Abstract
Background
Interleukin 35 (IL35) has been reported to play a role in acute lung injury (ALI); however, the current results regarding the relationship between IL35 and ALI are inconsistent. Therefore, we aimed to further determine the function of IL35 in ALI in mice and the potential mechanism in this paper.
Materials and methods
Hematoxylin-eosin (HE) staining and Masson staining were used to evaluate lung injury in mice. Immunohistochemical staining was used to evaluate the expression of IL35 p35, TLR4 and MD2 and the Bax/Bcl2 and p-P65/P65 ratios. The expression levels of IL35 EBi3, CD68, CD206 and MPO were assessed by immunofluorescence staining. RT–PCR was used to examine the expression levels of IL1β and IL6. Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining was performed to detect apoptotic cells.
Results
Overexpression of IL35 alleviated LPS-induced ALI in mice. IL35 overexpression decreased the expression of CD68 and increased the expression of CD206 in mice with ALI. Furthermore, upregulation of IL35 expression obviously reduced the expression of MPO, IL1β and IL6 in the lung tissues of mice with ALI. Mechanistically, IL35 suppressed the TLR4/NFκB-P65 pathway, leading to the promotion of the M1 to M2 macrophage transition and alleviation of inflammation in mice with ALI.
Conclusions
IL35 relieved LPS-induced inflammation and ALI in mice by regulating M1/M2 macrophage polarization and inhibiting the activation of the TLR4/NFκB-P65 pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute lung injury (ALI) is characterized by alveolar and capillary endothelial damage and an increase in the permeability of the alveolar-capillary membrane, which are noncardiac pathological processes that can result in hypoxic respiratory failure and even acute respiratory distress syndrome (ARDS) or death[1]. The main treatments for ALI/ARDS are antibacterial drugs, corticosteroids, lung protective ventilation strategy (LPVS) and extracorporeal membrane oxygenation (ECMO). Unfortunately, all of these approaches have some limitations, as they have no beneficial in some cases, have side effects or are expensive, and the morbidity and mortality rates of ALI/ARDS may still be high even when these methods are used [2, 3]. Therefore, exploration and elucidation the physiological and molecular mechanisms of ALI/ARDS are urgently needed.
An increasing number of studies have confirmed that inflammation plays a crucial role in the initiation and development of ALI[4, 5]. With the increase in the proinflammatory response and the decrease in the anti-inflammatory response in ALI, a large number of inflammatory cytokines, such as interleukin (IL)6 and TNF-α, are secreted, and resulting in the development of the cytokine storm, in which causes respiratory failure and ARDS[6]. Macrophage activation is considered the critical initial stage of the inflammatory response[7, 8]. Macrophages have high plasticity and can be polarized toward distinct functional phenotypes depending on the presence of different stimuli[7]. In particular, many studies have found that macrophages are polarized toward the M1 (proinflammatory) phenotype rather than the M2 (anti-inflammatory/reparative) phenotype in ALI, leading to aggravation of lung inflammation[9, 10]. Therefore, acceleration of the M1/M2 macrophage switching is the key to improving inflammatory lung damage.
Interleukin 35 (IL35), a newly identified cytokine of the IL12 heterodimeric cytokine family, is composed of Epstein–Barr virus-induced gene 3 (EBi3) and the IL12 p35 subunit (IL-12 A)[11, 12]. IL35 is primarily secreted by T cells and B cells and plays an important role in inflammatory and immune responses[11, 13]. In recent years, an increasing number of studies have provided insight into the relationship between IL35 and pulmonary inflammatory diseases and have found that IL35 can protect the lung from inflammation and autoimmune diseases, including asthma and respiratory infections[14, 15]. However, the results related to the role of IL35 in ALI/ARDS are inconsistent. Cao observed that IL35 was elevated in sepsis and mediated inflammation[16]. Pan and his colleagues reported that IL35 was downregulated in ALI and that overexpression of IL35 reduced inflammation[17]. Wang and his colleagues showed that IL35 expression was upregulated and alleviated endothelial injury in ALI[18]. However, whether IL35 regulates macrophage polarization in ALI remains unclear. Thus, we further explicated the role of IL35 and explored whether IL35 exerts its effect by regulating macrophage polarization in ALI.
In this study, we observed the expression and function of IL35 in an in vivo mouse model of ALI. Next, we detected the markers of M1 and M2 macrophages using immunofluorescence staining to determine the potential mechanism of IL35 in ALI. Our findings may provide a novel basis for the development of therapies for ALI/ARDS.
