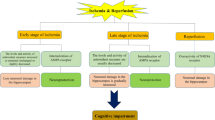
Abstract
Several mechanisms are involved in the loss of cellular integrity and tissue destructions in various brain regions during ischemic insult. The affected brain employs various self-repair mechanisms during the poststroke recovery. Therefore, the current study involves time course changes in different brain regions following ischemia in terms of inflammation, oxidative stress and apoptosis for which a bilateral common carotid arteries occlusion model was chosen. The development of oxidative stress was seen with a marked increase in ROS and NO levels with concomitant decrease in GSH levels and also the activities of anti-oxidant enzymes. These alterations were accompanied with decreased levels of neurotransmitters and motor and cognitive deficits at various time points. Increased expressions of various pro-inflammatory cytokines and a decline in BDNF levels in hippocampal regions on 7th day post ischemia, suggesting their role in its pathogenesis. The restoration of BDNF and neurotransmitter levels along with significant decline in inflammatory cytokine levels 14th day onwards following ischemia in hippocampus suggested poststroke recovery. The extent of neuronal damage was found to be increased significantly on 7th day post ischemia as indicated by TUNEL assay and hematoxylin and eosin staining depicting enhanced number of pyknotic neurons in cortical and hippocampal regions. Cortical regions of the ischemic brains were severely affected while hippocampal regions showed significant poststroke recovery, which might attributed to the normalization of BDNF and pro-inflammatory cytokine levels. In conclusion, the present study established the central role of BDNF and pro-inflammatory cytokines in the poststroke recovery. Also, the cortical and hippocampal regions were found to be more susceptible for ischemic injury. As our results indicated, full recovery after ischemic injury in different brain regions was not achieved, therefore further studies with long-term recovery time are required to be conducted.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is the third leading cause of death worldwide [1], which results either from occlusion of blood supply by a thrombus or by subsequent bleeding from ruptured vessel in a certain region of the brain. It can be classified as ischemic or haemorrhagic stroke. In transient global ischemia, there is a temporary loss of blood supply to whole brain resulting in the death of specific neuronal cell population [2]. Several factors such as impaired blood flow, free radical generation, energy failure, impaired calcium homeostasis, vascular leakages and inflammatory responses contribute to the ischemic insult. These effects are non-uniform in both neurons and glial cells, as well as, in different regions of the brain [3].
During restoration, a sudden flow of oxygenated blood leads to overproduction of Reactive Oxygen Species (ROS) such as superoxide anions, hydrogen peroxides and hydroxyl free radicals which are involved in the initiation of cell death [4]. The role of oxidative stress becomes more pronounced due to sudden increase in oxygen levels and consequent ROS/RNS (Reactive Nitrogen Species) production leads to enormous tissue damage [5]. Several studies have also linked ROS production during ischemia to neuronal cell death [5, 6].
Along with ROS, common inflammatory cascade is a major contributing factor for the development of stroke [7]. Several inflammatory cytokines such as IL-1 (Interleukin-1), IL-6 (Interleukin-6) and TNF-α (Tumour Necrosis Factor) are the common biomarkers of this pathway. This inflammatory insult leads to the development of atherosclerosis, which is mostly associated with the occurrence of ischemic stroke [8]. Also, the amino acid neurotransmission plays a critical role in the pathology of ischemic stroke citing the importance of various neurotransmitters and neurogenesis during poststroke recovery [9, 10]. Various monoamines such as Dopamine (DA), Norepinehprine (NE) and Serotonin (5 HT) are critically essential for the normal functioning of nervous system [11]. Similarly, neurotrophins such as Brain Derived Neurotrophic Factor (BDNF) plays a major role in the maintenance of neuronal plasticity. Alterations in the levels of these biogenic amines and neurotrophins can cause significant neuronal insults [12, 13]. Majority of the studies have discussed the role of these factors in context with the occurrence of ischemic stroke but the studies establishing their role in the poststroke recovery are scarce.
Mergenthaler et al. [14] showed that the various brain regions have different threshold for ischemic damage. There are many neurons which resist the ischemic environment while some selective neurons are vulnerable to these hypoxic conditions. For example, specific populations of neurons such as pyramidal neurons in the CA1 (Cornu Ammonis) region of hippocampus are selectively more vulnerable to ischemia [15], whereas CA3 and dentate granule neurons are resistant [2]. The underlying mechanism of this differential vulnerability and resistance of various neuronal population to ischemic injury is not well understood [16] and still remains a debated topic in studies which are focused on the variation in local vasculature [17], differential superoxide anion production [18] and neuronal-glial population ratio [16]. The other brain regions that are vulnerable to ischemia are cortex [19] and cerebellum [20]. Particularly, in cerebellum, some reports have suggested cerebellar granule cell layers are more vulnerable to hypoxia [21]. Considering the vulnerability of these regions cortical, hippocampal and cerebellar regions of the rat brain were observed in the present study.
