Abstract
In this study we are presenting the development of technetium-99m (99mTc) labeled ibuprofen for the imaging of aseptic inflammation. 99mTc-Ibuprofen complex was developed by optimizing the radiolabeling conditions such as reaction time, ligand and reducing agent concentration, pH, reaction time and temperature. Following the addition of 600 µg of ibuprofen, 4 µg of stannous chloride as reducing agent and 300 MBq 99mTc radioactivity; the pH of reaction mixture was adjusted to 11 and allowed to react for 15 min at room temperature. Chromatography analysis revealed > 94% 99mTc-ibuprofen complex formation with promising stability in saline and blood serum up to 6 h. Biodistribution study using normal and sterile inflammation induced mice indicated low accumulation of labeled compound in key body organs; however, kidneys (14.76 ± 0.87% ID/g organ) and bladder (31.6 ± 3.0% ID/g organ) showed comparatively higher radioactivity due to main excretory path. Inflamed to normal tissues ratio (T/NT), at 1 h post-injection, showed promising value (4.57 ± 0.56). The SPECT imaging of artificially inflammation induced rabbit model also verified the biodistribution results. In conclusion, radiochemical purity and biological evaluation of 99mTc-ibuprofen complex indicates the agent can be utilized for imaging of deep seated aseptic inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infection is serious illness which commonly originates as a result of invasion and multiplication of microorganisms such as bacteria, viruses, and parasites that are not normally present within the body. Inflammation of infected tissues is a primary indicator of invasion and multiplication of microorganisms—which is termed as septic inflammation. On contrary, aseptic inflammation relates to non-bacterial issues occurred in body e.g. physical injury or internal stress [1]. In medical setup it is important to diagnose both processes with quite sensitivity and accuracy to treat the roots of inflammation. The inflammatory process, although is an asymptomatic or shows non-specific symptoms but its early diagnosis allows well-in-time treatment which may prevent the onset of complications [2]. Nuclear medicine technique (NMT) helps in early diagnosis of abnormalities due to its specificity even at molecular level. Despite of the outstanding sensitivity and accuracy of state-of-the-art diagnostic radiological instruments such as magnetic resonance imaging (MRI) and computed tomography (CT), they do not work until some morphological and entomological changes not occur at infected site, while NMT shows the physiological function of the tissue or organ being investigated. Due to target specific detection at molecular level NMT are gaining ample intention in diagnosis of deep-seated inflammation, infections and many hard to detect/treat malignancies [3,4,5]. Recent advances in the understanding of the pathophysiological process of inflammation at cellular level and to diagnose them through NMT has boosted the development of radiopharmaceuticals [6]. The 99mTc-labeled white blood cells [7] and immunoglobulins [8] and gallium-67 labeled citrate [9] was studied to locate the inflammation-infection cites and reported its promising credibility in infection-inflammation imaging accuracy [10]. However, most of the imaging-agents showed poor function in discriminating the septic-inflammation from aseptic-inflammation, which created the confusion in selecting the therapy. The development of 99mTc-ciprofloxacin (the first antibiotic radiopharmaceutical) in 1993, helped to discriminate the both processes [11]. The agent was reported to diagnose the septic-inflammation with good accuracy by targeting the bacterial DNA gyrase-II enzyme [12, 13]. However, the enthusiasm was drained off when different contradictory reports were published regarding the imaging sensitivity and specificity of 99mTc-ciprofloxacin for septic-inflammation but still it is in practice to diagnose bacterial infection [14]. Differentiation between septic- and aseptic-inflammations can be made possible by developing aseptic-inflammation imaging radiopharmaceutical and then by adopting the nuclear medicine procedure in combination with 99mTc-ciprofloxacin (as septic-inflammation imaging agent) then both processes can be identified.
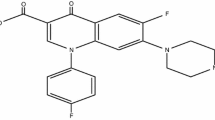
Ibuprofen (IBF), as shown in Fig. 1a, is an anti-inflammatory non-steroidal drug which targets cyclooxygenase enzyme, especially COX-2 at inflamed tissues with promising therapeutic efficacy [15, 16]. The drug can be developed as radiopharmaceutical for discrimination of septic-inflammation from aseptic-inflammation. The aim of this study is to develop 99mTc-IBF complex (proposed chemical structure is shown in Fig. 1b) with promising purity, yield, stability and biodistribution in aseptic-inflammation induced mice model.
