin treatment, the ratio of G/S phase was dramatically reduced in BMSCs from AA model (P < 0.05) (Fig. 4B). However, p38 agonist partially offset the effects of icariin on inhibiting the apoptosis and arresting cell cycle of BMSCs from AA rats (P < 0.05) (Fig. 4A, B).
Icariin suppresses the apoptosis of BMSCs in AA via inhibiting MAPK pathway A The apoptosis of BMSCs from AA rats was measured by flow cytometry. B The cell cycle of BMSCs from AA rats was measured by flow cytometry. AA rats (n = 6 per group) were treated with 10 µm icariin and/or p38 agonist. ***P < 0.001 vs. the control; #P < 0.05 and ###P < 0.001 vs. the AA model; ∆P < 0.05 vs. the icariin treatment
Icariin inhibits adipogenic differentiation of BMSCs in AA via retarding MAPK pathway
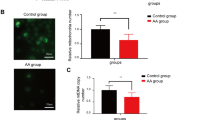
AA is manifested as the decreased hematopoietic function of bone marrow, mainly caused by adipocyte differentiation of BMCSs. Oil Red O staining showed that lipid droplets increased in BMSCs from AA model compared with that from control. Icariin inhibited the lipid droplet formation in BMSCs from AA model, whereas p38 agonist weakened the repressive effect of icariin (Fig. 5A). Moreover, PPARγ, C/EBP-α, and FABP4 are three adipogenic biomarkers closely related to adipocyte differentiation [23]. We found that the mRNA and protein expression of PPARγ, C/EBP-α, and FABP4 was significantly up-regulated in BMSCs from AA model compared to that from control (P < 0.01). Icariin down-regulated the expression of PPARγ, C/EBP-α, and FABP4 in BMSCs from AA model, whereas p38 agonist alleviated the effect of icariin (P < 0.05) (Fig. 5B, C).
Icariin retards the adipogenic differentiation of BMSCs in AA via inhibiting MAPK pathway A The mature adipocytes differentiated from BMSCs of AA rats were visualized using oil red O staining. Scale bar = 25 µm. B The relative mRNA expression of PPARγ, C/EBPα, and FABP4 was measured by qRT-PCR. C The relative protein levels of PPARγ, C/EBP-α, and FABP4 were determined by Western blotting. AA rats (n = 6 per group) were treated with 10 µm icariin and/or p38 agonist. **P < 0.01 and ***P < 0.001 vs. the control; ##P < 0.01 and ###P < 0.001 vs. the AA model; ∆P < 0.05, ∆∆P < 0.01, and ∆∆∆P < 0.001 vs. the icariin treatment
Discussion
AA is a bone marrow dysplasia disease induced by hematopoietic progenitor cell damage [24]. The adipogenic differentiation of BMSCs is one of the major pathological characteristics of AA [25]. Icariin, a flavonoid extracted from Epimedi herba, possesses the ability of affecting BMSC adipogenesis [26]. In this study, we found that icariin promoted the proliferation, and inhibited apoptosis and adipogenic differentiation of BMSCs from AA rats. Moreover, the effects of icariin on BMSCs proliferation and adipogenesis in AA were regulated via the inhibition of MAPK signaling pathway.
BMSCs play an essential role in bone marrow hematopoietic microenvironment that is involved in the pathogenesis of AA [8, 11]. AA is characterized by hypoplasia and pancytopenia with increasing fat cells in the bone marrow. BMSCs from AA are more susceptible to be induced into adipogenic differentiation, therefore, adipogenesis has become a critical feature of AA [27]. Previous studies have reported that BMSCs in AA present the decreased proliferation and the increased adipogenesis [28]. Luo et al. found that BMSCs from AA rats manifested as poor proliferation behavior and enhanced adipogenic differentiation [11]. Li et al. revealed that BMSCs from children with AA displayed a better adipogenic differentiation capacity [12]. Consistent with previous studies, we observed the reduced proliferation, and the increased apoptosis and adipogenesis in BMSCs isolated from AA rats after. These results suggest that BMSCs growth is inhibited, whereas its adipogenic differentiation is potentiated in AA.
