Abstract
Alzheimer's disease is a common neurodegenerative disease in the elderly population and a leading cause of dementia. Genetics and environmental risk factors were considered to play a major role in the onset of the disease. This study aimed to examine the correlation between different metals levels and the gene expression in Alzheimer's patients with age-matched control subjects. Non- essential metals were measured in the whole blood due to its higher concentration in red blood corpuscles (RBCs) and essential biometals in the serum samples of Alzheimer's disease (AD) by using Inductively coupled plasma optical emission spectroscopy (ICP-OES) that allows the analysis and detection of the different elements at low levels. Gene expression level was performed by quantitative real-time PCR (qRT-PCR). In this study, the levels of Lead and Arsenic metals were not detected in the AD patient samples. Cadmium, Mercury, and Aluminum were found higher in cases as compared to controls with 0.009240 ± 0.0007707 (P = < 0.0001), 0.02332 ± 0.001041 (P = < 0.0001), and 0.09222 ± 0.02804 (P = 0.0087) respectively. Essential biometal like copper was higher 0.1274 ± 0.02453 (P = 0.0254) in cases, while iron 0.1117 ± 0.009599 (P = 0.0304) and zinc 0.03800 ± 0.003462 mg/L were found significantly lower as compared to controls. All targeted genes such as APP, PSEN1, PSEN2, and APOE4 were found up-regulated in AD patients. We concluded that there was no significant correlation between metals dyshomeostasis and gene expressions in this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer's disease is characterized by neuronal cell death and synaptic loss of the brain with the accumulation of insoluble amyloid-beta (Aβ) protein and hyperphosphorylated tau protein [1, 2]. Many studies have focused on finding out reliable biomarkers in the peripheral blood for early clinical diagnosis, but facing problems due to the multifactorial nature of the disease onset [3, 4].
Genetics and environment, both may play an important role in an early and late-onset of the disease [5]. Metal ions such as zinc and copper have a key role in brain neurobiology. Its altered homeostasis may contribute to protein misfolding and influence protein energetics, with specific attention on conformational and structural changes [6,7,8]. Essential metals like Zn, Cu, and Fe were found to be excessively bound with amyloid-beta and nearby extracellular senile plaques [9, 10]. Some non-essential metals (As, Pb, Cd, and Hg) have also been reported to induce cognition impairment in the brain [11]. The four most common genes that have been associated with more than half of the genetic risk of Alzheimer's disease are amyloid precursor protein (APP) on chromosome 21, presenilin 1 on chromosome 14, presenilin 2 on chromosome 1, and apolipoprotein E on chromosome19. Apolipoprotein (ApoE) has the most important genetic role in the late-onset of the disease; this protein is important for the proper growth, maintenance, and transportation of cholesterol in synapses [12]. PSEN2 gene has been found significantly downregulated in the auditory cortex region of the brain by using real-time PCR [13]. However, many studies have used a blood sample to estimate the level of heavy metals for many years [14,15,16]. Furthermore, the inductively coupled plasma-optical emission spectrometry technique (ICP-OES) is the most commonly used technique for multi-element analysis in biological samples [17, 18]. In this context, wet digestion of various biological samples using mineral acids has been followed by many studies [19,20,21]. The microwave digestion technique is more reliable and efficient, currently been used widely for biological sample preparation [15, 22, 23]. This study was conducted to investigate the correlation of metals toxicity with gene expression levels in AD patients.
Material and method
This study was approved by the Ethics committee of King George's Medical University, Lucknow. Written informed consent forms were collected from all the subjects or primary caregivers. Probable Alzheimer's patients mainly from the Indian population n = 50 (32 females and 18 males) were enrolled with 50 age-matched non-demented control subjects from the department of the Geriatric Mental Health department. Four ml peripheral blood sample was collected from each patient into a plane and EDTA vial. The patients were clinically diagnosed by criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer Disease and Related Disorders Association (NINCDS-ADRDA). Patients were enrolled by following Mini-Mental State Examination criteria (MMSE ≤ 20) to understand the disease progression; the P-value was < 0.0001, which is statistically significant. Subsequently, the MMSE score for the non-demented control subject was between 26 and 30. The mean age for the Alzheimer's patient was 74.13 ± 1.683. The person suffering from Diabetes mellitus, hypertension, and other chronic illness were excluded from this study.