Experimental
Materials and methods
IBF and stannous chloride dihydrate was purchased from Sigma-Aldrich (Germany). 99mTc activity was obtained through elution of PAKGEN 99Mo/99mTc-Generator at Isotope Production Division (IPD), Pakistan Institute of Nuclear Science and Technology (PINSTECH), Islamabad, Pakistan. Sodium phosphate, Sodium hydroxide, hydrochloric acid, and methanol (HPLC grade) were purchased from Merck (Germany). All the chemicals were of analytical grade. The stock solution of IBF was prepared in methanol and stored at 4 °C for further use. Whatman No.3MM (W3) strip and Instant thin layer chromatography impregnated with silica gel (ITLC-SG) were obtained from Agilent (Singapore). Chromatographic strips were analyzed with Well-type NaI (Tl) detector (Genesys Gamma-1, USA) and 2π-Scanner (Berthold, Germany). Deluxe electrophoresis chamber (Gelman-Germany) system was used for the determination of charge of the complex. Sprague-Dowley mice were obtained from National Institute of Health (NIH), Islamabad, Pakistan. Animal ethics committee [ethical review committee (ERC)], Government College University, Faisalabad gave approval for animal study. All equipment and apparatus were calibrated before use.
Animal ethical review committee approval and informed consent from human volunteer
All protocols used for handling animals i.e. the induction of sterile inflammation, giving anesthesia, administration of 99mTc-IBF, and biodistribution studies, were carried out after institutional ERC approval (Document Nos. GCUF/ERC/16/03 & GCUF/ERC/18/06). The blood was taken from healthy human volunteer for harvesting blood serum after informed consent (Document No. GCUF/NRPU5612/08/17).
Radiosynthesis of 99mTc-IBF
Optimization of reaction conditions for the radiosynthesis of 99mTc-IBF was performed through series of experiments. For this took 300 to 700 µg of IBF in the form of methanol solution in five separate sterilized vials. Added 2 to 6 µg of SnCl2. 2H2O in each vial as reducing agent. The labeling was carried out at pH 5–13. After addition of reactants in each vial, added ~ 300 MBq of 99mTcO4−1 in saline solution and shaked well for 10–15 s. Then the reaction mixture was allowed to react at different temperatures and time i.e. 4 °C to 65 °C for 10–60 min. All experiments were performed by keeping the total volume 2.5 ± 0.2 mL in each vial.
Chromatographic analysis
The labeling purity and yield of 99mTc-IBF was determined with the help of paper chromatography and ITLC-SG systems in terms of percent radiochemical purity (%RCP). About ~ 2 µL of an aliquot of 99mTc-IBF reaction mixture was spotted on the base line of W3 and ITLC-SG strips having dimensions of (1.5 × 14) cm each. For the determination of free 99mTc, W3 paper was developed with acetone as mobile phase while for hydrolyzed 99mTc, ITLC-SG strip was developed with 0.5N NaOH as mobile phase. After the development, each strip was dried and cut into segments of 1 cm. The radioactive counts on each segment were recorded by Well-type NaI (Tl) gamma (γ) counter (Genesys Gamma-1, USA). The strips were also scanned by 2π-scanner (Berthold-Germany) for confirmation of radiochemical yield. The experiments were performed thrice a day and periodically during the course of work to confirm the results and reproducibility. The percent radiochemical purity was determined by using the following expressions;
Saline stability of 99mTc-IBF complex
Following the optimization of reaction conditions for the radiosynthesis of 99mTc-IBF complex, the stability of complex was determined in saline from 0.25 to 24 h at room. At predefined time intervals ~ 2µL aliquot was withdrawn from incubated reaction mixture and spotted at W3 and ITLC-SG strips. After developing and drying, the strips were analyzed for recording intact fraction of 99mTc-IBF.