Icariin is the major flavonoid constituent from Epimedi herba, which has been reported to affect BMSC proliferation and differentiation [29, 30]. A study conducted by Wu et al. showed that icariin dose-dependently promoted the proliferation of BMSCs [30]. Similarly, our study found that icariin exhibited a dose-dependent effect on facilitating the proliferation ability of BMSCs from AA rats. Icariin also reduced the apoptosis and cell cycle G/S ratio of BMSCs from AA rats, indicating that icariin has the efficacy of promoting BMSC proliferation in AA. In addition, some previous studies denoted that icariin can suppress the adipogenic differentiation of BMSCs [15, 31]. For instance, icariin inhibited the BMSCs differentiation into adipocyte in ovariectomized mice [15, 31]. In the current study, oil red O staining showed that oil droplet deposition was increased in BMSCs from AA rats after icariin treatment. PPARγ and C/EBPα are coordinated to control adipogenic differentiation, and FABP4 regulated late adipogenesis [32, 33]. Icariin also downregulated the expression of PPARγ, C/EBP-α, and FABP4 in BMSCs of AA rats. These results demonstrate that icariin exerts an anti-adipogenic effect on BMSCs in AA.
MAPK signaling pathway acts as a critical role in cell proliferation, apoptosis, and differentiation [34]. So far, several publications have reported that MAPK pathway is involved in the regulation of BMSCs proliferation and adipogenic differentiation [18, 35]. Li et al. unveiled that the activation of MAPK pathway can restrain the proliferation of BMSCs [35]. Cho et al. suggested that the enhanced adipogenic differentiation of BMSCs is modulated by the upregulating of MAPK pathway [18]. Therefore, we speculated that the effects of icariin on BMSCs in AA may be regulated by MAPK signaling pathway. p38, JNK, and ERK are three major members of MAPK pathway [34]. As expected, our study found that icariin downregulated the protein levels of p-p38/p38, p-JNK/JNK, and p-ERK/ERK in BMSCs from AA rats. However, p38 agonist weakened the inhibitory effects of icariin on the expression of p-p38/p38, p-JNK/JNK, and p-ERK/ERK, meanwhile, p38 antagonist enhanced the effects of icariin (Supplementary Fig. 1B). These results indicate that icariin can retard the activation of MAPK pathway in BMSCs derived from AA. To further confirmed whether icariin promotes the proliferation and suppresses the adipogenesis of BMSCs via downregulating MAPK pathway, AA rats were treated with icariin plus p38 agonist. Our results showed that p38 agonist diminished the inhibitory effects of icariin on the apoptosis, cell cycle G/S arrest, and adipogenic differentiation of BMSCs in AA. Meanwhile, we found that p38 antagonist potentiated the protective effect of icariin on BMSC proliferation in AA (Supplementary Fig. 1A). These findings demonstrate that icariin facilitates the proliferation and suppresses the adipogenesis of BMSCs in AA via deactivating MAPK pathway.
In conclusion, icariin is a promising therapeutic drug for AA via promoting BMSCs proliferation and adipogenic differentiation. The action mechanism of icariin on BMSCs in AA is involved in the downregulation of MAPK pathway. However, our results also showed that there was an increase in early apoptosis in BMSCs of AA rats treated with icariin or/and p38 agonist, thus, the therapeutic effect of icariin on AA is needed to be further evaluated in vivo and in clinical. The underlying mechanism of icariin on BMSCs in AA should be investigated in more depth. Our findings may provide an effective drug for AA treatment and reveal the potential therapeutic target.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Wang L, Liu H (2019) Pathogenesis of aplastic anemia. Hematology 24:559–566
Young NS (2013) Current concepts in the pathophysiology and treatment of aplastic anemia. Hematol Am Soc Hematol Educ Prog 2013:76–81
