Abstract
The literature has shown the beneficial effects of microcurrent (MC) therapy on tissue repair. We investigated if the application of MC at 10 μA/90 s could modulate the expression of remodeling genes transforming growth factor beta (Tgfb), connective tissue growth factor (Ctgf), insulin-like growth factor 1 (Igf1), tenascin C (Tnc), Fibronectin (Fn1), Scleraxis (Scx), Fibromodulin (Fmod) and tenomodulin in NIH/3T3 fibroblasts in a wound healing assay. The cell migration was analyzed between days 0 and 4 in both fibroblasts (F) and fibroblasts + MC (F+MC) groups. On the 4th day, cell viability and gene expression were also analyzed after daily MC application. Higher expression of Ctgf and lower expression of Tnc and Fmod, respectively, were observed in the F+MC group in relation to F group (p < 0.05), and no difference was observed between the groups for the genes Tgfb, Fn1 and Scx. In cell migration, a higher number of cells in the scratch region was observed in group F+MC (p < 0.05) compared to group F on the 4th day, and the cell viability assay showed no difference between the groups. In conclusion, MC therapy at an intensity/time of 10 μA/90 s with 4 daily applications did not affect cell viability, stimulated fibroblasts migration with the involvement of Ctgf, and reduced the Tnc and Fmod expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For decades, low-intensity electrical stimulation therapy, microcurrent (MC), has been reported as an auxiliary therapeutic method to promote wound healing [1], as it triggers multiple events such as increased cell proliferation, collagen synthesis, tissue contraction, neovascularization, cell membrane permeability and normalization of tissue bioelectricity [2]. MC activates and rearranges both ion channels as carrier proteins across the cell, regardless of external chemical gradient and ion flux, contributing to intracellular polarization and cellular response [3]. Each cell type exhibits specific behavior under electrostimulation that may exhibit significant reduction in cell viability or cytotoxic effect [3].
The MC effect has been tested in vitro on different cell types involved in wound healing, such as macrophages, fibroblasts, epidermal cells, endothelial cells and has shown beneficial effects on cell migration and viability, proliferation, orientation, protein augmentation and DNA synthesis [3]. Still in vitro, Uemura et al. [4] observed that MC with 200 μA intensity favored the migration of human dermal fibroblasts within 24 h after application. Data from Sugimoto et al. [5] demonstrated that migration of human dermal fibroblasts treated with 100 μA was greater than in controls. According to a study by Leppik et al. [6] involving osteoblast culture and application of MC therapy (10–150 mV/mm) for 1 h daily for 3 weeks increased cell viability. However, intracellular changes induced by MC are not completely defined.
The literature describes different protocols for applying MC in different tissues. De Campos et al. [7] investigated MC stimulation (20 μA) in the xiphoid cartilage repair process and the animals treated with MC showed an increase in the number of chondroblasts and proteoglycans from the 21st day, showing the effect of MC on the acceleration of healing process. In skin, Neves et al. [8] used MC therapy (10 μA/3 min) and observed a reduction in the number of inflammatory cells, an increase in fibroblasts and blood vessels. Data from Belli et al. [9] demonstrated that the treatment of burns with MC (10 μA/3 min) alone or alternating with laser therapy, decreased inflammation, increased angiogenesis and favored tissue reorganization. In bone tissue, Mendonça et al. [10] evaluated MC stimulation (10 μA/5 min) in rat calvary excision and the results demonstrated a reduction in inflammatory cells, an increase in blood vessels and fibroblasts, as well as an increase in the organization of collagen fibers. Zaniboni et al. [11] used MC (10 μA/5 min) during orthodontic movement and observed that MC decreased inflammation, increased angiogenesis and favored bone remodeling.