Materials and methods
Animal studies
Male C57BL/6 mice (aged 8–10 weeks, body weight 22 ± 3 g) were purchased from Beijing SiPeiFu Biotechnology Co., Ltd (China). All mice were housed conventionally at 22 °C under a 12-h light-dark cycle with access to water and food ad libitum. All animal experiments were performed in compliance with the National Institutes of Health (NIH) policies in the Guide for the Care and Use of Laboratory Animals and were approved by the Experimental Animal Ethics Committee of CHINA-JAPAN Friendship Hospital.
Eighteen mice were randomly divided into three groups of 6 mice each, including the control group, the LPS group and the LPS + plasmid DNA containing the IL35 gene (pcDNA-IL35) group. pcDNA-IL35 was purchased from Gene Pharma (Shanghai, China) and administered to the mice by intravenous (i.v.) injection into the tail according to the manufacturer’s instructions[19]. The experimental procedures are described below (Supplementary Fig. 1). Mice from the control group were given 2 ml of PBS by i.v. injection and received an intraperitoneal (i.p.) injection of normal saline after 7 days. Mice from the LPS group were given 2 ml of PBS by i.v. and received an i.p. injection of LPS (10 mg/kg, Sigma, USA) after 7 days. Mice from the LPS + pcDNA-IL35 group were given 2 ml of PBS containing 100 µg of pcDNA-IL35 by i.v. injection and received an i.p. injection of LPS (10 mg/kg) after 7 days. All mice were anesthetized by i.p. injection of an overdose of sodium pentobarbital at 12 h postinjury. Subsequently, the lungs were perfused and isolated for preparation of paraffin sections for histological examination and PCR analyses.
Hematoxylin-eosin (HE) staining
Lung tissues were collected and fixed in 4% paraformaldehyde. Then, 5-µm sections cut from paraffin tissue blocks were stained with HE and observed under an optical microscope (Olympus, Japan). The lung injury score was calculated by two researchers blinded to the group information as described previously[20]. Five independent variables, including neutrophils in the alveolar space, neutrophils in the interstitial space, the existence of hyaline membranes, proteinaceous debris filling the airspaces and alveolar septal thickening, were used to generate a lung injury score[20].
Masson staining
Lung tissues were stained with Ponceau-fuchsin solution, rinsed with glacial acetic acid and then immersed in phosphomolybdic acid. After the excess liquid was absorbed with filter paper, the sections were stained with aniline blue solution, soaked in glacial acetic acid, washed with water, air‐dried, treated with xylene three times and then mounted. An optical microscope (Olympus, Japan) was used to detect collagen deposition on the airway walls in lung tissues.
Immunofluorescence and immunohistochemical staining
Lung tissues were collected and fixed in 4% paraformaldehyde, and immunofluorescence staining was performed according to the manufacturer’s protocol[8]. Briefly, tissue specimens were cut into 5-µm serial sections and blocked with 10% blocking serum in PBS. The sections were then incubated with primary antibodies against IL35 EBi3 (Abclonal, A19613, China) and CD68 (Abcam, ab53444, USA) or CD206 (Santa Cruz, sc-58986, USA), MPO (Abcam, ab208670, USA) at 4 °C overnight. Next, the sections were incubated with Alexa Fluor 488-conjugated goat anti-rabbit IgG secondary antibody or Alexa Fluor Plus 555-conjugated goat anti-rabbit IgG secondary antibody or Alexa Fluor 488 -conjugated goat anti-rat IgG secondary antibody or Alexa Fluor 488 -conjugated goat anti-mouse IgG secondary antibody (Molecular Probes, Invitrogen, USA) for 1 h at room temperature. DAPI (Invitrogen, USA) was used for nuclear staining. The sections were observed with a fluorescence microscope (Olympus, Japan), and the images were analyzed using ImageJ (Bethesda, MD, USA).
Immunohistochemical staining was used to evaluate IL35 p35, Bax, Bcl2, TLR4, MD2 and p-P65/P65 expression in lung tissues according to the manufacturer’s protocol[21]. Briefly, tissue specimens were cut into 5-µm serial sections, deparaffinized, rehydrated, blocked and incubated with primary antibodies against IL35 p35 (R&D Systems, MAB6688, USA), Bax (Abcam, ab32503, USA), Bcl2 (Abcam, ab182858, USA), TLR4 (Abcam, ab13867, USA), MD2 (Abcam, ab24182, USA), p-P65 (Abclonal, AP0475, China) or P65 (Abcam, ab16502, USA) at 4 °C overnight. Next, the sections were incubated with biotinylated IgG (1:250) for 1 h and then with streptavidin-HRP for 30 min at room temperature. DAB was then added to each section for 5 min. The sections were observed with a microscope (Olympus, Japan), and the images were analyzed using Image-Pro Plus 6.0 (Media cybernetics, USA).
