Abstract
Human amniotic membrane mesenchymal stem cells-derived conditioned medium (hAM-MSCs-CM) has positive effects against myocardial ischemia/reperfusion (MI/R) injury. However, it needs further investigations how hAM-MSCs-CM leads to the cell survival under MI/R via modulation of autophagy. The purpose of this study is investigating the effects of hAM-MSCs-CM in a rat model of MI/R injury by focusing on the role of autophagy as one of its possible mechanisms. Male Wistar rats (44 rats, 175–200 g) were randomly divided into four groups: Sham, MI/R, culture media-receiving and conditioned medium-receiving. MI/R was induced by 30 min of left anterior descending coronary artery ligation. After 15 min reperfusion, culture media or hAM-MSCs-CM (150 μl) were injected intramyocardially. At the end of the experiment, CK-MB, autophagy markers, phosphorylated and total forms of mTOR and ULK1, cardiac function and fibrosis were measured. hAM-MSCs-CM significantly decreased CK-MB levels (P < 0.0001), and also the mRNA levels of Beclin1 (P < 0.0001), LC3 (P = 0.012) and p62 (P = 0.003). In addition, hAM-MSCs-CM significantly reduced Beclin1, LC3II/LC3I and p62 protein levels (P < 0.0001), and increased p-mTOR/mTOR (P = 0.022) and p-ULK1/ULK1 (P < 0.0001) expressions. Moreover, hAM-MSCs-CM improved cardiac function and decreased fibrosis (P < 0.0001). This study showed cardioprotective effects of hAM-MSCs-CM against MI/R injury through modulation of autophagy via mTOR/ULK1 pathway. Based on these findings, it can be concluded that hAM-MSCs-CM can be offered as an attractive candidate for attenuation of MI/R injury in future, but needs further investigations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic heart diseases (IHD) are known as common causes of cardiac dysfunction, heart failure (HF) and even death in the world. Although reperfusion of ischemic myocardium is a primary goal for treatment of acute myocardial infarction (MI) in patients, but it causes myocardial ischemia/reperfusion (MI/R) injury [1]. Since myocardial I/R injury mechanisms are not fully understood, evaluating signaling pathways and molecular mechanisms which effectively protect the myocardium against reperfusion injury may lead to identify new therapeutic targets to improve cardiac remodeling and HF following acute MI [1,2,3].
Autophagy is an intracellular metabolic self-digestive process in which lysosomes abolish impaired organelles and cytoplasmic misfolded proteins. It has been shown that autophagy takes part in energy production, metabolism and survival of the cell [4]. During myocardial I/R injury, autophagy can antagonize or contribute to further myocardial pathogenesis. But its associated mechanisms are still under debate. The debate emphasizes on the damaging effects of accelerated autophagy in reperfusion injury. Therefore, the therapeutic goal is suppressing excessive levels of autophagy, but maintaining its basal levels [5].
Studies have revealed that mesenchymal stem cells (MSCs) can repair heart tissue, improve cardiac function and limit the progression of HF [6]. Recent studies have reported that MSCs can secrete and release paracrine agents called conditioned medium (CM; consisting of different groups of soluble peptides and proteins) by which they can exert their therapeutic effects [7]. It has been confirmed that by CM therapy, it can be avoided from number of limitations related to stem cell therapy including ectopic tumors, immune incompatibility, budgets and waiting time for ex vivo development [8]. Amniotic membrane, as an easily accessible source of stem cells for treatment of many diseases, has attracted much attention in recent years [9]. An in vitro study showed that human amniotic membrane mesenchymal stem cells-derived CM (hAM-MSCs-CM) could protect cardiac-like cells against hypoxia/reoxygenation injury by activating ERK1/2 MAPK pathway and inhibiting SAPK/JNK and p38 MAPK pathways. Likewise, hAM-MSCs-CM prevented the activation of pro-apoptotic genes in cardiac cells. In line with these findings, in vivo experiment also showed that hAM-MSCs-CM reduced infarct size, as well as cardiac apoptosis [10]. Another study proved that dental pulp stem cells-derived CM decreased myocardial injury and improved heart function by reducing apoptosis and inflammation in mice model of myocardial I/R [11]. Besides, we recently examined the effects of hAM-MSCs-CM on oxidative stress in myocardial I/R injury in rats. We proved that hAM-MSCs-CM has cardioprotective effects through improving cardiac histological changes, decreasing malondialdehyde (MDA) and increasing superoxide dismutase (SOD) and glutathione peroxidase (GPx) levels [12]. Therefore, it can be said that CM therapy may be an appropriate candidate in the future researches for reducing cardiac remodeling and improving clinical outcomes.
