Abstract
Background
Tim-3/Galectin-9 is involved in the immune escape of many pathogens. However, the role of Tim-3/Galectin-9 in persistent infection of Echinococcus multilocularis (Em), which is related to immune escape, is still unclear.
Objective
To investigate the role of Tim-3/Galectin-9 and related cytokines in mice with persistent infection of Em.
Methods
Em infection model was established by injecting the protoscoleces. Serum was collected at days 2, 8, 30, 60, 90, 180 and 270 after infection. Lymphocytes were isolated from liver tissue samples with Ficoll. Tim-3 + CD4 + T percentage was analyzed by flow cytometry. CD4 + T cells were isolated from liver tissues of Em infected mice and cultured in vitro. The mRNA levels of Tim-3, Galectin-9, IFN-γ and IL-4 were detected by qRT-PCR. Cytokine levels in serum and culture supernatant (IFN-γ and IL-4) were analyzed by cytometric bead array.
Results
The expression of Tim-3 and Galectin-9 mRNA significantly increased after 30 days of infection, reached peak on day 90, and then decreased slightly on days 180–270. The expression of IFN-γ mRNA, increased on day 2 and 8 after infection, slightly decreased on days 30–60, and obvious decreased on days 90–270, but were still higher than those of the control group. The expression of IL-4 mRNA gradually increased along with the time of infection. In serum of Em infected mice, level of IFN-γ peaked at day 30 and then gradually decreased; whereas IL-4 level peaked at day 90 and then gradually decreased. In vitro experiment found that Tim-3/Galectin-9 directly caused the changes in the levels of IFN-γ and IL-4.
Conclusions
Tim-3/Galectin-9 signaling pathway may be involved in the development of persistent infection of Em by regulating the production of Th1 and Th2 cytokines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Echinococcosis is a zoonosis caused by cestodes of the genus Echinococcus. The incidence of echinococcosis is high in Xinjiang, Gansu, Tibet and the Ganzi Tibetan Autonomous Prefecture of Sichuan Province of China [1]. The two main types of this disease are cystic echinococcosis (CE) and alveolar echinococcosis (AE). AE caused by Echinococcus multilocularis (E. multilocularis) is extremely harmful and untreated patients may develop chronic liver erosions with prolonged asymptomatic phase, during which an invasive tumor-like multi-vesicular and exogenously budding mass-like lesion is developed [2, 3]. Symptoms are often manifested in a late infection stage that is too late to be treated by surgery [4]. More than 90% of patients die in 10–15 years without treatment [5]. Thus, AE is considered as one of the most deadly worm infections, which is also called parasite cancer [6].
The development, reproduction and long-term survival of E. multilocularis in the intermediate host all depend on effective immune escape mechanisms. Current studies found that the complex escaping mechanisms of E. multilocularis mainly include histological isolation, molecular simulation and immunosuppression [7,8,9,10]. T helper type 1 (Th1) / T helper type 2 (Th2) cytokines, interferon-γ (IFN-γ) and interleukine-4 (IL-4) can activate multiple immune-related signaling pathways, induce and regulate the activation, proliferation and differentiation of macrophages, B lymphocytes, and other lymphocytes [11]. These cytokines can mutually interact and inhibit each other and play an important role in regulating the normal functions and balances between Th1 and Th2 cells. These are the important basis for maintaining the normal immune function [12, 13]. In the preliminary study, the process of E. multilocularis infection in mice was divided into three stages: (1) early infection (2–30 days after infection), (2) mid-term infection (30–90 days after infection), and (3) late infection (90–270 days after infection). It was found that the immune status of E. multilocularis-infected mice changed significantly in these different stages of infection [14].
T cell immunoglobulin domain and mucin domain family 3 (Tim-3) is mainly an inhibitory regulator of immune response molecules and are expressed on the surface of T cells and a variety of immune cells. Galectin-9 (Gal-9), its ligand, has multiple functions. It can induce apoptosis of T cells, while can also activate resting T-cells in the absence of typical T-cell activating signals [15]. Especially, it can activate and expand human Th1 cells [15]. Gal-9 can specifically bind to Tim-3 and has important biological functions, such as induction of cell aggregation and adhesion, chemotaxis, cell activation, and apoptosis [16,17,18,19].
The interaction of Tim-3 and Gal-9 can result in the loss of Th1 cell function and reduced expression of IFN-γ (Th1-related cytokine). The established Tim-3/Gal-9 signaling pathway has negative regulation on Th1 type immune response and induction of immune tolerance [20,21,22,23]. However, the role of Tim-3/Galectin-9 signaling pathway in the pathogenesis of E. multilocularis infection is not yet clear.
In this study, an animal model of E. multilocularis infection was established. The effect of Tim-3/Gal-9 signaling pathway in E. multilocularis infection was analyzed and discussed.