Solomou EE (2019) Idiopathic aplastic anemia: an update. Clin Hematol Int 1:52–57
Zhu H, Luo RM, Luan Z, Lee V, Zhu YP, Luo CJ et al (2016) Unmanipulated haploidentical haematopoietic stem cell transplantation for children with severe aplastic anaemia. Br J Haematol 174:799–805
Fu R, Chen T, Song J, Wang G, Li L, Ruan E et al (2017) De-escalation empirical antibiotic therapy improved survival for patients with severe aplastic anemia treated with antithymocyte globulin. Medicine 96:e5905
van der Hem JGK, de Wreede LC, Brand A, Veelken H, Falkenburg JHF, Halkes CJM (2017) Long-term risk of cancer development in adult patients with idiopathic aplastic anemia after treatment with anti-thymocyte globulin. Haematologica 102:e382–e383
Shipounova IN, Petrova TV, Svinareva DA, Momotuk KS, Mikhailova EA, Drize NI (2009) Alterations in hematopoietic microenvironment in patients with aplastic anemia. Clin Transl Sci 2:67–74
Li H, Xu X, Wang D, Zhang Y, Chen J, Li B et al (2021) Hypermethylation-mediated downregulation of long non-coding rna meg3 inhibits osteogenic differentiation of bone marrow mesenchymal stem cells and promotes pediatric aplastic anemia. Int Immunopharmacol 93:107292
Sugimura R, Li L (2010) Shifting in balance between osteogenesis and adipogenesis substantially influences hematopoiesis. J Mol Cell Biol 2:61–62
Tripathy NK, Singh SP, Nityanand S (2014) Enhanced adipogenicity of bone marrow mesenchymal stem cells in aplastic anemia. Stem Cells Int 2014:276862
Luo S, Chen Y, Zhao L, Qi X, Miao X, Zhou H et al (2018) Effect of nutritional supplement on bone marrow-derived mesenchymal stem cells from aplastic anaemia. Br J Nutr 119:748–758
Li H, Xu X, Wang D, Zeng L, Li B, Zhang Y et al (2020) Mir-146b-5p regulates bone marrow mesenchymal stem cell differentiation by siah2/pparγ in aplastic anemia children and benzene-induced aplastic anemia mouse model. Cell Cycle 19:2460–2471
Chen Y, Huang JH, Ning Y, Shen ZY (2011) [icariin and its pharmaceutical efficacy: research progress of molecular mechanism]. Zhong Xi Yi Jie He Xue Bao 9:1179–1184
Zhang X, Chen Y, Zhang C, Zhang X, Xia T, Han J et al (2021) Effects of icariin on the fracture healing in young and old rats and its mechanism. Pharm Biol 59:1245–1255
Huang JM, Bao Y, Xiang W, Jing XZ, Guo JC, Yao XD et al (2017) Icariin regulates the bidirectional differentiation of bone marrow mesenchymal stem cells through canonical wnt signaling pathway. Evid Based Complement Alternat Med 2017:8085325
Qin S, Zhou W, Liu S, Chen P, Wu H (2015) Icariin stimulates the proliferation of rat bone mesenchymal stem cells via erk and p38 mapk signaling. Int J Clin Exp Med 8:7125–7133
Bost F, Aouadi M, Caron L, Binétruy B (2005) The role of mapks in adipocyte differentiation and obesity. Biochimie 87:51–56
Cho JH, Lee JH, Lee KM, Lee CK, Shin DM (2021) Bmp-2 induced signaling pathways and phenotypes: comparisons between senescent and non-senescent bone marrow mesenchymal stem cells. Calcif Tissue Int
Zhao Q, Lu Y, Gan X, Yu H (2017) Low magnitude high frequency vibration promotes adipogenic differentiation of bone marrow stem cells via p38 mapk signal. PLoS ONE 12:e0172954
Xu X, Li X, Yan R, Jiang H, Wang T, Fan L et al (2016) Gene expression profiling of human bone marrow-derived mesenchymal stem cells during adipogenesis. Folia Histochem Cytobiol 54:14–24
Shang Q, Yu X, Ren H, Shen G, Zhao W, Zhang Z et al (2019) Effect of plastrum testudinis extracts on the proliferation and osteogenic differentiation of rbmscs by regulating p38 mapk-related genes. Evid Based Complement Alternat Med 2019:6815620
Lee K, Seo I, Choi MH, Jeong D (2018) Roles of mitogen-activated protein kinases in osteoclast biology. Int J Mol Sci 19:3004
Xiao F, Tang CY, Tang HN, Wu HX, Hu N, Li L et al (2021) Long non-coding rna 332443 inhibits preadipocyte differentiation by targeting runx1 and p38-mapk and erk1/2-mapk signaling pathways. Front Cell Dev Biol 9:663959