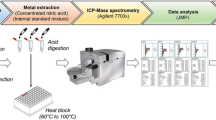
Metals estimation
Inductively coupled plasma optical emission spectroscopy (ICP-OES) was used for metals analysis such as lead, mercury, cadmium, aluminum, and arsenic (Pb, Hg, Cd, Al, and As) from blood samples of Alzheimer's disease. Blood samples were digested by using the Multiwave Reaction System (Multiwave 3000, Anton Paar, Perkin Elmer) with the rotor 16HF100 (100 ml PFA vessels, 40 bars) and pressure, temperature (p/T) sensor. All samples were appropriately homogenized by vortexing for 2 min before pipetting and then transferred into a closed vessel for the acid digestion following the (Hseu 2004) method [24]. The reaction mixture was: 0.5 ml whole blood, 1.5 ml of nitric acid, 0.5 ml perchloric acid, 1 ml hydrogen peroxide (Thermo Fisher Scientific), and 1 ml ultra-pure water from a milli-Q purification apparatus with one blank sample at each set simultaneously, according to the digestion program shown in the electronic supplementary material (Appendix S1).
Essential metals zinc, copper, and iron (Zn, Cu, and Fe) were analyzed from the serum samples diluted in a 1:3 ratio. The serum was isolated from the whole blood centrifuged at 2000 rpm for 15 min. Multi-element standard (Perkin Elmer Pure Plus, USA) stock solution 1000 mg/L was used for the preparation of calibration standard solution of different concentration levels 0.005, 0.05, 0.5, and 1 mg/L. The obtained clear solution after acid digestion was kept into fresh tubes after sufficient cooling of the vessels. The sample was analyzed in triplicate by ICP-OES (Optima 8000, Perkin Elmer) inbuilt with winlab32 software for elemental analysis at given instrumental conditions in Table 1.
Instrumentation
The instrument was equipped with a cyclonic spray chamber and concentric high solid nebulizer for the determination of metal ions. Samples were aspirated by a manual analysis control process.
Instrument detection limits were determined by measuring the emission intensities of seven blanks. The limits of detection (LOD) and limits of quantification (LOQ) were calculated from the formula CLOD = 3sb/m and CLOQ = 10sb/m respectively. Where Sb is the standard deviation of seven replicate blank measurements, and m is the slope of the calibration curve. The figures of merit of the instrumental conditions are available online in the supplementary material (Appendix S2). Before sample analysis, the linear calibration curve for the standard was done. The determined correlation coefficient (R2) means for all the metals was 0.997. The instrument was adjusted to measure the samples in triplicate, and the mean concentration of analyte and relative standard deviation were automatically calculated.
Isolation of total RNA and cDNA synthesis
Total RNA was isolated from whole blood by using the TRIZOL (Invitrogen) extraction method. The yield and quality of the isolated RNA were assessed by using a nanodrop (Thermo scientific nanodrop 2000 spectrophotometer).
Complementary DNA (cDNA) was synthesized by using Thermo scientific verso cDNA synthetic kit (AB-1453/B) by following reaction mixture preparation: 5 × cDNA synthesis buffer 4 µl, dNTP mixture 2 µl, RNA primer 1 µl, RT Enhancer 1 µl, Verso Enzyme mix 1 µl, RNA template 1 ng, and nuclease-free water for total 20 µl final reaction volume at the following PCR conditions: 1 cycle at 42 °C for 30 min and inactivated at 95 °C for 2 min as prescribed in the protocol. 10× diluted cDNA was used as a template for real-time PCR.
Oligonucleotide primers
Primers were designed from The National Center for Biotechnology Information (NCBI) by using the Primer Quest tool. The details of the primer sequence used in this study have been mentioned in the online supplementary material (Appendix S3).
Quantitative real-time PCR
Applied Biosystems 7500 Fast Real-Time PCR system (Applied Biosystems™, USA) instrument was used for the quantitative gene expression study by using SYBR- Green chemistry. All reactions were carried out in duplicate containing 10 µl volume per well with No Template control (NTC). Beta Actin (β-actin) was used as an endogenous control. Amplification conditions for PCR reaction were: 50 °C for 20 s, 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. A dissociation curve was generated to distinguish the specific amplicon from the unspecific amplicon. The amplified real-time PCR product was loaded onto agarose gel containing ethidium bromide dye and run by using the electrophoresis technique. Appeared bands were captured using image lab ™ software (version 5.1) associated with Gel Doc™ XR + and ChemiDoc™ XRS + System.