Serum stability of 99mTc-IBF complex
For in-vitro investigation of 99mTc-IBF complex stability in blood serum, the serum was harvested from fresh blood collected from healthy volunteer by centrifuging 3 mL blood at 3000 rpm for 15 min. Then took 0.2 mL of 99mTc-IBF complex in a sterilized reaction vial, added equal amount of blood serum, fit the vial in vortex machine for 30 s to shake the reaction mixture and then incubated for 24 h at 37 °C in 5% CO2 incubator. An aliquot of ~ 2µL were taken out from incubated mixture at specific time intervals, spotted on chromatographic strips, developed and analyzed to record percent fraction of intact 99mTc-IBF complex up to 24 h.
Electrophoresis analysis
The electrical charge on 99mTc-IBF complex was determined by paper electrophoresis. Whatman No. 1 paper was used as supporting medium. Phosphate buffer (0.02 M) of pH 6.8 was used as an electrophoretic electrolyte. An aliquot of ~ 10 µL of 99mTc-IBF complex was spotted at the center of strip (1 × 20 cm), dried and placed the strip in chamber of deluxe electrophoresis (Gelman-Germany) system to allow electrophoretic mobility for 1 h at 300 V direct current (DC). Following the completion of electrophoresis procedure the strip was dried and scanned from anode to cathode end using 2π-Scanner.
Biological distribution study
In-vivo biodistribution of 99mTc-IBF complex was investigated in Sprague-Dowley mice (~ 50–100 g) following the protocol reported previously and guidelines of ERC, Government College University, Faisalabad [17]. Biodistribution study of 99mTc-IBF was assessed in normal as well as in aseptic-inflammation induced mice at 30 min, 1 h and 4 h time points. At each time point the group of three mice was studied. Aseptic-inflammation was induced in right thigh muscles using turpentine oil (target tissues) under sterile condition and the normal left thigh muscles were used as non-target tissues. At each time point, about 200 µL of 99mTc-IBF saline solution (120–130 MBq activity) was injected intravenously through tail vein of mice and dissected at 30 min, 1 h and 4 h post-injection intervals. The chloroform anesthesia was given before dissection. During dissection procedure, 1 mL blood sample was collected by cardiac puncture, considering it 5% of the total body weight followed by separating the different organ such as heart, lungs, liver, stomach, kidney, spleen, intestine, bladder, femur, urine, carcass A, carcass B, thyroid and brain; washing with saline solution, dried on filter paper and placed each organ in pre-weighed vials to note the organ weight. Activity in each organ was measured through NaI (Tl) well type detector in connection with single channel gamma-counter (SR-7) and calculated the %ID/g organ of 99mTc-IBF by using the following expression;
SPECT scintigraphy
SPECT Scintigraphy study was performed using healthy male rabbits (~ 1.0 to 1.5 kg weight) artificially induced with sterile inflammation using turpentine oil at its left thigh muscle. After 36 h, when visible swelling was seen, the rabbit was anesthetized by injecting diazepam injection through rear ear vain followed by placing the rabbit under dual headed SPECT gamma camera (connected to an on-line dedicated computer system) by stretching the fore and rear legs out with polythene tape. Then, 99mTc-IBF (185 MBq) was administrated through rear ear vain of rabbit and SPECT images were taken at 5, 15 and 60 min.
Results
To optimize the reaction conditions for maximum purity and yield of 99mTc-IBF, a series of radiolabeling reactions were performed considering different quality control parameters such as amount of IBF, reducing agent, pH, time and temperature, which showed different results at different sets of parameters as shown in Fig. 2. The optimized set of reaction conditions resulted > 94% RCP, however, during the reaction optimization process a critical effect of each parameter was noted on the yield of radiolabeling.
Effect of ligand concentration on percent RCP
To optimize the ligand concentration for maximum RCP, hit-and-trial methodology were adopted by sequentially changing the concentration of IBF from 300 to 700 µg and fixing the other reaction parameters (reducing agent, pH, radioactivity and reaction time) at room temperature. At 300 µg, 45% RCP was recorded that was increased to 94% at 600 µg. Above 600 µg IBF concentration, the radiochemical yield graph declined to lower values as shown in Fig. 2a.
Effect of reducing agent on RCP
Figure 2b shows the effect of reducing agent on the RCP and yield. SnCl2·2H2O in acidic solution (2–6 µg) was used as a reducing agent in different radiolabeling reactions. SnCl2.2H2O solution was prepared in 1N HCl at boiling temperature. The maximum radiochemical yield (> 94%) was obtained with 4 µg SnCl2·2H2O, however, at lower and higher concentration the reaction resulted lower radiochemical yield.
