Abstract
Grain size is an important appearance quality trait in rice, which also affects grain yield. In this study, a recombinant inbred line (RIL) population derived from a cross between indica variety 9311 and japonica variety Cypress was constructed. And 181 out of 600 RILs were sequenced, and a high-density genetic map containing 2842 bin markers was constructed, with a total map length of 1500.6 cM. A total of 10 quantitative trait loci (QTL) related to grain length (GL), grain width (GW), grain length-to-width ratio (LWR), and 1000-grain weight (TGW) were detected under two environments. The genetic effect of qGL4, a minor QTL for GL and TGW, was validated using three heterogeneous inbred family (HIF) segregation populations. It was further dissected into two closed linked QTL, qGL4.1 and qGL4.2. By progeny testing, qGL4.1 and qGL4.2 were successfully delimited to intervals of 1304-kb and 423-kb, respectively. Our results lay the foundation for the map-based cloning of qGL4.1 and qGL4.2 and provide new gene resources for the improvement of grain yield and quality in rice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice is one of the most important food crops and provides energy for about half of the world’s population. Grain weight, a major component of rice yield, is directly influenced by grain size/shape, which can be further divided by grain length (GL), grain width (GW), grain thickness (GT), and length-to-width ratio (LWR) (Xing and Zhang 2010). Grain size is also an important appearance quality. Rice with good appearance quality will be accepted by more consumers and increase its market value.
Lots of previous studies demonstrated that grain size is a complex quantitative trait regulated by many quantitative trait loci (QTL). Till now, large numbers of QTL for grain size have been mapped, and some major QTL have been successfully cloned and functionally characterized. These genes are involved in multiple pathways in regulating spikelet hull cell proliferation and/or expansion and then influencing grain size. Among those, G protein signaling plays a role in controlling grain size. Such as GS3 and DEP1, which both encode G protein γ-subunits and antagonistically regulate grain size (Fan et al. 2006; Huang et al. 2009b; Sun et al. 2018; Takano-Kai et al. 2009). The ubiquitin–proteasome pathway is also involved in grain size control. Both GW2 and CLG1 encode E3 ubiquitin ligase and oppositely regulate grain size (Song et al. 2007; Yang et al. 2021). Phytohormone biosynthesis and signaling-related genes modulate grain size by controlling cell expansion and proliferation. GS5, GW5/GSE5, qGL3/qGL3.1, and GS9 all affect the BR signaling and consequently regulate grain size (Hu et al. 2012; Li et al. 2011; Liu et al. 2017; Qi et al. 2012; Shomura et al. 2008; Weng et al. 2008; Zhang et al. 2012; Zhao et al. 2018). BG1 and TGW6 affect grain size through the auxin signaling pathway (Ishimaru et al. 2013; Liu et al. 2015). GW6 regulates GA responses and biosynthesis and positively regulates grain width (Shi et al. 2020). Besides, several transcription factors have been shown to regulate grain size, such as the SQUAMOSA promotor binding protein-like (SPL) family members: GL7/GLW7/OsSPL13, GW8/OsSPL16, and OsSPL18 (Si et al. 2016; Wang et al. 2012, 2015b, 2015c; Yuan et al. 2019; Zhou et al. 2015), and GS2/GL2, which encodes the plant-specific transcription factor OsGRF4 (Che et al. 2015; Duan et al. 2015; Hu et al. 2015).
In this study, QTL mapping of rice grain size-related traits was conducted in 2 years using a RIL population derived from variety 9311 and Cypress. And 10 QTL affecting grain size and weight were identified. qGL4, a minor QTL for GL and 1000-grain weight (TGW), was repeatedly detected in 2 years. The genetic effect of qGL4 was confirmed using three HIF-F2 populations. It was further dissected into two closed linked minor QTL, qGL4.1 and qGL4.2. By progeny testing, qGL4.1 and qGL4.2 were successfully delimited to intervals of 1304-kb and 423-kb, respectively. The results of this study laid a foundation for cloning of qGL4.1 and qGL4.2 and would be helpful for breeding applications.
Materials and methods
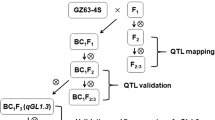
Population development and field experiments
Indica variety 9311 is an elite high-yield restorer line in China, which has reference genome (Gao et al. 2013; Yu et al. 2002). Cypress is a heat-resisting and good grain quality tropical japonica variety from the Philippines (Cooper et al. 2008). A RIL population consisting of 600 lines was derived from 9311 and Cypress by single-seed descent method. The parents and RILs of F8 and F9 generations were planted at the experiment paddy field of the Rice Institute of Anhui Academy of Agricultural Sciences in the summer of 2018 (Hefei, Anhui) and the spring of 2019 (Lingshui, Hainan).
In order to validate the genetic effect of qGL4, three independent HIFs of qGL4 were identified from the RILs. And the HIF-F2 populations were planted at the Lingshui experimental station in the spring of 2020.
To fine-map qGL4, progeny testing of 14 and 17 recombinants from HIF1 and HIF2 segregating populations, respectively, were conducted at the Hefei experimental station in the summer of 2020, with each line consisting of 36 individuals.