During healing processes, especially of connective tissues, some genes play fundamental role in the structural and functional tissue restoration, such as Tgfb (transforming growth factor-β1), Ctgf (connective tissue growth factor), Igf1 (insulin-like growth factor 1), Tnc (tenascin C), Fn1 (Fibronectin), Fmod (Fibromodulin), Scx (Scleraxis) and Tnmd (tenomodulin). Tgfb acts as a chemotactic agent for neutrophils, monocytes and fibroblasts [12], orchestrates many cellular responses during wound healing as fibroblast proliferation, angiogenesis, and increases extracelular matrix (ECM) production [13], including collagen and fibronectin [14]. Ctgf stimulates fibroblast proliferation, ECM production [15], cell adhesion and cell differentiation [16, 17]. Igf1 enhances healing process through cell proliferation and migration, activity of fibroblasts, collagen production, agiogenesis and re-epithelialization of skin wounds [18,19,20,21,22,23]. Tnc is involved in a range of processes such as mitogenic responses, cell migration, cell attachment, cell spreading, focal adhesion, cell survival, matrix assembly, pro-inflammatory cytokine synthesis [24], and tenascin-C can also interact with diverse growth factors [25]. Fn1 plays an essential role in development, angiogenesis and in the wound healing promotion [26], and contains a number of binding sites for growth factors [27]. Fmod is involved in cell migration, collagen fibrillogenesis [28], angiogenesis [29], osteoclastogenesis [30], myogenic differentiation [31] and modulation of growth-factors signaling [32]. Scx facilitates tenocyte mechanosensing [33], it is essential for tendon [34] and musculoskeletal [35] development, regulates proteoglycans expression [36] and fracture callus formation during bone healing [37], and Scx also activates Tnmd expression to regulate differentiation and maturation of tenocytes [38]. Tnmd expression enhances cellular adhesion [39], prevents adipocyte accumulation and fibrovascular scar formation during tendon healing [40], and affects self-renewal and senescence properties of tendon stem/progenitor cells [41].
In addition to the important roles of the previously described genes during development and tissue repair, a study of Zheng et al. [42] have demonstrated the interaction of Fmod with Tgfb signaling on the fibroblasts function during skin wound healing, with Ctgf as dowstream mediator of Tgfb signaling. In a previous work performed by our group [43], the MC (10 μA/4 min) therapy applied until the 14th day of tendon repair, increased the expression of Fmod, Ctgf, Tnc and Fn genes, suggesting activation of the pathway Tnmd–Tgfb1–Ctgf. Therefore, it would be possible to suggest that MC therapy, without systemic influence, could activate the pathway Tnmd–Tgfb1–Ctgf? Although the MC therapy at intensity of 10 μA has improved the repair process of various tissues, the molecular mechanisms activated by MC are partially elucidated. We investigated if the application of MC at 10 μA/90 s could modulate the expression of remodeling genes previously mentioned Tgfb, Ctgf, Igf1, Tnc, Fn1, Scx, Fmod and Tnmd in NIH/3T3 fibroblasts in a wound healing assay.
Materials and methods
NIH/3T3 fibroblast culture and experimental groups
NIH/3T3 mouse fibroblasts used in the present study were donated by the University of São Paulo-USP, Ribeirão Preto. The cultures were plated in 75 cm2 culture bottles with DMEM (Dulbecco’s Modified Eagle’s Medium) supplemented with 15% fetal bovine serum (FBS, Nutricell Nutrientes Celulares, Campinas, São Paulo, Brazil), under a humid atmosphere at 37 °C containing 5% CO2, until reaching 85% confluence. The culture passages were performed approximately every three days. On the 21st passage, the experimental groups were established: (F)-NIH/3T3 fibroblasts without MC application; and (F+MC)-NIH/3T3 fibroblasts + MC application. Thus, NIH/3T3 fibroblasts were plated at a density of 1 × 104 per well for both groups in 24-well culture plates. DMEM supplemented with 15% FBS were used for maintenance of cultures under a humid atmosphere at 37 °C containing 5% CO2, until reaching 70% confluence. Subsequently, the scratch assay was performed using a 200 μL pipette tip, which was slid over the median extension of the previously marked well, creating a scratch on the cell monolayer. The cultures were washed with DMPBS Flush (Dulbecco’s Modified Phosphate-Buffered Saline) to remove cell debris and kept for 4 days until scratch closure. Experimental procedures were performed according to approval of the Animal Use Ethics Committee (CEUA) of the University Center of Hermínio Ometto Foundation/FHO under number 013/2018.
MC application
The intensity of the MC application (10 μA) was based on pre-established studies obtained in the literature [10, 11, 44,45,46,47]. To define the application time, the cell viability test was performed by the [3-(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide] test (MTT) test, for the application times of 30, 90 and 150 s. The application of MC was performed by immersing two electrodes in the culture medium (DMEM supplemented with 15% FBS) present in each well of the culture plate, without maintaining contact with the attached cell monolayer. The device used for the application of MC (biphasic microgalvanic direct current) was the Physiotonus microcurrent© stimulator (Bioset-Rio Claro, São Paulo, Brazil).
