Abstract
In this paper, the inhibitory effects of 4-chlorocinnamaldehyde on mushroom tyrosinase were investigated. The results showed that 4-chlorocinnamaldehyde had a significant inhibitory effect on monophenolase and diphenolase in tyrosinase. 4-Chlorocinnamaldehyde could obviously prolong the lag phase of monophenolase and it decreased the steady-state rate of both monophenolase and diphenolase. The IC50 values were found to be 0.07 and 0.3 mM for monophenolase and diphenolase, respectively. The kinetic studies showed that the inhibitory mechanism of mushroom tyrosinase by 4-chlorocinnamaldehyde was the reversible competitive inhibition. The inhibition constant (K I) was measured to be 0.17 mM. The experimental research demonstrated that 4-chlorocinnamaldehyde was an effective tyrosinase inhibitor and the research results may offer the theoretical basis for designing and synthesizing safer and more efficient tyrosinase inhibitors in future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tyrosinase (1.14.18.1 EC) is an important oxidoreductase and it is an integral part of the metabolism of melanin in the body (Alijanianzadeh et al. 2012). Tyrosinase has both monophenolase and diphenolase activities and it catalyzes l-tyrosine oxidized into l-3,4-dihydroxyphenylalanine (L-DOPA), then further oxidized into dopaquinone. After a series of reactions, dopaquinone eventually becomes melanin (Chan et al. 2014; Chen et al. 2005). Tyrosinase is widely distributed in the epidermis, eyes and hair of human. Melanin as its end catalysate can protect the above parts of body from UV damage so it has a certain positive significance to the human body. But excessive deposition of melanin can lead to freckles, brown spots, and other pigmentation disorders (Khan 2007; Xing et al. 2016). In addition, tyrosinase also contributes to the enzymatic browning of vegetables and fruits (Chen et al. 2013). This undesired darkening finally leads to the unattractive appearance and nutrient loss in those fresh fruits and vegetables, which cause the reduction in economic value. Therefore, finding new safe and efficient tyrosinase inhibitors is of great social and economic significance. Due to the decrease in melanization, tyrosinase inhibitors have a wide range of application value, not only applied to food preservation, but also used in medicine and cosmetics whitening agent (Georgiev et al. 2012).
Many researchers have been searching for more efficient tyrosinase inhibitors. For example, 4-chlorosalicylic acid, α-cyano-4-hydroxycinnamic acid and some novel 4-hydroxybenzaldehyde derivatives have been discovered as tyrosinase inhibitors (Han et al. 2008; Qiu et al. 2009; Yi et al. 2010). Although there are many inhibitors in previous studies, inhibition efficiency not high enough or biological toxicity always making it difficult for their practical applications (Ashraf et al. 2015). The two characteristics have become the new driving force for the later research. The purpose of this study is to find novel efficient tyrosinase inhibitor in order to develop it as new potent food preservatives or cosmetic additives in future.
Materials and methods
Reagents and instruments
Mushroom tyrosinase (EC 1.14.18.1), 4-chlorocinnamaldehyde (purity ˃ 96%), l-tyrosine (purity ˃ 98.5%) and L-DOPA (purity ˃ 99%) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Dimethyl sulfoxide (DMSO), sodium dihydrogen phosphate and disodium hydrogen phosphate were of analytical grade. The deionized water was used throughout the all experiments. The absorbance of the product was measured by a Beckman UV-650 spectrophotometer.
Enzyme assay
Method for testing is based on absorbance measurement. Dopachrome as the tyrosinase catalytic oxidation product has maximum absorption at wavelength of 475 nm and its extinction coefficient is 3700 M−1cm−1. One unit of enzymatic activity can lead to dopachrome absorbance at the wavelength of 475 nm increased by 0.001 per min (Wang et al. 2004). This effective method has been widely used in previous studies (Chai et al. 2013; Zhu et al. 2011). According to this method, l-tyrosine and L-DOPA were designated as substrates in monophenolase and diphenolase assay. The mixed solution contained 2.8 mL substrate solution, 0.1 mL 4-chlorocinnamaldehyde solution and 0.1 mL tyrosinase aqueous solution. NaH2PO4–Na2HPO4 buffer (50 mM, pH 6.8) was used to dissolve the substrate. 4-Chlorocinnamaldehyde added in the reaction solution was dissolved in DMSO solution. In the experiment of monophenolase activity testing, the concentration of tyrosinase (570 U/mg) remained at 333.3 μg/mL, and it was turned to be 33.3 μg/mL in the diphenolase testing. The final concentration of the substrates was 0.5 mM and the temperature was kept at 30 °C (Hu et al. 2016). The enzyme activity could be calculated according to the absorbance curve of enzyme catalyzed product at 475 nm.