Materials and methods
Animals
A total of 112 SPF BALB/c mice were selected to establish the animal model of E. multilocularis infection. All mice were purchased from the animal center of Xinjiang Medical University (Urumqi, China), and they were all 6-week-old female mice weighing 20 ± 2 g. The experimental mice were randomly divided into model group (n = 56) and control group (n = 56). The E. multilocularis suspension was prepared and adjusted to 10,000 protoscoleces/mL as previously described [24]. Then, 100 µL suspension was injected into the anterior liver lobe of mice in the model group as previously described [25], and the mice in the control group were injected with equal volume of saline at the same position. The mice were normally cultured after inoculation. The two groups were divided into seven subgroups according to the days of infection: 2d, 8d, 30d, 60d, 90d, 180d and 270d groups.
Ethics
The animal model establishment and feeding were carried out in the Research Laboratory of Animal Science (ALLAC certification) of the First Affiliated Hospital of Xinjiang Medical University. All animal experiments were conducted according to the ethical guidelines of the Animal Care and Use Committee and were approved by the Ethical Committee of First Affiliated Hospital of Xinjiang Medical University (License number: A-20130216-155).
Sampling
At each time point, retro-orbital blood sample was collected, and serum was separated and stored at -80 °C for CBA assay. The mice were sacrificed by cervical dislocation immediately after the blood sampling. Liver tissues were collected to isolate lymphocytes.
Isolation and purification of mononuclear cells and CD4+T cells from liver
The liver tissues were grinded, digested and centrifuged. The supernatant was collected and centrifuged again (1500 r/min at 4 °C for 10 min) to collect the cell precipitates. The cell precipitates were re-suspended and subjected to Ficoll density gradient centrifugation to isolate liver lymphocytes or Percoll density gradient centrifugation to isolate liver mononuclear cells. After isolation, the cells were stained with 1% trypan blue and counted. CD4 + T cells were sorted from liver lymphocytes with magnetic cell sorting (MACS) (Miltenyi Biotech, Bergisch Gladbach, Germany).
Flow cytometry
Liver lymphocytes were adjusted to a cell concentration of 5 × 106 cells/mL with PBS and incubated with anti-CD3-PE-Cy7, anti-human-CD4-PerCP and anti-Tim-3-PE at 4 ° C for 20 min. After washing and re-suspension, the percentage of Tim-3 + CD4 + T cells was detected by flow cytometry. The gating strategies were as follows: monocytes were gated based on FSC and SSC. Then, CD3 + T cells were gated on CD3 positive monocytes. Finally, the Tim-3 + CD4 + T cells were gated on Tim-3 positive and CD4 positive cells.
Quantitative RT-PCR
CD4 + T cells were isolated from the livers of mice and the total RNA was extracted with Trizol. The PCR kit and Trizol regent were bought from Invitrogen Co. (Carlsbad, CA, US). The RNA was reverse-transcribed into cDNA using a reverse transcription kit (Revert Aid TM). First strand cDNA synthesis Kit was purchased from Fermentas Co. (Thermo Fisher Scientific, CA, US), and the target gene was amplified. The primer sequences of Tim-3, Galectin-9, IFN-γ, IL-4 and GAPDH genes were searched from GeneBank and designed by DNAMAN software (Version, 5.0, LynnonBiosoft, USA). The primers are listed in Table 1. All the primers were synthesized by Sangon Biotech Co. Ltd. (Shanghai, China).
The cDNAs of Tim-3, Galectin-9, IL-4, IFN-γ and GAPDH were used as templates of gene amplification and the PCR products were collected and stored at -20 °C for further use. For each sample, both the housekeeping gene (GAPDH) and the target genes were amplified in triplicate using the following procedure: initial denaturation at 95 ∘C for 1 min, 40 cycles of 95∘C for 5 s, 58∘C (or other) for 30s, and 72∘C for 30s. The results were analyzed using 2-ΔΔCt method to quantify the target genes.
In vitro experiments
Liver mononuclear cells were incubated with PBS or E. multilocularis vesicle fluid (Em-VF) (1 mg/mL). There was no endotoxin in the Em-VF as evaluated by the Limulus amebocyte assay kit (Dana Biotechnology, Tianjin, China; detection range: 0.02-5.00 EU/mL) for 72 h. Galectin-9 level in the supernatant was detected with CBA assay.
Liver CD4 + T cells were adjusted to a cell concentration of 1 × 108 cells/mL and seeded to a 96-well plate. Then, they were incubated with PBS, Em-VF, anti-Tim-3 (20 mg/mL; Cat# 566,346, eBioscience), and Galctin-9 (1.0 µg/m; Catalog #3535-GA-050, R&D Systems) for 72 h. IL-4 and IFN-γ levels in the supernatant were detected with CBA assay.