Along with vulnerability in the selective neuronal cell population, the damage following ischemia depends upon the different time windows following ischemia. Ouyang [22] also showed delayed neuronal death in CA1 of hippocampus at day 2–4 following ischemia which suggests that the time window is a very crucial factor following ischemia. In previous studies, the sequential alterations in the hippocampus following ischemia were well established only for small time durations i.e. from few hours to few days but much remains to be studied in other areas of brain. Also, a long duration study is required to establish the roles of various markers of ischemic stroke during poststroke recovery [16]. Therefore, the current study was designed to explore the susceptibility and resistance of various brain regions at day 1,7,14 and 21 following ischemic reperfusion injury using bilateral common carotid arteries occlusion model to induce transient global ischemia. This model was first established in dessert (gerbil) rats in 1984 by Kirino et al. [23], which resulted in pronounced injury in CA1 layer of hippocampal region in gerbil rats. This model was selected for the present study because a specific population of neurons was affected, which could be studied following ischemic insult and also, a therapeutic window was available to try-out various neuroprotection strategies to ameliorate or delay cerebral injury by interrupting the biochemical and cellular events.
Materials and methods
Chemicals
All the chemicals were of analytical grade. Chloral hydrate, 2′7′-dichlorofluoresceine diacetate (DCFH-DA), dopamine, nor-epinephrine and serotonin standards and anti-BDNF antibody were purchased from Sigma Chemical Co. (St. Louis, MO, USA). TTC (triphenyltetrazolium chloride) was purchased from Merck (Mumbai, India). DTNB (dithiobis2-nitrobenzoic acid) and NBT (nitroblue tetrazolium) were purchased from Sisco Research Laboratories Pvt. Ltd. (Mumbai, India). TUNEL kit was purchased from Calbiochem, USA.
Experimental animals
Male wistar rats (250–300 g) were procured from the Central Animal House, Panjab University, Chandigarh, India (45/GO/ReBi/s/99/CPCSEA). The animals (n = 5–7) were housed in polypropylene cages under ambient conditions of humidity and temp and acclimatized for 1 week. They were provided with food and water ad libtium throughout the experimental period. All the protocols were done in accordance with the ethical guidelines given by the Institutional Animal Ethics Committee (IAEC) of Panjab University, Chandigarh. The animals were divided into two groups, sham and Transient global cerebral ischemia (TGCI) and sacrificed at four different time intervals i.e. at day 1, 7, 14 and 21 after ischemic/reperfusion injury.
Induction of transient global ischemia in rats
Transient global cerebral ischemia was induced by the modified method of Jingtao [24]. For the surgical procedure, the rats were anesthetized by 10% chloral hydrate (300 mg/kg body weight, i.p.) and were fixed in supine position and middle incision was made in the neck. Both common carotid arteries were exposed and separated carefully from the vagus nerve. Ischemic insult was induced by occlusion of both the arteries by an occlusion clamp for a period of 15 min followed by recirculation by removing the clamps. The animals which were subjected to the same surgery without occlusion of both arteries served as sham. Animals were kept at approx. at 37 °C (by using heating pad) to recover from anaesthesia.
Infarct area measurement
Infarct area was estimated by the method of Himori [25]. The rat brains were extracted and were kept at – 20 °C for 5–10 min. The coronal slices (2 mm thickness) were obtained and incubated in 0.5% (w/v) TTC (triphenyltetrazolium chloride) at 37 °C for 30 min in dark. TTC reacts with metabolically active area and gives deep red or orange colour while inactive areas were depicted as non-stained. Images were captured by a digital camera and analysed by image J software (NIH).
Behavioural alterations
Behavioural tests were performed at day 0 (before surgery) and on 7th, 14th and 21st day post-surgery.
Morris water maze (MWM)
This test was performed for the spatial learning and memory [26]. MWM consists of a circular pool (140 cm in diameter and 50 cm in height) filled with water and is divided into four quadrants. Each animal was given four trials per day to reach a visible platform (with different start points) for consecutive 8–10 days until all the animals reached in ≤ 10 s (escape latency). After trial, escape latency (that is the time to reach the platform) was calculated at various time intervals.
Elevated plus maze (EPM)
This test was performed for the spatial short term memory [27]. EPM consists of two open and two closed arms which are elevated from the floor (50 cm). It is a two day procedure. On day one, animals were placed in one of the open arms and the time to enter in one of the closed arm was recorded. After 120 s, if the animal did not enter any of the arm, it was guided to the closed arm and called transfer latency (TL) assigned as 120 s. After 24 h, same procedure was followed and the TL was recorded. The percentage transfer latency was calculated by the following formula.
Actophotometer
The total locomotor activity was measured with the help of digital actophotometer (IMCORP, India) [28, 29]. Each animal was first habituated in the actophotometer chamber for 3–5 min on the given training day. Then counts were recorded for 180 s according to standard protocols [29]. These counts were called the rearing and ambulation movements of each animal.
Neurotransmitter level
The different brain regions (cortex, hippocampus and cerebellum) were separated and homogenised in buffer containing 0.1 M perchloric acid followed by centrifugation at 12,000×g for 5 min. The supernatant was taken and filtered through 0.25 µm pore size filter. The levels of dopamine, nor-epinephrine and serotonin were analysed by a high performance liquid chromatography (HPLC), attached with electrochemical detector (ECD) following the method of Church [30] at various time intervals. The area under the peaks was analysed with the help of Empower software.
Biochemical estimations
Preparation of sample
The brain was dissected and the different brain regions namely cortex, hippocampus and cerebellum were separated. A 10% (W/V) tissue homogenate was made in PBS (phosphate buffer saline; pH 7.4). The homogenate was then centrifuged at 10,000×g for 30 min and the supernatant was collected, i.e. post mitochondrial fraction (PMF).
Protein estimation
The protein contents in various sections of brain samples were estimated by the method of Lowry [31].