Effect of pH on percent RCP
The pH effect was exercised at different pH values i.e. from 5 to 13 in combination with variety of other sets of parameters. The pH 11was noted, the most compatible value, to achieve maximum radiochemical yield i.e. 94%, however at other pH values less than 94% radiochemical yield was noted as shown in Fig. 2c.
Effect of reaction time
The effect of reaction time on maximum formation of 99mTc-IBF complex was monitored by withdrawing an aliquot of 2 µL at 10, 15, 30, 40, 50 and 60 min from reaction vial and analyzed using chromatography. The chromatography analysis revealed that maximum 99mTc-IBF complex was formed at 15 min reaction period. Above this time no change was noted in radiochemical yield as shown in Fig. 2d.
Effect of temperature on RCP
Radiosynthesis was performed at different temperatures i.e. from 5 to 65 °C. Maximum radiochemical yield (> 94%) was obtained at 25 °C. Below and above this temperature slight decrease in yield was noted which was negligible. The overall temperature effect on radiochemical yield is shown in Fig. 2e.
Chromatographic analysis of 99mTc-IBF
The percent formation of bound, free and hydrolyzed 99mTc at experimental and optimized reaction conditions was analyzed using W3 paper and ITLC-SG chromatographic analysis. In case of former analysis (Fig. 3a) the free 99mTc was found to travel along with solvent front (Rf = 1; 2.76 ± 0.14%) leaving bound and hydrolyzed 99mTc at base line (Rf = 0.0–0.1; 96.56 ± 1.34%). While in case of later analysis (Fig. 3b), the hydrolyzed 99mTc remained at base line (Rf = 0.0; 2.14 ± 0.21%) and the bound and free 99mTc was found to travel along with solvent front (Rf = 0.9–1.0; 96.14 ± 1.71%). Through the calculations of counts on both strips > 94% 99mTc-IBF was recorded.
99mTc-IBF stability in saline solution
Stability of 99mTc-IBF complex in saline was determined by incubating the saline solution of 99mTc-IBF up to 24 h at room temperature. An aliquot of 2 µL was withdrawn from incubating saline solution for chromatography analysis at pre-defined time intervals. The 99mTc-IBF complex was remained intact up to 4 h followed by slight decomposition (i.e. 4–5%) was noted in next two hours as shown in Fig. 4a.
99mTc-IBF stability in blood serum
99mTc-IBF complex stability was evaluated at definite time intervals in freshly harvested human blood serum. The 99mTc-IBF complex was found quite stable i.e. more than 91% intact complex was recorded up to 24 h as shown in Fig. 4b.
Determination of electrical charge on 99mTc-IBF
Electrophoresis analysis showed that maximum activity i.e. > 93% of 99mTc-IBF complex remained at origin (point of sample introduction) of electrophoretogram and only a small portion (~ 7%) of free 99mTcO4− moved toward anode as shown in Fig. 5. The results revealed that the maximum fraction of reaction mixture (93%) is neutral while about 7% comprises of negatively charged species.
Biodistribution evaluation of 99mTc-IBF
Biodistribution of 99mTc-IBF complex was assessed in normal and inflammation induced Sprague-Dowley mice at 30 min, 1 h and 4 h post-injection, as shown in Fig. 6. It was counted that at 30 min post-injection interval the liver, stomach, kidneys and blood showed 5.3 ± 0.9, 6.8 ± 1.03, 5.16 ± 0.43 and 17.5 ± 2.5%ID/g organ, respectively which continuously increased in kidneys and liver but decreased in stomach and blood at 1 and 4 h post-injection periods. Inflammation induced mice showed significant higher accumulation at inflamed thigh muscles as compared to normal thigh muscles at all three post-injection time intervals as shown in Fig. 7.
SPECT scintigraphy
Figure 8 shows the SPECT images of 99mTc-IBF administrated inflammation induced rabbit model at 5, 15 and 60 min post-injection. The SPECT scintigraphy results at 5 min showed absence of activity at inflamed and healthy tissues while the activity started to accumulate at inflamed tissues at 15 min image which increased to considerable counts at 1 h post-injection scintigraphy study.