In order to prove that qGL4 region contained two closely linked minor QTL, 4 HIF-F2 populations (HIF1 qGL4.1, HIF1 qGL4.2, HIF2 qGL4.1, and HIF2 qGL4.2) each with 192 individuals were planted at the Lingshui experimental station in the spring of 2021. And progeny testing of 12 and 13 recombinants of qGL4.1 and qGL4.2, respectively, were conducted at the Hefei experimental station in the summer of 2021.
Seedlings of about 30 days after sowing were transplanted with a single plant spacing of 16.7 cm and 26.6 cm between rows in the field. Field cultivation and management followed normal local practices.
Trait evaluation
For the RILs, 181 out of 600 lines were randomly selected for phenotyping and genotyping. Nine individuals of each line with a similar field performance were mixed and harvested for measuring grain size and weight. For the HIF-F2 populations and recombinant progeny lines, each plant was genotyped and harvested for phenotyping.
Before phenotyping, harvested seeds were air-dried at room temperature for approximately 3 months to maintain a consistent water balance. At least 300 mature grains were used to measure the GL, GW, LWR, and TGW via a rice yield trait scorer (Yang et al. 2014).
Genotyping, genetic map construction, and QTL analysis
The 9311 genome sequences were downloaded from the Rice Information System database (http://rice.genomics.org.cn/rice). Genotyping of Cypress and the 181 RILs (F8) was carried out using genotyping-by-sequencing (GBS) technology on the Illumina HiSeq 2500 platform by the Beijing Genomics Institute (BGI). For Cypress, a total of 8.3 Gb of clean data with 19.1 × genome coverage was obtained. And the 181 RILs obtained a total of 53.8 Gb of clean data with an average of 0.7 × of genome coverage. The sequencing data were aligned to the Nipponbare reference genome (MSU v7.0, http://rice.uga.edu/). After SNP calling and filtering, a total of 33,799 high-quality and evenly distributed SNPs were selected to construct the genetic map. All the SNPs were used to identify the bin blocks by the sliding window method (Huang et al. 2009a). And a bin-based genetic linkage map was generated using R/qtl (Broman et al. 2003). QTL analysis was conducted by composite interval mapping (CIM) method using WinQTLCart2.5 (Wang et al. 2007) with logarithm of odds (LOD) values of 2.5; the QTL intervals were calculated by WinQTLCart2.5 with a confidence interval of 95%.
In the HIF-F2 populations, the genotyping was performed using Kompetitive Allele-Specific PCR (KASP) markers (LGC Group) and InDel markers, which were designed according to the variations between the parents. The genetic maps were constructed by MapMaker/Exp3.0 (Lander et al. 1987). And QTL analysis was performed by WinQTLCart2.5 (Wang et al. 2007).
Results
Phenotypic performance of grain size traits
The GL, GW, LWR, and TGW of the parents and 181 RILs were investigated in two environments. Compared with Cypress, 9311 showed larger values in GL, GW, and TGW, but smaller values in LWR (Fig. 1a–d). All these traits in the RIL population showed significant transgressive segregation and followed normal distribution in two years (Fig. 1a–d), indicating that these traits were controlled by multiple genes.
Performance of grain size-related traits in the RILs. a–d Frequency distribution of grain length, grain width, length–width ratio, and 1000-grain weight. Black bars and arrows, year of 2018. White bars and arrows, year of 2019. Single-headed and double-headed arrows indicate the trait values of Cypress and 9311, respectively
Correlation analysis was conducted for these traits. As expected, TGW was highly positively correlated with GL and GW. LWR displayed a significantly positive correlation with GL and a significantly negative correlation with GW (Table S1).
Construction of genetic linkage map
A total of 33,799 SNPs with polymorphism between 9311 and Cypress were employed to construct the linkage map. We constructed a linkage map that contained 2842 bins for the RIL population with a total length of 1500.6 cM and an average maker density of 0.53 bin per cM in the rice genome (Fig. 2a, Table S2). The chromosome length ranged from 60.2 cM (chromosome 11 with 112 bins) to 230.0 cM (chromosome 1 with 419 bins) (Fig. 2b, Table S2).
Bin map, genetic linkage map, and QTL of grain size-related traits in the RILs. a Bin map of 181 RILs. The x axis shows the 181 lines. The y axis shows the bins along the chromosomes (from bottom to top, chromosome 1 to chromosome 12). b Genetic linkage map and QTL of grain size-related traits. Red font, known genes. Black font, QTL detected in this study
QTL for grain size in the RIL population
In total, ten QTL for grain size and weight were identified on 5 chromosomes in the RIL population in 2 years. The phenotypic variation explained by each QTL ranged from 4.6 to 38.1% (Fig. 2b, Table 1).
For GL, four QTL were identified. And all of them were repeatedly detected in 2 years. A major QTL (qGL7b), located between 94.6 and 96.4 cM on chromosome 7, showed a high LOD score of 12.59 and 10.45 and phenotypic variation of 21.2% and 18.0% in 2018 and 2019, respectively. qGL7a was a minor QTL located at the end of the short arm of chromosome 7 and explained 5.8% and 6.5% of the phenotypic variation in 2018 and 2019, respectively. qGL4 had a LOD score of 6.82 in 2018 and 4.25 in 2019 and explained 10.8% and 6.7% of the phenotypic variation, respectively. qGL5 was located between 122.0 and 125.1 cM of chromosome 5 and contributed 6.8% and 6.6% of phenotypic variation in 2018 and 2019, respectively. qGL5 and qGL7b showed negative additive effects, indicating that alleles from Cypress increased GL, while qGL4 and qGL7a had positive additive effects, indicating that alleles from 9311 enhanced GL.
