Abstract
Penicillin V is a bacteriolytic β-lactam antibiotic drug. In the present work, we investigated the inhibitory effect of Penicillin V on the activity of mushroom tyrosinase for the first time. The molecular mechanism for the inhibition of tyrosinase by Penicillin V was investigated by means of kinetics analysis, fluorescence quenching and molecular docking techniques. The results showed that Penicillin V could inhibit both monophenolase and diphenolase activities with IC50 of 16.6 ± 0.5 and 11.0 ± 0.2 mmol/L, respectively. The inhibitory type of Penicillin V on mushroom was mixed type, and the values of KI and KIS were 13.46 and 17.26 mmol/L, respectively. The fluorescence quenching and molecular docking showed that Penicillin V could form static interaction near the catalytic pocket of the enzyme to hinder the transportation of substrate to the active site, as well as reduce the copper plasticity for catalysis. Our results contributed to the usage of Penicillin V as a novel tyrosinase inhibitor with dual effect in field of antimicrobial and food preservation and could also provide guidance for the design of novel tyrosinase inhibitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tyrosinase (EC 1. 14. 18. 1) is a poly-functional oxidase with two copper ions in the active center, which has been widely found in microorganisms, animals, plants and human beings [1]. It has monophenolase and diphenolase activities that can hydroxylate tyrosine and produce L-3,4-dihydroxyphenylalanine (l-DOPA) to oxidize dopaquinone to form melanin. De Castro et al. [2] found that the intrinsic tyrosinase activity is an important element to cause enzymatic browning and nutrient loss of fruits and vegetables during transportation and preservation. Thus, inhibition on the tyrosinase mediated browning is essential to keep fruit fresh and tyrosinase inhibitor has great values in the field of fresh fruit and vegetable preservation. In recent years, studies on tyrosinase inhibitor mainly focus on extraction from natural products and synthetic organic substances [3], such as C-glycosylated flavonoids [4], coumarin derivatives [5, 6]. However, few studies are reported on traditional antibiotics as tyrosinase inhibitor.
Penicillin V is a semi synthetic penicillin, which belongs to the penicillin type antibiotics. Its penam can destroy cell walls of bacteria in reproduction period so as to kill bacteria and it is a kind of antibiotics in β-lactams, whose structure contains a carboxyl and an azyl. Previous studies reported that compounds with carboxyl and azyl have good tyrosinase inhibition effects [7, 8]. But tyrosinase inhibition by Penicillin V has not been reported. Although the antimicrobial mechanism of Penicillin V is very clear, its inhibition mechanism on tyrosinase is still not figured out. Here, this study clarified inhibitory effect and action mechanism of Penicillin V on mushroom tyrosinase to lay a foundation for development of multiple functional fruit and vegetable fresh-keeping agents.
Materials and methods
Materials
Penicillin V in form of potassium salt was purchased from Shanghai Shenggong Bioengineering Company. Mushroom (Agaricus bisporus) tyrosinase, l-DOPA and l-tyrosine (l-Tyr) are products of Sigma-Aldrich (Germany). The specific activity of the mushroom tyrosinase was 1000 U/mg. Other reagents were domestic analytic grade reagents and redistilled and ion-free water. DU800 spectrophotometer by Beckman coulter and Cary Eclipse fluorescence spectrophotometer by Varian were used as optic analytical instruments.
Effect of Penicillin V on monophenolase activity of mushroom tyrosinase
The experiment was carried out according to the method of Plenge et al. [9] by taking 6 mmol/L l-Tyr as the substrate and adding 0.1 mL Penicillin V with different concentrations in the colorimetric cup, 2.8 mL substrate solution pre-heated in the 30 °C constant temperature water bath and 0.1 mL mushroom tyrosinase solution in the 3 mL assay system (containing 0.05 mol/L PBS, pH 6.8). The 475 nm light absorption value growth curve was traced over time at a constant temperature of 30 °C. The monophenolase’s steady-state activity was obtained by the slope of straight lines of the response curve. The intercept of the horizontal axis extrapolated to straight lines refered to the response lag time. The final concentration of mushroom tyrosinase was 16.67 μg/mL, which was detected by Beckman DU800 spectrophotometer.