TUNEL staining
Lung tissues were collected and fixed in 4% paraformaldehyde, and tissue specimens were cut into 5-µm serial sections. Terminal deoxynucleotide transferase-mediated dUTP nick end-labeling (TUNEL) staining was performed using an In Situ Cell Death Detection Kit (Roche, Mannheim, Germany) following the manufacturer’s instructions. The sections were observed with a fluorescence microscope (Olympus, Japan) with 10 fields of view randomly selected, and the rate of cell apoptosis was determined using ImageJ (Bethesda, MD, USA). Green nuclei were considered positive-apoptotic cells. The cells with blue nuclei were deemed to be normal, and the average value was determined accordingly. The ratio of the number of green cells to that of blue cells was regarded as the rate of cell apoptosis.
Quantitative real-time PCR
Total RNA was extracted from lung tissues using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA), and cDNA synthesis was performed using random hexamer primers and a reverse transcription kit (TaKaRa, Japan) according to the manufacturer’s protocol[11]. RT–PCR was performed using a SYBR® PremixEx Taq II Kit (TaKaRa, Japan) on a 7500 Fast Real Time PCR system from Applied Biosystems (Bio–Rad, USA). The relative gene expression levels were determined by the comparative critical threshold (ΔΔCT) method. Primer sequences for mouse genes were in Supplementary Table 1.
Statistical analysis
The data are presented as the means ± S.E.M.s. One-way ANOVA followed by the Dunnett or Bonferroni test was used to analyze statistical significance as appropriate. P < 0.05 was considered statistically significant. The statistical analyses were performed using Statistical Product and Service Solutions 19.0 (SPSS, Systat Software, San Jose, CA, USA).
Results
IL35 alleviates LPS-induced ALI
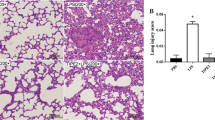
IL35 has been reported to play immunosuppressive and anti-inflammatory effects in the context of infections, inflammation, and autoimmune diseases[11]. We examined whether IL35 alleviated LPS-induced ALI in our study. The expression of IL35 p35 was first assessed by immunohistochemical staining. The results showed that LPS-induced ALI resulted in upregulation of IL35 p35 expression and that the expression of IL35 p35 was further overexpressed after pcDNA-IL35 transfection (P < 0.05, Fig. 1 A-B). HE staining and lung injury scores were then used to evaluate the severity of lung injury. As shown in Fig. 1 C, LPS treatment increased alveolar collapse and thickened the alveolar wall and septa; however, overexpression of IL35 obviously relieved LPS-stimulated lung tissue injury (P < 0.05, Fig. 1 C). A similar trend was observed for the lung injury score (P < 0.05, Fig. 1D). We next detected collagen deposition in lung tissues by Masson staining. As expected, LPS increased collagen deposition in lung tissues, and upregulation of IL35 expression inhibited collagen deposition in mice with ALI (P < 0.05, Fig. 1E-F). The above data suggested that IL35 may alleviate LPS-induced ALI.
IL35 alleviates LPS-induced ALI in mice
A Immunohistochemical staining for IL35 p35 in each group (original magnification ×10 and ×40). B Quantification of positive staining of IL35 p35 in each group. C Hematoxylin and eosin (HE) staining of lung tissues (original magnification ×10 and ×40). D Quantification of the lung injury score. E Masson staining of lung tissues (original magnification ×10 and ×40). F Quantification of collagen deposition. * P < 0.05 vs. Control. # P < 0.05 vs. LPS + pcDNA-IL35
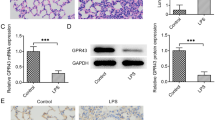
IL35 regulates macrophage polarization in mice with ALI
Some studies have found that IL35 ameliorates tissue injury by stimulating macrophage polarization[22, 23]. Therefore, we hypothesized that IL35 reduced ALI by regulating macrophage polarization. To explore whether IL35 decreased the levels of M1 macrophages and increased the levels of M2 macrophages in mice with ALI, we detected the expression of IL35 EBi3, the other subunit of IL35, and markers of M1/M2 macrophages (CD68 and CD206) in lung tissues by immunofluorescence staining. The results showed that IL35 overexpression led to a decrease in the levels of CD68 and an increase in the levels of CD206 in mice with ALI (P < 0.05, Fig. 2 A-E).