Reactive oxygen species (ROS)
Intracellular ROS levels were estimated by method of Best [32]. It was based on deacetylation of 2′7′-dichlorofluoresceine diacetate (DCFH-DA) following ROS mediated oxidation which further gives a fluorescent product, i.e. DCF. The fluorescence measured with the help of a Perkin Elmer spectrofluorimeter at an excitation/emission wavelength of 488/525 nm, respectively. The units were expressed as AFU/mg of protein where AFU: Arbitrary fluorescence units.
Levels of nitric oxide (NO)
NO was measured by the method of Raddassi [33], where the stable product, nitrite reacted with Griess reagent. This resulted in purple color azo dye which has absorbance at 540 nm on ELISA reader. The NO content was expressed as nmoles of nitrite/mg of protein.
Reduced glutathione (GSH)
GSH levels were estimated with the method of Ellman [34]. It was based upon the reduction of DTNB (dithiobis2-nitrobenzoic acid) with free –SH groups to form thio-2-nitrobenzoic acid which was yellow in color and absorbance was read at 412 nm. The levels of GSH were calculated by a standard plot formed by using GSH as standard and the results were expressed as μmoles of GSH/mg of protein.
Superoxide dismutase activity (SOD)
SOD activity was measured following the method of Kono [35]. It was based upon the inhibitory effect of SOD on the reduction of NBT (nitroblue tetrazolium) dye by superoxide anion generated from hydroxylamine hydrochloride. The change in kinetics was observed at wavelength of 560 nm for 3 min. Units were calculated as 50% inhibitory concentration of SOD and the results were expressed as the units/mg of protein.
Catalase activity (CAT)
Catalase activity was estimated following the method of Luck [36]. The change in absorbance of H2O2 buffer after adding the enzyme was observed and the activity was calculated using extinction coefficient of 39.4 mM−1 cm−1. The catalase units were expressed as µmoles H2O2 decomposed/min/mg of protein.
Histopathology
Histopathology was done according to the procedure as described by Pearse [37]. Animals were anesthetised and were perfused intracardially with normal saline (0.9% NaCl). Then brain was dissected and fixed in 10% formaldehyde (V/V) for 4–5 days till it get hardened. The tissue was then embedded in the paraffin blocks and sections (5 μm thickness) were cut with a hand microtome. Qualitative analysis was done by Hematoxylin and eosin staining [37].
Reverse transcriptase polymerase chain reaction (RT-PCR)
Total RNA was isolated from different brain regions with TRI-reagent. For the RT-PCR analysis primers for the following genes: IL-1α, IL-1β, TNF-α, BDNF and β-actin were designed either on NCBI or their sequences were obtained from literature, and custom synthesized by Sigma Aldrich (USA). The primers designed for various genes are mentioned in Table 1. The mRNA expression was evaluated by RT-PCR using the standard protocol described in one step RT-PCR kit (Invitrogen). PCR products were separated on 1.2% agarose gels. Densitometric analysis of bands was done by using the Image J software (NIH). The densitometric values were first normalized with β-actin of the same sample, and then the relative differences between the sham and treatment groups were calculated and expressed as relative change.
TUNEL assay
The procedure was carried out according to the standard procedure provided in the TUNEL kit (cat#QIA33; Calbiochem, USA). This assay is based on the labelling of DNA nicks by terminal deoxynucleotidyl transferase, an enzyme that catalyses the addition of dUTPs labelled with a marker. Slides from each time period groups were used to estimate the number of TUNEL positive nuclei. Cell counting was done using Image J software by counting the cells in different coronal sections.
Immunohistochemistry for BDNF
Paraffin sectioning of the tissue preparation was done with the same procedure as that for H&E staining. These were dewaxed in xylene and then hydrated through a graded series of alcohol. For antigen retrieval, slides were incubated in sodium citrate buffer (pH 6.0). Blocking was done using 2% BSA in tris-buffered saline (TBS) for 30 min in a moist chamber. Sections were incubated with primary antibody against BDNF (1:1000) (Sigma-Aldrich, St. Louis, USA) in 1% BSA for 2 h at 37 °C. Briefly, the slides were washing twice with TBST (TBS containing 0.05% Tween-20), and incubated with the secondary antibody conjugated with alkaline phosphatase in 1% BSA for 2 h at 37 °C. Color development was done using NBT/BCIP solution (Genei, Bangalore, India). Eosin was used to counter stain the NBT/BCIP treated slides.
Statistical analysis
Data was expressed as mean ± standard deviation (SD) (n = 5–7) and the results were subjected to one way analysis of variance (ANOVA) followed by the LSD (post hoc comparison of means) test using SPSS (14.0 for window evaluation version). Values corresponding to p ≤ 0.05 were considered statistically significant.
Results
Infarct area measurement
The infarct is the marker of injury in ischemic stroke and the non-stained area depicts the damage. The infarct area was estimated at various time intervals following ischemia. The percentage infarct area was found to be higher at day 1(p ≤ 0.001) and 7 (p ≤ 0.001) as compared to other time points following ischemia (Fig. 1).
Infarct visualised by TTC staining at different time intervals (a–e). The histograms showed the percentage infarct area at different time intervals at the successive coronal sections of the brain (f). The data represented was the mean ± SD (n = 7). Statistical significance: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 significant when compared with sham
Behavioural analysis
Different behavioural tests were done to analyse the cognitive and motor impairments.