Determination of the inhibitory pattern
Generally, enzyme inhibitors are classified into two types, reversible and irreversible. To further explore the inhibitory pattern of 4-chlorocinnamaldehyde, a new measurement system had been established. The substrate (L-DOPA) concentration was maintained at 0.5 mm, while the tyrosinase amount was changed. Under each tyrosinase amount, different concentrations of 4-chlorocinnamaldehyde were added in the reaction solution to observe the effect of 4-chlorocinnamaldehyde on the relationship between the catalytic reaction rate and the amount of tyrosinase (Xie et al. 2016).
Determination of inhibitory type and constant
In this test system, the enzymatic activity was measured by maintaining concentration of tyrosinase at 33.3 μg/mL and changing substrate concentration (L-DOPA) from 0.25 to 1.0 mM. The inhibitory types of 4-chlorocinnamaldehyde on tyrosinase activity were determined by analyzing the change of the kinetic parameters from Lineweaver–Burk double reciprocal plots (Liu et al. 2015). By secondary plot of the intercept (1/V max) or slope (Km/V max) versus the concentrations of inhibitor, the inhibition constant was obtained from intersection point of straight line and the abscissa axis (Bao et al. 2010).
Results and discussion
Inhibition on tyrosinase
To investigate inhibition of 4-chlorocinnamaldehyde to tyrosinase deeply, the effects of 4-chlorocinnamaldehyde on the monophenolase and diphenolase activities were individually studied.
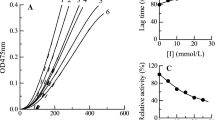
For monophenolase, the absorbance curve of the enzyme catalyzed product was shown in Fig. 1. As was shown in the graph, there was a marked lag phase in the enzymatic reaction of monophenolase. After lag period, the reaction reached stable state. By pushing forward the linear part of the curve, its intersection with the X-axis represented the lag time (Jiménez et al. 2001). The slope of the linear part could be used to test the steady-state rate of monophenolase. It was found that the lag time increased with the increase of 4-chlorocinnamaldehyde amount, but the steady-state rate decreased with the increase of inhibitor concentration (Figs 2 and 3). The IC50 value of 4-chlorocinnamaldehyde to monophenolase was measured to be 0.07 mM.
The inhibition of 4-chlorocinnamaldehyde on diphenolase was investigated with L-DOPA as substrate. When L-DOPA was added into assay medium, the reaction immediately reached a steady-state. The stable activity of diphenolase decreased with increasing the inhibitor concentration (Fig. 4), which suggested that 4-chlorocinnamaldehyde was a concentration-dependent inhibitor. The IC50 value of 4-chlorocinnamaldehyde to diphenolase was measured to be 0.3 mM.
Inhibitory pattern of 4-chlorocinnamaldehyde on tyrosinase
The relationship between the activity and quantity of tyrosinase was studied by changing inhibitor concentrations. With the increasing 4-chlorocinnamaldehyde concentration, the slope of the straight line decreased (Fig. 5), which revealed that 4-chlorocinnamaldehyde inhibited tyrosinase in a reversible way. Adding 4-chlorocinnamaldehyde couldn’t reduce the amount of tyrosinase, but just inhibited its activity.
Inhibitory type and constant of 4-chlorocinnamaldehyde on tyrosinase
If the inhibitor combines only with the free enzyme, it will be the competitive inhibitor; if the inhibitor binds only with the enzyme-substrate complex, it will belong to the noncompetitive inhibitor; if the inhibitor not only combines with free enzyme but also binds with enzyme-substrate complex, it will be classified as the mixed-type inhibitor.
