Abstract
Background
The enhancement of fish immune system and growth performance throughout the administration of bio-friendly agents such as diet supplements (taurine) is considered a promising alternative in farmed aquatic species.
Materials and methods
The present study was aimed to examine the effect of supplementation of dietary taurine (0, 5 g-TAU5 and 10 g-TAU10) in crystalline form (99% purity) in L. calcarifer juveniles, taking into account growth performance, general health indices and immune related-genes mRNA transcript abundance.
Results
The results confirmed that the supplementation of taurine enhances significantly all the growth parameters and a better flesh quality. While the blood biochemical and immunological factors didn’t present any significant differences, the expression of growth-related genes showed that IGF-1 was almost 3 times higher in fishes fed diet Tau 5 and Tau 10 compared to the control group.
Conclusions
Finally, it can be concluded that at the maximum dose tested (10 g) the treatment was effective for Asian seabass. In addition, Tau inclusion in an FM-based diet can increase the productivity parameters along with raising the antioxidant status.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fish health management and improving diet formulations in large production practices are major factors in the aquaculture industry and for this reason, with the increased development of the aquaculture industry; different strategies such as natural additive components have been used to handle these factors [1, 2]. Among different classes of supplements, taurine is a β-amino sulfur amino acid and its essentiality in fish metabolism could be conditionally related to the species, the developmental stages and the nutrient behavior [3]. Some studies reported that its deficiencies in marine fish, fed plant protein (PP)-rich diets, could have symptoms of anemia and green liver syndrome while manifesting a slowed growth and reduced hepatic and plasma taurine concentrations [3].
Unfortunately, taurine (Tau) concentration in several kinds of FM including jack mackerel, white, menhaden and brown has been respectively reported as 0.23%, 0.34%, 0.5%, 0.6%. Since, 50% FM is usually included in a FM-based diet for carnivorous fish, the mentioned concentrations of Tau in FM in these diets would reduce to half [4, 5]. Moreover, there are two different types of FM including high and low grades in the industry, which are different in terms of nutritional quality and composition [6].
On the other hand, there are several enzymes in fish, whose activity is vital for the endogenous synthesis of Tau, such as cysteamine dioxy-genase (CDO), cysteine sulphinate decarboxylase (CSD) or cysteic acid decarboxylase (CAD). In most marine fish, the activity of these enzymes is limited as reviewed by El-Sayed [3] and Salze and Davis [7]. However, due to low levels of Tau in commercial FM-based diets, Tau requirement in a marine carnivorous species, whose determined range is between 1 and 15 g kg−1 diets, is not met by FM in a formulated diet and is often added to compensate for this reduction.
Some previous studies showed positive effects on growth performance, digestive enzyme activity, immunity status, antioxidative capacity and, in general, the stress resistance for example in Anoplopoma fimbria [8], Scophthalmus maximus juveniles [9], Epinephelus Aeneus [10], Dicentrarchus labrax [11], Argyrosomus regius [12]) and Takifugu obscurus [13]. It seems that different duration of Tau administration, life stage and/or different fish species and dosage are the main factors that influence the beneficial effect of dietary Tau on fish. Hence, recommending this amino acid as a feed additive in aquaculture relies on the prior determination of its metabolic effects on different aquatic species under specific rearing conditions [14].
The species chosen for this experiment was the Asian seabass (Lates calcarifer), because it is considered a very interesting species for developing marine cage culture projects in tropical and semitropical areas. The aquaculture of this species due to high growth, survival and market demand and its ability to adapt to culture condition fluctuations has been started from a decade ago in the coastal saline waters as well as in cage culture in the Persian Gulf and Oman Sea [15].
Despite there are some studies on Tau’s effects in aquatic species related to growth indices, feed efficiency and body composition, however very limited information is available about the supplementation of Tau in FM-based diets and its impact on fish health, immunity and antioxidant status and gene expression. So, the present study was aimed to examine the effect of Tau integration in a FM-based diet for L. calcarifer juveniles, taking into account growth performances, general health indices and immune-related and growth-related genes mRNA transcript abundance.