The application time of MC for 90s in the F+MC group was selected due to the higher cell viability observed in the 24 h MTT assay. Thus, MC (10 μA/90 s) was applied on days 0, 1, 2 and 3 after scratch assay (Fig. 1). For F group, the disconnected electrodes were placed as described for group F+MC. 24 h after the last application, samples were collected, that is, they were collected on the 4th day. Samples of groups F and F+MC were analyzed for cell viability and gene expression by the Real Time Polymerase Chain Reaction (RT-qPCR) technique.
Microcurrent application (10 μA/90 s): two metal electrodes were placed on the left and right side of the well for half of time (15, 45 and 75 s). Then, the position of electrodes was inverted and the current applied for another half of time (15, 45 and 75 s), considering that total time of application was 30, 90 and 150 s
MTT Test [3-(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide] for cell viability assay
NIH/3T3 fibroblasts at 21st passage (P) were plated in quadruplicate at 1 × 104 density per well in 24-well culture plates. Cultures were maintained with culture medium (DMEM supplemented with 15% FBS) under a humid atmosphere at 37 °C containing 5% CO2 for 24 h for cell adhesion. Control wells were established: (1) Only culture medium to be considered as “blank” in absorbance reading; (2) Positive control: NIH/3T3 fibroblasts and culture medium; (3) Negative control: NIH/3T3 fibroblasts, culture medium and 50% DMSO (dimethylsufoxide). On the 4th day, complete culture medium was removed and a solution of 0.5% MTT (Sigma-Aldrich, St. Louis, MO, USA) with DMEM medium without phenol red was used for incubation for 4 h protected from light in a 37 °C oven containing 5% CO2. After MTT removal, 200 μL of DMSO was added and the absorbance was measured at 570 nm in a microplate reader. For cell viability calculation, the following formula was used: Cell viability (%) = (sample absorbance/positive control absorbance) × 100.
Cell migration assay
Images from the scratch on the cell monolayer were documented daily using inverted microscope (Carl Zeiss—Cousin Vert) and Microscope Software ZEN 2012 blue edition. Six wells were used per group, and images were captured in 3 regions (one central and two peripheral) of each well, to analyze cell migration towards the scratch region. Images were captured on day 0 and were used to measure scratch size (cm) to obtain an average value (cm) of scratch width. This average was used to cut the images from days 1, 2, 3 and 4, to isolate only the cells that migrated to the scratch region. After the standardization of the photos, only the scratch area cells were counted by ImageJ 1.46r (“Cell Counter” Plugin) and the average values were calculated for each period.
Real time polymerase chain reaction
Each sample from groups F and F+MC was collected from a 24-well plate, totaling 4 samples per group with 3.1 × 106 cells in each sample. After cells trypsinization and following centrifugation at 1800 rpm for 10 min, the RNA extraction from each sample was performed.
The RNeasy® Plus Mini Kit (Qiagen®, cat. No. 74104) was used for total RNA extraction, according to the manufacturer’s instructions. The analysis of quality of the samples was evaluated by reading the absorbance ratios 260/280 nm and 260/230 nm in nanophotometer and the integrity of the RNA was verified by 1% agarose gel electrophoresis under denaturing conditions.
Complementary DNA (cDNA) synthesis was performed from 2.0 μg total RNA using the Applied Biosystems High-Capacity cDNA Reverse Transcription ™ kit (cat. 4368814) following the manufacturer’s instructions. The cDNA produced was used as a sample for real time polymerase chain reaction reactions. Reactions, always performed in triplicate, were performed using: cDNAs produced, TaqMan™Gene Expression Master Mix (Life Technologies—PN 4369016), RNase free water and Taqman assays (primer + hydrolysis probes) for the genes contained in Table 1 making a final volume of 20 μL. The entire RT-qPCR procedure was performed on the MX3005P (GE) instrumentation platform under 95 °C (10 minutes) and 45 cycles of 95 °C (15 s) and 60 °C (1 min). The results were analyzed by the MxPro (GE) program and relative gene expression was obtained by the 2−ΔΔCT method. Ppia gene was used as endogenous for data normalization.
Statistical analysis
All data presented normal distribution (Gaussian distribution) and parametric tests were employed: for comparisons between groups F and F+MC, data were analyzed using the Student t test (p < 0.05); while for the cell viability test after the 30, 90 and 150 s of MC application, the data of the different groups were analyzed using Analysis of Variance (ANOVA One-way), followed by Tukey test (p < 0.05). The values were represented by the mean ± standard deviation (SD), using GraphPad Prism® (GraphPad Software, La Jolla, CA, USA), version 3.0 was used.