Ct value was calculated by using Data assist software version 3.01. The fold change was calculated by using the comparative Ct method (2−ΔΔCT), where ΔΔCT was the difference between ΔCT and the ΔCT calibrator value.
Statistical calculations
Graph pad Prism software 8.4 version was used for statistical analysis. Heatmapper tool was used for the gene expression data. The non-parametric test was used to perform the data analysis. All results were expressed as Mean ± SEM for all the parameters. P-value < 0.05 was considered as statistically significant.
Results
In this study, the levels of Lead and Arsenic metals were not detected (ND*) in the blood sample of AD patients. The levels of cadmium, mercury, and aluminum were found higher in cases as compared to controls. Non- essential metals such as cadmium and mercury were estimated significantly higher with a P-value < 0.0001 in AD.
At the same time, the levels of essential biometal like copper were higher in the serum samples of cases. In contrast, iron and zinc were found lower in cases as compared to controls. Essential metals analysis suggested that zinc was significantly lower with a P-value < 0.0001 in serum samples of Alzheimer's disease (Fig. 1, Table 2). Metals concentration was measured by ICP-OES in the mg/L unit.
The expression levels of selected genes such as APP, PSEN1, PSEN2, and APOE4 were found up-regulated in AD patients. PSEN2 gene was found significantly up-regulated with a P-value of 0.0002 in cases as compared to controls (Fig. 2, Table 3). Normalized gene expression of AD patients has been shown in (Fig. 3) by using a heatmap. The correlation between metals level and gene expression was not found significant in Alzheimer's disease (Fig. 4).
Discussion
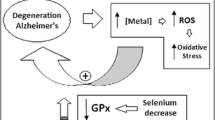
Essential biometals like Cu, Zn, and Fe were also found concentrated in and around amyloid-beta plaques in the AD brain [9]. Many studies reported a decreased level of Zn2+ in serum and blood but increased in the cerebrospinal fluid [25,26,27]. Increased Cu2+ level may enhance the dimerization of amyloid-beta precursor protein that leads to the overproduction of extracellular Aβ [28].
In our study, we have also found a higher level of Cu and lower levels of Zn and Fe in the serum samples of AD. Similarly, a low level of zinc has been found in serum samples of the patients [29].
Moreover, it has been found that Apo E isoform can bind to metals such as iron, copper, and zinc that are commonly involved in the onset of AD showed a maximum affinity with copper and minimum for zinc [30]. Zinc and apolipoprotein E (Apo E) have been associated with the amyloid-beta pathology involved in Alzheimer’s disease. Apolipoprotein E4 (apoE4) is the most prevalent genetic risk factor expressed in more than half of patients and is thus considered as an important possible therapeutic target in AD [31, 32].
Different studies have shown the association among non-essential metals such as Cd, Pb, and MeHg as a risk factors for cognitive functions [33, 34]. A growing amount of evidence has shown that Cd is able to initiate neuronal apoptosis [35, 36]. We have found a higher level of Cd, Al, and Hg in this study. Pb and As were not detected in the blood samples of AD. The correlation between metals level and gene expression was not found significant in Alzheimer's disease (Fig. 5).
Correlation matrix plot illustrating correlation coefficients between Metals and Gene expression level in AD. Correlation coefficient + 1 indicates a perfect positive relationship, Correlation Coefficient 0 indicates No relationship, and Correlation Coefficient − 1 indicates a perfect negative relationship
A higher concentration of mercury levels has been reported in isolated subcellular fractions of AD brains [37]. One study proposed that inorganic arsenic associated with cognitive impairment may be due to activation of pro-inflammatory responses, which may further contribute to neurodegenerative disease [38].
A higher level of aluminum accumulation was found in AD and more pronounced in the hippocampus and entorhinal cortex region of the brain [39,40,41,42]. Aluminum may induce conformational changes in amyloid-beta and increased its aggregation in vitro [43]. Several in vivo studies, showed that aluminum might involve in the up-regulation of the APP gene that leads to the deposition of amyloid-beta plaque in the cerebral cortex and hippocampus region of the brain [44, 45]. Aluminum is also able to accelerate the aberrant splicing isoform of PSEN2 that occurs in the neural tissue of AD [46].