Cytometric bead array (CBA) assay
The concentrations of IL-4, IFN-γ and Galectin-9 were quantitatively determined by BD™CBA Mouse Cytokine Kit (BD Biosciences, San Jose, CA, USA). Cytokine standards were prepared, mixed with capture beads, vortexed and aliquoted into 50 µL. The samples were diluted and added to each sample tubes. The diluted standards (50 µL) were added to the standard tubes. All test tubes were added with 50 µL mouse cytokine PE detection reagent and incubated in dark at room temperature for 3 h. Mouse PE anti-Tim-3 was purchased from eBioscience Co. (San Diego, CA, USA). After centrifugation at 200 g for 5 min, the supernatant was carefully removed and the beads were re-suspended. The CBA samples were freshly prepared on the day of detection.
Data analysis
The statistical analysis was performed with the statistical software SPSS 17.0 (IBM Co., NY, US). Data are expressed as mean ± standard deviation (SD). Independent samples t-test was used for comparison between two groups (such as the difference in Tim-3 + CD4 + T cells). Multiple comparisons were performed with one-way ANOVA. P value P < 0.05 was considered as statistically significant.
Results
The mRNA expressions of Tim-3, Galectin-9, IFN-γ and IL-4 in the E. multilocularis infected mice
To determine the expression of Tim-3 and Galectin-9 mRNAs in the mice infected by E. multilocularis at different stages, qRT-PCR was performed. After 2 to 8 days of infection, the mean expressions of both Tim-3 (day 2:1.26 ± 0.54; day 8: 1.48 ± 0.46) and Galectin-9 mRNAs (day 2: 0.98 ± 0.35; day 8: 1.08 ± 0.20) (Fig. 1 A and 1B) in lymphocytes showed no significant changes compared to the control group (Tim-3, day 2: 0.98 ± 0.35, day 8:1.08 ± 0.20; Galectin-9, day 2: 0.98 ± 0.35, day 8:1.06 ± 0.34) (P > 0.05). After 30 days of infection, Tim-3 and Galectin-9 mRNAs in the lymphocytes began to increase (Tim-3: 3.31 ± 0.84; Galectin-9: 2.51 ± 0.40) (P < 0.05). After 60 to 90 days of infection, the mean expressions of Tim-3 and Galectin-9 mRNAs significantly increased and reached the peak (P < 0.05) (Tim-3, day 60: 6.51 ± 1.02, day 90: 8.91 ± 1.70; Galectin-9, day 60: 5.98 ± 1.23, day 90: 6.95 ± 1.22). After 180 to 270 days of infection, the expressions of Tim-3 and Galectin-9 mRNAs decreased but still maintained at high levels, which were significantly higher than those in the control group (P < 0.05) (Tim-3, day 180, infection group: 5.79 ± 1.42; control group, 1.17 ± 0.38; day 270, infection group: 5.09 ± 1.14; control group: 1.48 ± 0.76; Galectin-9, day 180, infection group: 5.09 ± 0.74; control group: day 270: 5.04 ± 1.02; control group: 0.67 ± 0.13).
Analysis of mRNA expression. The mRNA expression levels in the lymphocytes of E. multilocularis-infected or non-infected mice were detected with qRT-PCR, using GAPDH as the control. The mean relative expression of each gene was normalized to that of GAPDH. Relative expression of Tim-3 (A), Galectin-9 (B), IFN-γ (C), and IL-4 (D) mRNA. At each time point, an independent sample t test was used to analyze the difference between the infection and control groups. ** P < 0.01
To determine the expressions of IFN-γ and IL-4 mRNAs in the mice infected by E. multilocularis at different stages, qRT-PCR was performed. After 2 days of infection, the IFN-γ mRNA was 3.21 ± 0.62, the IFN-γ mRNA after 8 days of infection was 3.19 ± 0.96. After 30 to 60 days of infection, IFN-γ mRNA expression continued to increase from 4.00 ± 0.72 to 4.24 ± 1.03, which has statistically significant difference from those of the corresponding time points in the control group (P < 0.05). After 90 days of infection, the IFN-γ mRNA was 2.05 ± 0.46, which has statistically significant difference from those of the corresponding time points in the control group (P < 0.05). After 180 to 270 days of infection, the expressions of IFN-γ mRNA had no significant changes compared to that at 90 days but were still higher than that in the control group (P < 0.05, Fig. 1 C).
After 2 to 8 days of infection, the mean expression of IL-4 mRNA began to increase from 0.26 ± 0.05 to 0.29 ± 0.06, but the increase was not significant (P > 0.05). After 30 days of infection, the IL-4 mRNA was 0.61 ± 0.12. The IL-4 mRNA after 60 days infection was 0.80 ± 0.11. It showed an increasing trend over time, and it was significantly higher than that of the control group (P < 0.05). After 180 days of infection, the IL-4 mRNA was 1.12 ± 0.19. The IL-4 mRNA after 270 days infection was 1.14 ± 0.15. There was no significant difference in the IL-4 mRNA at these two time points in the infection group (P > 0.05), but they were significantly higher than those of the control group at the corresponding time points (P < 0.05, Fig. 1D).