Determination of inhibition effect and inhibition mechanism of Penicillin V on diphenolase activity of tyrosinase
An aliquot of 0.05 mol/L (pH 6.8) PBS contained 0.5 mmol/L l-DOPA was as the substrate solution and 0.1 mL Penicillin V of different concentrations were added into the colorimetric cup. An aliquot of 2.85 mL substrate solution that was pre-heated in the 30 °C constant temperature water bath was added. Finally, 0.05 mL mushroom tyrosinase solution was added to be final concentration of 3.33 μg/mL and mixed immediately. The 475 nm light absorption value kinetic curve was determined over time at 30 °C. The enzyme activity was obtained from the slope of straight lines, of which the extinction coefficient was 3700 (mol/L·cm)−1. The dose–effect curve of Penicillin V inhibition on tyrosinase activity was obtained based on enzyme relative residual activity. When remained activity was 50%, the corresponding concentration of Penicillin V was the value of IC50. If the amount of enzyme was changed in the assay system, the activity of mushroom tyrosinase catalyzing l-DOPA oxidation could be detected under different concentrations Penicillin V. The addition of enzyme was plotted by taking the remained enzyme activity as the factor.
Determination of inhibition type and inhibition constant of Penicillin V on diphenolase activity of tyrosinase
The influence of inhibitors at different concentrations on enzyme activity was determined by fixing enzyme’s concentration and changing l-DOPA concentration in the activity assay system. A group of curves concerning the concentration of l-DOPA could be obtained based on initial reaction rate, which indicated that the enzymatic reaction observed Michaelis–Menten kinetic equation. The inhibition mechanism of diphenolase was determined from the change of kinetic parameters of enzymatic catalytic reaction, including the apparent Michaelis constant (Km) and the maximum reaction velocity (Vm) by taking Lineweaver–Burk double-reciprocal plot of initial reaction rate versus substrate concentration. Finally, the slope and intercept of straight lines were obtained via the double-reciprocal plot for the second plotting regarding the concentration of Penicillin V. Then, the free enzyme inhibition constant (KI) and the enzyme–substrate complex inhibition constant (KIS) were obtained from the plots.
Influences of Penicillin V on tyrosinase catalyzing l-DOPA/l-Tyr oxidation reaction
To track UV–Vis spectra within 240–700 nm at varying reaction times, the method of Espin et al. [10] was adopted to scan the absorption spectra in the presence of Penicillin V following tyrosinase catalysis in the reaction system as described above.
Fluorescence quenching of mushroom tyrosinase by Penicillin V
The fluorescence quenching experimental method was referred to Zhao et al. [11]. The changes of intrinsic fluorescence intensity of mushroom tyrosinase were determined with Cary Eclipse spectrofluorophotometer. The excitation wavelength was set as 280 nm with slit width of 5 nm. The intrinsic fluorescence emission spectrum was scanned within the range of 300–400 nm. Different concentrations of penicillin V were added into 2 mL 0.2 mg/mL mushroom tyrosinase to scan fluorescence emission spectrums after 1 min of mixing.
Molecular docking of Penicillin V and mushroom PPO3
MOE software was used to simulate protein molecule and small-molecule ligand docking. The mushroom tyrosinase in this research was in an oxidation state. The PPO3 crystal structure (2Y9 W) in Agaricus bisporus was found on PDB for further re-construction [12]. The energy minimization was conducted on PPO3 and ligands before docking. The virtual docking sites were searched in order to find out one with the highest score. The docking parameters were set by referring to reports by Chen et al. [13] and the force field was set as 10 with others as default values.
Results and analyses
Influence of Penicillin V on monophenolase activity of tyrosinase
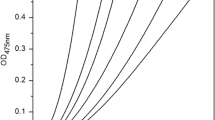
The effect of Penicillin V on monophenolase activity of mushroom tyrosinase was shown in Fig. 1. The Fig. 1a referred to the kinetic curve of reaction concerning monophenolase of mushroom tyrosinase catalyzing l-Tyr. At the beginning, the product formation grew slowly; after a while, it started to go up rapidly until in a straight line. The constant slope indicated a steady state. It could be observed from Fig. 1b that with the increasing concentration of Penicillin V, the reaction lag time of monophenlase was prolonged from the initial 80.3 to 101.86 s. In terms of steady-state activity of monophenolase, the enzyme activity was decreased to a half at the time of 16.6 mmol/L Penicillin V, namely, IC50 was 16.6 mmol/L; comprehensively speaking, the inhibition effect of Penicillin V on monophenolase activity of tyrosinase was achieved by prolonging enzyme’s reaction lag time and decreasing enzyme’s steady-state activity.