Three QTL for GW were detected, of which two were repeatedly detected in both years. The major QTL qGW7 had a LOD score of 10.66 and 15.44, an additive effect of 0.08 mm and 0.11 mm, and explained 21.1% and 31.0% of the phenotypic variation in 2018 and 2019, respectively. And the 9311 allele of qGW7 enhanced GW. qGW2, a minor QTL, explained 4.6% and 5.3% of the phenotypic variation in 2018 and 2019, respectively. qGW3 was only identified in 2018 with a LOD score of 2.54.
qLWR7 was the only QTL controlling LWR. It shared the same interval with the major QTL qGL7b and qGW7. qLWR7 had a LOD score of 20.35 and 19.68 and explained 38.1% and 35.5% of the phenotypic variation in 2018 and 2019, respectively. And the Cypress allele of qGW7 increased LWR.
For TGW, two QTL were identified. qTGW4 was repeatedly detected in 2 years. It had an additive effect of 1.40 g in 2018 and 1.29 g in 2019 and explained 23.1% and 15.5% of the phenotypic variation, respectively. qTGW4 shared the same interval with the GL QTL qGL4, and the 9311 alleles enhanced trait values. qTGW3 was only identified in 2019; nevertheless, it shared the same interval with the GW QTL qGW3.
The 94.6–96.4 cM interval on chromosome 7 had significant effects on GL, GW, and LWR in 2 years. The cloned gene GW7/GL7 was located to the interval of this major QTL (Wang et al. 2015b, 2015c; Zhou et al. 2015) (Fig. 2b). Three novel minor QTL for GL, qGL4, qGL5, and qGL7a, were identified in this study. And qGL4 co-located to the interval of grain weight QTL, qTGW4. Therefore, qGL4 was selected for further validation and fine mapping.
Validation of qGL4 in HIFs
Based on the results of QTL mapping, three HIFs (HIF1, HIF2, and HIF3) of qGL4 were identified. HIF1 came from the 181 RILs for preliminary mapping, and HIF2 and HIF3 were identified from the rest 419 RILs. HIF1 and HIF2 were heterozygous in the primary mapping region of qGL4 (chromosome 4, M350-M363), while HIF3 was heterozygous from marker M350 to K4-4 and 9311 homozygous from marker K4-5 to M363 (Fig. 3a, Table S3). HIF1, HIF2, and HIF3 segregating populations consisted of 176, 173, and 166 individuals, respectively, were planted to validate the genetic effect of qGL4. The effects of qGL4 in HIF1 and HIF2 populations were similar, explained 34.2–56.1% of the TGW and GL variations, with the 9311 allele increasing TGW and GL by 0.87 g and 0.75 g, and 0.23 mm and 0.28 mm, respectively. In HIF3, the additive effects of qGL4 were 0.59 g for TGW and 0.12 mm for GL, respectively, which were much lower than those in HIF1 and HIF2 (Fig. 3b–d, Table 2). The varied effects were supposed to be caused by the differences in genetic backgrounds. No QTL for GW was detected in HIF1, HIF2, and HIF3 populations (Table 2), indicating that qGL4 influenced TGW by controlling GL. The frequency distribution of GL and TGW in HIF1 and HIF2 segregating populations were nearly bimodal, and GL and TGW of heterozygous plants were between those of Cypress and 9311 homozygous, indicating that a semidominant allele of qGL4 from 9311 controlled GL and TGW (Fig. 3e–h, Table 2).