Materials and methods
Fish and husbandry settings
One hundred and eighty (n = 180) juveniles of Asian seabass (L. calcarifer) with average weight of 13.7 ± 0.5 g were stocked into 9 fiberglass tanks (3 treatments in triplicates) of 300 L cylindrical polyethylene tanks (with more than 50% of daily recycling). The water physicochemical parameters including water temperature, pH and salinity were recorded 28.6 ± 0.5 °C, 8.2 ± 0.2 and 40.0 ± 1.0 ppt, respectively and the photoperiod was 12 L:12 D (Light:Darkness). Fish were fed on the experimental diets twice a day (08h00 and 16h00) to apparent satiation for 6 weeks.
Experimental feeds
Three experimental diets were provided from a commercial pellet diet (Beyza, Iran, crud protein = 51%, lipid = 14%, ash = 15% and fiber = 2%) containing 0 (Control), 5 g (Tau 5) and 10 g (Tau 10) Tau (crystalline form, 99% purity, Sumchun Pure Chemical, South Korea).
Sampling
At the end of the experiment, biometric indices were determined measuring individually 20 fish of each tank. In order to assess the body composition and liver antioxidant enzymes activity, 6 fish of each tank were randomly euthanasia with overdose of an anaesthetic (2-phenoxyethanol) and the blood samples for plasma biochemical assays were drawn from the caudal vein and instantly eviscerated on an ice surface.
Chemical composition
The analyses of proximate composition of fish’s whole body including protein, lipid, moisture and ash were determined using standard methods of AOAC [16].
Antioxidant enzymes
The homogenate samples for antioxidant enzymes activity prepared according to Regoli et al. [17]. Glutathione reductase (GR) activity was measured in liver from 3 fish in each replicate, according to Cohen and Duvel [18]. Glutathione S-transferase (GST) activity was measured by the absorbance increase at 340 nm as described by Habig et al. [19]. The soluble protein of crude enzyme extracts was measured according to the method described by Bradford [20]. Glutathione (GSH) and malondialdehyde (MDA) concentration were measured in the homogenate according to Paglia and Valentine [21] and Buege and Aust [22], respectively.
Blood biochemical parameters
Biochemical parameters including glucose, albumin, total cholesterol and triglyceride were analyzed by means of an auto-analyzer, using commercial and standard kits (Pars Azmun, Tehran, Iran). The total protein content was assessed using bovine serum albumin as a standard according to the method described by Bradford [20].
Mucosal immune parameters
At the end of the experimental period, skin mucus samples were collected from 9 fish per group using a polyethylene bag containing 50 mMNaCl [23]. After mucus collection, fish were returned to the corresponding tank. The skin mucus lysozyme activity was measured according to a method based on the lysis ability of the skin mucus, using the lysozyme sensitive bacterium Micrococcus luteus (obtained from IBRC microorganism’s collection) [24]. The total Ig level in mucus samples was gauged according to the method that considers immunoglobulin precipitation by means of polyethylene glycol [25]. The alternative complement pathway hemolytic activity (ACH50) was estimated following the procedure of Bagni et al. [26].
Gene expression
Elongation factor 1α (Ef1a with accession number GQ507427.1 and Sequences of primers Forward: AAATTGGCGGTATTGGAAC and Reverse: GGGAGCAAAGGTGACGAC) was applied for internal housekeeping gene. The genes determined in the present study included Insulin-like Growth Factor I (IGF-I with accession number XM_018697285.1 and Sequences of primers Forward: ACGCTGCAGTTTGTATGTGG and Reverse: CCTTAGTCTTGGGAGGTGCA) and Interleukin-1β (IL-1β with accession number XM_018669006.1 and Sequences of primers Forward: CCTGTCGCATTTCAGTACGG and Reverse: ATTTCCACCGGCTTGTTGTC). All protocol and real-time quantitative RT‐PCR was performed according to Zeynail et al. [27].
Statistics
The analysis of variance by means of the Statistical Analysis System (version 9.4, SAS Institute, Cary, NC, USA) was undertaken on fish growth performance and physiological responses data. A Tukey’s post-test was used for multiple comparisons at significance of 0.05.
Results
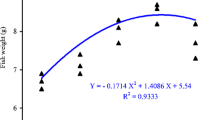
Growth and feeding performance of the fishes of Asian seabass juveniles (L. calcarifer) are reported in Table 1. While the final weight was significantly higher with diet Tau 10, the SGR and WG were similar in diet Tau 5 and Tau 10 (3.2442, 3.3786 and 290.62, 313.50, respectively, vs. 3.0608 and 261.70 in control) (P < 0.05). FCF and DFI were significantly better in Tau 10 (P < 0.05). Food conversion ratio (FCR) and protein efficiency ratio (PER) showed similar better values in Tau 5 e Tau 10 (P < 0.05).