Inhibition effect and mechanism of Penicillin V on diphenolase activity of tyrosinase
The influence of Penicillin V on reaction of mushroom tyrosinase catalyzed l-DOPA oxidation was assayed. The progress curve of the oxidation reaction of l-DOPA by mushroom tyrosinase was a line passing through the origin, meaning the formation of product was in proportion to the reaction time (data no shown). It could be seen from Fig. 2a that the slope of the straight line of enzymatic reaction was decreased obviously as concentration of Penicillin V increased, indicating that Penicillin V could inhibit diphenolase activity of tyrosinase. Further, the dose–effect course presented IC50 of Penicillin V on diphenolase activity of tyrosinase was 11.0 ± 0.05 mmol/L.
The inhibition mechanism of Penicillin V on diphenolase activity was studied by changing the amount of enzyme under constant substrate concentration. As shown in Fig. 2b, a group of lines through the origin were obtained by taking the remaining enzyme activity as the factor. The slope of straight lines were deceased with the increasing concentration of inhibitors, which showed that its inhibition effect on enzyme activity was reversible. The decrease on its oxidation rate would not lead to enzyme denaturation and deactivation.
Determination of inhibition type and constant of Penicillin V on tyrosinase activity
The inhibition type of Penicillin V on mushroom tyrosinase was studied in the assay system by fixing the concentration of enzyme and changing the concentration of substrate l-DOPA. The relative inhibition constants were also determined (Fig. 3). Figure 3a was plotted based on Lineweaver–Burk method to judge inhibition types of Penicillin V. A group of lines intersecting in the second quadrant were obtained, which indicated that as a kind of tyrosinase inhibitor, Penicillin V could not only affect Vm, but Km. It would increase Km but decrease Vm, showing as a mixed-type inhibitor. The Penicillin V could not only be bound with free form enzyme, but ES with different constants. The second plotting took slope (Fig. 3b) and intercepted at the vertical axis (Fig. 3c) as factors of concentration of Penicillin V so as to obtain its inhibition constant of E (KI) and ES (KIS) of 13.46 mmol/L and 17.26 mmol/L respectively.
Inhibition types of Penicillin V on mushroom tyrosinase for the catalysis of l-DOPA. a Lineweaver–Burk plots for diphenolase activity. The concentrations of Penicillin V for curves 1–5 are 0, 5, 10, 15 and 20 mmol/L. b Plot of slope versus the concentration of inhibitor for determining the inhibition constants KI. c Plot of intercept versus the concentration of inhibitor for determining the inhibition constants KIS
Influence of Penicillin V on tyrosinase catalyzed l-Tyr or l-DOPA oxidation
The UV–Vis spectra after the tyrosinase catalyzed oxidation of l-Tyr and l-DOPA in the absence (Fig. 4a, b) and presence (Fig. 4c, d) of Penicillin V were obtained. Figure 4a, b referred to UV–Vis absorption spectra of tyrosinase catalyzing l-Tyr or l-DOPA. It could be found from the results that the light absorption value was increased continuously at 475 nm in the catalytic oxidation process and the presence of Penicillin V caused the absorption at 475 nm decreased. For the oxidation of l-Tyr, at 12 min after the addition of the enzyme the presence of Penicillin V reduced the peak intensity by 53.5%. For l-DOPA, at 5 min after the addition of the enzyme the peak intensity was reduced by 45.1%.
Interaction profile of Penicillin V on mushroom tyrosinase determined with fluorescence quenching
The intrinsic fluorescence method was used to evaluate the interaction of Penicillin V with mushroom tyrosinase. The fluorescence emission spectra of mushroom tyrosinase were recorded from a range of 300–450 nm with excitation at 290 nm and the enzyme showed a strong fluorescence emission with a peak at 337 nm. The Penicillin V itself had no fluorescence intensity, but it could quench fluorescence emission of tyrosinase at different concentrations (Fig. 5). The results in Fig. 5a showed that the peak of the fluorescence emission remained the same at 337 nm without shift even as the increasing concentration of Penicillin V. However its intrinsic fluorescence intensity was decreased inversely (Fig. 5b), which indicated that the interaction was formed between Penicillin V and the enzyme [11].