IL35 regulates macrophage polarization in mice with ALI
A-B Immunofluorescence staining for IL35 EBi3 and CD68 or CD206 in each group (original magnification ×10 and ×40). C Quantification of positive staining for IL35 EBi3 in each group. D Quantification of positive staining for CD68 and CD206in each group. E Quantification of the ratio of CD206/CD68 in each group. * P < 0.05 vs. Control. # P < 0.05 vs. LPS + pcDNA-IL35
IL35 mitigates inflammation in mice with ALI
An imbalance between the activation and regulation of macrophage polarization may accelerate the development of ALI/ARDS[10, 24]. We observed that IL35 may regulate macrophage polarization in mice with ALI. We then explored whether IL35 reduced inflammation in ALI. As expected, the expression levels of MPO protein in lung tissues of mice with LPS intervention were found to be higher than those in the LPS + pcDNA-IL35 group (P < 0.05, Fig. 3 A-B). RT–PCR was used to check the levels of inflammatory cytokines. Similarly, the levels of IL1β and IL6 were decreased in the lung tissues from the LPS + pcDNA-IL35 group compared with those in the LPS group (P < 0.05, Fig. 3 C-D). These results demonstrated that IL35 may mitigate inflammation by regulating macrophage polarization.
IL35 mitigates inflammation in mice with ALI
A Immunofluorescence staining for MPO in each group (original magnification ×10 and ×40). B Quantification of positive staining for MPO in each group. C RT–PCR analysis for IL1β. D RT–PCR analysis for IL6. * P < 0.05 vs. Control. # P < 0.05 vs. LPS + pcDNA-IL35
IL35 reduces apoptosis in mice with ALI
To further confirm whether IL35 improved lung injury, TUNEL staining and immunohistochemical staining were used to assess apoptosis in the lung tissues of mice with ALI. The results showed that IL35 overexpression decreased the rate of cell apoptosis induced by LPS (P < 0.05, Fig. 4 A-B). Consistently, upregulation of IL35 expression reduced the ratio of Bax/Bcl2 in the lung tissues of mice, which was high after LPS treatment (P < 0.05, Fig. 4 C-D).
IL35 reduces apoptosis in mice with ALI
A TUNEL staining of apoptotic cells (original magnification ×10 and ×40). B Quantification of apoptotic ALI cells in each group. C Immunohistochemical staining for Bax and Bcl2 in each group (original magnification ×10 and ×40). D Quantification of the ratio of Bax/Bcl2 in each group. * P < 0.05 vs. Control. # P < 0.05 vs. LPS + pcDNA-IL35
IL35 regulates macrophage polarization by the TLR4/NFκB-P65 pathway
A compelling body of evidence has shown that the TLR4/NFκB-P65 pathway is a critical signal involved in macrophage polarization[10, 25, 26]. However, whether IL35 regulates macrophage polarization in ALI by inhibiting the TLR4/NFκB-P65 pathway remains unclear. To gain insight into the mechanism by which IL35 alleviated ALI, we detected the expression of TLR4, MD2 and p-P65/P65 by immunohistochemical staining. High levels of TLR4 and MD2 expression were observed in LPS group, but IL35 overexpression stopped them from rising (P < 0.05, Fig. 5 A-D). The same trend was observed for the ratio of p-P65/P65 expression (P < 0.05, Fig. 5E-F). Furthermore, we found that the expression of TLR4 and MD2 and the ratio of p-P65/P65 in pulmonary macrophages were higher in the LPS group than in the control group, and that overexpression of IL35 prevented the increases in these measures in pulmonary macrophages in mice with ALI. All these results suggested that IL35 may regulate macrophage polarization by the TLR4/NFκB-P65 pathway in mice with LPS-induced ALI.