These results indicate that IFN-γ mRNA expression increases significantly in the mice at early infection of E. multilocularis while it decreases at late stages of infection. The IL-4 mRNA expression keeps increasing after the infection of E. multilocularis.
Serum cytokine levels in mice infected with E. multilocularis
In order to determine serum IFN-γ and IL-4 levels in mice after infected with E. multilocularis at different stages, CBA assay was performed. After 2 to 8 days of infection, the serum IFN-γ level significantly increased (P < 0.05). After 30 to 60 days of infection, serum IFN-γ level was further increased, and it was significantly higher than that in the control group (P < 0.05). After 90 days of infection, the IFN-γ level was significantly lower than before (P < 0.05). After 180 to 270 days of infection, the IFN-γ level decreased but had no significant changes compared to that of the 90th day (P < 0.05, Fig. 2 A).
Analysis of cytokine levels. Serum cytokine levels in E. multilocularis-infected or non-infected mice were measured by CBA assay. (A) IFN-γ serum level. (B) IL-4 serum level. At each time point, an independent sample t test was used to analyze the difference between the infection and control groups. ** P < 0.01
After 2 to 8 days of infection, the IL-4 level did not change and showed no statistical difference from that in the control group (P > 0.05). After 30 days of infection, the IL-4 level increased significantly (P < 0.05). The IL-4 level continued to increase over time and was significantly higher than that in the control group (P < 0.05). After 90 days of infection, the serum IL-4 level reached the peak (P < 0.05). After 180 to 270 days of infection, the serum IL-4 level maintained steady, and it was significantly higher than that in the control group (P < 0.05, Fig. 2B).
These results indicate that peripheral level of IFN-γ increases significantly at early infection of E. multilocularis while decreases in the late infection. The IL-4 level continuously increases after infection.
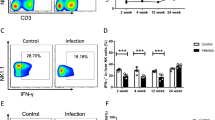
SPercentage of Tim-3 + CD4 + T cells in mice infected with E. multilocularis and Galectin-9 level in the supernatant
Since the level of Tim-3 mRNA were the highest in the liver of E. multilocularis infected mice at day 90, we isolated Tim-3 + CD4 + T cells from the liver of mice with infection for 90 days. Flow cytometry was used to detect the percentage of Tim-3 + CD4 + T cells. As shown in Fig. 3 A, the E. multilocularis infected mice had significantly higher percentage of Tim-3 + CD4 + T cells than control mice (P < 0.01). Moreover, the isolated liver mononuclear cells were incubated with PBS or Em-VF for 72 h. Then, Galectin-9 level in the supernatant was detected with CBA assay. The result showed that Galectin-9 level in the supernatant was significantly increased after Em-VF stimulation (Fig. 3B).
Tim-3 expression on liver CD4 + T cells and Galectin-9 secretion by liver mononuclear cells. (A) Liver lymphocytes of control and E. multilocularis infected mice at 90 days of infection were detected with flow cytometry. Quantitative results of Tim-3 + CD4 + T in liver lymphocytes were shown. (B) Liver mononuclear cells were incubated with PBS or Em-VF for 72 h. Then, Galectin-9 level in the supernatant was detected with CBA assay. Independent sample t test, **P < 0.01
Regulation of Tim-3/Galectin-9 on production of IFN-γ and IL-4
To investigate the effect of the Tim-3/Galectin-9 on the level of CD4 + T cell-associated cytokines in E. multilocularis infection, the isolated CD4 + T cells were respectively incubated with PBS, Em-VF, anti-Tim3 and Galctin 9 for 72 h, and then the levels of IL-4 and IFN-γ in the culture supernatant were measured with CBA assay. The results showed that the levels of IFN-γ in the Em-VF and Em-VF + Anti-Tim3 + Galctin 9 culture groups were significantly higher than those in the PBS group and Em-VF + Galectin-9 group (P < 0.05) (Fig. 4 A). IFN-γ level in Em-VF + anti-Tim3 group was significantly higher than that in PBS group (P < 0.05). The highest IL-4 level was found in Em-VF + Galectin-9 group and Em-VF + Anti-Tim-3, significantly higher than other groups (P < 0.05) (Fig. 4B). Em-VF and Em-VF + Anti-Tim3 + Galctin 9 groups had significantly higher IL-4 level than PBS control group (P < 0.05). These data indicate that the Tim-3/Galectin-9 pathway regulates the production of IFN-γ and IL-4.
Cytokine changes caused by Tim-3 and Galectin-9. The liver CD4 + T cells of the E. multilocularis infected mouse were sorted and incubated respectively with PBS, Em (E. multilocularis)-VF, Galectin-9, and anti-Tim-3 mAb for 72 h. Then the cytokine levels in the supernatant were detected by CBA. (A) IFN-γ; (B) IL-4. One-way ANOVA, *P < 0.05, **P < 0.01
Discussion
In this study, for the first time, we report that elevated Tim-3/Galectin-9 participates in the persistent infection of AE, and induces the imbalance of the Th1/Th2-related cytokines IFN-γ and IL-4 in the development of AE.