Results
Cell viability
The protocol for applying MC therapy (10 μA) was defined 24 h after the 30, 90 and 150 s times (Fig. 2). All times presented higher cell viability (p < 0.05) in comparison to positive control. However, lower cell viability (p < 0.05) was observed after the application of MC in the 30 s time in relation to 90 and 120 s times.
On the 4th day after scratch, there was no difference in cell viability between groups F and F+MC (Fig. 3).
Cell migration
Cell migration analysis was performed on days 0, 1, 2, 3 and 4 by counting the number of cells in the scratch region. On day 0, no cells were observed in the scratch region. On the 1st day, few cells were observed in both groups. On the 2nd day, group F+MC presented lower number of cells in the scratch region compared to group F, however, no statistical differences were observed. On the 3rd day, both groups presented similar number of cells and, on the 4th day, scratch closure was observed in both groups, but with a larger number of cells in the F+MC group (p < 0.001) (Fig. 4a, b).
(a) Images showing cell migration (→) to the scratch region on days 0, 1, 2, 3, and 4 in groups F and F+MC. Notice higher number of cells in group F+MC on the 4th day. Bar = 200 μm. (b) Cell migration: Until day 3, no marked difference was observed between groups F and F+MC. On the 4th day, there was an increase in cell migration to the scratch region in group F+MC compared to group F. a = difference between groups (p < 0.05). Values represented by the mean ± SD
Gene expression
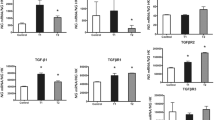
Higher expression of Ctgf and lower expression of Tnc and Fmod, respectively, were observed in the F+MC group in relation to F group (p < 0.05). No difference was observed between the groups for the genes Tgfb, Fn1 and Scx on the 4th day (Fig. 5). Regarding Tnmd and Igf1, no gene expression was observed in both groups under the experimental conditions studied.
Discussion
The literature has demonstrated the effectiveness of MC therapy in vivo during the repair of different tissues, such as tendon [43], skin [8], bone [48], heart [49] and cartilage [7]. Thus, the present study aimed to analyze the influence of MC on the modulation of remodeling genes in a wound healing assay, as well to correlate the possible activation of the pathway Tnmd–Tgfb1–Ctg, as described by Zheng et al. [42] during wound healing. In a previous work performed by our group [43], the MC (10 μA/4 min) therapy applied until the 14th day of tendon repair, increased the expression of Fmod, Ctgf, Tnc and Fn genes. In the present study, higher expression of Ctgf and lower expression of Tnc and Fmod were observed after MC daily application during 4 days, and no difference was observed between the groups for the genes Tgfb, Fn1 and Scx. Zheng et al. [42, 50] stated that the wound repair process can be initiated by the activation of Fmod expression that can act on the modulation of Tgfb1 and Ctgf genes, inducing cell migration and myofibroblast differentiation, resulting in improved wound healing. Although a reduced expression of Fmod was observed, our results point for the involvement of Ctgf after MC therapy due to the higher cell migration. Fmod and Ctgf have other important roles during tissue repair, in addition to participating in cell migration, such as collagen fibrillogenesis [28], angiogenesis [29], and modulation of other growth-factors signaling [32]. Regarding Tgfb, Neves et al. [8] have demonstrated the influence of MC stimulating TGF-β1 expression during wound closure in vivo, while Fonseca et al. [48] reported that MC therapy in the bone reconstruction model reduced TGF-β1 expression and modulated the inflammatory process.
Surprisingly, our results showed a reduced Tnc expression after MC therapy on the 4th day, when the closure of scratch occurred. As reviewed by Giblin and Midwood [25], tenascin-C has a diverse range of functions such as the stimulus of the cell migration, attachment and survival, matrix assembly, diverse cytokines synthesis, among others. Tenascin-C transcription may be influenced by a variety of growth factors, cytokines, ECM proteins, biomechanical stimulus [25, 51, 52], and according to our results, also in response to the MC therapy.
An in vivo study of Zuzzi et al. [53] showed that MC 20 μA/5 min accelerated cartilage repair by increasing the deposition of ECM components such as proteoglycans [53], glycosaminoglycans and collagen [54]. It was expected in the present study to show the activation of the Igf1 and Fn1 expression, which activate essential signaling pathways that increase deposition and remodeling of ECM components, as well as cell adhesion and migration [55]. However, our results demonstrated the absence of Igf1 expression and no difference in the expression of Fn1 after MC therapy on the 4th day.