IL35 regulates the TLR4/NFκB-P65 pathway
A Immunohistochemical staining for TLR4 or MD2 in each group (original magnification ×10 and ×40). B Quantification of positive staining for TLR4 in each group. C Immunohistochemical staining for MD2 in each group (original magnification ×10 and ×40). D Quantification of positive staining for MD2 in each group. E Immunohistochemical staining for p-P65 or P65 in each group (original magnification ×10 and ×40). F Quantification of the ratio of p-P65/P65 in each group. * P < 0.05 vs. Control. # P < 0.05 vs. LPS + pcDNA-IL35
Discussion
ALI/ARDS is an acute and life-threatening pulmonary inflammatory disease with a mortality rate of up to 40–60% [10, 27]. An imbalance in macrophage polarization is crucial for initiating the inflammatory response in ALI. IL35 is a novel cytokine of the IL12 family and has been reported to reduce the progression of some inflammatory diseases [11,12,13]. Here, we observed that the expression of IL35 in lung tissues was upregulated in mice with ALI and that IL35 overexpression promoted the transformation of M1 microphages to M2 macrophages and relieved inflammation, resulting in alleviation of LPS-induced ALI in mice. Our results and previous studies consistently suggest that IL35 has a protective effect and may be a key therapeutic target for ALI/ARDS [17, 18].
Accumulating evidence has shown that IL35 may be produced by T cells, dendritic cells (DCs), B cells and even nonimmune cells [28,29,30]. However, IL35 is not constitutively expressed in normal tissues [28]. The expression of IL35 is low or undetectable in some immune cells and can be triggered under inflammatory conditions [28, 31]. Thus, IL35 constitutes an important mediator of inflammation and contributes to regulating inflammatory disease progression. For example, Li et al. confirmed that IL35 suppressed allergic airway inflammation in asthma [15]. Hu suggested that IL35 alleviates LPS-induced acute kidney injury in mice by inhibiting proinflammatory cytokine production [19]. Pan and his colleagues reported that IL35 protected against cigarette smoke-induced lung inflammation in mice [11]. In addition, researchers have recently gained insight into the role of IL35 in ALI/ARDS. Our study and previous reports revealed that overexpression of IL35 reduced inflammation and ameliorated ALI[17, 18]. However, Ju and his colleagues observed lower bacterial loads in septic mice after treatment with anti-IL35 p35 antibodies [16]. This is in line with a previous report showing that IL23 p35 knockout enhanced the acute immune response during Staphylococcus aureus infection [32]. IL12 p35 is a mutant subunit of IL35 and regulates the activity of IL35 [11, 12, 16]. Ye et al. observed obvious differences in the expression of IL12 p35 in macrophages, B lymphocytes, T lymphocytes and DCs after stimulation by harmful substances [13]. The differential expression of IL12 p35 in different cells may indicate that it plays different roles in inflammatory diseases. The conflicting findings regarding whether IL35 mediates or alleviates inflammation in ALI suggest that more research is needed to confirm the current results and potential mechanism[16,17,18].
M1/M2 macrophage polarization contributes to the development of ALI[8, 33]. A growing amount of data has verified that macrophage polarization toward the M1 phenotype leads to aggravation and progression of ALI/ARDS[8, 33, 34]. Li revealed that HSF1 attenuates LPS-induced ALI by suppressing macrophage infiltration and CCR2 expression[8]. Cui found that lncRNA Malat1 knockdown attenuated LPS-induced M1 macrophage activation and enhanced M2 differentiation, resulting in alleviation of lung injury [33]. Zhang showed that MCP-induced protein 1 attenuated sepsis-induced ALI by decreasing the M1/M2 macrophage ratio[34]. In this study, our results showed that IL35 overexpression decreased the expression of the M1 phenotype-specific marker CD68 and increased the expression of the M2 phenotype-specific marker CD206, leading to relief of inflammation and pulmonary injury in mice with ALI. Our data are in line with previous results showing that overexpression of IL35 protects pulmonary tissue from LPS-induced ALI by promoting the M1 to M2 macrophage switch.
We further explored how IL35 regulated macrophage polarization to ameliorate pulmonary inflammatory damage in mice with ALI. LPS is an important component of gram-negative bacteria and a ligand of TLR4[25]. TLR4, an important transmembrane pattern-recognition receptor of the innate immune system, is recruited and activated after LPS stimulation, leading to the activation of the NFκB-P65 signaling pathway and the release of proinflammatory cytokines, such as IL1β and IL6[26, 35]. In our study, upregulation of IL35 expression obviously suppressed the expression of TLR4 and MD2, as well as p-P65/P65. This trend was also observed in pulmonary macrophages in mice with ALI. Previous studies have shown that IL35 responds to TLR4 agonists [30]. Thus, we suspected that IL35 may interact with TLR4 to inhibit the TLR4/NFκB-P65 pathway, relieving inflammatory lung injury. More studies are needed in the future to further confirm the underlying mechanism.