A previous study has found that immune responses differed significantly in different stages of AE infection [14], but it is unclear whether this difference is related to negative regulation of immune response. Based on this, this study investigated the changes of the negative regulatory molecules Tim-3 and Galectin-9 at different stages. The infection stages were designed according to previous study [14], with small modification. We found that the expressions of Tim-3 and Galectin-9 increased at the early infection stage and decreased slightly at the late stage of infection. Negative co-stimulatory molecules, Tim-3 and Galectin-9, can inhibit or down-regulate the activation of T cells, and promote immunosuppression [26,27,28]. Previous studies of Tim-3/Galectin-9 focused mainly on autoimmune diseases, transplant immunology and infectious diseases. In chronic hepatitis C virus patients, Tim-3 was highly expressed in CD8 + T cells, which may contribute to the mechanism of infection chronicity[29]. In patients with SLE (Systemic Lupus Erythematosus), the expression of Tim-3 in CD4 + and CD8 + T cells and the serum Galectin-9 level were also higher than those in the healthy controls [30]. Further studies demonstrated that the immune responses of peripheral blood monouclear cells induced by CD3 in patients with SLE were inhibited by Galectin-9 blocking antigens. Moretto et al. [31] also showed increased Tim-3 expression in peripheral T cells in patients infected with chronic Toxoplasmosis.
This study investigated the role of Tim-3 in the pathogenesis of AE by comparing the expression of Tim-3 in the liver lymphocytes of normal mice and mice at different infection stages. Tim-3 was almost not expressed in the control group. For mice infected with E. multilocularis, the expression of Tim-3 changed accompanying with the progression of liver infections. According to the literature, most of the inflammatory cells surrounding the liver lesion during AE infection are T-lymphocytes [32, 33]. In this study, the expression of Tim-3 mRNA in the liver lymphocytes of E. multilocularis infected mice was increased after 30 days of infection, indicating that E. multilocularis infection can lead to increased expression of Tim-3 in liver lymphocytes.
Galectin-9, a natural ligand of Tim-3, can specifically bind to the glycosyl side chain of Tim-3 molecule that is expressed on the surface of activated Th1 cells and thus plays an immunomodulatory role [34,35,36]. Galectin-9 induces intracellular calcium efflux and Th1 cell aggregation and death in a Tim-3-dependent manner in vitro, while it results in selective reduction of IFN-γ, thereby suppressing the immune response of Th1-type cells in vivo [37].
Dembele et al. [38] found that Galectin-9 significantly increased in malaria infection and the increasing level was associated with the stages of malaria infection. In mouse malaria infection, the expression of Galectin-9 also increased [39]. Steichen et al. [40] revealed that the expression of Galectin-9 increased in the lungs of mouse infected with Francisella novicida, and the inflammatory response was enhanced after the infected mouse was administered with Galectin-9. In this study, the expression of Galectin-9 mRNA in the liver lymphocytes of E. multilocularis infected mice was increased after 30 days of infection, indicating that E. multilocularis infection can also lead to increased expression of Galectin-9 in liver lymphocytes. Consistently, in vitro experiments showed that Galectin-9 level was significantly increased in the supernatant of liver mononuclear cells after Em-VF stimulation.
Studies also show that inhibition of the interaction between Tim-3 and Galectin-9 can significantly increase the clinical manifestations of immune system diseases, which is achieved by regulating cellular immunity [41]. Hou et al. showed that block of Tim-3 can recover the exhausted lymphocytes in plasmodium infection and enhance the clearance of Malaria. This study found that mice with E. multilocularis infection had altered expressions of Th1 and Th2-related cytokines. In the early stage of immune response, the growth and development of Th1 and Th2 cells are impacted by surrounding environment, in which IL-4 plays dominant roles [42,43,44]. IL-4 can induce Th0 to Th2 cell differentiation [45,46,47]. The qRT-PCR results of this study showed that the expressions of IFN-γ mRNA in mice significantly increased at the early infection of E. multilocularis. At the middle stage of infection, IFN-γ mRNA levels remained high. However, at the late stage of infection, the expression of IFN-γ mRNA significantly decreased. For the expression of IL-4 mRNA, the results showed no change at the early stage of infection, it began to increase at the middle stage and was maintained at high level in the late stage. CBA assay showed that the changes of IFN-γ and IL-4 were similar to those of the qRT-PCR results. We suppose that IFN-γ may be involved in the inhibition of the growth of E. multilocularis in the early stage. However, in the late stage, IL-4 may play a major role inhibiting a robust immune response and promoting the growth of E. multilocularis in vivo while also producing anti-inflammatory effects. Binding of Galectin-9 to Tim-3 can lead to intracellular calcium loss and Th1 cell aggregation and death in vitro, while it can result in the reduction of IFN-γ in vivo [48]. In this study, the low expressions of Tim-3 and Galectin-9 and the elevated Th1 type cytokines such as IFN-γ with inflammatory reaction of liver tissues were observed in the early stage of E. multilocularis infection. In the late stage of infection, the expressions of Tim-3 and Galectin-9 significantly increased. The level of IFN-γ decreased and the level of Th2 cytokine IL-4 significantly increased. Thus, it is hypothesized that Tim-3 and Galectin-9 could regulate IFN-γ and IL-4 immune function. To verify whether Tim-3 and Galectin-9 can affect the level of CD4 + T cell-associated cytokines in vitro, CD4 + T cells were isolated from the liver of E. multilocularis infected mouse and then co-cultured with Em-VF, anti-Tim-3, and Galectin-9 for 72 h. The results showed that the Tim-3/Galentin-9 pathway led to the changes in cytokines during E. multilocularis infection.