Changes in intrinsic tyrosinase fluorescence at different concentrations of Penicillin V. a Emission spectra of tyrosinase with excitation by 280 nm. b Relative fluorescence intensity of tyrosinase at 337 nm in the presence of Penicillin V. c Stern–Volmer plot. d Plot of lg [(F0−F)/F] against lg [I]
According to Stern–Volmer equation [14]: F0/F = 1 + Kqτ0[I] = 1 + KSV[I], the Stern–Volmer quenching curve of tyrosinase fluorescence was obtained by making the plot of the concentration of Penicillin V (Fig. 5c). F0/F was increased gradually with the rising concentration of Penicillin V, which also presented a good linear relation. The rate constant KSV was calculated as 182 L/mol in the fluorescence quenching process. The maximum rate constant of biomacromolecules KSV < 100 L/mol in the dynamic quenching process, while the speed constant in the quenching process of nonconvalent binding of the above obtained tyrosinase and Penicillin V was larger than that of the maximal dynamic quenching process, which indicated that the quenching process of Penicillin V on the intrinsic fluorescence of tyrosinase is a static interaction instead of dynamic process controlled by diffusion.
The Scatchard equation [15]: lg[(F0−F)/F] = lgKA + nlg[I] was used in the static quenching process to determine the binding constant (KA) and binding site (n). Based on this equation, the equation of linear regression in Fig. 5d was obtained as follows: lg[(F0−F)/F] = 2.13 + 0.93 lg[I], R2 = 0.9994. The binding constant KA and binding site n were 134.89 ± 12.1 L/mol and 0.93 ± 0.05, respectively.
Studies on molecular docking of Penicillin V and mushroom PPO3 model
The molecular simulation docking was performed to study the molecular mechanism of Penicillin V on mushroom (Agaricus bisporus) tyrosinase inhibition. The docking model was shown in Fig. 6 . The crystal structure of Agaricus bisporus PPO3 was validated as Ramachandran plot suggested (Fig. 6a). It could be seen from the 3D structure of the complex (Fig. 6b) that Penicillin V could bind with the active center of mushroom PPO3. The two-dimension diagram of Fig. 6c showed that Penicillin V did not react with copper ion directly. It would not react with dual nuclear copper ion and there existed steric hindrance on combination of substrate to enzyme active center after the binding of Penicillin V. Besides, hydrophobic amino acids, such as Phe292, Phe264, Val283 and Val248 were found near the docking site of Penicillin V in mushroom tyrosinase, which implied that hydrophobicity was crucial for the combination of Penicillin V and tyrosinase. More importantly, the results also indicated that Penicillin V could react with histidine residues near the active center, including His61, His85, His94, His244, His259, His263 and His296.
Discussion
This study investigated the inhibition mechanism of Penicillin V on tyrosinase, which is responsible for the formation of melanin during preservation of fruit and vegetables [16]. Penicillin V, an over the counter antibiotic drug, showed obvious anti-tyrosinase effect in this study, owning good potential as dual functional tyrosinase inhibitor with antimicrobial ability. And it could also serve as a new core structure for design of novel anti-tyrosinase agent in fresh-keeping field. This paper showed it could prolong reactions lag time and decrease enzyme’s steady-state activity so as to inhibit monophenolase activity of tyrosinase. In terms of diphenolase activity, Penicillin V was presented as a reversible mixed inhibitor with KIS < KI. That mean the activity of enzyme–substrate complex was affected by Penicillin V in a smaller extent than free enzyme, which was also found other study on tyrosinase inhibition [17]. Compared to reported tyrosinase inhibitors, such inhibition mechanism was similar with that of synthetic methylsalicylic acid and some plant condensed tannins [3, 18,19,20], but different from 3′, 5′-di-C-β-glucopyranosylphloretin, which was a competitive type tyrosinase inhibitor [4].
The UV–Vis spectra verified that Penicillin V could inhibit mushroom tyrosinase activity drastically. After 10- and 5-min reaction, the absorption values of oxidation products of l-Tyr and l-DOPA catalyzed by the enzyme were decreased meaning the decrease of the amount of oxidation products. As reported, the active site of tyrosinase has three oxidation states: reduced (Emet), deoxidized (Edeoxy) and oxidized (Eoxy) states [6]. During the formation of melanin pigments, three forms of tyrosinase with different binuclear copper structures of the active site are involved. Meanwhile, in-silicon docking showed the required energy for binding was − 69.01 kcal/mol between Penicillin V and mushroom PPO3, which indicated the complex was enough stable to make the enzyme activity be inhibited. The molecular docking result indicated that Penicillin V did not directly interact with copper ions in the tyrosinase active center. Instead, it combined with amino acid residues near copper ions in the active center, which showed that its inhibition on enzyme activity was realized through blocking the enzyme active center rather than insider the active center, thus impeded substrate to enter the catalytic pocket. According to above structural analysis, the binding site of Penicillin V in the tyrosinase might be involved in transportation of substrate to copper ions in the active site of tyrosinase. Moreover, the responding amino acid residues of tyrosinase to Penicillin V are overall close to the copper-binding histidine residues, which weaken the plasticity of copper ions and thus decrease the electron transmission efficiency between oxygen atoms and copper ions [21,22,23]. Meanwhile, the enzyme kinetics indicated static binding of Penicillin V to tyrosinase could retain effective inhibition on enzyme activity and decrease cumulated amount of oxidation products for an enough long time (up to 10 min), even though the inhibition is reversible, that is similar with the effects of some reported over the counter drugs [13, 24, 25].