Moister (%) showed lower levels in diet Tau 5 and Tau 10 than the control (P < 0.05, Table 2). Protein content (%) of the carcasses was higher in diet Tau 5 e Tau 10 (P < 0.05, Table 2). Lipid (%) presented a different trend, in fact their level was lower with diet Tau 10 (3.835), intermediate in control (4.500) and higher in diet Tau 5 (5.2103) (P < 0.05, Table 2). No significant differences were observed for the % of ash in the carcasses (P > 0.05, Table 2).
After 6 weeks of experimental feeding, no significant differences in terms of mucus immune response such as lysozyme, complement activity (ACH50), total immunoglobulin (total Ig), total protein, albumin, triglyceride, cholesterol and glucose in Asian seabass (P > 0.05; Table 3).
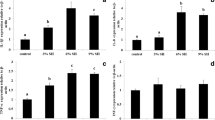
The investigation about the expression of immune-related and growth-related genes permitted to observe that IGF-1 in almost 3 times higher in fishes fed diet Tau 5 and Tau 10 (P < 0.05). It was considered also IL1B, but no significant differences among the three treatments were observed (Fig. 1; P > 0.05).
Glutathione S-transferase (GST) presented similar concentrations in the 3 different treatments (Fig. 2; P > 0.05). GR levels were about 8.07 and 8.77 in diet Tau 5 and Tau 10, respectively, vs. 7.15, observed in diet control (Fig. 2; P < 0.05). Great differences were reported for GSH, in fact the value for control (6.96) was significantly lower (almost a half) towards the concentration in diet Tau 5 (15.97) and Tau 10 (15.62) (Fig. 2; P < 0.05). The MDA had the lower concentration in diet Tau 5 (0.17), intermediate in Tau 10 (0.38) and higher in control (0.66) (Fig. 2; P < 0.05).
Discussion
In fish tissues, Tau is present in high concentrations in various organs and for these reasons, recently, a number of studies have concentrated on evaluating and demonstrating the essentiality of its inclusion in the diet of many commercially teleosts fish [3]. It is interesting to underline that, actually, in the past, Tau had not been considered essential in fish nutrition, but plenty of recent literatures indicate that its metabolic synthesis widely differs between fish species. In particular, some species lack at all of the ability of its endogenous synthesis or they are not able to produce it in a sufficient amount. Furthermore, Tau is commonly applied in diets in which fish meal (FM) is replaced with plant source protein to cover the reduction of FM in these diets. However, Tau levels in FM-based diet are also low compared to the base requirement and fresh food [28]. Therefore, the present study aimed to determine whether a FM-based diet requires to supplementing Tau and it could improve the productivity parameters of Asian seabass juvenile (D. labrax).
The studies about productive parameters conducted by Kim et al. [5] reported that a dietary Tau supplementation in FM-based diets stimulated the feed intake and the final body weight of Japanese flounder (Paralichthys olivaceus). Similarly, inclusion of Tau different levels in FM-based diets showed that growth performance and feed efficiency enhanced in European sea bass (D. labrax) juveniles when fish fed 0.7 and 1% Tau diets compared to the control group [29]. These juveniles showed a better growth performance, due by the addition of 0.2% of this amino acid when fish meal and soybean meal were used as the primary sources of protein [30]. Some studies that underlines the fact that Tau had no significant effects on growth performance in juvenile Japanese flounder (FM-based diet with 0.25, 0.5, 1, and 1.5% Tau) [5], in red sea bream (Pagrus major), (FM-based diet with 0.3, 0.6, and 1% Tau and casein-based diet) [28, 31], and Atlantic salmon (Salmo salar) [32] are also valuable, confirming that species, Tau dose, test duration and experimental conditions play an important role on enhancing a positive effect of dietary Tau on growth performance of the fish.