Conclusions
In conclusion, Tim-3/Galectin-9 signaling pathway caused the changes in IFN-γ and IL-4, which may play important roles in the process of AE. This study provides important information on the Th1/Th2 imbalance theory of parasitic infection and the immunoregulatory mechanism of AE.
Availability of data and material
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Wen H, Xu MQ (2007) Practical Science of Echinococcosis. Science Press, Beijing, pp 15–19
Liance M, Bresson-Hadni S, Vuitton D, Bretagne S, Houin R (1990) Comparison of the viability and developmental characteristics of Echinococcus multilocularis isolates from human patients in France. Int J Parasitol 20:83–86
Koziol U, Rauschendorfer T, Zanon Rodríguez L, Krohne G, Brehm K (2014) The unique stem cell system of the immortal larva of the human parasite Echinococcus multilocularis. Evodevo 5:10
Kern P (2010) Clinical features and treatment of alveolar echinococcosis. Curr Opin Infect Dis 23:505–512
Wen H, Vuitton L, Tuxun T, Li J, Vuitton DA, Zhang W, McManus DP (2019) Echinococcosis: Advances in the 21st Century.Clin Microbiol Rev32
Xu K, Ahan A (2020) A new dawn in the late stage of alveolar echinococcosis “parasite cancer”. Med Hypotheses 142:109735
Cho JL, Roche MI, Barry S, Brian S, Xavier RJ, Medoff BD (2012) Enhanced Tim3 activity improves survival after influenza infection. J Immunol 189:2879–2889
Li Y, Liu X, Zhu Y, Zhou X, Cao C, Hu X, Ma H, Wen H, Ma X, Ding JB (2013) Bioinformatic prediction of epitopes in the Emy162 antigen of Echinococcus multilocularis. Exp Ther Med 6:335–340
Mejri N, Hassen IE, Knapp J, Saidi M (2017) Impairment of Macrophage Presenting Ability and Viability by Echinococcus granulosus Antigens. Iran J Immunol 14:35–50
Pang N, Zhang F, Ma X, Zhu Y, Zhao H, Xin Y, Wang S, Chen Z, Wen H, Ding J (2014) TGF-beta/Smad signaling pathway regulates Th17/Treg balance during Echinococcus multilocularis infection. Int Immunopharmacol 20:248–257
Infante-Duarte C, Kamradt T (1999) Th1/Th2 balance in infection. Springer Semin Immunopathol 21:317–338
Bellanger AP, Mougey V, Pallandre JR, Gbaguidi-Haore H, Godet Y, Millon L (2017) Echinococcus multilocularis vesicular fluid inhibits activation and proliferation of natural killer cells.Folia Parasitol (Praha)64
Liu X, Zhao H, Cao W, Liu Y, Zhang C, Lan X, Peng S, Wen H, Ding J, Ma X (2016) Bioinformatic prediction of the antigenic epitopes of recombinant ferritin of Echinococcus granulosus. Mol Med Rep 13:888–894
Zhang C, Wang J, Lü G, Li J, Lu X, Mantion G, Vuitton DA, Wen H, Lin R (2012) Hepatocyte proliferation/growth arrest balance in the liver of mice during E. multilocularis infection: a coordinated 3-stage course. PLoS ONE 7:e30127
Gooden MJ, Wiersma VR, Samplonius DF, Gerssen J, van Ginkel RJ, Nijman HW, Hirashima M, Niki T, Eggleton P, Helfrich W, Bremer E (2013) Galectin-9 activates and expands human T-helper 1 cells. PLoS ONE 8:e65616
La X, Zhang F, Li Y, Li J, Guo Y, Hui Z, Pang N, Ma X, Hao W, Fan H (2015) Upregulation of PD-1 on CD4 + CD25 + T cells is associated with immunosuppression in liver of mice infected with Echinococcus multilocularis. Int Immunopharmacol 26:357–366
Sánchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, Manlongat N, Bender O, Kamradt T, Kuchroo VK (2003) Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol 4:1093–1101
Tang ZH, Liang S, Potter J, Jiang X, Mao HQ, Li Z (2013) Tim-3/galectin-9 regulate the homeostasis of hepatic NKT cells in a murine model of nonalcoholic fatty liver disease. J Immunol 190:1788–1796
Wu M, Zhu Y, Zhao J, Ai H, Gong Q, Zhang J, Zhao J, Wang Q, La X, Ding J (2015) Soluble costimulatory molecule sTim3 regulates the differentiation of Th1 and Th2 in patients with unexplained recurrent spontaneous abortion. Int J Clin Exp Med 8:8812–8819