The fluorescence quenching was commonly used to elucidate the mutual interaction between small-molecule ligands and proteins. This research adopted this method to investigate influences of Penicillin V on conformational change of tyrosinase. The used excitation wavelength was 280 nm, so 337 nm of fluorescence peak was contributed by Trp, Tyr residues in the hydrophobic domain [26]. The results indicated that with the increasing concentration of Penicillin V, the fluorescence intensity was reduced, which might be due to that Penicillin V could react with Trp or Tyr residues on the surface of enzyme molecules to form hydrophobic bonds so as to lead to decrease of fluorescence emission peak [27]. However, Penicillin V at various concentrations did not cause redshift or blueshift, which implied that the whole conformation of enzyme molecules had no detectable change under the determination condition, meaning the whole structure of the enzyme remained intact with combination of Penicillin V, which is consistent with many other tyrosinase inhibitors [28, 29].
In conclusion, Penicillin V exhibited both monophenolase and diphenolase inhibitory activities on mushroom tyrosinase. The kinetic analysis revealed that Penicillin V was a mixed type inhibitor of mushroom tyrosinase. It could attenuate l-Tyr/l-DOPA oxidation catalyzed by mushroom tyrosinase so as to reduce melanin production and accumulation. Fluorescence quenching and molecular docking study showed that the combination of Penicillin V on tyrosinase did not induce change of the tertiary structure of the enzyme. The inhibition of Penicillin V on mushroom tyrosinase realized might be due to Penicillin V could bind to key amino acid residues around the tyrosinase active center and block the enzyme active center to hinder substrate enter the pocket to decrease enzyme activity.
References
Bao K, Dai Y, Zhu ZB, Tu FJ, Zhang WG, Yao XS (2010) Design and synthesis of biphenyl derivatives as mushroom tyrosinase inhibitors. Bioorg Med Chem 18:6708–6714
Castro E, Barrett DM, Jobling J, Mitcham EJ (2008) Biochemical factors associated with a CO2-induced flesh browning disorder of Pink Lady apples. Postharvest Biol Technol 48:182–191
Zhang JP, Chen QX, Song KK, Xie JJ (2006) Inhibitory effects of salicylic acid family compounds on the diphenolase activity of mushroom tyrosinase. Food Chem 95:579–584
Lou SN, Yu MW, Ho CT (2012) Tyrosinase inhibitory components of immature calamondin peel. Food Chem 135:1091–1096
Liu J, Wu F, Chen L et al (2012) Biological evaluation of coumarin derivatives as mushroom tyrosinase inhibitors. Food Chem 135:2872–2878
Song YM, Ha YM, Kim JA et al (2012) Synthesis of novel azo-resveratrol, azo-oxyresveratrol and their derivatives as potent tyrosinase inhibitors. Bioorg Med Chem Lett 22:7451–7455
Chang TS (2009) An updated review of tyrosinase inhibitors. Int J Mol Sci 10:2440–2475
Si YX, Yin SJ, Park D et al (2011) Tyrosinase inhibition by isophthalic acid: kinetics and computational simulation. Int J Biol Macromol 48:700–704
Plenge T, Dillinger R, Santagostini L et al (2003) Catecholate adducts of binuclear copper complexes modelling the type 3 copper active site: spectroscopic characterization and relevance to the tyrosinase reaction. Z Anorg Allg Chem 629:2258–2265
Espin JC, Wichers HJ (2001) Effect of captopril on mushroom tyrosinase activity in vitro. Biochim Biophysica Acta 1544:289–300
Kim D, Park J, Kim J, Han C, Yoon J, Kim N, Seo J, Lee C (2006) Flavonoids as mushroom tyrosinase inhibitors: a fluorescence quenching study. J Agric Food Chem 54:935–941
Ismaya WT, Rozeboom HJ, Weijn A et al (2011) Crystal structure of Agaricus bisporus mushroom tyrosinase: identity of the tetramer subunits and interaction with tropolone. Biochem 50:5477–5486