The present results confirm that the supplementation of Tau enhances significantly all the productive parameters compared to the control group. A part for the final weight, FCF and DFI, that were significantly better for diet Tau 10, the other measurements including WG, SGR, FCR and PER didn’t showed differences for the two levels of supplement (5 or 10 g of Tau added in diet). The positive effects of dietary Tau supplementation may be due to different reasons as:
-
(i)
Although Tau have little role as a feed attractant, it seems to increase fish growth performance [7, 30];
-
(ii)
An increase in feed intake due to the appetite stimulating effect as observed in our study can interpret the increased growth;
-
(iii)
It is known that inclusion of dietary Tau can be associated with feed utilization efficiency, which is in line with our result regarding FCR and PER;
-
(iv)
Have a number of essential biological functions such as bile salt conjugation and bile acid metabolism to anti-oxidation, membrane stabilization, osmoregulation, calcium-signaling and transport, neuro-protection, immunomodulation, myocardial contractility, retina development and several endocrine functions, such as detoxification [7];
Due to the carcass analysis, it can be stated that also the quality of the whole body in the present study was influenced by the diets Tau 5 and Tau 10. In juvenile European sea bass (D. labrax), dietary Tau supplementation increased protein value, but decreased lipid content compared to the control group [29]. Similar results regarding body composition have been reported by Li et al. [33] for yellow cat fish (Pelteobagrus fulvidraco) and Yun et al. [34] and Qi et al. [35] for turbot (S. maximus) that fed the Tau-supplemented diets. The Asian seabass (L. calcarifer) juveniles fed with the control diet resulted more fat and with low protein levels in comparison with the other two treatments, that were probably affected positively by the enhancement of the fat metabolism and the feed utilization efficiency by this sulfur compound like Tau (as reported by El-Sayed, [3]). It has been demonstrated that Tau has a significant effect on lipid metabolism and increases lipid absorption in the gut through improving bile acid secretion and cholesterol catabolism [36].
Since Tau has the numerous physiological and metabolic activities, it can be affirmed that deficiency symptoms has a negative impact on animal welfare and consequently on commercial production. Plasma glucose is usually used as a stress indicator in fish and its increase is often associated with the increased stress status [37]. In our results, plasma glucose remained unchanged among different treatments that indicating L. calcarifer fed with or without Tau diet did not induce physiological stress to fish. Similar to our results on blood biochemical parameters, the study of Han et al. [37] showed that plasma total protein, total cholesterol and lysozyme activity were not significantly changed with supplementing Tau. However, some experiments have stated that blood biochemical parameters and innate immune response are also improved by the Tau supplementation on olive flounder (P. olivaceus) [38] and on yellowfin seabream (Acanthopagrus latus) [39]. Gunathilaka et al. [40] in red seabream (P. major) juvenile have reported that dietary Tau did not change glucose and cholesterol values and total immunoglobulin (total Ig) when fish fed Tau-supplemented diet compared to fish fed the control diet. In the present study, although fish in the control group and Tau-supplemented groups did not show significant differences in terms of immune response, the trend of total Ig lysozyme and ACH50 tended to increase, but this difference was not significant. The result of non-specific immune response and some biochemical parameters such as total protein and albumin indicated that the disease resistance of Asian seabass (L. calcarifer) may not change with the Tau supplementation under the current conditions.
The IGF-1 mRNA expression was measured in order to determine the hypothetic effect of Tau supplementation on gene expression. It was observed that IGF-1 was in almost 3 times higher in fishes fed diet Tau 5 and Tau 10. This peak of IGF-1 confirms that it can be up-regulated by the Tau supplementation in diets. Similar conclusions were reported by Kim et al. [38] and by Gunathilaka et al. [40] that observed the enforcement enhancement of IGF-1 mRNA expression by dietary Tau supplement, especially in waters characterized by low temperature, in the olive flounder and red seabream, respectively. For these reason, Tau could increase the tolerance to low temperature stress [12]. Furthermore, since IGF-1 production in fishes is stimulated by the growth hormone (GH) and modulated by nutritional immunity of fish and shellfish, this can explain the present growth performances observed [40].
Considering the pattern of the antioxidant enzymes of the present paper and the significant differences observed for GSH and GR, with a value in the control diet almost an half towards the other 2 experimental treatments for the first enzyme, it can be affirmed that they are consistent with the fact that Tau has a similar function in human, mammals and fish. Its role is important in exerting nutritional regulation in fish hepatic activity by eliminating different free radicals or by improving and/or recovering the antioxidant enzyme activities [41]. Vanitha et al. [42] described the modulatory effect of Tau on the liver mitochondrial enzyme system, considering the mitochondrial lipid peroxidation (LPO), antioxidants, major tricarboxylic acid cycle enzymes and electron transport chain enzymes. Fang at al. also reported that Tau can directly scavenge free radicals [43]. The maintaining of cellular homeostasis and the protection of multiple tissues from systemic responses and damage is essentially assured by the redox system. Intensive breeding and rearing of fish generally exposed the animals to numerous stressors that can affect their productivity and the reproductive sphere.