Hirashima M, Kashio Y, Nishi N, Yamauchi A, Imaizumi TA, Kageshita T, Saita N, Nakamura T (2002) Galectin-9 in physiological and pathological conditions. Glycoconj J 19:593–600
Li X, Chen Y, Xu L, Jin Z, Xu H, Teng G, Yu D (2017) Tim3/Gal9 interactions between T cells and monocytes result in an immunosuppressive feedback loop that inhibits Th1 responses in osteosarcoma patients. Int Immunopharmacol 44:153–159
Sabatos CA, Chakravarti S, Cha E, Schubart A, Sanchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ, Kuchroo VK (2003) Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol 4:1102–1110
Wang F, He W, Yuan J, Wu K, Zhou H, Zhang W, Chen ZK (2008) Activation of Tim-3-Galectin-9 pathway improves survival of fully allogeneic skin grafts. Transpl Immunol 19:12–19
Zhang C, Shao Y, Yang S, Bi X, Li L, Wang H, Yang N, Li Z, Sun C, Li L, Lü G, Aji T, Vuitton DA, Lin R, Wen H (2017) T-cell tolerance and exhaustion in the clearance of Echinococcus multilocularis: role of inoculum size in a quantitative hepatic experimental model. Sci Rep 7:11153
Zhang F, Li S, Zhu Y, Zhang C, Li Y, Ma H, Pang N, An M, Wang H, Ding J (2018) Immunization of mice with egG1Y162-1/2 provides protection against Echinococcus granulosus infection in BALB/c mice. Mol Immunol 94:183–189
Bresson-Hadni S, Mantion GA, Vuitton DA (2007) From basic science to clinical practice. Echinococcosis of the liver. Blackwell Publishing Inc, Oxford-Malden, pp 1047–1057
Qi Y, Song XR, Shen JL, Xu YH, Shen Q, Luo QL, Zhong ZR, Wang W, Chu DY, Song WJ (2012) Tim-2 up-regulation and galectin-9-Tim-3 pathway activation in Th2-biased response in Schistosoma japonicum infection in mice. Immunol Lett 144:60–66
Zhang Y, Zhang Y, Gu W, He L, Sun B (2014) Th1/Th2 cell’s function in immune system. Adv Exp Med Biol 841:45–65
Barathan M, Mohamed R, Vadivelu J, Li YC, Vignesh R, Krishnan J, Sigamani P, Saeidi A, Ram MR, Velu V (2017) CD8 + T cells of chronic HCV-infected patients express multiple negative immune checkpoints following stimulation with HCV peptides. Cell Immunol 313:1–9
Jiao Q, Qian Q, Zhao Z, Fang F, Hu X, An J, Jian W, Liu C (2016) Expression of human T cell immunoglobulin domain and mucin-3 (TIM-3) and TIM-3 ligands in peripheral blood from patients with systemic lupus erythematosus. Arch Dermatol Res 308:1–9
Moretto MM, Hwang S, Khan IA (2017) Downregulated IL-21 Response and T Follicular Helper Cell Exhaustion Correlate with Compromised CD8 T Cell Immunity during Chronic Toxoplasmosis. Front Immunol 8:1436
Ricken FJ, Nell J, Grüner B, Schmidberger J, Kaltenbach T, Kratzer W, Hillenbrand A, Hennebruns D, Deplazes P, Möller P (2017) Albendazole increases the inflammatory response and the amount of Em2-positive small particles ofEchinococcus multilocularis(spems) in human hepatic alveolar echinococcosis lesions.Plos Neglected Tropical Diseases11
Sada-Ovalle I, Chavez-Galan L, Torre-Bouscoulet L, Nava-Gamino L, Barrera L, Jayaraman P, Torres-Rojas M, Salazar-Lezama MA, Behar SM (2012) The Tim3-galectin 9 pathway induces antibacterial activity in human macrophages infected with Mycobacterium tuberculosis. J Immunol 189:5896–5902
Dai SY, Nakagawa R, Itoh A, Murakami H, Kashio Y, Abe H, Katoh S, Kontani K, Kihara M, Zhang SL, Hata T, Nakamura T, Yamauchi A, Hirashima M (2005) Galectin-9 induces maturation of human monocyte-derived dendritic cells. J Immunol 175:2974–2981
Yang S, Jin W, Chen F, Liu G, Weng Z, Chen J (2017) Elevated Galectin-9 Suppresses Th1 Effector Function and Induces Apoptosis of Activated CD4 + T Cells in Osteoarthritis. Inflammation 40:1–10
Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK (2005) The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol 6:1245–1252