Effects on escape latency in Morris water maze (MWM)
This test was performed to analyse the spatial learning and memory, i.e. lesser the time animals took to reach the platform, better the cognition. Following ischemia, there was a significant increase in escape latency (time to reach the platform) at day 7 (2.43-fold), 14 (2.28-fold) and 21 (1.74-fold) as compared to sham (Fig. 2a).
Behavioural parameters at different time intervals: plus maze (a); Morris water maze (b); total locomotor activity by actophotometer (c); day 0, 1, 7, 14 and 21. The “day 0” means the day before surgery was performed. Statistical significance: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 significant when compared with sham. Each value was mean ± SD (n = 7)
Effects on percentage escape latency in elevated plus maze (EPM)
This test was performed to analyse the short term memory on the basis of transfer latency. The term transfer latency indicated the percentage of memory retention in 24 h. A significant decrease was observed in percentage transfer latency at day 7 (94.2% decrease) and day 14 (38.55% decrease) as compared to the sham following ischemic insult (Fig. 2b).
Effect on total locomotor activity by actophotometer
It was performed to evaluate the rearing and ambulation movements of the animal in 180 s. There was a significant reduction in counts/180 s at day 7 (70.8% decrease), 14 (58.8% decrease) and 21(47.41% decrease) day post-surgery as compared to sham but the counts were minimum at day 7 (Fig. 2c).
Alterations in the level of neurotransmitters
The dopamine (DA) levels were declined significantly in cortex and hippocampus at all the time intervals following ischemia but the maximum decline was observed at day 7 (68.98% and 80.21% decrease, respectively) as compared to sham (Fig. 3a, b). The norepinephrine (NE) levels were reduced at day 7, 14 and 21. The lowest levels of serotonin were observed at day 7 (56.4% decrease in cortex and 96.7% decrease in hippocampus) as compared to sham (Fig. 3a, b). The levels of dopamine were altered in cerebellum at day 7 but other neurotransmitters remained unaltered in cerebellum (Fig. 3c).
Alterations in neurotransmitter levels: dopamine (DA), norepinehprine (NE) and serotonin (5 HT) in cortex (a), hippocampus (b) and cerebellum (c) at day 1, 7, 14 and 21. Statistical significance: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 significant when compared with sham. Data represented was mean ± SD (n = 5)
Oxidative stress markers
Oxidative stress was evaluated by the levels of reactive oxygen species, nitric oxide, reduced glutathione and the enzyme activity of catalase and SOD.
Elevation of ROS levels
In cortex, hippocampus and cerebellum, intracellular ROS levels were measured at different time intervals following ischemia. It was found that in cortex and hippocampus, ROS levels elevated significantly at all the time points, i.e. at day 1, 7, 14 and 21 following ischemia as compared to sham. There was no significant change in ROS levels in cerebellum (Table 2).
Nitric oxide levels
Nitric oxide (NO) levels were found to be increased at day 7 following ischemia as compared to sham (Table 2). Levels were significantly high in cortex and hippocampus at day 1, 7 and 14 following ischemia with maximum levels at day 7 (36% and 53.12% increase, respectively in cortex and hippocampus) as compared to sham (Table 2). But in cerebellum, the change was significant at day 7 (18.36% increase) as compared to sham (Table 2).
Reduced glutathione
The reduced glutathione (GSH) levels were depleted significantly in cortex (55.17% decrease) and hippocampus (49.21% decrease) at day 7 following ischemia. The levels of GSH remain unaltered in cerebellum at all time frames following injury as compared to sham (Table 2).
Superoxide dismutase (SOD) activity
In cortex and hippocampus, the SOD activity was found to decrease appreciably at day 7 and 14 as compared to sham (Table 2). In cerebellum, the enzyme activity was not altered significantly as compared to sham (Table 2).
Catalase activity
Catalase activity was found to be significantly decreased in cortex and hippocampus at day 7 (42.9% and 31.11% decrease, respectively) following ischemia as compared to sham (Table 2). In cerebellum, there was no significant change in activity as compared to sham (Table 2).
Histopathological alterations
Neuronal damage was observed in 3rd, 4th, 5th layer of cortex, pyramidal cells of CA1 region of hippocampus and granular cells of cerebellum (Fig. 4). The pyknotic and darkly stained neurons were observed at day 7 and 14 in various regions of brain but histopathological alterations were less at day 1 and day 21 following ischemia as shown in Figs. 4 and 5. The neuronal population of dentate gyrus were spared of the hypoxic damage as shown in Fig. 5. The damage was evident in cortical layers and CA1 region of hippocampus as compared to cerebellum.
Hematoxylin and eosin staining in cortex, hippocampus and cerebellum. a–e Represent the cortical sections; f–j represents the hippocampus and k–o represent the cerebellum at day 1, 7, 14 and 21. The pyknotic and darkly stained neurons were marked by open arrows which are clear indicators of inflammation and oxidative stress. The bar graphs (p) represents the number of pyknotic cells in various regions at different intervals of time. Statistical significance: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 significant when compared with sham. Data represented was mean ± SD (n = 5)
Hematoxylin and eosin stating in dentate gyrus of hippocampus. a–e Represents the dentate gyrus at day 1, 7, 14 and 21. The bar graphs (f) represents the number of pyknotic neurons at different intervals of time. Statistical significance: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 significant when compared with the sham. Data represented was mean ± SD (n = 5)
Apoptotic study
TUNEL assay was used to detect DNA fragmentation. TUNEL positive cells were counted in cortex, hippocampus and cerebellum at day 1, 7, 14 and 21. The numbers of TUNEL positive cells were found to be maximum at day 7 in various brain regions as shown in Fig. 6. The change in number of TUNEL positive cells was higher in hippocampus and cortex followed by cerebellum.