Life stage of fish is important to influence the level of oxidative stress, probably because juveniles are physiologically characterized by incomplete development of the endogenous antioxidant defense system and/or a delayed response of this one [44]. The effect on the reduction of the MDA was more evident in diet Tau 5 than in Tau 10, while the control group had the highest level of MDA. MDA is commonly considered as a marker of lipid peroxidation in fish; its presence in the muscle indicates muscular oxidative distress and it is probably related to the muscular dystrophy. But several investigations report that levels of MDA in the hepatic tissue are higher than the whole body, which may be determined by the significant liver storage of vitamin E. Oxidative stress can often be associated with slow growth and feed utilization [44], which is consistent with the results obtained with the control group in our study.
In conclusion, the results obtained confirmed that the supplementation of Tau in an FM-based diet exerts a positive effect on growth and feeding performances for Asian seabass juvenile. Also the antioxidant system activity reflected a positive effect. The dose of Tau (5 or 10 g) did not seem to exert a significant difference for all the parameters measured such as blood biochemical and mucus immunological factors. For this reason, it can be stated that at the maximum dose tested (10 g) the treatment was effective for Asian seabass (L. calcarifer). In addition, Tau inclusion in an FM-based diet can increase the productivity parameters along with raising the antioxidant status.
References
Ahmadifar E, Pourmohammadi Fallah H, Yousefi M, Dawood MA, Hoseinifar SH, Adineh H, Yilmaz S, Paolucci M, Doan HV (2021) The gene regulatory roles of herbal extracts on the growth, immune system, and reproduction of fish. Animals 11(8):21–67
FAO (2020) The State of World Fisheries and Aquaculture 2020. Sustainability in action. FAO, Rome. https://doi.org/10.4060/ca9229en
El-Sayed A-FM (2014) Is dietary taurine supplementation beneficial for farmed fish and shrimp? A comprehensive review. Rev Aquacult 6:241–255
Kim SK, Takeuchi T, Akimoto A, Furuita H, Yamamoto T, Yokoyama M, Murata Y (2005) Effect of taurine supplemented practical diet on growth performance and taurine contents in whole body and tissues of juvenile Japanese flounder Paralichthys olivaceus. Fish Sci 71:627–632
Kim SK, Matsunari H, Takeuchi T, Yokoyama M, Furuita H, Murata Y, Goto T (2008) Comparison of taurine biosynthesis ability between juveniles of Japanese flounder and common carp. Amino Acids 35:161–168
Hardy RW (1995) Nutrition and utilization technology in aquaculture. AOCS Press, Champaign, IL
Salze G, Davis DA (2015) Taurine: a critical nutrient for future fish feeds. Aquaculture 437:215–229. https://doi.org/10.1016/j.aquaculture.2014.12.006
Johnson RB, Kim S-K, Watson AM, Barrows FT, Kroeger EL, Nicklason PM, Goetz GW, Place AR (2015) Effects of dietary taurine supplementation on growth, feed efficiency, and nutrient composition of juvenile sablefish (Anoplopoma fimbria) fed plant based feeds. Aquaculture 445:79–85
Liu Y, Yang P, Hu H, Li Y, Dai J, Zhang Y (2018) The tolerance and safety assessment of taurine as additive in a marine carnivorous fish, Scophthalmus maximus L. Aquacult Nutr 24(1):461–471
Koven W, Peduel A, Gada M, Nixon O, Ucko M (2016) Taurine improves the performance of white grouper juveniles (Epinephelus Aeneus) fed a reduced fish meal diet. Aquaculture 460:8–14
Martins N, Estevão-Rodrigues T, Diógenes AF, Diaz-Rosales P, Oliva-Teles A, Peres H (2018) Taurine requirement for growth and nitrogen accretion of European seabass (Dicentrarchus labrax, L.) juveniles. Aquaculture 494:19–25. https://doi.org/10.1016/j.aquaculture.2018.05.007