Despite the clinical success of coronary revascularization in order to save damaged myocardium, there are rarely effective treatment options for prevention of myocardial necrosis following MI. Even with the best and most effective drugs in many patients, increased infarct size and left ventricular remodeling have been developed [10]. Due to the limited time window to prevent cardiac I/R injury, CM therapy has attracted a lot of attention [6]. Despite studies and advances in the therapeutic potentials of hAM-MSCs-CM in heart diseases, it is clinically important to study specific mechanisms by which hAM-MSCs-CM leads to the cardioprotective effects under I/R injury conditions. Although hAM-MSCs-CM has been shown to act through several signaling pathways, the exact mechanism of its therapeutic effects on autophagy flux in myocardial I/R injury setting is still unclear. So we thought to examine the effects of intramyocardial injection of hAM-MSCs-CM on autophagy flux by focusing on the mTOR/ULK1 pathway in a rat model of myocardial I/R injury with assessment of clinically relevant end points.
Methods
Selection of animals
Adult male Wistar rats (44 rats, 175–200 g) were purchased from the animal laboratory of Iran University of Medical Sciences. Rats had free access to water ad libitum and standard pellet chow in an animal room with controlled temperature (22 ± 2 °C) and humidity (55%) with 12 h dark–light cycles. This study was performed in consistent with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (8th Edition, NRC 2011) and were approved by the local animal care committee. Animal Ethical Committee of Iran University of Medical Sciences approved all the experiments and protocols (Ethical code: IR.IUMS.FMD.REC.1397.234).
Preparation of hAM-MSCs-CM
MSCs isolating was done in accordance with our previous work [13]. Amniotic membranes (AMs) were isolated from decidua tissues. For several times, the AMs were washed with cold phosphate-buffered saline (PBS), so vessels and blood clots were eliminated. Then AMs were fragmented by means of a mechanical technique. After homogenizing and centrifuging small pieces of AMs (400×g, 5 min), the supernatant was removed and 30 ml of medium supplemented with type 1 collagenase (Sigma-Aldrich, St. Louis, MO, USA) was added to the pellet and then was incubated at 37 °C in a humidified 5% CO2 incubator for 60 min. Thereafter, by centrifuging the samples (400×g, 5 min), the supernatant was removed and trypsin (0.25% containing 1 ml EDTA) was added to the pellet. The suspension was incubated at 37 °C and 5% CO2 for 30 min, then washed several times. Finally, the cell pellet containing hAM-MSCs was resuspended in an adequate volume of dulbecco's modified eagle media (DMEM, Gibco Company, New York, USA), supplemented with 10% fetal bovine serum (FBS, Gibco Company, New York, USA). MSCs were cultured at 1 × 106 cells/cm2 in a medium consist of α-MEM supplemented with 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin and 10% FBS overnight. At passage 3, the medium was replaced with DMEM without antibiotics and FBS. Thereafter, cells were incubated for 48 h at the optimal conditions. For removing detached cells, cell culture supernatant was centrifuged (400×g, 10 min) and filtered through 0.22 μm membrane.
MSCs identification
Analyzing of cultured MSCs was done by fluorescence-activated cell sorting (FACS). Briefly, antibodies in PBS per 1 × 106 cells were added to the trypsinized MSCs and then were incubated in the dark for 20 min at 25 °C. The antibodies were fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD105, phycoerythritin (PE)-conjugated mouse anti-human CD73 and PE-conjugated mouse anti-human CD34 (Dako Company, USA). Finally, after fixation of samples in 1% paraformaldehyde solution (Sigma-Aldrich, St. Louis, MO, USA), a flow cytometer (Partec Pas III, Germany) was used for quantification of FACS results.
Rat model of myocardial I/R
First, animals were anesthetized by intraperitoneally (i.p) injection of a combination of ketamine (60 mg/kg) and xylazine (5 mg/kg). Next, animals were positioned in supine. A thermal pad and heating lamp were used for maintaining the body temperature as close as possible to 37 °C. After intubation of rats, they were connected to a small animal ventilator (2–3 ml tidal volume and 65–70/min respiratory rate; Harvard Apparatus VentElit, USA), and ventilated by room air. Under sterile conditions, a lateral incision at the level between the 4th and 5th ribs on the left side of the sternum was performed. Then the chest was opened. With minimal manipulation, the pericardium was removed and the left anterior descending (LAD) coronary artery was ligated by 6.0 silk suture. Successful occlusion of LAD was approved by the pale discoloration of the affected myocardium and elevation of ST segment in lead II ECG. 30 min after LAD occlusion, the LAD ligature was removed and reperfusion to the ischemic region was confirmed. Finally, the chest was closed in layers with 2.0 silk suture. The lungs were inflated by increasing positive end expiratory pressure. When animals start making attempts to breathe spontaneously, they were dissociated from the ventilator, allowed to recover. Sham-operated rats experienced the above operation, without LAD ligation.