The changes of kinetic parameters were studied in reaction catalyzed by tyrosinase. As shown in Fig. 6a, all straight lines intersected at Y-axis. With the increasing concentrations of inhibitor, the value of Vm remained unchanged, but the value of Km increased. The experimental results strongly suggested that 4-chlorocinnamaldehyde was a competitive inhibitor of tyrosinase (Radhakrishnan et al. 2015). 4-Chlorocinnamaldehyde could compete with the substrate for the active site of tyrosinase. The value of K I was obtained from Fig. 6b to be 0.17 mM.
Conclusions
The inhibition of tyrosinase by 4-chlorocinnamaldehyde was studied in this work. There are both monophenolase and diphenolase activity in tyrosinase. According to the results of the research, 4-chlorocinnamaldehyde had a significant inhibitory effect to two enzymatic activities of tyrosinase. For monophenolase, 4-chlorocinnamaldehyde could obviously prolong its lag phase and decrease its steady-state rate. The inhibitor had obvious effect on diphenolase activity too. The IC50 values of inhibitor to two enzymatic activities were measured to be 0.07 and 0.3 mM, respectively. 4-Chlorocinnamaldehyde could compete with the substrate for the active site of tyrosinase and the inhibition process was reversible. And the K I value was found to be 0.17 mM. 4-Chlorocinnamaldehyde has an aldehyde group and a chloride group in para-position of benzene ring. The amino group of tyrosinase can form the Schiff base structure with the aldehyde group. Chlorine has electron attracting ability, which can increase the bare nucleus carbon, so that the nucleophilic addition reaction is more likely to occur. Therefore, the chloride group in para-position can promote the combination of 4-chlorocinnamaldehyde and tyrosinase. 4-Chlorocinnamaldehyde is a competitive inhibitor of tyrosinase. Once 4-chlorocinnamaldehyde binds with the active site of tyrosinase, the substrate will not be able to bind with the enzyme. So 4-chlorocinnamaldehyde can competitively restrain the activity of tyrosinase and the chloride group in para-position can obviously enhance the activity of inhibition.
The results of this research indicated that 4-chlorocinnamaldehyde was an effective tyrosinase inhibitor, and its practical application in food and cosmetics fields still need to be further studied.
References
Alijanianzadeh M, Saboury AA, Ganjali MR, Hadi-Alijanvand H, Moosavi-Movahedi AA (2012) The inhibitory effect of ethylenediamine on mushroom tyrosinase. Int J Biol Macromol 50:573–577
Ashraf Z, Rafiq M, Seo S-Y, Kwon K-S, Babar MM, Zaidi N-u-SS (2015) Kinetic and in silico studies of novel hydroxy-based thymol analogues as inhibitors of mushroom tyrosinase. Eur J Med Chem 98:203–211
Bao K, Dai Y, Zhu Z-B, Tu F-J, Zhang W-G, Yao X-S (2010) Design and synthesis of biphenyl derivatives as mushroom tyrosinase inhibitors. Bioorgan Med Chem 18:6708–6714
Chai W-M, Liu X, Hu Y-H, Feng H-L, Jia Y-L, Guo Y-J, Zhou H-T, Chen Q-X (2013) Antityrosinase and antimicrobial activities of furfuryl alcohol, furfural and furoic acid. Int J Biol Macromol 57:151–155
Chan C-F, Lai S-T, Guo Y-C, Chen M-J (2014) Inhibitory effects of novel synthetic methimazole derivatives on mushroom tyrosinase and melanogenesis. Bioorgan Med Chem 22:2809–2815
Chen Q-X, Song K-K, Qiu L, Liu X-D, Huang H, Guo H-Y (2005) Inhibitory effects on mushroom tyrosinase by p-alkoxybenzoic acids. Food Chem 91:269–274