The change in TUNEL positive cells after recirculation: all the sections are at ×400 magnification. a–e Showing the cortical regions; f–j represents the CA1 region of hippocampus; k–o represents the cerebellum at day 1, 7, 14 and 21. The TUNEL positive cells were stained brown by DAB as indicated by arrows. Counterstaining is done with methyl green. The bar graphs (p) represents the number of TUNEL positive cells in various regions at different intervals of time. Statistical significance: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 significant when compared with sham. Data represented was mean ± SD (n = 5)
The mRNA expression of cytokines
In cortex and hippocampus, the mRNA expressions of various cytokines (IL-1α, IL-1β and TNF-α) were found to be maximum at day 7 and 14 as compared to sham. In hippocampus, the expressions remained elevated till day 21 following ischemia as shown in Fig. 7. In cerebellum, the gene expression of IL-1β was altered significantly at day 1, while the expression of IL-1α and TNF-α remain unaltered at all-time points following ischemia.
The change of cytokine mRNA levels after reperfusion by RT-PCR. a Showing the mRNA expressions of IL-1α, IL-1β and TNF-α at day 1, 7, 14 and 21. b Showing histograms represent quantitative densitometry analysis (by image J) of IL-1 α, IL-1β and TNF-α which is normalised by β-actin. Statistical significance: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 significant when compared with sham. Data represented was mean ± SD (n = 5)
The mRNA expressions of BDNF in hippocampus
In dentate gyrus region of hippocampus of rat brain, neurogenesis was initiated. Neurogenesis was confirmed by an increase in both gene and protein expressions of BDNF, which were found to be increased at day 14 and 21 significantly, as shown in Fig. 8.
a Showing the protein expression BDNF by immunohistochemistry at day 1, 7, 14 and 21. The expression was seen with intensely blue coloured BDNF positive cells at ×400 magnification. b Showing the mRNA expression of BDNF and histograms represent quantitative densitometric analysis (by image J) of BDNF which is normalised by β-actin. Statistical significance: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 significant when compared with sham. Data represented was mean ± SD (n = 5)
Discussion
Various regions of brain are not only functionally discrete but also have distinct anatomical and neurochemical features which make them differently susceptible to various insults. Also, heterogeneous vascular system and differential neuronal/glial population ratio in various brain areas make it susceptible to oxidative stress [16, 38]. In previous studies, the alterations within hippocampus were well studied at very early time points but the sequential alterations at longer interval in different brain areas has not been reported and therefore being focused in the present study. Briefly, the current study was undertaken to explore the susceptibility and resistance of various regions of brain to ischemia at different time intervals, i.e. at day 1, 7, 14, 21.
At day 1 and 7, a marked increase in infarct area was observed by TTC staining [39]. This may be due to the inactivation of succinic dehydrogenases (SDH) in the ischemic area [40]. The levels of ROS and NO were significantly increased in both cortex and hippocampus at day 1 and 7 post ischemia with substantial low levels at 14 and 21 day. This is due to sudden flow of oxygenated blood into the ischemic tissue upon reperfusion which leads to the production of ROS [41, 42]. The diminution in antioxidant defence system was observed at day 7 following reperfusion injury. The glutathione levels were markedly reduced in hippocampus followed by cortex and cerebellum. Similar changes in the ischemic brains were reported by Bragin et al. in a previous study [43].
Morphological alterations were most evident on 7th day in all the three regions (cortex, hippocampus and cerebellum). Though the alterations varied in various neuronal populations such as pyramidal cells in CA1 region of hippocampus, granular cells of cerebellum and cortical neurons in 3rd, 5th and 6th layer of cortex, the hippocampal neurons were most vulnerable to ischemia. It was observed in the present study that CA1 region of hippocampus was specifically affected in hypoxia while the dentate gyrus and CA3 region remain unaffected. CA1 region of hippocampus has specific function in memory and learning. These results are in correlation with many other studies [15, 16, 44] which showed the selective neuronal vulnerability of CA1 region of hippocampus following ischemia. Thus damage in hippocampus could be responsible for the alterations in cognitive function as evident by the water maze and elevated plus maze tests. The cognitive decline was seen maximum on 7th day following ischemia and thereafter a recovery was seen both in cognitive functions as well in neuronal population following reperfusion. The neurons in cortex and cerebellum, both have an important role in sensory nerve input and output timing, sequencing and motor functions [45]. Also the cortical neurons play an important role in motor activities and can control the intensity of muscle contraction. Further, the decrease in granular cell layer of cerebellar region could be correlated with the decline of total locomotor activity in the present study. Similar findings were also reported by Hara et al. [46]. The decline in dopamine and serotonin levels (in cortex and hippocampus) was well associated with post stroke behavioural changes [47, 48]. It has been demonstrated that there is a massive release of dopamine (DA) into extracellular space within few minutes of reperfusion which is followed by a marked decline in dopamine [49]. Dopamine may also act as neurotoxin when released excessively [50]. The down regulation of nor-epinephrine levels at day 1, may also correlated to the motor dysfunction and biochemical alteration following the ischemic stroke [51].