Experimental design
Animals were randomly placed in 4 groups (n = 11 per group): Sham, MI/R, MI/R + Culture media (MI/R + receiving 150 µl culture media following 15 min of reperfusion) and MI/R + CM (MI/R + receiving 150 µl conditioned medium following 15 min of reperfusion). Culture media or CM were injected into three different sites of infarct border zone. After 24 h reperfusion, 5 rats per group were sacrificed for biochemical assessments. The remaining rats (n = 6 per group) were examined by echocardiography on the 28th day of reperfusion for evaluation of cardiac function and then were sacrificed for evaluation of cardiac fibrosis (Supplementary Fig. 1).
Blood sampling and tissue preparation for biochemical assessments
After 24 h reperfusion, anesthetization of rats (n = 5 per group) were performed by i.p injection of ketamine (60 mg/kg) and xylazine (5 mg/kg). After collecting cardiac blood samples, the hearts were rapidly removed and washed in normal saline. Next, left ventricle (LV) of Sham, and peri-infarct region of LV in other groups were rapidly frozen in liquid nitrogen, then stored in − 80 °C for further biochemical assessments. After centrifuging blood samples (5000 rpm, 15 min, 4 °C), serums were collected and stored at − 80 °C until biochemical assessments.
CK-MB assay
CK-MB serum levels were evaluated by colorimetric method with specific kit (Pars Azmoon, Tehran, Iran) by means of auto analyzer (Roche Hitachi Modular DP Systems; Mannheim, Germany) in accordance with the instructions of manufacturer. Values were presented in I.U/L.
Real-time polymerase chain reaction (real-time PCR)
Beclin1, LC3 and p62 mRNA levels in cardiomyocytes were examined by real-time PCR. In order to extract total RNA, frozen samples were powdered and then were homogenized in Trizol Reagent (Invitrogen Company, San Diego, CA, USA) on ice. By means of Dart cDNA kit (EURx Company, Poland), RNA series were converted to cDNA in accordance with manufacturer’s protocol. For running real-time PCR on a Rotor-Gene Q 5plex HRM System, the SYBR® Premix Ex Taq ™ II (Tli RNaseH Plus, RR820Q) was used. The internal control for this study was Glyceraldehyde 3-phosphate dehydrogenase (GAPDH). 2−∆∆CT method was used for calculation of relative changes in gene expression [14]. The specific primers sequences of the present work are listed in Supplementary Table 1.
Western blotting
For western blotting analysis, fresh-frozen samples were dissected and homogenized in radio-immunoprecipitation assay (RIPA) lysis buffer (Sigma-Aldrich, St. Louis, MO, USA). After centrifuging the resulting solutions (13,000×g, 20 min) and collecting the supernatants, concentration of total protein was determined by UV 3000 ultraviolet spectrophotometer (NanoDrop, Wilmington, DE). Next, equal amounts of proteins (50 μg) were loaded into the electrophoresis chamber in 10–15% SDS-PAGE. Thereafter, transferring of separated soluble proteins to a polyvinylidene difluoride membrane (PVDF, Sigma-Aldrich, St. Louis, MO, USA) was performed. In order to block non-specific bindings, membranes were incubated for 2 h at room temperature with 5% non-fat dry milk solution in Tris buffered saline-Tween 20 (TBST, pH 7.4). Then, the membranes were incubated with primary antibodies at 1:1000 against Beclin1, p62, phospho-mTOR (Ser2448) and mTOR (Abcam, Cambridge, MA, USA), LC3B (Sigma-Aldrich, St. Louis, MO, USA), phospho-ULK1 (Ser757) and ULK1 (Cell Signaling Technology, USA), diluted in blocking buffer overnight at 4 °C on a shaker. To control for equal loading, membranes were also incubated for GAPDH antibody (1:1000, Cell Signaling Technology, USA), diluted in TBST overnight at 4 °C. Then blots were incubated with goat anti-rabbit horseradish peroxidase conjugated IgG for 1 h at room temperature. Following incubation with enhanced chemiluminescence (ECL) substrate (Millipore) for detecting the immunoreactivity, the bands were exposed by the ChemiDoc Imaging System. Quantification of protein bands intensity in the blots was performed by densitometry analysis and normalized with GAPDH band intensity as loading control. The measured values are presented in arbitrary unit (AU).
Evaluation of cardiac function
Evaluation of cardiac function was done by echocardiography on the 28th day of reperfusion. Animals were anesthetized with i.p injection of ketamine (10–20 mg/kg). M-mode transthoracic echocardiography by an echocardiographic apparatus equipped with 12 MHz transducer connected to an ultrasound system (SSD-5500; Aloka, Tokyo, Japan) was done in all rats by an echocardiographist who was blinded to the experimental groups. Ejection fraction (EF), fractional shortening (FS), left ventricular inner diameter in end systole (LVIDs), left ventricular inner diameter in end diastole (LVIDd), left ventricular posterior wall thickness in end systole (LVPWs), and left ventricular posterior wall thickness in end diastole (LVPWd) were evaluated from three cardiac cycles.