In conclusion, IL35 relieved LPS-reduced inflammation and ALI in mice by regulating M1/M2 macrophage polarization and inhibiting the activation of the TLR4/NFκB-P65 pathway (Supplementary Fig. 2). Our findings may provide new insight into the potential to treat ALI/ARDS by targeting IL35 expression regulation.
Data Availability
The datasets supporting the conclusions of this article are included within the article.
References
Wheeler AP, Bernard GR (2007) Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 369:1553–1564. doi: https://doi.org/10.1016/S0140-6736(07)60604-7
Hotchkiss RS, Monneret G, Payen D (2013) Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis 13:260–268. doi: https://doi.org/10.1016/S1473-3099(13)70001-X
Wenzel RP, Edmond MB (2012) Septic shock–evaluating another failed treatment. N Engl J Med 366:2122–2124. doi: https://doi.org/10.1056/NEJMe1203412
Fowler AA 3, Truwit JD, Hite RD, Morris PE, DeWilde C, Priday A, Fisher B, Thacker LR 2, Natarajan R, Brophy DF, Sculthorpe R, Nanchal R, Syed A, Sturgill J, Martin GS, Sevransky J, Kashiouris M, Hamman S, Egan KF, Hastings A, Spencer W, Tench S, Mehkri O, Bindas J, Duggal A, Graf J, Zellner S, Yanny L, McPolin C, Hollrith T, Kramer D, Ojielo C, Damm T, Cassity E, Wieliczko A, Halquist M (2019) Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA 322:1261–1270. doi: https://doi.org/10.1001/jama.2019.11825
Tong Y, Yu Z, Chen Z, Zhang R, Ding X, Yang X, Niu X, Li M, Zhang L, Billiar TR, Pitt BR, Li Q (2021) The HIV protease inhibitor Saquinavir attenuates sepsis-induced acute lung injury and promotes M2 macrophage polarization via targeting matrix metalloproteinase-9. Cell Death Dis 12:67. doi: https://doi.org/10.1038/s41419-020-03320-0
Zhang X, Zhang Z, Ju M, Li J, Jing Y, Zhao Y, Gu C, Dong M, Li G, Liu Y (2020) Pretreatment with interleukin 35-engineered mesenchymal stem cells protected against lipopolysaccharide-induced acute lung injury via pulmonary inflammation suppression. Inflammopharmacology 28:1269–1281. doi: https://doi.org/10.1007/s10787-020-00696-5
Syed MA, Bhandari V (2013) Hyperoxia exacerbates postnatal inflammation-induced lung injury in neonatal BRP-39 null mutant mice promoting the M1 macrophage phenotype. Mediators Inflamm 2013:457189. doi: https://doi.org/10.1155/2013/457189
Li T, Xiao G, Tan S, Shi X, Yin L, Tan C, Gu J, Liu Y, Deng H, Liu K, Liu M, Zhang H, Xiao X (2020) HSF1 Attenuates LPS-Induced Acute Lung Injury in Mice by Suppressing Macrophage Infiltration. Oxid Med Cell Longev 2020:1936580. doi: https://doi.org/10.1155/2020/1936580
Kumar V (2020) Pulmonary Innate Immune Response Determines the Outcome of Inflammation During Pneumonia and Sepsis-Associated Acute Lung Injury. Front Immunol 11:1722. doi: https://doi.org/10.3389/fimmu.2020.01722
Wang L, Zhang H, Sun L, Gao W, Xiong Y, Ma A, Liu X, Shen L, Li Q, Yang H (2020) Manipulation of macrophage polarization by peptide-coated gold nanoparticles and its protective effects on acute lung injury. J Nanobiotechnol 18:38. doi: https://doi.org/10.1186/s12951-020-00593-7
Pan X, Xu K, Li Y, Wang X, Peng X, Li M, Li Y (2019) Interleukin-35 expression protects against cigarette smoke-induced lung inflammation in mice. Biomed Pharmacother 110:727–732. doi: https://doi.org/10.1016/j.biopha.2018.12.028
Ye J, Huang Y, Que B, Chang C, Liu W, Hu H, Liu L, Shi Y, Wang Y, Wang M, Zeng T, Zhen W, Xu Y, Shi L, Liu J, Jiang H, Ye D, Lin Y, Wan J, Ji Q (2018) Interleukin-12p35 Knock Out Aggravates Doxorubicin-Induced Cardiac Injury and Dysfunction by Aggravating the Inflammatory Response, Oxidative Stress, Apoptosis and Autophagy in Mice. EBioMedicine 35:29–39. doi: https://doi.org/10.1016/j.ebiom.2018.06.009