Validation of qGL4 in three HIF-F2 populations. a Genotypes of qGL4 region in the HIFs. Black and gray blocks indicate 9311 homozygous and heterozygous genotypes, respectively. b–d Observation of grain length of 9311 and Cypress homozygous plants in the HIFs. Bar = 1 cm. e, f Frequency distribution of grain length and 1000-grain weight in HIF1-F2 population. g, h Frequency distribution of grain length and 1000-grain weight in HIF2-F2 population. Black, white, and gray columns indicate 9311 homozygous, Cypress homozygous, and heterozygous genotypes, respectively
qGL4 contained two closely linked QTL, qGL4.1 and qGL4.2
As GL explained higher phenotypic variation than TGW in the HIFs (Table 2), we used GL as the target trait to fine-map qGL4. Due to a much lower genetic effect in HIF3, 14 and 17 recombinants from HIF1 and HIF2 segregating populations were selected to conduct fine mapping of qGL4. Thirty-six progenies of each recombinant were planted, genotyped, and phenotyped. Student’s t-test was performed to compare the GL differences between two types of homozygous progenies isolated from the recombinant. If there was a significant difference, the candidate gene was considered to be heterozygous; otherwise, it was considered to be homozygous. Results of progeny testing showed that the GL was segregated in recombinants HIF1-7, HIF1-42, HIF1-131, HIF2-78, and HIF2-172, indicating that qGL4 was heterozygous, and it should be located in the region downstream of marker M350 (Fig. 4). However, progeny testing of recombinants HIF1-95, HIF2-37, and HIF2-112 also proved that qGL4 was heterozygous, and it should be located in the region upstream of marker K4-5 (Fig. 4). For each recombinant, the average value of GL of 9311 homozygous progenies was about 0.2 mm longer that of Cypress homozygous progenies (Fig. 4), indicating that the additive effect of the candidate gene on GL was about 0.1 mm, which was the same level in HIF3 (0.12 mm), and about half of those in HIF1 (0.23 mm) and HIF2 (0.28 mm) (Table 2). Moreover, grains of line HIF1-42 (9311 homozygous in the region upstream of marker K4-5) were longer than those of line HIF1-7 (Cypress homozygous in the region upstream of marker K4-5); grains of line HIF2-112 (9311 homozygous in the region downstream of marker M350) were also longer than those of line HIF2-37 (Cypress homozygous in the region downstream of marker M350) (Fig. 4). These results indicated that qGL4 might contained two closely linked minor QTL, one was located downstream of marker M350 (named qGL4.1), and the other was located upstream of marker K4-5 (named qGL4.2) (Fig. 4).
Fine mapping of qGL4. The progeny test results represented the genotypes reflected by the grain length differences between two types of homozygous progenies isolated from the recombinant; if the P value was less than 0.05, the candidate gene was considered to be located in the heterozygous fragment; otherwise, it was considered to be located in the homozygous fragment. HIF, heterogeneous inbred family. Black, white, and grey blocks represent the genotypes of homozygous 9311, homozygous Cypress, and heterozygous genotype, respectively. Data are means ± SD. **P < 0.01, *P < 0.05, based on Student’s two-tailed t-test
In order to separately confirm the genetic effects of qGL4.1 and qGL4.2, HIF1 qGL4.1, HIF1 qGL4.2, HIF2 qGL4.1, and HIF2 qGL4.2 were developed from HIF1-42, HIF1-95, HIF2-172, and HIF2-112, respectively. In these HIFs, one locus was heterozygous, and the other was homozygous (Fig. 4). And segregating populations of each HIFs consisting of 192 individuals were planted. A QTL analysis showed that the genetic effects of qGL4.1 and qGL4.2 for GL were similar, had an additive effect of 0.10–0.14 mm, and explained 31.3–39.6% of the variations in the HIF segregating populations (Table 3). qGL4.1 had an additive effect of 0.26 g and 0.43 g for TGW in HIF1 qGL4.1 and HIF2 qGL4.1, respectively. And the additive effects of qGL4.2 for TGW were 0.74 g and 0.61 g in HIF1 qGL4.2 and HIF2 qGL4.2, respectively (Table 3). For qGL4.1 and qGL4.2, the alleles from 9311 increased both GL and TGW (Table 3). Furthermore, significant differences were found in GL and TGW between the 9311 and Cypress homozygous plants in all the HIF populations, and both qGL4.1 and qGL4.2 were semidominant on GL and TGW (Fig. 5a–h, Table 3). These results proved that qGL4 contained two closely linked minor QTL, qGL4.1 and qGL4.2. And qGL4.1 was heterozygous, and qGL4.2 was homozygous in HIF3, which could explain why the genetic effect of qGL4 in HIF3 was much lower than in HIF1 and HIF2 (Fig. 3a–d, Table 2).
Grain size difference among three genotype plants in the HIF-F2 populations of qGL4.1 and qGL4.2. a, b 1000-grain weight and grain length differences in HIF1 qGL4.1. c, d 1000-grain weight and grain length differences in HIF1 qGL4.2. e, f 1000-grain weight and grain length differences in HIF2 qGL4.1. g, h 1000-grain weight and grain length differences in HIF2 qGL4.2. A, B, and H represent 9311 homozygous, Cypress homozygous, and heterozygous genotypes, respectively. Data are means ± SD. P, Student’s t-test
Fine mapping of qGL4.1 and qGL4.2
To fine-map qGL4.1, three new polymorphic markers (C4-1, C4-3, and C4-7) were developed, and the progeny testing of 12 recombinants from HIF1 qGL4.1 and HIF2 qGL4.1 segregating populations were conducted. Results of progeny testing showed that recombinants YP27, YP19, and YP22 carried homozygous genotype of qGL4.1, proving that qGL4.1 should be located upstream of marker C4-1. YP26 carried Cypress homozygous, and YP18 carried heterozygous genotype of qGL4.1, indicating that qGL4.1 should be located downstream of C4-7. Therefore, qGL4.1 should be located in the 1304-kb region flanked by markers C4-1 and C4-7 (Fig. 6a, Table S3).