At the onset of ischemia, the glial activation could be protective or deleterious which depends on the time of ischemic insult. Initially, an over activation may lead to the production of the cytokines resulting in inflammatory response. The inflammatory cytokines, such as IL-1α, IL-1β and TNF-α play a pivotal role in neurodegeneration and are generally associated with initiation of apoptosis [52]. Furthermore, astrocytic dysfunction may induce the over activation of glutamate receptors, which results in the aggravation of detrimental effects of ischemia/reperfusion injury. At day 14 and 21 following ischemia, a significant decline in the levels of ROS and an improvement in the antioxidant enzyme activity was observed as compared to the early time points. Also, fewer TUNEL positive apoptotic cells were observed at day 21 indicating the poststroke recovery.
Pro-inflammatory cytokines such as IL-1α and IL-1β activate microglia and lead to leukocyte infiltration. These activated microglia release various factors such as endothelial growth factor (EGF-1) in the sub-ventricular zone and sub granular zone and initiate neurogenesis [53]. Many studies suggest that after ischemic insult, neurogenesis occurs even in those areas which were not previously considered as neurogenic [54,55,56]. The reason of this neurogenesis is the activation of neurotrohic factors in subventricular and subgranular zone which resulted in stimulation of progenitor proliferation and migration of new born neurons to replace the damaged neurons. In the current study, the increased BDNF expression in hippocampal region depicted the recovery following ischemia at later stages of ischemia. Earlier studies also showed the neuroprotective role of BDNF following ischemia [57, 58]. Also, in hippocampus the inflammatory cytokines were found down regulated at day 14 but again interestingly elevated at day 21, which may be linked to repair mechanisms following ischemia [56]. While opposite changes in the BDNF levels were observed as BDNF expression was elevated on day 14 and down regulated on day 21. The down regulation of hippocampal levels of BDNF play a major role in the development of chronic stress, inflammation and depression [59]. So, decreased levels of BDNF might have led to a surge in the levels of inflammatory cytokines on day 21 in the hippocampal region of the rat brain. The increased levels of BDNF and neurotransmitters in late phase as compared to early time windows following reperfusion injury may promote recovery, further, in correlation with the improved cognitive and motor activity [58]. Interestingly, the alterations in neurochemical levels and inflammatory cytokines in the present study were observed maximum in hippocampus followed by cortex and cerebellum.
Conclusion
The cascade of ischemic injury following reperfusion was found varied temporally and regionally in term of oxidative stress and inflammation. The maximum changes in biochemical, neurochemical, histopathological parameters as well as the functional brain damage were observed in hippocampus and cortex followed by cerebellum at day 7 following ischemia. A considerable improvement in the cellular and molecular damage at day 21 of ischemic injury established the major role of neurotransmitters and BDNF in the poststroke recovery. Our results suggested a significant poststroke recovery in the ischemic animals but full recovery was still not achieved. Therefore further long-term studies are required to understand and establish the mechanisms of complete mitigation of alterations induced by ischemic stroke.
Abbreviations
- TGCI:
-
Transient global cerebral ischemia
- ROS/RNS:
-
Reactive oxygen species/reactive nitrogen species
- DA:
-
Dopamine
- NE:
-
Norepinephrine
- 5-HT:
-
Sertonin
- DTNB:
-
5,5′-Dithiobis-(2-nitrobenzoic acid)
- TTC:
-
Triphenyltetrazolium chloride
- ECD:
-
Electrochemical detector
- TL:
-
Transfer latency
- BDNF:
-
Brain derived neurotrophic factor
- LSD:
-
Least significant difference
References
Simats A, García-Berrocoso T, Montaner J (2016) Neuroinflammatory biomarkers: from stroke diagnosis and prognosis to therapy. Biochim Biophys Acta 1862:411–424. https://doi.org/10.1016/j.bbadis.2015.10.025
Woodruff TM, Thundyil J, Tang S-C et al (2011) Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol Neurodegener 6:11. https://doi.org/10.1186/1750-1326-6-11
Lee J-M, Grabb MC, Zipfel GJ, Choi DW (2000) Brain tissue responses to ischemia. J Clin Invest 106:723–731. https://doi.org/10.1172/JCI11003
Lee J-C, Won M-H (2014) Neuroprotection of antioxidant enzymes against transient global cerebral ischemia in gerbils. Anat Cell Biol 47:149–156. https://doi.org/10.5115/acb.2014.47.3.149
Kalogeris T, Baines CP, Krenz M, Korthuis RJ (2012) Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 298:229–317. https://doi.org/10.1016/B978-0-12-394309-5.00006-7
Kalogeris T, Bao Y, Korthuis RJ (2014) Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol 2:702–714. https://doi.org/10.1016/j.redox.2014.05.006
Esenwa CC, Elkind MS (2016) Inflammatory risk factors, biomarkers and associated therapy in ischaemic stroke. Nat Rev Neurol 12:594–604. https://doi.org/10.1038/nrneurol.2016.125