Measurement of myocardial fibrosis
After echocardiography, animals were sacrificed under deep anesthesia. Sternotomies were performed, then hearts were quickly removed and washed in normal saline. After fixation of hearts in 10% neutral buffered formalin for 2 days, the hearts were embedded in paraffin. Then paraffin embedded segments were cut into transverse Sects. (5 µm) by a microtome. After deparaffinization of sections, they were stained with Masson's trichrome (Sigma-Aldrich, St. Louis, MO, USA) and were observed under a light microscope with high magnification (Labomed Inc., USA). Representative areas were photographed using a DeltaPix microscope camera. Photoshop software (Version 7.0, Adobe System, San Jose, CA, USA) was used for quantification of collagen volume fraction of LV. Cardiac fibrosis was presented as a mean percentage of fibrotic zone to LV area in 5 sections per heart. All these assays were performed in a blinded manner.
Statistical analysis
Values were presented as mean ± standard deviation (SD). To compare differences among the experimental groups, one-way analysis of variance (ANOVA) followed by Tukey post hoc test was used. P value less than 0.05 (P < 0.05) was considered to be statistically significant.
Results
Characterization of MSCs
As shown in Supplementary Fig. 2, FACS analysis showed that the MSCs markers (CD105 and CD73) were highly expressed in cultured cells at 4th passage. Whereas hematopoietic progenitor marker (CD34) did not show significant expression. These results showed a highly purified isolating of MSCs.
Effects of hAM-MSCs-CM on CK-MB serum levels
As shown in Fig. 1, CK-MB levels in MI/R and MI/R + Culture media groups were significantly higher than Sham group (P < 0.0001 for both). Treatment with hAM-MSCs-CM decreased CK-MB levels as compared to MI/R and MI/R + Culture media groups (P < 0.0001 for both). In addition, there was a significant difference between MI/R + CM and Sham groups (P = 0.006). Furthermore, treatment with culture media couldn’t significantly decrease CK-MB levels as compared to MI/R group (P = 0.473).
The effects of hAM-MSCs-CM on CK-MB serum levels in different groups (n = 5 per group). The data were expressed as Mean ± SD. **P < 0.01 and ***P < 0.0001 vs. Sham group, ####P < 0.0001 vs. MI/R group, &&&&P < 0.0001 vs. MI/R + Culture media group). hAM-MSCs-CM Human amniotic membrane mesenchymal stem cells-derived conditioned medium, CK-MB Creatine kinase-MB, MI/R myocardial ischemia/reperfusion, CM conditioned medium
Effects of hAM-MSCs-CM on Beclin1, LC3 and p62 mRNA levels in the heart
Statistical analysis showed significant elevation of Beclin1 mRNA levels in MI/R and MI/R + Culture media groups as compared to Sham group (P < 0.0001 for both), and hAM-MSCs-CM treatment significantly reduced Beclin1 mRNA levels in comparison with MI/R and MI/R + Culture media groups (P < 0.0001 for both). There was no significant difference between MI/R + CM and Sham groups (P = 0.933). Treatment with culture media couldn’t markedly reduce Beclin1 mRNA levels as compared to MI/R group (P = 0.853, Fig. 2a).
Real-time PCR analysis of myocardial mRNA levels of Beclin1 (a); LC3 (b); and p62 (c) in different groups (n = 5 per group). The data were expressed as Mean ± SD. ( **P < 0.01 and**** P < 0.0001 vs. Sham group, #P < 0.05, ##P < 0.01 and ####P < 0. 0001 vs. MI/R group, &P < 0.05, &&P < 0.01 and&&&& P < 0.0001 vs. MI/R + Culture media group). MI/R myocardial ischemia/reperfusion; CM conditioned medium
The mRNA levels of LC3 were significantly increased in MI/R and MI/R + Culture media groups as compared to Sham group (P = 0.003 and P = 0.009 respectively). LC3 mRNA levels in MI/R + CM group were significantly lower as compared to MI/R (P = 0.012) and MI/R + Culture media (P = 0.037) groups. Furthermore, there was no significant difference between MI/R + CM and Sham groups (P = 0.892). Treatment with culture media couldn’t markedly reduce mRNA levels of LC3 as compared to MI/R group (P = 0.939, Fig. 2b).
The mRNA levels of p62 were significantly increased in MI/R and MI/R + Culture media groups as compared to Sham group (P = 0.001 and P = 0.002 respectively). While hAM-MSCs-CM treatment significantly decreased mRNA levels of p62 as compared to MI/R (P = 0.003) and MI/R + Culture media (P = 0.006) groups. There was no significant difference between MI/R + CM and Sham groups (P = 0.882). Also there was no significant difference between MI/R and MI/R + Culture media treated animals in mRNA levels of p62 (P = 0.982, Fig. 2c).