In the present study, no alteration in the Scx expression was observed after MC therapy on the 4th day, and absence of Tnmd expression was observed in both groups. According to a study of Shukunami et al. [56], Scx activates Tnmd expression in vitro to regulate differentiation and maturation of tenocytes, although the Scx expression and absence of Tnmd expression were observed from the 20th passage of cells. Still in the previously mentioned study [56], no expression of Scx and Tnmd was found in the NIH/3T3 fibroblasts, partially corroborating our results, in which was observed the expression of Scx though no difference after MC application. Kague et al. [35] reported that Scx is also related to secretion of ECM components, including some types of collagen, and Tnmd is also related to the formation of cytoplasmic extensions that support the organization of ECM.
In vitro studies have shown that electrical currents can influence migration, proliferation, viability and cell function [3, 4]. Our results showed that the application of MC at 10 μA/90 s on the 4th day did not affect the cell viability, corroborating with the in vitro analysis performed by Uemura et al. [4], that demonstrated no difference in the viability of dermal fibroblasts after 24 h of electrostimulation application between 100 and 300 μA (for 8 h). Lin et al. [57] used the intensity of 0.05 to 1.5 mA of MC application in equine tenocytes culture for 8 min and observed no apoptosis after 24 h, and an increase in apoptosis after 48 and 72 h. Thus, the effects of MC on cell viability depends on the intensity and time of application.
Regarding cell migration, a higher number of cells in the scratch region on the 4th day observed in our results, demonstrated a stimulatory effect of the MC therapy corroborating with the literature. Our results also showed the involvement of the increased Ctgf gene expression with the enhanced cell migration after MC application. Uemura et al. [4] analyzed the migration of dermal fibroblasts 24 h after the application of electrostimulation at 100, 200 and 300 μA intensities, and observed increased migration only using electrostimulation at the intensity of 200 μΑ. Tai et al. [2], showed that electrical fields activate multiple cellular signaling mechanisms, such as the PI3K-AKT (phosphoinositide-3 kinase-AKT serine/threonine kinase) mechanism, which alter cell migration, survival and proliferation. It is important to emphasize that, in the present study, on the 2nd day after the application of MC, there was cellular reorientation, since the cells partially retracted to the scratch edges and returned to migrate towards the scratch center from the 3rd day. Corroborating with our results, Rouabhia et al. [58] reported that dermal fibroblasts when electrically stimulated (50 and 200 mV/mm for up to 6 h) showed a retractable behavior of fibroblasts in a collagen gel matrix. Our study has a translational relevance since research on understanding the molecular mechanisms by which MC regulates cell response, can contribute to the development of protocols for this therapy’s clinical use.
Conclusion
The present study demonstrated that the application of MC at an intensity of 10 μA/90 s modulated the expression of some important remodeling genes in a wound healing assay, differently of those results observed in a tendon healing model, and with apparently no involvement of the pathway Tnmd–Tgfb1–Ctgf activation on the 4th day. It is important to emphasize that in the in vitro experiments, there is no molecular signaling from other cell types that also respond to the effects of MC therapy, nor cellular interactions and therefore genes expression and protein turnover may be different. Thus, these variables should be considered in development of new protocols using MC, since the effects of the MC depend on the intensity/time of application, of the tissue or cells lineage and of period of the repair process analyzed. In conclusion, MC therapy at an intensity/time of 10 μA/90 s with 4 daily applications did not affect cell viability, stimulated fibroblast migration with the involvement of Ctgf, and reduced the Tnc and Fmod expression.