Chen XX, Zhang J, Chai WM et al (2013) Reversible and competitive inhibitory kinetics of amoxicillin on mushroom tyrosinase. Int J Biol Macromol 62:726–733
Eftink MR, Ghiron CA (1981) Fluorescence quenching studies with proteins. Anal Biochem 114:199–227
Zhao Y, Cao Y, Han FM et al (2008) Study on the interaction between cinnamic acid and human serum albumin by fluorescence quenching method. Spectroscop Spectr Anal 28:904–907
Dam TK, Brewer CF (2003) Carbohydrate-lectin cross-linking interactions: structural, thermodynamic, and biological studies. Method Enzymol 362:455–486
Zhang L, Zhao X, Tao GJ, Chen J, Zheng ZP (2017) Investigating the inhibitory activity and mechanism differences between norartocarpetin and luteolin for tyrosinase: a combinatory kinetic study and computational simulation analysis. Food Chem 223:40–48
Xie LP, Chen QX, Huang H, Wang HZ, Zhang RQ (2003) Inhibitory effects of some flavonoids on the activity of mushroom tyrosinase. Biochemistry (Moscow) 68:487–491
Chai WM, Wei QM, Deng WL, Zheng YL, Chen XY, Huang Q, Ouyang C, Peng YY (2019) Anti-melanogenesis properties of condensed tannins from Vigna angularis seeds with potent antioxidant and DNA damage protection activities. Food Funct 10:99–111
Chai WM, Huang Q, Lin MZ, Ouyang C, Huang WY, Wang YX, Xu KL, Feng HL (2018) Condensed tannins from longan bark as inhibitor of tyrosinase: structure, activity, and mechanism. J Agric Food Chem 66:908–917
Chai WM, Ouyang C, Huang Q, Lin MZ, Wang YX, Xu KL, Pang DD (2018) Antityrosinase and antioxidant properties of mung bean seed proanthocyanidins: novel insights into the inhibitory mechanism. Food Chem 260:27–36
Guo NH, Wang CL, Shang C, You X, Zhang LY, Liu WB (2018) Integrated study of the mechanism of tyrosinase inhibition by baicalein using kinetic, multispectroscopic and computational simulation analyses. Int J Biol Macromol 118:57–68
Fan MH, Zhang GW, Hu X, Xu XM, Gong DM (2017) Quercetin as a tyrosinase inhibitor: inhibitory activity, conformational change and mechanism. Food Res Int 100:226–233
Goldfeder M, Kanteev M, Fishman A (2015) Structure-function correlations in tyrosinases. Protein Sci 24:1360–1369
Lin MZ, Chai WM, Zheng YL, Huang Q, Ou-Yang C (2019) Inhibitory kinetics and mechanism of rifampicin on α-glucosidase: insights from spectroscopic and molecular docking analyses. Int J Biol Macromol 122:1244–1252
Lin MZ, Chai WM, Ou-Yang C, Huang Q, Xu XH, Peng YY (2018) Antityrosinase mechanism of omeprazole and its application on the preservation of fresh-cut Fuji apple. Int J Biol Macromol 117:538–545
Penttinen L, Rutanen C, Saloheimo M, Kruus K, Rouvinen J, Hakulinen N (2018) A new crystal form of Aspergillus oryzae catechol oxidase and evaluation of copper site structures in coupled binuclear copper enzymes. PLoS ONE 13:1–7
Garcia-Jimenez A, García-Molina F, Teruel-Puche JA, Saura-Sanmartin A, Garcia-Ruiz PA, Ortiz-Lopez A, Rodríguez-López JN, Garcia-Canovas F, Munoz-Munoz J (2018) Catalysis and inhibition of tyrosinase in the presence of cinnamic acid and some of its derivatives. Int J Biol Macromol 119:548–554
Rodríguez-López JN, Ros R, Varón JR, García-Cánovas F (1993) Oxygen Michaelis constants for tyrosinase. Biochem J 293:859–866
Acknowledgement
This work was supported by Natural Science Foundation of Fujian Province (2017J01446).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Human and animal rights
There is no human participants and/or animals in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dong, X., Wang, S., Xu, L. et al. Inhibitory mechanism of Penicillin V on mushroom tyrosinase. Mol Biol Rep 47, 967–975 (2020). https://doi.org/10.1007/s11033-019-05188-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-05188-6