Ye J, Que B, Huang Y, Lin Y, Chen J, Liu L, Shi Y, Wang Y, Wang M, Zeng T, Wang Z, Hu H, Xu Y, Shi L, Ye D, Liu J, Jiang H, Wan J, Ji Q (2019) Interleukin-12p35 knockout promotes macrophage differentiation, aggravates vascular dysfunction, and elevates blood pressure in angiotensin II-infused mice. Cardiovasc Res 115:1102–1113. doi: https://doi.org/10.1093/cvr/cvy263
Branchett WJ, Lloyd CM (2019) Regulatory cytokine function in the respiratory tract. Mucosal Immunol 12:589–600. doi: https://doi.org/10.1038/s41385-019-0158-0
Li Y, Pan X, Peng X, Li S, Zhou Y, Zheng X, Li M (2015) Adenovirus-mediated interleukin-35 gene transfer suppresses allergic airway inflammation in a murine model of asthma. Inflamm Res 64:767–774. doi: https://doi.org/10.1007/s00011-015-0858-1
Cao J, Xu F, Lin S, Tao X, Xiang Y, Lai X, Zhang L (2015) IL-35 is elevated in clinical and experimental sepsis and mediates inflammation. Clin Immunol 161:89–95. doi: https://doi.org/10.1016/j.clim.2015.08.016
Pan W, Xu X, Wang Y, Song X (2020) Interleukin-35 reduces inflammation in acute lung injury through inhibiting TLR4/NF-kappaB signaling pathways. Exp Ther Med 19:1695–1700. doi: https://doi.org/10.3892/etm.2020.8407
Sha X, Meng S, Li X, Xi H, Maddaloni M, Pascual DW, Shan H, Jiang X, Wang H, Yang XF (2015) Interleukin-35 Inhibits Endothelial Cell Activation by Suppressing MAPK-AP-1 Pathway. J Biol Chem 290:19307–19318. doi: https://doi.org/10.1074/jbc.M115.663286
Hu L, Chen C, Zhang J, Wu K, Zhang X, Liu H, Hou J (2017) IL-35 Pretreatment Alleviates Lipopolysaccharide-Induced Acute Kidney Injury in Mice by Inhibiting NF-kappaB Activation. Inflammation 40:1393–1400. doi: https://doi.org/10.1007/s10753-017-0582-9
Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, Kuebler WM, Acute Lung Injury in Animals Study G (2011) An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol 44:725–738. doi: https://doi.org/10.1165/rcmb.2009-0210ST
Yu LL, Wang Y, Xiao ZK, Chen SS (2021) Heat shock protein B8 promotes proliferation and migration in lung adenocarcinoma A549 cells by maintaining mitochondrial function. Mol Cell Biochem 476:187–197. doi: https://doi.org/10.1007/s11010-020-03896-3
Peng M, Qiang L, Xu Y, Li C, Li T, Wang J (2019) IL-35 ameliorates collagen-induced arthritis by promoting TNF-alpha-induced apoptosis of synovial fibroblasts and stimulating M2 macrophages polarization. FEBS J 286:1972–1985. doi: https://doi.org/10.1111/febs.14801
Liu X, Zhang R, Hou J, Wu J, Zhang M, Fang S, Wang X, Huang X, Tian J, Li H, Sun Y, Yu B (2019) Interleukin-35 promotes early endothelialization after stent implantation by regulating macrophage activation. Clin Sci (Lond) 133:869–884. doi: https://doi.org/10.1042/CS20180879
Tu GW, Shi Y, Zheng YJ, Ju MJ, He HY, Ma GG, Hao GW, Luo Z (2017) Glucocorticoid attenuates acute lung injury through induction of type 2 macrophage. J Transl Med 15:181. doi: https://doi.org/10.1186/s12967-017-1284-7
Pinheiro NM, Santana FP, Almeida RR, Guerreiro M, Martins MA, Caperuto LC, Camara NO, Wensing LA, Prado VF, Tiberio IF, Prado MA, Prado CM (2017) Acute lung injury is reduced by the alpha7nAChR agonist PNU-282987 through changes in the macrophage profile. FASEB J 31:320–332. doi: https://doi.org/10.1096/fj.201600431R