Effects of hAM-MSCs-CM on Beclin1, LC3II/LC3I, p62, p-mTOR/mTOR, and p-ULK1/ULK1 protein expressions in the heart
Expressions of Beclin1, LC3II/LC3I and p62 were significantly higher in MI/R (P < 0.0001 for all) and MI/R + Culture media (P < 0.0001 for all) groups as compared to Sham group. Treatment with hAM-MSCs-CM significantly decreased Beclin1, LC3II/LC3I and p62 expressions as compared to MI/R (P < 0.0001 for all) and MI/R + Culture media (P < 0.0001 for all) groups. Statistical analysis also showed that there was a significant difference between MI/R + CM and Sham groups in Beclin1 and p62 expressions (P < 0.0001 for both), but there was no significant difference between MI/R + CM and Sham groups in LC3II/LC3I expression (P > 0.999). Furthermore, Beclin1, LC3II/LC3I and p62 expressions were still high in treatment group with culture media and there was no significant difference between MI/R and MI/R + Culture media groups (P = 0.960, P = 0.234 and P = 0.933 respectively, Fig. 3a–c).
Western blot bands analysis and densitometry of Beclin1 (a); LC3II/LC3I (b); p62 (c); p-mTOR/mTOR (d); and p-ULK1/ULK1 (e) correlated to the GAPDH band in different groups (n = 5 per group). The data were expressed as Mean ± SD. (**P < 0.01 and **** P < 0.0001 vs. Sham group, #P < 0.05 and ####P < 0.0001 vs. MI/R group, &P < 0.05 and &&&& P < 0.0001 vs. MI/R + Culture media group). MI/R myocardial ischemia/reperfusion, CM conditioned medium
Figure 3d shows that protein expression of p-mTOR/mTOR was significantly decreased in MI/R and MI/R + Culture media groups as compared to Sham group (P = 0.006 and P = 0.009 respectively). hAM-MSCs-CM treatment could increase the expression of p-mTOR/mTOR in MI/R + CM group as compared to MI/R (P = 0.022) and MI/R + Culture media (P = 0.033) groups. There was no significant difference between MI/R + CM and Sham groups (P = 0.755). Treatment with culture media couldn’t significantly increase the expression of p-mTOR/mTOR as compared to MI/R group (P = 0.991).
Figure 3e shows that protein expression of p-ULK1/ULK1 was significantly decreased in MI/R and MI/R + Culture media groups as compared to Sham group (P < 0.0001 for both). hAM-MSCs-CM treatment could increase the expression of p-ULK1/ULK1 in MI/R + CM group as compared to MI/R and MI/R + Culture media groups (P < 0.0001 for both). Furthermore, there was a significant difference between MI/R + CM and Sham groups (P = 0.001). Treatment with culture media couldn’t significantly increase the expression of p-ULK1/ULK1 as compared to MI/R group (P = 0.893).
Effects of hAM-MSCs-CM on cardiac function
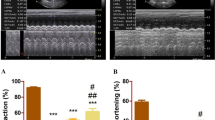
Figure 4a shows representative echocardiogram recordings from different groups. Figure 4b–g shows changes in echocardiographic parameters in different groups. Statistical analysis showed that EF and FS were significantly decreased in MI/R (P < 0.0001 for both) and MI/R + Culture media (P < 0.0001 for both) groups as compared to Sham group. hAM-MSCs-CM treatment increased EF and FS in comparison with MI/R (P < 0.0001 for both) and MI/R + Culture media (P < 0.0001 for both) groups. In addition, our results showed that there were no significant differences between MI/R + CM and Sham groups in EF (P = 0.881) and FS (P = 0.791) parameters. Also, there were no significant differences between MI/R and MI/R + Culture media groups in EF (P = 0.368) and FS (P = 0.109) parameters (Fig. 4b and c).
Representative echocardiogram recordings in different groups. Similar results were observed in at least 20/24 rats (a). The effects of hAM-MSCs-CM on EF (b); FS (c); LVIDs (d); LVIDd (e); LVPWs (f); and LVPWd (g) in different groups (n = 6 per group). The data were expressed as Mean ± SD. (****P < 0.0001 vs. Sham, ####P < 0.0001 vs. MI/R group, &&&&P < 0.0001 vs. MI/R + Culture media group). hAM-MSCs-CM: Human amniotic membrane mesenchymal stem cells-derived conditioned medium; EF ejection fraction; FS fractional shortening, LVIDs left ventricular inner diameter in end systole, LVIDd left ventricular inner diameter in end diastole, LVPWs left ventricular posterior wall thickness in end systole, LVPWd left ventricular posterior wall thickness in end diastole, MI/R myocardial ischemia/reperfusion, CM Conditioned medium
Figure 4d and e show that LVIDs and LVIDd were significantly increased in MI/R (P < 0.0001 for both) and MI/R + Culture media (P < 0.0001 for both) groups compared with Sham group. In addition, our results showed that LVIDs and LVIDd in MI/R + CM group were lower than MI/R (P < 0.0001 for both) and MI/R + Culture media (P < 0.0001 for both) groups. There were no significant differences between MI/R + CM and Sham groups in LVIDs (P = 0.243) and LVIDd (P = 0.426). Also, there were no significant differences between MI/R and MI/R + Culture media groups in LVIDs (P = 0.101) and LVIDd (P = 0.263).