Fine mapping of qGL4.1 and qGL4.2. a Fine mapping of qGL4.1. b Fine mapping of qGL4.2. The progeny test results represented the genotypes reflected by the grain length differences between two types of homozygous progenies isolated from the recombinant; if the P value was less than 0.05, the candidate gene was considered to be heterozygous; otherwise, it was considered to be homozygous. Black, white, and gray blocks represent 9311 homozygous, Cypress homozygous, and heterozygous genotypes, respectively
To fine-map qGL4.2, the progeny testing of 13 recombinants from HIF1 qGL4.2 and HIF2 qGL4.2 segregating populations were conducted. Genotyping of newly designed polymorphic markers finally narrowed down qGL4.2 to a 423-kb region between markers C4-17 and C4-10 (Fig. 6b, Table S3).
Discussion
QTL for grain size and weight
Rice grain size is a trait of great application value. In the past two decades, significant progress has been achieved in the map-based cloning of QTL for grain size and weight (Fan and Li 2019; Li et al. 2018; Ren et al. 2023). In this study, QTL mapping for grain size and weight was carried out using a RIL population derived from a cross between indica variety 9311 and japonica variety Cypress. A total of 10 QTL on 5 chromosomes were found to control grain size-related traits, and 8 of them were repeatedly detected in 2 years. Three major QTL, qGL7b, qGW7, and qLWR7, were co-located at the same locus, and the allele from Cypress increased GL and LWR and decreased GW (Table 1). And the cloned GL7/GW7/SLG7 was found to be located in this interval (Fig. 2b). Highly-expressed alleles of GL7/GW7/SLG7 produce slender grains with low chalkiness. And it has few negative effects on grain yield-related traits (Wang et al. 2015b, 2015c; Zhou et al. 2015). Qiu et al. (2012) identified a QTL (qSS7) for GL, GW, and LWR, using a segregating population derived from a cross between an indica variety Zhenshan97 and a chromosomal segment substitution line of a japonica variety Cypress within the genetic background of Zhenshan97. And qSS7 was fine-mapped to a 23-kb region containing the GL7/GW7/SLG7 gene. These indicated that qGL7b, qGW7, and qLWR7 were GL7/GW7/SLG7. It positively controls grain length and negatively regulates grain width and, as a result, does not influence grain weight (Qiu et al. 2012; Wang et al. 2015b, 2015c; Zhou et al. 2015). In addition, several minor QTL for grain size and weight were found on chromosomes 2, 3, 4, 5, and 7. qGW2, qGL5, and qGL7a only involved grain shape changes without affecting grain weight in primary mapping population (Table 1). qGL4 had a relatively large effect on GL and TGW, and it was further validated and dissected. The genetic effects of other QTL need to be further verified. It is worth noticing that the GL of 9311 and Cypress were similar (Fig. 1a). However, four QTL for GL were identified, and both the parents carried two QTL that enhanced GL (Table 1).
Validation of QTL in HIFs
Many traits are controlled by multiple QTL and affected by the environment. In order to verify the genetic effect of QTL and conduct fine-mapping, it is required to produce as isogenic a background as possible to avoid the statistical noise caused by variations in non-critical parts of the genome. Near isogenic lines (NILs) are ideal genetic materials for this purpose. However, their generation is time-consuming, especially for the minor QTL. NIL-F2 populations developed from HIFs of the RIL population are also ideal materials for validating minor QTL (Tuinstra et al. 1997). In this study, a total of 600 RILs were developed. And 181 lines were randomly selected for primary mapping (F8 for genotyping, F8 and F9 for phenotyping). Based on the results of QTL mapping, three HIFs (HIF1, HIF2, and HIF3) of qGL4 were identified. HIF1 came from the 181 RILs for preliminary mapping, and HIF2 and HIF3 were identified from the rest 419 RILs. The genetic effect of qGL4 was validated independently using the HIF-F2 populations. We believe developing a relatively large RIL population and conducting primary mapping in appropriately early generations would ensure the identification of HIFs.
Candidate genes underlying qGL4.1 and qGL4.2
The genetic effect of qGL4 was confirmed using three HIF-F2 populations. qGL4 was a stably expressed QTL with relatively large effects on GL and TGW. It was further dissected into two closed linked minor QTL, qGL4.1 and qGL4.2. Clustering distribution of QTL controlling grain size and weight has been frequently reported (Cheng et al. 2021; Guo et al. 2013; Li et al. 2011; Wang et al. 2015a; Zhang et al. 2016). qGL4.1 was further delimited to the 1304-kb region flanked by markers C4-1 and C4-7 (Chr4, 29.07–30.37 Mb) (Fig. 6a). And qGL4.2 was narrowed down to a 423-kb region between markers C4-17 and C4-10 (Chr4, 31.71–32.12 Mb) (Fig. 6b).
Rice NARROW LEAF 1 (NAL1) is a key gene regulating leaf size, chlorophyll content, adventitious root formation, grain number per panicle, and grain yield (Fujita et al. 2013; Jiang et al. 2015; Xu et al. 2015; Zhai et al. 2023; Zhang et al. 2014). NAL1 (Chr4, 31.20 Mb) is located between qGL4.1 (Chr4, 29.07–30.37 Mb) and qGL4.2 (Chr4, 31.71–32.12 Mb) (Fig. 6a, b). It was reported that the grain weight of 9311 was significantly higher than a near isogenic line carrying the NAL1 region from Nipponbare (Zhang et al. 2014). An integrated genome-wide association study also regarded NAL1 as the gene that underlay a GL QTL on chromosome 4 (Wei et al. 2021). However, grain weight alterations were not reported in nal1 mutants, overexpression lines, and knockdown lines (Fujita et al. 2013; Jiang et al. 2015; Xu et al. 2015; Zhai et al. 2023; Zhang et al. 2014). From others and our data, we concluded that NAL1 was not a QTL for grain size and weight, and the grain weight difference between the NILs reported by Zhang et al. (2014) was possibility caused by the introduction of qGL4, but not the closed linked NAL1.