Vergadi E, Vaporidi K, Theodorakis EE, Doxaki C, Lagoudaki E, Ieronymaki E, Alexaki VI, Helms M, Kondili E, Soennichsen B, Stathopoulos EN, Margioris AN, Georgopoulos D, Tsatsanis C (2014) Akt2 deficiency protects from acute lung injury via alternative macrophage activation and miR-146a induction in mice. J Immunol 192:394–406. doi: https://doi.org/10.4049/jimmunol.1300959
Hu L, Chen Z, Li L, Jiang Z, Zhu L (2019) Resveratrol decreases CD45(+) CD206(-) subtype macrophages in LPS-induced murine acute lung injury by SOCS3 signalling pathway. J Cell Mol Med 23:8101–8113. doi: https://doi.org/10.1111/jcmm.14680
Pylayeva-Gupta Y (2016) Molecular Pathways: Interleukin-35 in Autoimmunity and Cancer. Clin Cancer Res 22:4973–4978. doi: https://doi.org/10.1158/1078-0432.CCR-16-0743
Du WX, He Y, Jiang HY, Ai Q, Yu JL (2016) Interleukin 35: A novel candidate biomarker to diagnose early onset sepsis in neonates. Clin Chim Acta 462:90–95. doi: https://doi.org/10.1016/j.cca.2016.09.005
Dasgupta S, Dasgupta S, Bandyopadhyay M (2020) Regulatory B cells in infection, inflammation, and autoimmunity. Cell Immunol 352:104076. doi: https://doi.org/10.1016/j.cellimm.2020.104076
Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, Rehg JE, Jones ML, Ni HT, Artis D, Turk MJ, Vignali DA (2010) IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol 11:1093–1101. doi: https://doi.org/10.1038/ni.1952
Held J, Preusse C, Doser A, Richter L, Heppner FL, Stenzel W (2013) Enhanced acute immune response in IL-12p35-/- mice is followed by accelerated distinct repair mechanisms in Staphylococcus aureus-induced murine brain abscess. J Infect Dis 208:749–760. doi: https://doi.org/10.1093/infdis/jit126
Cui H, Banerjee S, Guo S, Xie N, Ge J, Jiang D, Zornig M, Thannickal VJ, Liu G (2019) Long noncoding RNA Malat1 regulates differential activation of macrophages and response to lung injury. JCI Insight 4. doi: https://doi.org/10.1172/jci.insight.124522
Zhang Y, Huang T, Jiang L, Gao J, Yu D, Ge Y, Lin S (2019) MCP-induced protein 1 attenuates sepsis-induced acute lung injury by modulating macrophage polarization via the JNK/c-Myc pathway. Int Immunopharmacol 75:105741. doi: https://doi.org/10.1016/j.intimp.2019.105741
Ju M, Liu B, He H, Gu Z, Liu Y, Su Y, Zhu D, Cang J, Luo Z (2018) MicroRNA-27a alleviates LPS-induced acute lung injury in mice via inhibiting in fl ammation and apoptosis through modulating TLR4/MyD88/NF-kappaB pathway. Cell Cycle 17:2001–2018. doi: https://doi.org/10.1080/15384101.2018.1509635
Funding
This work was supported by the National Natural Science Foundation of China (81870072).
Author information
Authors and Affiliations
Contributions
Conception and design: Sheng-Song Chen, Jin-Gen Xia, Yi Zhang, Qing-Yuan Zhan; Conduct experiment: Sheng-Song Chen, Jin-Gen Xia; Data analysis: Sheng-Song Chen, Yi Zhang; Drafting manuscript: Sheng-Song Chen, Qing-Yuan Zhan; Interpretation and revised manuscript: Sheng-Song Chen, Jin-Gen Xia, Yi Zhang, Qing-Yuan Zhan.
Corresponding authors
Ethics declarations
Conflict of interest
All the authors declare no confict of interest.
Compliance with Ethical Standards
All applicable CHINA-JAPAN Friendship Hospital guidelines for the care and use of animals were followed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.


Rights and permissions
About this article
Cite this article
Chen, S., Xia, J., Zhang, Y. et al. IL35 attenuated LPS-induced acute lung injury by regulating macrophage polarization. Mol Biol Rep 49, 5811–5820 (2022). https://doi.org/10.1007/s11033-022-07293-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07293-5