Presenilin 1 (PSEN1) and presenilin 2 (PSEN2) genes are the major components of ϒ-secretase. Any mutation in these genes can alter the amyloid-beta protein synthesis [47,48,49]. One study conducted on PSEN1 and PSEN2 genes by using 150 postmortem tissue samples from the different regions of the brains showed no difference in PSEN1 expression, but PSEN2 was significantly downregulated in AD patients [13]. Furthermore, contrary to this increased PSEN1 expression alone can increase the activity of ϒ secretase and elevate the accumulation of Aβ in vivo [50].
Nevertheless, no correlation between metals level and gene expression was established by this study. It may be that only 50 AD cases were examined in this study compared to the many millions of AD cases worldwide that have not been studied.
Conclusion
Environmental exposure and genetic risk factors may play the role of disease onset independently in AD. Essential biometals such as copper were higher, while iron and zinc were found lower in the serum sample of AD, and its imbalance may lead to neurodegeneration. Further studies are required using a large sample size of AD patients in the future. We concluded that there was no significant correlation between metals dyshomeostasis and gene expressions in this study.
Data availability
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.
References
Selkoe DJ (2001) Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid\beta-protein. J Alzheimers Dis 3(1):75–82
Bradley-Whitman MA, Lovell MA (2013) Epigenetic changes in the progression of Alzheimer’s disease. Mech Aging Dev 134(10):486–495
Park JH, Lee DW, Park KS, Joung H (2014) Serum trace metal levels in Alzheimer’s disease and normal control groups. Am J Alzheimer’s Dis Other Dement 29(1):76–83
Gruenblatt E, Bartl J, Zehetmayer S, Ringel TM, Bauer P, Riederer P, Jacob CP (2009) Gene expression as peripheral biomarkers for sporadic Alzheimer’s disease. J Alzheimers Dis 16(3):627–634
Yadav J, Verma AK (2018) The genetic and environmental risk factors of Alzheimer’s disease. Int J Aging Res 1(4):16
Bush AI (2003) The metal biology of Alzheimer’s disease. Trends Neurosci 26(4):207–214
Leal SS, Botelho HM, Gomes CM (2012) Metal ions as modulators of protein conformation and misfolding in neurodegeneration. Coord Chem Rev 256(19–20):2253–2270
Pfaender S, Grabrucker AM (2014) Characterization of biometal profiles in neurological disorders. Metallomics 6(5):960–977
Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR (1998) Copper, iron, and zinc in Alzheimer’s disease senile plaques. J Neurol Sci 158(1):47–52
Zatta P, Drago D, Bolognin S, Sensi SL (2009) Alzheimer’s disease, metal ions, and metal homeostatic therapy. Trends Pharmacol Sci 30(7):346–355
Breydo L, Uversky VN (2011) Role of metal ions in aggregation of intrinsically disordered proteins in neurodegenerative diseases. Metallomics 3(11):1163–1180
Bu G (2009) Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci 10(5):333–344
Delabio R, Rasmussen L, Mizumoto I, Viani GA, Chen E, Villares J, Costa IB, Turecki G, Linde SA, Smith MC, Payao SL (2014) PSEN1 and PSEN2 gene expression in Alzheimer’s disease brain: a new approach. J Alzheimers Dis 42(3):757–760
Lemos VA, de Carvalho AL (2010) Determination of cadmium and lead in human biological samples by spectrometric techniques: a review. Environ Monit Assess 171(1–4):255–265
Yahaya MI, Shehu A, Dabai FG (2013) Efficiency of extraction of trace metals from blood samples using wet digestion and microwave digestion techniques. J Appl Sci Environ Manage 17(3):365–369
Gajek R, Barley F, She J (2013) Determination of essential and toxic metals in blood by ICP-MS with calibration in synthetic matrix. Anal Methods 5(9):2193–2202
Massadeh A, Gharibeh A, Omari K, Al-Momani I, Alomari A, Tumah H, Hayajneh W (2010) Simultaneous determination of Cd, Pb, Cu, Zn, and Se in human blood of Jordanian smokers by ICP-OES. Biol Trace Elem Res 133(1):1