A recent study indicated that OsMADS17 simultaneously increasing grain number, GL, GW, and TGW through decreased translation efficiency caused by a 65-bp deletion in the 5’ UTR region (Li et al. 2023). OsMADS17 (Chr4, 29.31 Mb) was located in the 1304-kb interval of qGL4.1 (Chr4, 29.07–30.37 Mb) (Fig. 6a). However, the 65-bp deletion in the 5’ UTR region of OsMADS17 was not found in 9311 and Cypress. And qGL4.1 only affected GL, but not GW, which was different from OsMADS17 (Li et al. 2023). So, we speculated that OsMADS17 should not be the functional gene underlying qGL4.1.
As the mapping interval of qGL4.1 was relatively large, we checked the candidate genes of qGL4.2. According to the Rice Genome Annotation Project (RGAP) website, 38 expressed genes were identified in the 423-kb region of qGL4.2. Based on the gene annotation information and expression pattern, three genes were preferentially selected as the candidates: LOC_Os04g53310 encodes a soluble starch synthase, LOC_Os04g53510 encodes an F-box domain containing protein, and LOC_Os04g53700 encodes a zinc finger protein. Further fine mapping and transgenic studies are required to identify the candidate genes of qGL4.1 and qGL4.2.
Conclusion
Rice grain size is a key quality and yield trait. In this study, using a RIL population derived from a cross between indica variety 9311 and japonica variety Cypress, we identified 10 QTL affecting grain size and weight in 2 years. Including a major QTL simultaneously controlled GL, GW, and LWR, and it should be the cloned gene GL7/GW7/SLG7. In addition, several minor QTL that affect grain size and weight were identified. And the genetic effect of qGL4 was confirmed using three HIF-F2 populations. It was further dissected into two closed linked minor QTL, qGL4.1 and qGL4.2. By progeny testing, qGL4.1 and qGL4.2 were successfully delimited to the intervals of C4-1 ~ C4-7 (1304-kb) and C4-17 ~ C4-10 (423-kb), respectively. The closely linked InDel markers, such as C4-1, C4-7, C4-17, and C4-10, could be used in the marker assistant selection of qGL4.1 and qGL4.2 to improve rice yield and quality. These findings laid a foundation for map-based cloning of qGL4.1 and qGL4.2.
Data availability
The data used in this study are available from the corresponding author on reasonable request.
Abbreviations
- QTL :
-
Quantitative trait loci
- RIL :
-
Recombinant inbred line
- GL :
-
Grain length
- GW :
-
Grain width
- LWR :
-
Grain length-to-width ratio
- TGW :
-
1000-Grain weight
- SNP :
-
Single nucleotide polymorphism
- GBS :
-
Genotyping-by-sequencing
- HIF :
-
Heterogeneous inbred family
- KASP :
-
Kompetitive allele-specific PCR
- LOD :
-
Logarithm of odds
References
Broman KW, Wu H, Sen S, Churchill GA (2003) R/qtl: QTL mapping in experimental crosses. Bioinformatics 19(7):889–890. https://doi.org/10.1093/bioinformatics/btg112
Che R, Tong H, Shi B, Liu Y, Fang S, Liu D, Xiao Y, Hu B, Liu L, Wang H, Zhao M, Chu C (2015) Control of grain size and rice yield by GL2-mediated brassinosteroid responses. Nat Plants 2:15195. https://doi.org/10.1038/nplants.2015.195
Cheng YC, Li G, Yin M, Adegoke TV, Wang YF, Tong XH, Zhang J, Ying JZ (2021) Verification and dissection of one quantitative trait locus for grain size and weight on chromosome 1 in rice. Sci Rep 11(1):18252. https://doi.org/10.1038/s41598-021-97622-8
Cooper NTW, Siebenmorgen TJ, Counce PA (2008) Effects of nighttime temperature during kernel development on rice physicochemical properties. Cereal Chem 85(3):276–282. https://doi.org/10.1094/CCHEM-85-3-0276
Duan P, Ni S, Wang J, Zhang B, Xu R, Wang Y, Chen H, Zhu X, Li Y (2015) Regulation of OsGRF4 by OsmiR396 controls grain size and yield in rice. Nat Plants 2:15203. https://doi.org/10.1038/nplants.2015.203
Fan Y, Li Y (2019) Molecular, cellular and Yin-Yang regulation of grain size and number in rice. Mol Breeding 39(12):163. https://doi.org/10.1007/s11032-019-1078-0