Discussion
The present study was designed to develop 99mTc-IBF radiopharmaceutical (Fig. 1b, proposed structure [18, 19]) for the imaging of aseptic-inflammation and possible assistance in discriminating from septic-inflammation in clinical setups. Inflammation is an ultimate indicator of septic and aseptic events occur in human body. The discrimination between the septic- and aseptic-inflammation always remained a challenge to clinicians, radiologist and physicians [20]. The state-of-the-art instrumental diagnostic procedures i.e. MRI and CT work poorly to diagnose physiological abnormalities that not associated with change in anatomy or structure of tissues or organs [10]. Nuclear medicine technique non-invasively offers wide range of procedures to meet the clinical diagnostic and therapeutic demands. An important clinical issue which remained a priority order challenge in clinical setup is to discriminate the septic-inflammation from aseptic-inflammation for making right therapeutic decision. Although variety of radiopharmaceuticals such as 99mTc-labeled antibiotics were developed to make difference between the two processes but appeared less effective especially in case of osteomyelitis inflammation. Ibuprofen which specifically bind to cyclooxygenase enzyme especially COX-2 at inflamed tissues with excellent efficacy [21], is an ideal candidate that can be selected as labeling ligand with 99mTc to specifically bind at inflamed tissues and to discriminate the two processes in combination with the radiopharmaceutical that can specifically accumulate at infected foci.
For the radiosynthesis of 99mTc-IBF; as a result of serial of experiments, a set of optimized reaction parameters were obtained which resulted maximum radiochemical yield i.e. 94.23 ± 1.34% (Fig. 2)—such that subsequent addition of 600 µg IBF, 4 µg of SnCl2. 2H2O, 300 MBq of 99mTc, adjusting the pH at 11, and gently shaking reaction mixture periodically for few seconds up to 25 min at room temperature. The 99mTc-IBF complex stability in saline and freshly harvested blood serum (93.83 ± 1.71 and 93.17 ± 1.69%, respectively) at 4 h time period ensures safe administration to carry out successful nuclear medicine procedures [22]. Electrophoretic analysis revealed the complex is electrically neutral—IBF itself a weak acid (pKa = 4.91) and binds with cyclooxygenase enzyme I or II in its undissociated form (neutral form); results in least formation of precursors of prostaglandins and thromboxane, thereby inhibit platelet aggregation. So the neutral nature certainly intact the binding potential of 99mTc-IBF complex with cyclooxygenase enzyme I or II [15, 21].
The biodistribution study, as shown in Fig. 6, reveals low accumulation of 99mTc-IBF in most of the body organs. Stomach, at 30 min post-injection interval showed higher uptake (6.8 ± 1.03%ID/g organ) which on 4 h time point showed 3.6 ± 0.53%ID/g organ. Two organs showed increasing trend up to 4 h, i.e. liver and kidneys—the former associated with metabolic excretory pathway and the latter organ is main excretory organ. As long as the toxicity of IBF concern, IBF is known among the safest NSAIDs and is generally well tolerated but can, nevertheless, rarely cause clinically apparent and serious acute liver injury [23]. But the administration of 99mTc-IBF for nuclear medicine procedure requires nano-molar quantity so the chance of acute liver injury not possible. The 99mTc-IBF complex showed promising uptake at inflamed tissues (target) as compared to normal thigh tissues (non-target) which was further indicated by the scintigraphy images taken at different time intervals. The promising target-to-nontarget (T/NT) ratio i.e. 2.58 ± 0.67, 4.57 ± 0.56 and 3.71 ± 0.31 at 30 min, 1 h and 4 h post-injection time intervals showed its admirable target specificity. Base on the biodistribution profile both in normal and inflamed animals, the 99mTc-IBF could safely be administrated for the localization of septic- and aseptic-inflammation.