Szynkowska MI, Pawlaczyk A, Wojciechowska E, Sypniewski S, Paryjczak T (2009) Human hair as a biomarker in assessing exposure to toxic metals. Pol J Environ Stud 18(6):1151–1161
Puchyr RF, Bass DA, Gajewski R, Calvin M, Marquardt W, Urek K, Druyan ME, Quig D (1998) Preparation of hair for measurement of elements by inductively coupled plasma-mass spectrometry (ICP-MS). Biol Trace Elem Res 62(3):167–182
Goullé JP, Mahieu L, Castermant J, Neveu N, Bonneau L, Lainé G, Bouige D, Lacroix C (2005) Metal and metalloid multi-elementary ICP-MS validation in whole blood, plasma, urine and hair: reference values. Forensic Sci Int 153(1):39–44
Abdulrahman FI, Akan JC, Chellube ZM, Waziri M (2012) Levels of heavy metals in human hair and nail samples from Maiduguri Metropolis, Borno State, Nigeria. World Environ 2(4):81–89
Amartey EO, Asumadu-Sakyi AB, Adjei CA, Quashie FK, Duodu GO, Bentil NO (2011) Determination of heavy metals concentration in hair pomades on the Ghanaian market using atomic absorption spectrometry technique. Br J Pharmacol Toxicol 2(4):192–198
Pengping S, Kungwankunakorn S (2014) Determination of some heavy metals in human hair by ultrasonic acid digestion and atomic absorption spectrophotometry. Chiang Mai J Sci 1(41):148–155
Hseu ZY (2004) Evaluating heavy metal contents in nine composts using four digestion methods. Biores Technol 95(1):53–59
Stelmashook EV, Isaev NK, Genrikhs EE, Amelkina GA, Khaspekov LG, Skrebitsky VG, Illarioshkin SN (2014) Role of zinc and copper ions in the pathogenetic mechanisms of Alzheimer’s and Parkinson’s diseases. Biochem Mosc 79(5):391–396
Brewer GJ, Kanzer SH, Zimmerman EA, Molho ES, Celmins DF, Heckman SM, Dick R (2020) Subclinical zinc deficiency in Alzheimer’s disease and Parkinson’s disease. Am J Alzheimer’s Dis Other Dement 25(7):572–575
Hozumi I, Hasegawa T, Honda A, Ozawa K, Hayashi Y, Hashimoto K, Yamada M, Koumura A, Sakurai T, Kimura A, Tanaka Y (2011) Patterns of levels of biological metals in CSF differ among neurodegenerative diseases. J Neurol Sci 303(1–2):95–99
Noda Y, Asada M, Kubota M, Maesako M, Watanabe K, Uemura M, Kihara T, Shimohama S, Takahashi R, Kinoshita A, Uemura K (2013) Copper enhances APP dimerization and promotes Aβ production. Neurosci Lett 28(547):10–15
Wang ZX, Tan L, Wang HF, Ma J, Liu J, Tan MS, Sun JH, Zhu XC, Jiang T, Yu JT (2015) Serum iron, zinc, and copper levels in patients with Alzheimer’s disease: a replication study and meta-analyses. J Alzheimers Dis 47(3):565–581
Miyata M, Smith JD (1996) Apolipoprotein E allele–specific antioxidant activity and effects on cytotoxicity by oxidative insults and β–amyloid peptides. Nat Genet 14(1):55–61
Oh SB, Kim JA, Park S, Lee JY (2020) associative interactions among zinc, apolipoprotein E, and amyloid-β in the amyloid pathology. Int J Mol Sci 21(3):802
Safieh M, Korczyn AD, Michaelson DM (2019) ApoE4: an emerging therapeutic target for Alzheimer’s disease. BMC Med 17(1):1–7
Bakulski KM, Rozek LS, Dolinoy DC, Paulson HL, Hu H (2012) Alzheimer’s disease and environmental exposure to lead: the epidemiologic evidence and potential role of epigenetics. Curr Alzheimer Res 9(5):563–573
Karri V, Schuhmacher M, Kumar V (2016) Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: a general review of metal mixture mechanism in brain. Environ Toxicol Pharmacol 1(48):203–213
Yuan Y, Jiang CY, Xu H, Sun Y, Hu FF, Bian JC, Liu XZ, Gu JH, Liu ZP (2013) Cadmium-induced apoptosis in primary rat cerebral cortical neurons culture is mediated by a calcium signaling pathway. PLoS ONE 8(5):e64330
Yuan Y, Zhang Y, Zhao S, Chen J, Yang J, Wang T, Zou H, Wang Y, Gu J, Liu X, Bian J (2018) Cadmium-induced apoptosis in neuronal cells is mediated by Fas/FasL-mediated mitochondrial apoptotic signaling pathway. Sci Rep 8(1):1–1
Wenstrup D, Ehman WD, Markesbery WR (1990) Trace element imbalances in isolated subcellular fractions of Alzheimer’s disease brains. Brain Res 533(1):125–131