Kelly PJ, Murphy S, Coveney S et al (2018) Anti-inflammatory approaches to ischaemic stroke prevention. J Neurol Neurosurg Psychiatry 89:211–218. https://doi.org/10.1136/jnnp-2016-314817
Amantea D, Bagetta G (2017) Excitatory and inhibitory amino acid neurotransmitters in stroke: from neurotoxicity to ischemic tolerance. Curr Opin Pharmacol 35:111–119. https://doi.org/10.1016/j.coph.2017.07.014
Gu W, Gu C, Jiang W, Wester P (2010) Neurotransmitter synthesis in poststroke cortical neurogenesis in adult rats. Stem Cell Res 4:148–154. https://doi.org/10.1016/j.scr.2009.12.001
Berg C, Backström T, Winberg S et al (2013) Developmental exposure to fluoxetine modulates the serotonin system in hypothalamus. PLoS ONE 8:e55053. https://doi.org/10.1371/journal.pone.0055053
Alcantara CC, García-Salazar LF, Silva-Couto MA et al (2018) Post-stroke BDNF concentration changes following physical exercise: a systematic review. Front Neurol 9:637. https://doi.org/10.3389/fneur.2018.00637
Ji X-W, Wu C-L, Wang X-C et al (2014) Monoamine neurotransmitters and fibroblast growth factor-2 in the brains of rats with post-stroke depression. Exp Ther Med 8:159–164. https://doi.org/10.3892/etm.2014.1674
Mergenthaler P, Dirnagl U, Meisel A (2004) Pathophysiology of stroke: lessons from animal models. Metab Brain Dis 19:151–167
Bartsch T, Döhring J, Reuter S et al (2015) Selective neuronal vulnerability of human hippocampal CA1 neurons: lesion evolution, temporal course, and pattern of hippocampal damage in diffusion-weighted MR imaging. J Cereb Blood Flow Metab 35:1836–1845. https://doi.org/10.1038/jcbfm.2015.137
Wang X, Michaelis EK (2010) Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci 2:12. https://doi.org/10.3389/fnagi.2010.00012
Cavaglia M, Dombrowski SM, Drazba J et al (2001) Regional variation in brain capillary density and vascular response to ischemia. Brain Res 910:81–93
Wang X, Pal R, Chen X-W et al (2005) High intrinsic oxidative stress may underlie selective vulnerability of the hippocampal CA1 region. Brain Res Mol Brain Res. https://doi.org/10.1016/j.molbrainres.2005.07.018
Sieber FE, Palmon SC, Traystman RJ, Martin LJ (1995) Global incomplete cerebral ischemia produces predominantly cortical neuronal injury. Stroke 26:2091–2095 (discussion 2096)
Hara A, Yoshimi N, Hirose Y et al (1995) DNA fragmentation in granular cells of human cerebellum following global ischemia. Brain Res 697:247–250. https://doi.org/10.1016/0006-8993(95)00902-3
Wang K, Damjanov I, Wan Y-JY (2010) The protective role of pregnane X receptor in lipopolysaccharide/D-galactosamine-induced acute liver injury. Lab Investig 90:257–265. https://doi.org/10.1038/labinvest.2009.129
Ouyang Y-B, Voloboueva LA, Xu L-J, Giffard RG (2007) Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J Neurosci 27:4253–4260. https://doi.org/10.1523/JNEUROSCI.0211-07.2007
Kirino T, Sano K (1984) Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol 62:201–208. https://doi.org/10.1007/BF00691853
Jingtao J, Sato S, Yamanaka N (1999) Changes in cerebral blood flow and blood brain barrier in the gerbil hippocampal CA1 region following repeated brief cerebral ischemia. Med Electron Microsc 32:175–183. https://doi.org/10.1007/s007959900012
Himori N, Watanabe H, Akaike N et al (1990) Cerebral ischemia model with conscious mice. Involvement of NMDA receptor activation and derangement of learning and memory ability. J Pharmacol Methods 23:311–327
Morris RG, Garrud P, Rawlins JN, O’Keefe J (1982) Place navigation impaired in rats with hippocampal lesions. Nature 297:681–683
Itoh J, Nabeshima T, Kameyama T (1991) Utility of an elevated plus-maze for dissociation of amnesic and behavioral effects of drugs in mice. Eur J Pharmacol 194:71–76
Gaur V, Aggarwal A, Kumar A (2009) Protective effect of naringin against ischemic reperfusion cerebral injury: possible neurobehavioral, biochemical and cellular alterations in rat brain. Eur J Pharmacol 616:147–154. https://doi.org/10.1016/j.ejphar.2009.06.056
Bishnoi M, Chopra K, Kulkarni SK (2006) Involvement of adenosinergic receptor system in an animal model of tardive dyskinesia and associated behavioural, biochemical and neurochemical changes. Eur J Pharmacol 552:55–66. https://doi.org/10.1016/j.ejphar.2006.09.010
Church WH (2005) Column chromatography analysis of brain tissue: an advanced laboratory exercise for neuroscience majors. J Undergrad Neurosci Educ 3:A36–A41
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Best TM, Fiebig R, Corr DT et al (1999) Free radical activity, antioxidant enzyme, and glutathione changes with muscle stretch injury in rabbits. J Appl Physiol 87:74–82
Raddassi K, Berthon B, Petit J-F, Lemaire G (1994) Role of calcium in the activation of mouse peritoneal macrophages: induction of NO synthase by calcium ionophores and thapsigargin. Cell Immunol 153:443–455. https://doi.org/10.1006/cimm.1994.1041
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Kono Y (1978) Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 186:189–195
Luck H (1963) Catalase. In: Bergmeyer HW (ed) Methods of enzymatic analysis, section 3. Academic Press, New York, pp 885–894