No significant differences were found between different groups in LVPWs and LVPWd changes (Fig. 4f and g).
Effects of hAM-MSCs-CM on cardiac fibrosis
Figure 5a shows ventricular collagen deposition by Masson's trichrome staining in different groups. We also calculated interstitial fibrosis percentage compared with whole LV area (Fig. 5b). Fibrosis was significantly increased in MI/R and MI/R + Culture media groups as compared to Sham group (P < 0.0001 for both). Treatment with hAM-MSCs-CM markedly reduced fibrosis compared to MI/R and MI/R + Culture media groups (P < 0.0001 for both). Furthermore, there was a significant difference between MI/R + CM and Sham groups (P < 0.0001). However, treatment with culture media couldn’t significantly reduce fibrosis compared to MI/R group (P = 0.122).
Cross sections of the middle part of left ventricle tissue stained with Masson’s trichrome in different groups (Original magnification from upper row to lower row 40 × and 400 × respectively). Collagen fibers in blue are well detected (a). Quantification of interstitial fibrosis (n = 6 per group). The data were expressed as mean ± SD. (****P < 0.0001 vs. Sham group, ###P < 0.0001 vs. MI/R group, &&&&P < 0.0001 vs. MI/R + Culture media group) (b). MI/R myocardial ischemia/reperfusion, CM conditioned medium
Discussion
The present study revealed the therapeutic potentials of hAM-MSCs-CM in a rat model of cardiac I/R injury. By examining clinically relevant end points, we proved that hAM-MSCs-CM is able to reduce cardiac interstitial fibrosis and improve left ventricular function following I/R. Besides, decreased CK-MB serum level is another evidence for the therapeutic potentials of hAM-MSCs-CM. It seems that the above mentioned effects are partly mediated by the effect of hAM-MSCs-CM on autophagy flux. In details, our data showed that these beneficial effects of hAM-MSCs-CM may be caused by suppression of excessive autophagy flux through reduction of Beclin1, LC3 and p62 at both mRNA and protein levels. Moreover, hAM-MSCs-CM was found to suppress excessive autophagy flux by activating upstream mTOR/ULK1 signaling pathway.
Positive properties of CM derived from different MSCs in MI models have been carried out in previous experimental studies [6, 8, 10,11,12]. However, we need to comprehensively study the effects of hAM-MSCs-CM on intracellular parameters to achieve clinical goals in the future. Although several studies have reported cardioprotective effects of MSCs-derived CM by evaluation of different cellular signaling pathways, but the mechanism of therapeutic effects of hAM-MSCs-CM on autophagy flux via mTOR/ULK1 signaling pathway in cardiac I/R requires further investigations in depth. It seems that specific modulation of autophagy by hAM-MSCs-CM might be a novel strategy to increase survival under I/R injury and protect against myocardial death from reperfusion injury. By evaluating clinically relevant end points, we found that I/R led to the enhancement of CK-MB levels, cardiac fibrosis, cardiomyocyte damage, and finally development of systolic and diastolic dysfunctions. Interestingly, intramyocardial injection of hAM-MSCs-CM reduced I/R injury, which was confirmed by improving all of the above mentioned indexes. So it can be demonstrated that hAM-MSCs-CM can increase cardiac resistance against reperfusion injury, and plays an important role in prevention of post-ischemic remodeling after I/R injury. Our study revealed that these beneficial effects of hAM-MSCs-CM in I/R injury were accompanied by its positive effects on autophagy flux through downregulation of autophagy-related factors such as Beclin1, LC3 and p62 at both mRNA and protein levels, as well as elevation of p-mTOR and p-ULK1 expressions as autophagy upstream regulators.
It has been demonstrated that examinations of LC3II and p62 are useful tools in order to monitor the whole autophagy flux. p62 has been suggested to interact with ubiquitinated substrates and LC3, and serves as a link between them, and is involved in the aggregation of proteins. LC3 plays an important role in autophagosome elongation step. The ratio of conversion of LC3I to LC3II is associated with autophagosome formation and is often used as an indicator of autophagy induction [15, 16]. Studies have revealed that when LC3II and p62 are enhanced, initiation of autophagy is elevated, but impairment of autophagosome-lysosome fusion is occurred, and the clearance of autophagosome is blocked [17]. LC3 operates as downstream of Beclin1. It has been demonstrated that Beclin1 has an important role in autophagosome formation, especially during the reperfusion phase [5]. In consistent with previous studies, our data revealed that I/R led to excessive autophagy activity via elevation of Beclin1, LC3II/LC3I and p62 levels [17,18,19], and although the autophagy flux was not evaluated in our study because of some limitations, but according to our related data it seems indirectly that I/R injury could increase autophagosome formation and block autophagosome clearance and impair autophagy flux. Therefore, it can be concluded that this accelerated autophagy flux caused an increase in myocardial damage following I/R, or in other words, a decrease in cardiac resistance to reperfusion injury. Interestingly, injection of hAM-MSCs-CM could prevent cardiac I/R-induced elevation of Beclin1, LC3II/LC3I and p62 levels.