Barrueto L, Caminero F, Cash L, Makris C, Lamichhane P, Deshmukh RR (2020) Resistance to Checkpoint Inhibition in Cancer Immunotherapy. Transl Oncol 13:100738
Dembele BP, Chagan-Yasutan H, Niki T, Ashino Y, Tangpukdee N, Shinichi E, Krudsood S, Kano S, Hattori T (2016) Plasma levels of Galectin-9 reflect disease severity in malaria infection. Malar J 15:403
Xiao S, Liu J, Huang S, Lu F (2016) Increased Gal-9 and Tim-3 expressions during liver damage in a murine malarial model. Parasitol Res 115:663–672
Steichen AL, Simonson TJ, Salmon SL, Metzger DW, Mishra BB, Sharma J (2015) Alarmin function of galectin-9 in murine respiratory tularemia. PLoS ONE 10:e0123573
Pang N, Zhang F, Ma X, Zhang Z, Zhao H, Xin Y, Wang S, Zhu Y, Wen H, Ding J (2014) Th9/IL-9 profile in human echinococcosis: their involvement in immune response during infection by Echinococcus granulosus. Mediators Inflamm 2014: 781649
Curran SA, Romano E, Kennedy MG, Hsu KC, Young JW (2014) Phenotypic and functional activation of hyporesponsive KIRnegNKG2Aneg human NK-cell precursors requires IL12p70 provided by Poly(I:C)-matured monocyte-derived dendritic cells. Cancer Immunol Res 2:1000–1010
Dong J, Yang XF, Wang LX, Wei X, Wang AH, Hao CQ, Shen HJ, Huang CX, Zhang Y, Lian JQ (2017) Modulation of Tim-3 Expression by Antigen-Dependent and -Independent Factors on T Cells from Patients with Chronic Hepatitis B Virus Infection. Front Cell Infect Microbiol 7:98
Rubinstein MP, Su EW, Suriano S, Cloud CA, Andrijauskaite K, Kesarwani P, Schwartz KM, Williams KM, Johnson CB, Li M, Scurti GM, Salem ML, Paulos CM, Garrett-Mayer E, Mehrotra S, Cole DJ (2015) Interleukin-12 enhances the function and anti-tumor activity in murine and human CD8(+) T cells. Cancer Immunol Immunother 64:539–549
Jeon WY, Shin IS, Shin HK, Lee MY (2015) Samsoeum water extract attenuates allergic airway inflammation via modulation of Th1/Th2 cytokines and decrease of iNOS expression in asthmatic mice. BMC Complement Altern Med 15:47
Zhang F, Ma X, Zhu Y, Wang H, Liu X, Zhu M, Ma H, Wen H, Fan H, Ding J (2014) Identification, expression and phylogenetic analysis of EgG1Y162 from Echinococcus granulosus. Int J Clin Exp Pathol 7:5655–5664
Zhang F, Pang N, Zhu Y, Zhou D, Zhao H, Hu J, Ma X, Li J, Wen H, Samten B, Fan H, Ding J (2015) CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells are positively correlated with levels of IL-21 in active and transitional cystic echinococcosis patients. BMC Infect Dis 15:457
Zhang F, Lu X, Guo N, Zhang Y, Ji P, Hu J, Zhang Z, Li J, Li F, Ding J (2016) The prediction of T- and B-combined epitope of Ag85B antigen of Mycobacterium tuberculosis. Int J Clin Exp Med 9:1408–1421
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers 81960373, 81460307, 81660343, 81160200, 81160202, 81060135] and Xinjiang Institute of Hydatid Disease [grant number XJDX0202-2010-04].
Author information
Authors and Affiliations
Contributions
JBD and HNF conceived and designed the experiments. SYL, FBZ, YJZ and YJL performed the experiments. HNF, NNP and MTA analyzed the data. SYL, FBZ, HNF Fan and JBD wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethics approval
All animal experiments were conducted according to the ethical guidelines of the Animal Care and Use Committee and were approved by the Ethical Committee of First Affiliated Hospital of Xinjiang Medical University (License number: A-20130216-155).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shaoyu Li, Yuejie Zhu and Song Wang authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, S., Zhu, Y., Wang, S. et al. Tim-3/Galectin-9 signaling pathway is involved in the cytokine changes in mice with alveolar echinococcosis. Mol Biol Rep 49, 7497–7506 (2022). https://doi.org/10.1007/s11033-022-07554-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07554-3