Fan C, Xing Y, Mao H, Lu T, Han B, Xu C, Li X, Zhang Q (2006) GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor Appl Genet 112(6):1164–1171. https://doi.org/10.1007/s00122-006-0218-1
Fujita D, Trijatmiko KR, Tagle AG, Sapasap MV, Koide Y, Sasaki K, Tsakirpaloglou N, Gannaban RB, Nishimura T, Yanagihara S, Fukuta Y, Koshiba T, Slamet-Loedin IH, Ishimaru T, Kobayashi N (2013) NAL1 allele from a rice landrace greatly increases yield in modern indica cultivars. Proc Natl Acad Sci U S A 110(51):20431–20436. https://doi.org/10.1073/pnas.1310790110
Gao ZY, Zhao SC, He WM, Guo LB, Peng YL, Wang JJ, Guo XS, Zhang XM, Rao YC, Zhang C, Dong GJ, Zheng FY, Lu CX, Hu J, Zhou Q, Liu HJ, Wu HY, Xu J, Ni PX, Zeng DL, Liu DH, Tian P, Gong LH, Ye C, Zhang GH, Wang J, Tian FK, Xue DW, Liao Y, Zhu L, Chen MS, Li JY, Cheng SH, Zhang GY, Wang J, Qian Q (2013) Dissecting yield-associated loci in super hybrid rice by resequencing recombinant inbred lines and improving parental genome sequences. Proc Natl Acad Sci U S A 110(35):14492–14497. https://doi.org/10.1073/pnas.1306579110
Guo L, Wang K, Chen J, Huang D, Fan Y, Zhuang J (2013) Dissection of two quantitative trait loci for grain weight linked in repulsion on the long arm of chromosome 1 of rice (Oryza sativa L.). Crop J 1(1):70–76. https://doi.org/10.1016/j.cj.2013.07.008
Hu Z, He H, Zhang S, Sun F, Xin X, Wang W, Qian X, Yang J, Luo X (2012) A Kelch motif-containing serine/threonine protein phosphatase determines the large grain QTL trait in rice. J Integr Plant Biol 54(12):979–990. https://doi.org/10.1111/jipb.12008
Hu J, Wang Y, Fang Y, Zeng L, Xu J, Yu H, Shi Z, Pan J, Zhang D, Kang S, Zhu L, Dong G, Guo L, Zeng D, Zhang G, Xie L, Xiong G, Li J, Qian Q (2015) A rare allele of GS2 enhances grain size and grain yield in rice. Mol Plant 8(10):1455–1465. https://doi.org/10.1016/j.molp.2015.07.002
Huang X, Feng Q, Qian Q, Zhao Q, Wang L, Wang A, Guan J, Fan D, Weng Q, Huang T, Dong G, Sang T, Han B (2009a) High-throughput genotyping by whole-genome resequencing. Genome Res 19(6):1068–1076. https://doi.org/10.1101/gr.089516.108
Huang X, Qian Q, Liu Z, Sun H, He S, Luo D, Xia G, Chu C, Li J, Fu X (2009b) Natural variation at the DEP1 locus enhances grain yield in rice. Nat Genet 41(4):494–497. https://doi.org/10.1038/ng.352
Ishimaru K, Hirotsu N, Madoka Y, Murakami N, Hara N, Onodera H, Kashiwagi T, Ujiie K, Shimizu B, Onishi A, Miyagawa H, Katoh E (2013) Loss of function of the IAA-glucose hydrolase gene TGW6 enhances rice grain weight and increases yield. Nat Genet 45(6):707–711. https://doi.org/10.1038/ng.2612
Jiang D, Fang J, Lou L, Zhao J, Yuan S, Yin L, Sun W, Peng L, Guo B, Li X (2015) Characterization of a null allelic mutant of the rice NAL1 gene reveals its role in regulating cell division. PLoS One 10(2):e0118169. https://doi.org/10.1371/journal.pone.0118169
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newberg LA (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1(2):174–181. https://doi.org/10.1016/0888-7543(87)90010-3
Li Y, Fan C, Xing Y, Jiang Y, Luo L, Sun L, Shao D, Xu C, Li X, Xiao J, He Y, Zhang Q (2011) Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nat Genet 43(12):1266–1269. https://doi.org/10.1038/ng.977
Li N, Xu R, Duan P, Li Y (2018) Control of grain size in rice. Plant Reprod 31(3):237–251. https://doi.org/10.1007/s00497-018-0333-6
Li Y, Wu S, Huang Y, Ma X, Tan L, Liu F, Lv Q, Zhu Z, Hu M, Fu Y, Zhang K, Gu P, Xie D, Sun H, Sun C (2023) OsMADS17 simultaneously increases grain number and grain weight in rice. Nat Commun 14(1):3098. https://doi.org/10.1038/s41467-023-38726-9
Liu L, Tong H, Xiao Y, Che R, Xu F, Hu B, Liang C, Chu J, Li J, Chu C (2015) Activation of Big Grain1 significantly improves grain size by regulating auxin transport in rice. Proc Natl Acad Sci U S A 112(35):11102–11107. https://doi.org/10.1073/pnas.1512748112
Liu J, Chen J, Zheng X, Wu F, Lin Q, Heng Y, Tian P, Cheng Z, Yu X, Zhou K, Zhang X, Guo X, Wang J, Wang H, Wan J (2017) GW5 acts in the brassinosteroid signalling pathway to regulate grain width and weight in rice. Nat Plants 3:17043. https://doi.org/10.1038/nplants.2017.43