De Moura LB, Diógenes AF, Campelo DAV, De Almeidak FLA, Pousão-Ferreira PM, Furuya WM, Oliva-Teles A, Peres H (2018) Taurine and methionine supplementation as a nutritional strategy for growth promotion of meagre (Argyrosomus regius) fed high plant protein diets. Aquaculture 497:389–395. https://doi.org/10.1016/j.aquaculture.2018.07.038
Cheng CH, Guo ZX, Wang AL (2018) The protective effects of taurine on oxidative stress, cytoplasmic free-Ca2+ and apoptosis of pufferfish (Takifugu obscurus) under low temperature stress. Fish Shellfish Immunol 77:457–464. https://doi.org/10.1016/j.fsi.2018.04.022
Sampath H, Group GBW, Rathnayake RMDS, Yangm M, Zhang W (2020) Roles of dietary taurine in fish nutrition. Mar Life Sci Technol 2:360–375. https://doi.org/10.1007/s42995-020-00051-1
Ashouri G, Soofiani NM, Hoseinifar SH, Jalali SAH, Morshedi V, Van Doan H, Mozanzadeh MT (2018) Combined effects of dietary low molecular weight sodium alginate and Pediococcus acidilactici MA18/5 M on growth performance, haematological and innate immune responses of Asian seabass (Lates calcalifer) juveniles. Fish Shellfish Immunol 79:34–41
AOAC (2005) Official method of analysis, 18th edn. Association of Officiating Analytical Chemists, Washington, DC
Regoli F, Bocchetti R, Filho DW (2012) Spectrophotometric assays of antioxidants. In: Abele D, Vázquez-Medina JP, Zenteno-Savín T (eds) Oxidative stress in aquatic ecosystems. Wiley, Chichester, pp 367–380
Cohen MB, Duvel DL (1988) Characterization of the inhibition of glutathione reductase and the recovery of enzyme activity in exponentially growing murine leukemia (11210) cells treated with 1, 3-bis (2-chloroethyl)-1-nitrosourea. Biochem Pharmacol 37:3317–3320
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Hoseinifar SH, Sohrabi A, Paknejad H, Jafari V, Paolucci M, Van Doan H (2019) Enrichment of common carp (Cyprinus carpio) fingerlings diet with Psidium guajava: the effects on cutaneous mucosal and serum immune parameters and immune related genes expression. Fish Shellfish Immunol 86:688–694. https://doi.org/10.1016/j.fsi.2018.12.001
Ellis AE (1990) Lysozyme assays. In: Stolen JS, Fletcher TC, Rowley AF, Zelikoff J, Kaattari S, Smith S (eds) Techniques in fish immunology. SOS Publications, Cambridge, pp 101–103
Siwicki A (1993) Nonspecific defense mechanisms assay in fish. II. Potential killing activity of neutrophils and macrophages, lysozyme activity in serum and organs and total immunoglobulin (Ig) level in serum. In: Olsztyn P (ed) Disease diagnosis and prevention methods. FAO, Rome, pp 105–112
Bagni M, Romano N, Finoia MG, Abelli L, Scapigliati G, Tiscar PG, Sarti M, Marino G (2005) Short-and long-term effects of a dietary yeast β-glucan (Macrogard) and alginic acid (Ergosan) preparation on immune response in sea bass (Dicentrarchus labrax). Fish Shellfish Immunol 18(4):311–325
Zeynali M, Nafisi Bahabadi M, Morshedi V, Ghasemi A, Torfi Mozanzadeh M (2020) Replacement of dietary fishmeal with Sargassum ilicifolium meal on growth, innate immunity and immune gene mRNA transcript abundance in Lates calcarifer juveniles. Aquacult Nutr 26(5):1657–1668
Kato K, Yamamoto M, Peerapon K, Fukada H, Biswas A, Yamamoto S, Takii K, Miyashita S (2014) Effects of dietary taurine levels on epidermal thickness and scale loss in red sea bream, Pagrus major. Aquacullt Res 45(11):1818–1824
Saleh NE, Wassef EA, Ashry AM (2020) Is a taurine supplement necessary in fishmeal-based feeds for juvenile European sea bass (Dicentrarchus labrax)? Aquacult Int 28:321–333. https://doi.org/10.1007/s10499-019-00464-5
Martinez JB, Chatzifotis S, Divanach P, Takeuchi T (2004) Effect of dietary taurine supplementation on growth performance and feed selection of sea bass Dicentrarchus labrax fry fed with demand-feeders. Fish Sci 70:74–79