mTOR, as a gate-keeper in autophagy, is the major inhibitor of autophagy [16]. It has been demonstrated that overexpression of mTOR can inhibit cardiomyocyte necrosis and inflammatory response in I/R injury and is sufficient to protect the heart against I/R [20]. mTOR complex 1 negatively regulates autophagy by direct phosphorylation of ULK1 [21]. Our study revealed that I/R caused a significant decrease in expressions of p-mTOR/mTOR and p-ULK1/ULK1 as upstream proteins in autophagy flux, which were essential step for autophagic cell death in cardiomyocytes. So it can be said that I/R caused excessive autophagy activity by reducing mTOR/ULK1 inhibitory effect on Beclin1, LC3II/LC3I and p62 levels, which was associated with the increased cardiac injury in comparison with healthy hearts. However, treatment with hAM-MSCs-CM led to increased phosphorylations of mTOR and ULK1. Thus, it seems that the inhibitory effect of hAM-MSCs-CM on autophagy flux was associated with increased mTOR and ULK1 phosphorylations as its cardioprotective effect. So it can be said that the positive effect of hAM-MSCs-CM on cardiac I/R injury may be attributed in part to prevented excessive autophagy via mTOR/ULK1 activation.
Conforming to our study, Timmers et al. reported that human embryonic stem cells-derived CM has cardioprotective effects through reducing acaspase-3 and modulating TGF-β signaling in a porcine model of I/R [6]. In another study they demonstrated that MSCs-derived CM treatment enhanced capillary density, reduced myocardial infarct size and preserved systolic and diastolic functions following MI [8]. Moreover, Yamaguchi et al. showed that CM secreted by MSCs can improve heart function by reducing apoptosis and decreasing IL-6, IL-1β and TNF-α expressions in cardiomyocytes [11]. In general, increasing evidences suggest that CM is able to protect the myocardium against IR injury. In agreement with these studies, our work showed that hAM-MSCs-CM promoted cardioprotection in myocardial IR injury partly by preventing accelerated autophagy flux, as confirmed by improved end points following I/R injury. In the present study, the involvement of autophagy flux in the cardioprotective effects of hAM-MSCs-CM was investigated. Evaluating the contribution of the other signaling pathways may lead to the accurate understanding of hAM-MSCs-CM effects in cardioprotection.
Finally, it should be noted that despite the success of many drugs in clinic for MI treatment, however myocardial infarct size has been increased and cardiac remodeling has been occurred in many patients. In addition, many therapeutic interventions for cardiac ischemia such as angioplasty, coronary artery bypass surgery and thrombolytic drugs may result in I/R damage [10]. Therefore, hAM-MSCs-CM as an attractive potential therapeutic agent can be considered a promising therapeutic candidate for reducing cardiac I/R damage. In addition, its protective effects have been revealed in several in vitro and in vivo experimental studies [10, 12]. Therefore, hAM-MSCs-CM can be recommended as a promising therapeutic option for future clinical applications, but needs more investigations. In the present study, hAM-MSCs-CM was injected directly into myocardial infarct border zone. However, intramyocardial injection of hAM-MSCs-CM has limitations in clinical setting. So we recommend evaluating the protective effects of intravenous infusion of hAM-MSCs-CM in the future studies.
Conclusion
Our study disclosed that IR-induced autophagy and myocardial dysfunction were attenuated by mTOR/ULK1 activation after hAM-MSCs-CM treatment. hAM-MSCs-CM has cardioprotective effect which is partly mediated through modulation of autophagy flux including downregulation of Beclin1, LC3II and p62 via activation of mTOR/ULK1 pathway. So hAM-MSCs-CM can be proposed as a potential and promising therapeutic candidate for reduction of myocardial I/R injury severity in the future, but needs more researches.