References
Yu C, Hu ZQ, Peng RY (2014) Effects and mechanisms of a microcurrent dressing on skin wound healing: a review. Mil Med Res 1:24
Tai G, Tai M, Zhao M (2018) Electrically stimulated cell migration and its contribution to wound healing. Burns Trauma 6:20
Hunckler J, de Mel A (2017) A current affair: electrotherapy in wound healing. J Multidiscip Healthc 10:179–194
Uemura M, Maeshige N, Koga Y, Ishikawa-Aoyama M, Miyoshi M, Sugimoto M, Terashi H, Usami M (2016) Monophasic pulsed 200-μA current promotes galvanotaxis with polarization of actin filament and integrin α2β1 in human dermal fibroblasts. Eplasty 16:e6
Sugimoto M, Maeshige N, Honda H, Yoshikawa Y, Uemura M, Yamamoto M, Terashi H (2012) Optimum microcurrent stimulation intensity for galvanotaxis in human fibroblasts. J Wound Care 21:5–6, 8,10; discussion 10–11
Leppik L, Zhihua H, Mobini S, Thottakkattumana Parameswaran V, Eischen-Loges M, Slavici A, Helbing J, Pindur L, Oliveira KMC, Bhavsar MB et al (2018) Combining electrical stimulation and tissue engineering to treat large bone defects in a rat model. Sci Rep 8:6307
de Campos CC, Zuzzi DC, Neves LM, Mendonça JS, Joazeiro PP, Esquisatto MA (2013) Effects of microcurrent stimulation on hyaline cartilage repair in immature male rats (Rattus norvegicus). BMC Complement Altern Med 13:17
Neves LMG, Matheus RL, Santos GMT, Esquisatto MAA, Amaral MEC, Mendonça FAS (2013) Effects of microcurrent application and 670 nm InGaP low-level laser irradiation on experimental wound healing in healthy and diabetic Wistar rats. Laser Phys 23:35604–35615
Belli M, Fernandes CR, Neves LMG, Mourão V, Barbieri R, Esquisatto MAA, Amaral MEC, Santos GMT, Mendonça FAS (2015) Application of 670 nm InGaP laser and microcurrent favors the healing of second degree burns in Wistar rats. Laser Phys 25:11–24
Mendonça JS, Neves LMG, Esquisatto MAM, Mendonça FAS, Santos GMT (2013) Comparative study of the application of microcurrent and AsGa 904 nm laser radiation in the process of repair after calvaria bone excision in rats. Laser Phys 23:1–8
Zaniboni E, Bagne L, Camargo T, do Amaral MEC, Felonato M, de Andrade TAM, Dos Santos GMT, Caetano GF, Esquisatto MAM, Santamaria M, Mendonça FAS (2019) Do electrical current and laser therapies improve bone remodeling during an orthodontic treatment with corticotomy? Clin Oral Investig 23:4083
Hu L, Zhao J, Liu J, Gong N, Chen L (2013) Effects of adipose stem cell-conditioned medium on the migration of vascular endothelial cells, fibroblasts and keratinocytes. Exp Ther Med 5:701–706
Midwood KS, Valenick LV, Hsia HC, Schwarzbauer JE (2004) Coregulation of fibronectin signaling and matrix contraction by tenascin-C and syndecan-4. Mol Biol Cell 15:5670–5677
Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M (2008) Growth factors and cytokines in wound healing. Wound Repair Regen 16:585–601
Daniels A, van Bilsen M, Goldschmeding R, van der Vusse GJ, van Nieuwenhoven FA (2009) Connective tissue growth factor and cardiac fibrosis. Acta Physiol (Oxford) 195:321–338
Yosimichi G, Nakanishi T, Nishida T, Hattori T, Takano-Yamamoto T, Takigawa M (2001) CTGF/Hcs24 induces chondrocyte differentiation through a p38 mitogen-activated protein kinase (p38MAPK), and proliferation through a p44/42 MAPK/extracellular-signal regulated kinase (ERK). Eur J Biochem 268:6058–6065
Bradham DM, Igarashi A, Potter RL, Grotendorst GR (1991) Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol 114:1285–1294
Balaji S, LeSaint M, Bhattacharya SS, Moles C, Dhamija Y, Kidd M, Le LD, King A, Shaaban A, Crombleholme TM et al (2014) Adenoviral-mediated gene transfer of insulin-like growth factor 1 enhances wound healing and induces angiogenesis. J Surg Res 190:367–377
Semenova E, Koegel H, Hasse S, Klatte JE, Slonimsky E, Bilbao D, Paus R, Werner S, Rosenthal N (2008) Overexpression of mIGF-1 in keratinocytes improves wound healing and accelerates hair follicle formation and cycling in mice. Am J Pathol 173:1295–1310
Haase I, Evans R, Pofahl R, Watt FM (2003) Regulation of keratinocyte shape, migration and wound epithelialization by IGF-1- and EGF-dependent signalling pathways. J Cell Sci 116:3227–3238