Chiu ML, Hsu YL, Chen CJ, Li TM, Chiou JS, Tsai FJ et al (2021) Chinese herbal medicine therapy reduces the risks of overall and anemia-related mortalities in patients with aplastic anemia: a nationwide retrospective study in taiwan. Front Pharmacol 12:730776
Deng S, Zeng Y, Wu L, Hu Z, Shen J, Shen Y et al (2019) The regulatory roles of vegf-notch signaling pathway on aplastic anemia with kidney deficiency and blood stasis. J Cell Biochem 120(2):2078–2089
Xu Y, Jiang Y, Jia B, Wang Y, Li T (2021) Icariin stimulates osteogenesis and suppresses adipogenesis of human bone mesenchymal stem cells via mir-23a-mediated activation of the wnt/β-catenin signaling pathway. Phytomedicine 85:153485
Liu L-L, Liu L, Liu H-H, Ren S-S, Dou C-Y, Cheng P-P et al (2018) Levamisole suppresses adipogenesis of aplastic anaemia-derived bone marrow mesenchymal stem cells through zfp36l1-ppargc1b axis. J Cell Mol Med 22:4496–4506
Chao YH, Peng CT, Harn HJ, Chan CK, Wu KH (2010) Poor potential of proliferation and differentiation in bone marrow mesenchymal stem cells derived from children with severe aplastic anemia. Ann Hematol 89:715–723
Wang Z, Wang D, Yang D, Zhen W, Zhang J, Peng S (2018) The effect of icariin on bone metabolism and its potential clinical application. Osteoporos Int 29:535–544
Wu Y, Xia L, Zhou Y, Xu Y, Jiang X (2015) Icariin induces osteogenic differentiation of bone mesenchymal stem cells in a mapk-dependent manner. Cell Prolif 48:375–384
Liu H, Xiong Y, Zhu X, Gao H, Yin S, Wang J et al (2017) Icariin improves osteoporosis, inhibits the expression of pparγ, c/ebpα, fabp4 mrna, n1icd and jagged1 proteins, and increases notch2 mrna in ovariectomized rats. Exp Ther Med 13:1360–1368
Ge C, Cawthorn WP, Li Y, Zhao G, Macdougald OA, Franceschi RT (2016) Reciprocal control of osteogenic and adipogenic differentiation by erk/map kinase phosphorylation of runx2 and pparγ transcription factors. J Cell Physiol 231:587–596
Gao Q, Jiang Y, Dai S, Wang B, Gao F, Guo C, et al (2012) Interleukin 17a exacerbates atherosclerosis by promoting fatty acid-binding protein 4-mediated er stress in macrophages. Circ Res
Yue J, López JM (2020) Understanding mapk signaling pathways in apoptosis. Int J Mol Sci 21:2346
Li Z, Wang X, Hong TP, Wang HJ, Gao ZY, Wan M (2021) Advanced glycosylation end products inhibit the proliferation of bone-marrow stromal cells through activating mapk pathway. Eur J Med Res 26:94
Acknowledgements
Not applicable.
Funding
The work was supported by [Zhejiang Province Natural Science Foundation] [Grant numbers LQ18H080003]; and [National Natural Science Foundation of China] [Grant numbers 81903959].
Author information
Authors and Affiliations
Contributions
Study conception and design by SD, JS and YZ, Obtaining funding by SD, data analysis was performed by JX, the first draft of the manuscript was written by SL and YZ, revision of manuscript for important intellectual content by JS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of The First Affiliated Hospital of Zhejiang Chinese Medical University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11033_2022_7645_MOESM1_ESM.tif
Supplementary file1 Supplementary Fig. 1 Icariin exerts the protective effect on BMSC proliferation in AA via inhibiting MAPK pathway (A) The proliferation of BMSCs from AA rats was measured by CCK-8 assay. (B) The relative protein levels of p-p38/P38, p-JNK/JNK, and p-ERK/ERK were determined by Western blotting. AA rats (n = 6 per group) were treated with 10 µm icariin, p38 agonist, and/or p38 antagonist. *P < 0.05, **P < 0.01. (TIF 1816 kb)
Rights and permissions
About this article
Cite this article
Deng, S., Zeng, Y., Xiang, J. et al. Icariin protects bone marrow mesenchymal stem cells in aplastic anemia by targeting MAPK pathway. Mol Biol Rep 49, 8317–8324 (2022). https://doi.org/10.1007/s11033-022-07645-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07645-1