Limitations and suggestions
It is important to mention that our study had some limitations. Besides mTOR/ULK1 dependent mechanism, other possible candidates may also trigger autophagy inhibition by hAM-MSCs-CM, which needs further investigations. We also suggest to examine the effect of hAM-MSCs-CM on the chronic phase of myocardial ischemia. As well as, we recommend to evaluate the effects of hAM-MSCs-CM in the presence of cardiovascular risk factors which may impact on MI outcome.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
References
Bayrami G, Karimi P, Agha-Hosseini F, Feyzizadeh S, Badalzadeh R (2018) Effect of ischemic postconditioning on myocardial function and infarct size following reperfusion injury in diabetic rats pretreated with vildagliptin. J Cardiovasc Pharmacol Ther 23:174–183
Najafi M, Noroozi E, Javadi A, Badalzadeh R (2018) Anti-arrhythmogenic and anti-inflammatory effects of troxerutin in ischemia/reperfusion injury of diabetic myocardium. Biomed Pharmacother 102:385–391
Badalzadeh R, Azimi A, Alihemmati A, Yousefi B (2017) Chronic type-I diabetes could not impede the anti-inflammatory and anti-apoptotic effects of combined postconditioning with ischemia and cyclosporine A in myocardial reperfusion injury. J Physiol Biochem 73:111–120
Bélanger M, Rodrigues PH, Dunn WA Jr, Progulske-Fox A (2006) Autophagy: a highway for Porphyromonas gingivalis in endothelial cells. Autophagy 2:165–170
Ma S, Wang Y, Chen Y, Cao F (2015) The role of the autophagy in myocardial ischemia/reperfusion injury. Biochim Biophys Acta Mol Basis Dis 1852:271–276
Timmers L et al (2008) Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res 1:129–137
Spees JL, Lee RH, Gregory CA (2016) Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. https://doi.org/10.1186/s13287-016-0363-7
Timmers L et al (2011) Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res 6:206–214
Francisco JC et al (2013) Amniotic membrane as a potent source of stem cells and a matrix for engineering heart tissue. J Biomed Sci Eng. https://doi.org/10.4236/jbise.2013.612147
Danieli P et al (2015) Conditioned medium from human amniotic mesenchymal stromal cells limits infarct size and enhances angiogenesis. Stem Cells Transl Med 4:448–458
Yamaguchi S et al (2015) Dental pulp-derived stem cell conditioned medium reduces cardiac injury following ischemia-reperfusion. Sci Rep. https://doi.org/10.1038/srep16295
Mokhtari B et al (2020) Human amniotic membrane mesenchymal stem cells-conditioned medium attenuates myocardial ischemia-reperfusion injury in rats by targeting oxidative stress. Iran J Basic Med Sci 23:1453–1461
Razavi Tousi SMT et al (2017) A rapid and cost-effective protocol for isolating mesenchymal stem cells from the human amniotic membrane. Galen Med J 6:217–225
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. https://doi.org/10.1093/nar/30.9.e36
Obara K, Sekito T, Niimi K, Ohsumi Y (2008) The Atg18-Atg2 complex is recruited to autophagic membranes via phosphatidylinositol 3-phosphate and exerts an essential function. J Biol Chem 283:23972–23980
Xiao-Fang T, Shi-Wei Y, Yu-Jie Z (2017) Autophagy, dysglycemia and myocardial infarction. IJC Metab Endocr 14:40–44
Ma X et al (2012) Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation 125:3170–3181
Fan G et al (2016) Danshensu alleviates cardiac ischaemia/reperfusion injury by inhibiting autophagy and apoptosis via activation of mTOR signalling. J Cell Mol Med 20:1908–1919
Wang J-L et al (2020) Postconditioning with calreticulin attenuates myocardial ischemia/reperfusion injury and improves autophagic flux. Shock 53:363–372
Aoyagi T et al (2012) Cardiac mTOR protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 303:H75–H85
Ouyang C, You J, Xie Z (2014) The interplay between autophagy and apoptosis in the diabetic heart. J Mol Cell Cardiol 71:71–80
Acknowledgements
This study was part of a Ph.D. thesis of Behnaz Mokhtari and financially supported by Physiology Research Center in Iran University of Medical Sciences, Tehran, Iran.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work has been supported by a grant (Grant Number: 97-2-3-33054) from Iran University of Medical Sciences, Tehran-Iran.
Author information
Authors and Affiliations
Contributions
BM and NA did the study design. BM performed experimental tests, gathered and analyzed the data, and drafted the manuscript. RB contributed in interpretation of the results and finalized the manuscript editing and critically revised the manuscript. NA supervised the whole project and revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
Animal care procedures, as well as all experimental protocols were approved by the Institutional Animal Ethical Committee of Iran University of Medical Sciences (Ethical code: IR.IUMS.FMD.REC.1397.234).
Informed consent
Amniotic membranes were supplied from Shahid Akbar Abadi Hospital under informed agreement from each participants in accordance with ethical standards of the institutional and/or national research committee and with the 1975 Declaration of Helsinki as reflected in a prior approval by the institution's human research committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mokhtari, B., Badalzadeh, R. & Aboutaleb, N. Modulation of autophagy as the target of mesenchymal stem cells-derived conditioned medium in rat model of myocardial ischemia/reperfusion injury. Mol Biol Rep 48, 3337–3348 (2021). https://doi.org/10.1007/s11033-021-06359-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06359-0