Molloy T, Wang Y, Murrell G (2003) The roles of growth factors in tendon and ligament healing. Sports Med 33:381–394
Ando Y, Jensen PJ (1993) Epidermal growth factor and insulin-like growth factor I enhance keratinocyte migration. J Invest Dermatol 100:633–639
Granot I, Halevy O, Hurwitz S, Pines M (1991) Growth hormone and insulin-like growth factor I regulate collagen gene expression and extracellular collagen in cultures of avian skin fibroblasts. Mol Cell Endocrinol 80:1–9
Midwood KS, Chiquet M, Tucker RP, Orend G (2016) Tenascin-C at a glance. J Cell Sci 129:4321–4327
Giblin SP, Midwood KS (2015) Tenascin-C: form versus function. Cell Adhes Migr 9:48–82
Hielscher A, Ellis K, Qiu C, Porterfield J, Gerecht S (2016) Fibronectin deposition participates in extracellular matrix assembly and vascular morphogenesis. PLoS One 11:e0147600
Johnson MB, Pang B, Gardner DJ, Niknam-Benia S, Soundarajan V, Bramos A, Perrault DP, Banks K, Lee GK, Baker RY et al (2017) Topical fibronectin improves wound healing of irradiated skin. Sci Rep 7:3876
Tillgren V, Mörgelin M, Önnerfjord P, Kalamajski S, Aspberg A (2016) The tyrosine sulfate domain of fibromodulin binds collagen and enhances fibril formation. J Biol Chem 291:23744–23755
Adini I, Ghosh K, Adini A, Chi ZL, Yoshimura T, Benny O, Connor KM, Rogers MS, Bazinet L, Birsner AE et al (2014) Melanocyte-secreted fibromodulin promotes an angiogenic microenvironment. J Clin Invest 124:425–436
Kram V, Kilts TM, Bhattacharyya N, Li L, Young MF (2017) Small leucine rich proteoglycans, a novel link to osteoclastogenesis. Sci Rep 7:12627
Kim T, Ahmad K, Shaikh S, Jan AT, Seo MG, Lee EJ, Choi I (2019) Dermatopontin in skeletal muscle extracellular matrix regulates myogenesis. Cells 8:332
Mendes FA, Coelho Aguiar JM, Kahn SA, Reis AH, Dubois LG, Romão LF, Ferreira LS, Chneiweiss H, Moura Neto V, Abreu JG (2015) Connective-tissue growth factor (CTGF/CCN2) induces astrogenesis and fibronectin expression of embryonic neural cells in vitro. PLoS One 10:e0133689
Nichols AEC, Settlage RE, Werre SR, Dahlgren LA (2018) Novel roles for scleraxis in regulating adult tenocyte function. BMC Cell Biol 19:14
Killian ML, Thomopoulos S (2016) Scleraxis is required for the development of a functional tendon enthesis. FASEB J 30:301–311
Kague E, Hughes SM, Lawrence EA, Cross S, Martin-Silverstone E, Hammond CL, Hinits Y (2019) Scleraxis genes are required for normal musculoskeletal development and for rib growth and mineralization in zebrafish. FASEB J 33:9116–9130
Barnette DN, Hulin A, Ahmed AS, Colige AC, Azhar M, Lincoln J (2013) Tgfβ-Smad and MAPK signaling mediate scleraxis and proteoglycan expression in heart valves. J Mol Cell Cardiol 65:137–146
McKenzie JA, Buettmann E, Abraham AC, Gardner MJ, Silva MJ, Killian ML (2017) Loss of scleraxis in mice leads to geometric and structural changes in cortical bone, as well as asymmetry in fracture healing. FASEB J 31:882–892
Shukunami C, Takimoto A, Oro M, Hiraki Y (2006) Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev Biol 298:234–247
Komiyama Y, Ohba S, Shimohata N, Nakajima K, Hojo H, Yano F, Takato T, Docheva D, Shukunami C, Hiraki Y, Chung UI (2013) Tenomodulin expression in the periodontal ligament enhances cellular adhesion. PLoS One 8:e60203
Lin D, Alberton P, Caceres MD, Volkmer E, Schieker M, Docheva D (2017) Tenomodulin is essential for prevention of adipocyte accumulation and fibrovascular scar formation during early tendon healing. Cell Death Dis 8:e3116
Alberton P, Dex S, Popov C, Shukunami C, Schieker M, Docheva D (2015) Loss of tenomodulin results in reduced self-renewal and augmented senescence of tendon stem/progenitor cells. Stem Cells Dev 24:597–609
Zheng Z, James AW, Li C, Jiang W, Wang JZ, Chang GX, Lee KS, Chen F, Berthiaume EA, Chen Y et al (2017) Fibromodulin reduces scar formation in adult cutaneous wounds by eliciting a fetal-like phenotype. Signal Transduction Targeted Ther 2:e17050
Bortolazzo FO, Lucke LD, de Oliveira FL, Marqueti RC, Vieira Ramos G, Theodoro V, Bombeiro AL, Felonato M, Dalia RA, Carneiro GD et al (2020) Microcurrent and adipose-derived stem cells modulate genes expression involved in the structural recovery of transected tendon of rats. FASEB J 34:10011