Conclusion and prospects
The discrimination of septic-inflammation from aseptic-inflammation is a serious issue in clinical setup. Currently, the available radiopharmaceuticals, which are being used to diagnose inflammation, are unable to detect the roots of inflammation i.e. either due to bacteria, cancer, viral, chemical or mechanical injury. The need to discriminate the cause of inflammation looks more important in case of orthopedic infections, osteomyelitis, arthritis and endocarditis. Different macromolecules, for example, antimicrobial peptides (AMPs) [24], antibiotics [22], monoclonal antibodies [25], cytokines [26], polyclonal and immunoglobulins (IgG) [27], etc. labeled with various gamma radiation emitter radionuclides like Gallium-67, Indium-111, Iodine-131, 99mTc, Fluorine-18 have been developed to discriminate between two events, but commonly these agents, either due to one or more reasons, showed poor results. In this study, the introduction of NSAIDs based 99mTc-IBF new radiopharmaceutical is being developed with high chemical yield, purity, promising stability in saline and blood serum, electrically neutral complex, admirable favorable biodistribution and scintigraphy results and high inflamed to non-inflamed muscle ratio revealed that agent is an intelligent candidate to image inflammation. However, as prospects, the agent can be evaluated in combination with 99mTc-ciprofloxacin to discriminate between septic- from aseptic-inflammation that can provide the ground to clinicians in deciding therapeutic strategies for successful therapeutic outcome.
References
Martínez-Rodríguez I, Carril J (2013) Update on the use of PET radiopharmaceuticals in inflammatory disease. Revista Española de Medicina Nuclear e Imagen Molecular (English Edition) 32:378–386
Kyprianidou P, Tsoukalas C, Chiotellis A, Papagiannopoulou D, Raptopoulou CP, Terzis A, Pelecanou M, Papadopoulos M, Pirmettis I (2011) First example of well-characterized Re and 99 m Tc tricarbonyl complexes of ciprofloxacin and norfloxacin in the development of infection-specific imaging agents. Inorg Chim Acta 370:236–242
Rasheed R, Naqvi SAR, Gillani SJH, Zahoor AF, Jielani A, Saeed N (2017) 99 m) Tc-tazobactam, a novel infection imaging agent: radiosynthesis, quality control, biodistribution, and infection imaging studies. J Label Compd Radiopharm 60:242–249
Motaleb MA, Ibrahem IT, Ayoub VR, Geneidi AS (2016) Preparation and biological evaluation of (99 m)Tc-ropinirole as a novel radiopharmaceutical for brain imaging. J Label Compd Radiopharm 59:147–152
Fazli A, Salouti M, Ahmadi G, Mirshojaei SF, Mazidi M, Heidari Z (2012) Radiolabeling of ceftriaxone with 99 m Tc as a Targeting radiopharmaceutical for Staphylococcus aureus detection in mouse model. Iran J Med Phys 9:103–110
Chianelli M, Mather SJ, Martin-Comin J, Signore A (1997) Radiopharmaceuticals for the study of inflammatory processes: a review. Nucl Med Commun 18:437–455
Larikka MJ, Ahonen AK, Niemela O, Junila JA, Hamalainen MM, Britton K, Syrjala HP (2002) Comparison of 99mTc ciprofloxacin, 99mTc white blood cell and three-phase bone imaging in the diagnosis of hip prosthesis infections: improved diagnostic accuracy with extended imaging time. Nucl Med Commun 23:655–661
Asli IN, Javadi H, Seddigh H, Mogharrabi M, Hooman A, Ansari M, Jalallat S, Assadi M (2011) The diagnostic value of 99mTc-IgG scintigraphy in the diabetic foot and comparison with 99mTc-MDP scintigraphy. J Nucl Med Technol 39:226–230
Hughes DK (2003) Nuclear medicine and infection detection: the relative effectiveness of imaging with 111In-oxine-, 99mTc-HMPAO-, and 99mTc-stannous fluoride colloid-labeled leukocytes and with 67 Ga-citrate. J Nucl Med Technol 31:196–201 (quiz 203–194)
Das SS, Hall AV, Wareham DW, Britton KE (2002) Infection imaging with radiopharmaceuticals in the 21st century. Braz Arch Biol Technol 45:25–37