Pearse AG (1960) This week’s citation classic. R Postgrad Mcd Sch 998
Hoesch RE, Koenig MA, Geocadin RG (2008) Coma after global ischemic brain injury: pathophysiology and emerging therapies. Crit Care Clin 24:25–44. https://doi.org/10.1016/j.ccc.2007.11.003
Zhang X, Deguchi K, Yamashita T et al (2010) Temporal and spatial differences of multiple protein expression in the ischemic penumbra after transient MCAO in rats. Brain Res 1343:143–152. https://doi.org/10.1016/j.brainres.2010.04.027
Li F, Irie K, Anwer MS, Fisher M (1997) Delayed triphenyltetrazolium chloride staining remains useful for evaluating cerebral infarct volume in a rat stroke model. J Cereb Blood Flow Metab 17:1132–1135. https://doi.org/10.1097/00004647-199710000-00016
Adibhatla RM, Hatcher JF (2010) Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 12:125–169. https://doi.org/10.1089/ars.2009.2668
Raghavendra Rao VL, Rao AM, Dogan A et al (2000) Glial glutamate transporter GLT-1 down-regulation precedes delayed neuronal death in gerbil hippocampus following transient global cerebral ischemia. Neurochem Int 36:531–537
Bragin DE, Zhou B, Ramamoorthy P et al (2010) Differential changes of glutathione levels in astrocytes and neurons in ischemic brains by two-photon imaging. J Cereb Blood Flow Metab 30:734–738. https://doi.org/10.1038/jcbfm.2010.9
Candelario-Jalil E, Mhadu NH, Al-Dalain SM et al (2001) Time course of oxidative damage in different brain regions following transient cerebral ischemia in gerbils. Neurosci Res 41:233–241
Manto M, Bower JM, Conforto AB et al (2012) Consensus paper: roles of the cerebellum in motor control—the diversity of ideas on cerebellar involvement in movement. The Cerebellum 11:457–487. https://doi.org/10.1007/s12311-011-0331-9
Hara A, Yoshimi N, Hirose Y et al (1995) DNA fragmentation in granular cells of human cerebellum following global ischemia. Brain Res 697:247–250
Bello EP, Casas-Cordero R, Galiñanes GL et al (2016) Inducible ablation of dopamine D2 receptors in adult mice impairs locomotion, motor skill learning and leads to severe parkinsonism. Mol Psychiatry. https://doi.org/10.1038/mp.2016.105
Martín A, Szczupak B, Gómez-Vallejo V et al (2013) PET imaging of serotoninergic neurotransmission with [11C]DASB and [18F]altanserin after focal cerebral ischemia in rats. J Cereb Blood Flow Metab 33:1967–1975. https://doi.org/10.1038/jcbfm.2013.156
Cao W, Drumheller A, Zaharia M et al (1993) Effects of experimentally induced ischemia on dopamine metabolism in rabbit retina. Invest Ophthalmol Vis Sci 34:3140–3146
Oliva I, Fernández M, Martín ED (2013) Dopamine release regulation by astrocytes during cerebral ischemia. Neurobiol Dis 58:231–241. https://doi.org/10.1016/j.nbd.2013.06.007
Shimizu-Sasamata M, Yamamoto M, Okada M et al (1991) Effects of indeloxazine hydrochloride on behavioral and biochemical changes in the chronic phase of focal cerebral ischemia in rats. Arch Int Pharmacodyn Ther 314:74–89
Ferrari RS, Andrade CF, Ferrari RS, Andrade CF (2015) Oxidative stress and lung ischemia-reperfusion injury. Oxid Med Cell Longev 2015:1–14. https://doi.org/10.1155/2015/590987
Stamenkovic S, Sekeljic V, Radenovic LAP (2012) Ischemia and neurogenesis: link between neurodegeneration and repair. In: ICG, AW (eds) Neurogenesis research: new developments. NOVA Science Publishers, Inc. New York, pp 115–136
Lichtenwalner RJ, Parent JM (2006) Adult neurogenesis and the ischemic forebrain. J Cereb Blood Flow Metab 26:1–20. https://doi.org/10.1038/sj.jcbfm.9600170
Vandenbosch R, Borgs L, Beukelaers P et al (2009) Adult neurogenesis and the diseased brain. Curr Med Chem 16:652–666
Ziemka-Nałęcz M, Zalewska T (2012) Endogenous neurogenesis induced by ischemic brain injury or neurodegenerative diseases in adults. Acta Neurobiol Exp (Wars) 72:309–324
Chen A, Xiong L-J, Tong Y, Mao M (2013) The neuroprotective roles of BDNF in hypoxic ischemic brain injury. Biomed Rep 1:167–176. https://doi.org/10.3892/br.2012.48
Ploughman M, Windle V, MacLellan CL et al (2009) Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke 40:1490–1495. https://doi.org/10.1161/STROKEAHA.108.531806
Xie Z-M, Wang X-M, Xu N et al (2017) Alterations in the inflammatory cytokines and brain-derived neurotrophic factor contribute to depression-like phenotype after spared nerve injury: improvement by ketamine. Sci Rep 7:3124. https://doi.org/10.1038/s41598-017-03590-3
Acknowledgements
The study was carried out with the funds provided by Department of Science & Technology/Innovation in Science Pursuit for Inspired Research (DST/INSPIRE), India with IF no. IF130058.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflict of interest in the manuscript.
Ethical approval
The authors have read and abided by the statement of the ethical standards for manuscripts submitted to this journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kapoor, M., Sharma, S., Sandhir, R. et al. Temporal changes in physiological and molecular markers in various brain regions following transient global ischemia in rats. Mol Biol Rep 46, 6215–6230 (2019). https://doi.org/10.1007/s11033-019-05060-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-05060-7