Escudero-Lourdes C (2016) Toxicity mechanisms of arsenic that are shared with neurodegenerative diseases and cognitive impairment: Role of oxidative stress and inflammatory responses. Neurotoxicology 1(53):223–235
Kowall NW, Pendlebury WW, Kessler JB, Perl DP (1989) Aluminum-induced neurofibrillary degeneration affects a subset of neurons in rabbit cerebral cortex, basal forebrain and upper brainstem. Neuroscience 29(2):329–337
Walton JR (2006) Aluminum in hippocampal neurons from humans with Alzheimer’s disease. Neurotoxicology 27(3):385–394
Matyja E (2000) Aluminum enhances glutamate-mediated neurotoxicity in organotypic cultures of rat hippocampus. Folia Neuropathol 38(2):47–53
Kandimalla R, Vallamkondu J, Corgiat EB, Gill KD (2016) Understanding aspects of aluminum exposure in Alzheimer’s disease development. Brain Pathol 26(2):139–154
Kawahara M, Kato M, Kuroda Y (2001) Effects of aluminum on the neurotoxicity of primary cultured neurons and on the aggregation of β-amyloid protein. Brain Res Bull 55(2):211–217
Exley C (2005) The aluminium-amyloid cascade hypothesis and Alzheimer’s disease. Alzheimer’s disease. Springer, Boston, pp 225–234
Walton JR, Wang MX (2009) APP expression, distribution and accumulation are altered by aluminum in a rodent model for Alzheimer’s disease. J Inorg Biochem 103(11):1548–1554
Matsuzaki S, Manabe T, Katayama T, Nishikawa A, Yanagita T, Okuda H, Yasuda Y, Miyata S, Meshitsuka S, Tohyama M (2004) Metals accelerate production of the aberrant splicing isoform of the presenilin-2. J Neurochem 88(6):1345–1351
Cacquevel M, Aeschbach L, Houacine J, Fraering PC (2012) Alzheimer’s disease-linked mutations in presenilin-1 result in a drastic loss of activity in purified γ-secretase complexes. PLoS ONE 7(4):e35133
Wu L, Rosa-Neto P, Hsiung GY, Sadovnick AD, Masellis M, Black SE, Jia J, Gauthier S (2012) Early-onset familial Alzheimer’s disease (EOFAD). Can J Neurol Sci 39(4):436–445
Żekanowski C, Styczyńska M, Pepłońska B, Gabryelewicz T, Religa D, Ilkowski J, Kijanowska-Haładyna B, Kotapka-Minc S, Mikkelsen S, Pfeffer A, Barczak A (2003) Mutations in presenilin 1, presenilin 2 and amyloid precursor protein genes in patients with early-onset Alzheimer’s disease in Poland. Exp Neurol 184(2):991–996
Li T, Li YM, Ahn K, Price DL, Sisodia SS, Wong PC (2011) Increased expression of PS1 is sufficient to elevate the level and activity of γ-secretase in vivo. PLoS ONE 6(11):e28179
Acknowledgement
I would also like to acknowledge Dr. Satish Yadav for their support and guidance.
Funding
This study was funded by the Junior Research Fellowship (JRF) Scheme of University Grant Commission (India), UGC – Ref. No.: 3359/(OBC) (NET-DEC.2015) to Jyoti Yadav. A fraction of this study was also funded under the “Intramural Seed (Faculty) Fellowship (2018) proposal”, Ref. code: 95th ECM II B IMR-F/P2 from King George’s Medical University, Lucknow to Dr. Shiuli.
Author information
Authors and Affiliations
Contributions
Conception or design of the work by Prof. Anoop Kumar Verma, Data collection, Data analysis and interpretation by Jyoti Yadav, Drafting the article and Critical revision of the article by Dr. MD. Kaleem Ahmad, Prof. R. K. Garg, Dr. Shiuli, and Prof Abbas Ali Mahdi. Final approval of the version to be published by Prof. Shrikant Srivastava.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
This study was approved by the Ethics committee of King George's Medical University, Lucknow by the Ref. code- 95th ECM II B IMR-F/P2. Written informed consent forms were collected from all participating subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yadav, J., Verma, A.K., Ahmad, M.K. et al. Metals toxicity and its correlation with the gene expression in Alzheimer's disease. Mol Biol Rep 48, 3245–3252 (2021). https://doi.org/10.1007/s11033-021-06386-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06386-x