Matsunari H, Yamamoto T, Kim SK, Goto T, Takeuchi T (2008) Optimum dietary taurine level in casein-based diet for juvenile red sea bream Pagrus major. Fish Sci 74:347–353
Espe M, Ruohonen K, El-Mowafi A (2012) Effect of taurine supplementation on the metabolism and body lipid-to-protein ratio in juvenile Atlantic salmon (Salmo salar). Aquacult Res 43:349–360
Li M, Lai H, Li Q, Gong S, Wang R (2016) Effects of dietary taurine on growth, immunity and hyperammonemia in juvenile yellow catfish Pelteobagrus fulvidraco fed all-plant protein diets. Aquaculture 450:349–355
Yun B, Ai Q, Mai K, Xu W, Qi G, Luo Y (2012) Synergistic effects of dietary cholesterol and taurine on growth performance and cholesterol metabolism in juvenile turbot (Scophthalmus maximus L.) fed high plant protein diets. Aquaculture 324:85–91
Qi G, Ai Q, Mai K, Xu W, Liufu Z, Yun B, Zhou H (2012) Effects of dietary taurine supplementation to a casein-based diet on growth performance and taurine distribution in two sizes of juvenile turbot (Scophthalmus maximus L.). Aquaculture 358:122–128
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72:101–163
Han YZ, Koshio S, Jiang ZQ, Ren TJ, Ishikawa M, Yokoyama S, Gao J (2014) Interactive effects of dietary taurine and glutamine on growth performance, blood parameters and oxidative status of Japanese flounder Paralichthys olivaceus. Aquaculture 434:348–354
Kim J-M, Malintha GHT, Gunathilaka GLBE, Lee C, Kim M-G, Lee B-J, Kim J-D, Lee K-J (2017) Taurine supplementation in diet for olive flounder at low water temperature. Fish Aquat Sci 20(1):1–8
Dehghani R, Oujifard A, Mozanzadeh MT, Morshedi V, Bagheri D (2020) Effects of dietary taurine on growth performance, antioxidant status, digestive enzymes activities and skin mucosal immune responses in yellowfin seabream, Acanthopagrus latus. Aquaculture 517:734–795
Gunathilaka GLBE, Kim M-G, Lee C, Shin J, Lee BJ, Lee K-J (2019) Effects of taurine supplementation in low fish meal diets for red seabream (Pagrus major) in low water temperature season. Fish Aquat Sci 22:1–10
Duan C (1998) Nutritional and developmental regulation of insulin-like growth factors in fish. J Nutr 128:3056–3145
Vanitha MK, Priya KD, Baskaran K, Periyasamy K, Saravanan D, Venkateswari R, Mani BR, Ilakkia A, Selvaraj S, Menaka R, Geetha M, Rashanthy N, Anandakumar P, Sakthisekaran D (2015) Taurine regulates mitochondrial function during 7,12-dimethyl benz[a]anthracene induced experimental mammary carcinogenesis. J Pharmacopuncture 18(3):68–74. https://doi.org/10.3831/KPI.2015.18.027
Fang YZ, Sheng Yang S, Wu G (2002) Free radicals, antioxidants, and nutrition. Nutrition 18(10):872–879. https://doi.org/10.1016/s0899-9007(02)00916-4
Biller JD, Takahashi LS (2018) Oxidative stress and fish immune system: phagocytosis and leukocyte respiratory burst activity. An Acad Bras Ciênc 90(4):3403–3414. https://doi.org/10.1590/0001-3765201820170730
Acknowledgements
We are grateful to the director and staff of Hounam Abziparvaran Naji Company, Shiraz, Iran for providing the necessary facilities for the experiment.
Author information
Authors and Affiliations
Contributions
All persons listed as authors have read, contributed to preparing the manuscript as given below: SH and DB: fish maintenance, sample collection and some analyses; VM: experimental design, wrote the manuscript and statistical analyses; AG: gene expression analyses; SR: wrote the manuscript; RG: data interpretation.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed by the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Morshedi, V., Rainis, S., Hamedi, S. et al. Effects of dietary taurine amino acid on growth performance, mucosal and immune response, gene expression and antioxidant defence of asian seabass (Lates calcarifer). Mol Biol Rep 49, 3503–3510 (2022). https://doi.org/10.1007/s11033-022-07187-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07187-6