Castro FC, Magre A, Cherpinski R, Zelante PM, Neves LM, Esquisatto MA, Mendonça FA, Santos GM (2012) Effects of microcurrent application alone or in combination with topical Hypericum perforatum L. and Arnica montana L. on surgically induced wound healing in Wistar rats. Homeopathy 101:147–153
de Gaspi FO d G, Foglio MA, de Carvalho JE, Santos GM, Testa M, Passarini JR, de Moraes CP, Esquisatto MA, Mendonça JS, Mendonça FA (2011) Effects of the topical application of hydroalcoholic leaf extract of oncidium flexuosum sims. (Orchidaceae) and microcurrent on the healing of wounds surgically induced in Wistar rats. Evid Based Complement Alternat Med 2011:950347
Mendonça FA, Passarini Junior JR, Esquisatto MA, Mendonça JS, Franchini CC, Santos GM (2009) Effects of the application of Aloe vera (L.) and microcurrent on the healing of wounds surgically induced in Wistar rats. Acta Cir Bras 24:150–155
Passarini Junior JR, Gaspi FO, Neves LM, Esquisatto MA, Santos GM, Mendonça FA (2012) Application of Jatropha curcas L. seed oil (Euphorbiaceae) and microcurrent on the healing of experimental wounds in Wistar rats. Acta Cir Bras 27:441–447
Fonseca JH, Bagne L, Meneghetti DH, Dos Santos GMT, Esquisatto MAM, de Andrade TAM, do Amaral MEC, Felonato M, Caetano GF, Santamaria M, Mendonça FAS (2019) Electrical stimulation: complementary therapy to improve the performance of grafts in bone defects? J Biomed Mater Res B Appl Biomater 107:924–932
Macfelda K, Kapeller B, Holly A, Podesser BK, Losert U, Brandes K, Goettel P, Mueller J (2017) Bioelectrical signals improve cardiac function and modify gene expression of extracellular matrix components. ESC Heart Failure 4:291–300
Zheng Y, Xu QF, Chen HY, Ye CX, Lai W, Maibach HI (2017) Inhibition of MMPs Cat G and downregulates the signaling of TGF-β/Smad in chronic photodamaged human fibroblasts. Eur Rev Med Pharmacol Sci 21:5160–5165
Jones FS, Jones PL (2000) The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev Dyn 218:235–259
Udalova IA, Ruhmann M, Thomson SJ, Midwood KS (2011) Expression and immune function of tenascin-C. Crit Rev Immunol 31:115–145
Zuzzi DC, Ciccone CEC, Neves LM, Mendonça JS, Joazeiro PP, Esquisatto MA (2013) Evaluation of the effects of electrical stimulation on cartilage repair in adult male rats. Tissue Cell 45:275–281
Tangerino Filho EP, Fachi JL, Vasconcelos IC, Dos Santos GM, Mendonça FA, de Aro AA, Pimentel ER, Esquisatto MA (2016) Effects of microcurrent therapy on excisional elastic cartilage defects in young rats. Tissue Cell 48:224–234
Lipson KE, Wong C, Teng Y, Spong S (2012) CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair 5:S24
Shukunami C, Takimoto A, Nishizaki Y, Yoshimoto Y, Tanaka S, Miura S, Watanabe H, Sakuma T, Yamamoto T, Kondoh G, Hiraki Y (2018) Scleraxis is a transcriptional activator that regulates the expression of Tenomodulin, a marker of mature tenocytes and ligamentocytes. Sci Rep 8:3155
Lin YL, Moolenaar H, van Weeren PR, van de Lest CH (2006) Effect of microcurrent electrical tissue stimulation on equine tenocytes in culture. Am J Vet Res 67:271–276
Rouabhia M, Park H, Meng S, Derbali H, Zhang Z (2013) Electrical stimulation promotes wound healing by enhancing dermal fibroblast activity and promoting myofibroblast transdifferentiation. PLoS One 8:e71660
Acknowledgements
To the Biomedical Sciences Graduate Program of the University Center of Herminio Ometto Foundation/FHO, for the scholarship and “Sylvio Moreira” Citriculture Center Agronomic Institute-IAC for the technical training.
Funding
PIC/Institutional of the University Center of Herminio Ometto Foundation/FHO (445-2018), for the funding of the research and the scholarship granted to the master student Daniela Fernanda Dezotti Silva.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Silva, D.F.D., de Oliveira Fujii, L., Chiarotto, G.B. et al. Influence of microcurrent on the modulation of remodelling genes in a wound healing assay. Mol Biol Rep 48, 1233–1241 (2021). https://doi.org/10.1007/s11033-021-06135-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06135-0