Chen XX, Zhang J, Chai WM, Feng HL, Xiang ZH, Shen DY, Chen QX (2013) Reversible and competitive inhibitory kinetics of amoxicillin on mushroom tyrosinase. Int J Biol Macromol 62:726–733
Georgiev L, Chochkova M, Totseva I, Seizova K, Marinova E, Ivanova G, Ninova M, Najdenski H, Milkova T (2012) Anti-tyrosinase, antioxidant and antimicrobial activities of hydroxycinnamoylamides. Med Chem Res 22:4173–4182
Han P, Chen C-Q, Zhang C-L, Song K-K, Zhou H-T, Chen Q-X (2008) Inhibitory effects of 4-chlorosalicylic acid on mushroom tyrosinase and its antimicrobial activities. Food Chem 107:797–803
Hu Y-H, Zhuang J-X, Yu F, Cui Y, Yu W-W, Yan C-L, Chen Q-X (2016) Inhibitory effects of cefotaxime on the activity of mushroom tyrosinase. J Biosci Bioeng 121(4):385–389
Jiménez M, Chazarra S, Escribano J, Cabanes J, García-Carmona F (2001) Competitive inhibition of mushroom tyrosinase by 4-substituted benzaldehydes. J Agric Food Chem 49:4060–4063
Khan MTH (2007) Molecular design of tyrosinase inhibitors: a critical review of promising novel inhibitors from synthetic origins. Pure Appl Chem 79(12):2277–2295
Liu X, Jia Y-l, Chen J-w, Liang G, Guo H-y, Hu Y-h, Shi Y, Zhou H-T, Chen Q-X (2015) Inhibition effects of benzylideneacetone, benzylacetone, and 4-phenyl-2-butanol on the activity of mushroom tyrosinase. J Biosci Bioeng 119(3):275–279
Qiu L, Chen Q-H, Zhuang J-X, Zhong X, Zhou J-J, Guo Y-J, Chen Q-X (2009) Inhibitory effects of α-cyano-4-hydroxycinnamic acid on the activity of mushroom tyrosinase. Food Chem 112:609–613
Radhakrishnan S, Shimmon R, Conn C, Baker A (2015) Integrated kinetic studies and computational analysis on naphthyl chalcones as mushroom tyrosinase inhibitors. Bioorg Med Chem Lett 25:4085–4091
Wang Q, Shi Y, Song K-K, Guo H-Y, Qiu L, Chen Q-X (2004) Inhibitory effects of 4-halobenzoic acids on the diphenolase and monophenolase activity of mushroom tyrosinase. Protein J 23(5):303–308
Xie J, Dong H, Yu Y, Cao S (2016) Inhibitory effect of synthetic aromatic heterocycle thiosemicarbazone derivatives on mushroom tyrosinase: insights from fluorescence, 1H NMR titration and molecular docking studies. Food Chem 190:709–716
Xing R, Wang F, Dong L, Zheng AP, Wang L, Su W-J, Lin T (2016) Inhibitory effects of Na7PMo11CuO40 on mushroom tyrosinase and melanin formation and its antimicrobial activities. Food Chem 197:205–211
Yi W, Cao R, Peng W, Wen H, Yan Q, Zhou B, Ma L, Song H (2010) Synthesis and biological evaluation of novel 4-hydroxybenzaldehyde derivatives as tyrosinase inhibitors. Eur J Med Chem 45:639–646
Zhu Y-J, Zhou H-T, Hu Y-H, Tang J-Y, Su M-X, Guo Y-J, Chen Q-X, Liu B (2011) Antityrosinase and antimicrobial activities of 2-phenylethanol,2-phenylacetaldehyde and 2-phenylacetic acid. Food Chem 124:298–302
Acknowledgements
This work was supported by the Innovation Team of Scientific Research Platform in Anhui Universities, the Key Laboratory of Bioresource Protection and Utilization of Anhui Province, the Key Laboratory of Biotic Environment and Ecological Safety of Anhui Province.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Si, H., Wang, X., Li, L. et al. Inhibitory effects of 4-chlorocinnamaldehyde on the activity of mushroom tyrosinase. Med Chem Res 26, 1377–1381 (2017). https://doi.org/10.1007/s00044-017-1861-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1861-6