Britton K, Wareham D, Das S, Solanki K, Amaral H, Bhatnagar A, Katamihardja A, Malamitsi J, Moustafa H, Soroa V (2002) Imaging bacterial infection with 99mTc-ciprofloxacin (Infecton). J Clin Pathol 55:817–823
Naqvi SAR, Drlica K (2017) Fluoroquinolones as imaging agents for bacterial infection. Dalton Trans (Cambridge, England: 2003). 46:14452–14460
Naqvi SAR, Roohi S, Iqbal A (2018) Ciprofloxacin: from infection therapy to molecular imaging. Mol Biol Rep 45:1457–1468
Sarda L, Crémieux A-C, Lebellec Y, Meulemans A, Lebtahi R, Hayem G, Génin R, Delahaye N, Huten D, Guludec L, D (2003) Inability of 99mTc-ciprofloxacin scintigraphy to discriminate between septic and sterile osteoarticular diseases. J Nucl Med 44:920–926
Whittle B (2000) COX-1 and COX-2 products in the gut: therapeutic impact of COX-2 inhibitors. Gut 47:320–325
Yadav MR, Pawar VP, Marvaniya SM, Halen PK, Giridhar R, Mishra AK (2008) Site specific chemical delivery of NSAIDs to inflamed joints: Synthesis, Biological activity and γ-imaging studies of quaternary ammonium salts of tropinol esters of some NSAIDs or their active metabolites. Bioorganic Med Chem 16:9443–9449
Iqbal A, Naqvi SAR (2018) Radiosynthesis and Biodistribution of (99 m)Tc-Metronidazole as an Escherichia coli Infection. Imaging Radiopharm 185:127–139
Berry DJ, Torres M, de Rosales R, Charoenphun P, Blower PJ (2012) Dithiocarbamate complexes as radiopharmaceuticals for medical imaging. Mini Rev Med Chem 12:1174–1183
Motaleb MA, Ibrahem IT, Ayoub VR, Geneidi AS (2016) Preparation and biological evaluation of 99mTc–ropinirole as a novel radiopharmaceutical for brain imaging. J Label Compd Radiopharm 59:147–152
Ettinger M, Calliess T, Kielstein JT, Sibai J, Brückner T, Lichtinghagen R, Windhagen H, Lukasz A (2015) Circulating biomarkers for discrimination between aseptic joint failure, low-grade infection, and high-grade septic failure. Clin Infect Dis 61:332–341
Bushra R, Aslam N (2010) An overview of clinical pharmacology of Ibuprofen. Oman Med J 25:155–1661
Lambrecht FY (2011) Evaluation of 99 m Tc-labeled antibiotics for infection detection. Ann Nucl Med 25:1–6
Shahnazarian V, Ramai D, Reddy M (2018) A rare case of ibuprofen-induced acute liver injury. Cureus 10:e3225
Welling MM, Paulusma-Annema A, Balter HS, Pauwels EK, Nibbering PH (2000) Technetium-99m labelled antimicrobial peptides discriminate between bacterial infections and sterile inflammations. Eur J Nucl Med 27:292–301
Welling M, Feitsma HI, Calame W, Pauwels EK (1997) Detection of experimental infections with 99mTc-labeled monoclonal antibodies against TNF-α and interleukin-8. Nucl Med Biol 24:649–655
Chianelli M, Signore A, Ronga G, Fritzberg A, Mather S (1994) Labeling, purification and biodistribution of 99 m Tc-interleukin-2: a new radiopharmaceutical for in vivo detection of activated lymphocytes. Eur J Nucl Med 21:807
Welling M, Feitsma H, Calame W, Pauwels E (1997) Localization of a bacterial infection with 99Tcm-labelled human IgG: further improvement with enriched IgG subclass preparations. Nucl Med Commun 18:1057–1064
Acknowledgements
The study is a part of HEC funded Project No. 5612/Punjab/NRPU/R&D/HEC/2016, thanks to HEC, GCU Faisalabad, and PINSTECH Islamabad for providing resources and platform to conduct this research.
Funding
This study was funded by Higher Education Commission (HEC), Islamabad, Pakistan under Grant No. 5612/Punjab/NRPU/R&D/HEC/2016.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The article is in compliance with ethical standards with approval from ethical review committee.
Informed consent
Informed consent was taken from all co-authors before submission of manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, NUH., Naqvi, S.A.R., Sohail, H. et al. Technetium-99m labeled Ibuprofen: Development and biological evaluation using sterile inflammation induced animal models. Mol Biol Rep 46, 3093–3100 (2019). https://doi.org/10.1007/s11033-019-04762-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04762-2