Qi P, Lin YS, Song XJ, Shen JB, Huang W, Shan JX, Zhu MZ, Jiang L, Gao JP, Lin HX (2012) The novel quantitative trait locus GL3.1 controls rice grain size and yield by regulating Cyclin-T1;3. Cell Res 22(12):1666–1680. https://doi.org/10.1038/cr.2012.151
Qiu X, Gong R, Tan Y, Yu S (2012) Mapping and characterization of the major quantitative trait locus qSS7 associated with increased length and decreased width of rice seeds. Theor Appl Genet 125(8):1717–1726. https://doi.org/10.1007/s00122-012-1948-x
Ren D, Ding C, Qian Q (2023) Molecular bases of rice grain size and quality for optimized productivity. Sci Bull (Beijing) 68(3):314–350. https://doi.org/10.1016/j.scib.2023.01.026
Shi CL, Dong NQ, Guo T, Ye WW, Shan JX, Lin HX (2020) A quantitative trait locus GW6 controls rice grain size and yield through the gibberellin pathway. Plant J 103(3):1174–1188. https://doi.org/10.1111/tpj.14793
Shomura A, Izawa T, Ebana K, Ebitani T, Kanegae H, Konishi S, Yano M (2008) Deletion in a gene associated with grain size increased yields during rice domestication. Nat Genet 40(8):1023–1028. https://doi.org/10.1038/ng.169
Si L, Chen J, Huang X, Gong H, Luo J, Hou Q, Zhou T, Lu T, Zhu J, Shangguan Y, Chen E, Gong C, Zhao Q, Jing Y, Zhao Y, Li Y, Cui L, Fan D, Lu Y, Weng Q, Wang Y, Zhan Q, Liu K, Wei X, An K, An G, Han B (2016) OsSPL13 controls grain size in cultivated rice. Nat Genet 48(4):447–456. https://doi.org/10.1038/ng.3518
Song XJ, Huang W, Shi M, Zhu MZ, Lin HX (2007) A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat Genet 39(5):623–630. https://doi.org/10.1038/ng2014
Sun S, Wang L, Mao H, Shao L, Li X, Xiao J, Ouyang Y, Zhang Q (2018) A G-protein pathway determines grain size in rice. Nat Commun 9(1):851. https://doi.org/10.1038/s41467-018-03141-y
Takano-Kai N, Jiang H, Kubo T, Sweeney M, Matsumoto T, Kanamori H, Padhukasahasram B, Bustamante C, Yoshimura A, Doi K, McCouch S (2009) Evolutionary history of GS3, a gene conferring grain length in rice. Genetics 182(4):1323–1334. https://doi.org/10.1534/genetics.109.103002
Tuinstra MR, Ejeta G, Goldsbrough PB (1997) Heterogeneous inbred family (HIF) analysis: a method for developing near-isogenic lines that differ at quantitative trait loci. Theor Appl Genet 95(5):1005–1011. https://doi.org/10.1007/s001220050654
Wang S, Wu K, Yuan Q, Liu X, Liu Z, Lin X, Zeng R, Zhu H, Dong G, Qian Q, Zhang G, Fu X (2012) Control of grain size, shape and quality by OsSPL16 in rice. Nat Genet 44(8):950–954. https://doi.org/10.1038/ng.2327
Wang LL, Chen YY, Guo L, Zhang HW, Fan YY, Zhuang JY (2015a) Dissection of qTGW1.2 to three QTLs for grain weight and grain size in rice (Oryza sativa L.). Euphytica 202(1):119–127. https://doi.org/10.1007/s10681-014-1237-7
Wang S, Li S, Liu Q, Wu K, Zhang J, Wang S, Wang Y, Chen X, Zhang Y, Gao C, Wang F, Huang H, Fu X (2015b) The OsSPL16-GW7 regulatory module determines grain shape and simultaneously improves rice yield and grain quality. Nat Genet 47(8):949–954. https://doi.org/10.1038/ng.3352
Wang Y, Xiong G, Hu J, Jiang L, Yu H, Xu J, Fang Y, Zeng L, Xu E, Xu J, Ye W, Meng X, Liu R, Chen H, Jing Y, Wang Y, Zhu X, Li J, Qian Q (2015c) Copy number variation at the GL7 locus contributes to grain size diversity in rice. Nat Genet 47(8):944–948. https://doi.org/10.1038/ng.3346
Wang S, Basten CJ, Zeng ZB (2007) Windows QTL Cartographer 2.5 Department of Statistics, North Carolina State University, Raleigh, NC. http://statgen.ncsu.edu/qtlcart/WQTLCart.htm
Wei X, Qiu J, Yong K, Fan J, Zhang Q, Hua H, Liu J, Wang Q, Olsen KM, Han B, Huang X (2021) A quantitative genomics map of rice provides genetic insights and guides breeding. Nat Genet 53(2):243–253. https://doi.org/10.1038/s41588-020-00769-9
Weng J, Gu S, Wan X, Gao H, Guo T, Su N, Lei C, Zhang X, Cheng Z, Guo X, Wang J, Jiang L, Zhai H, Wan J (2008) Isolation and initial characterization of GW5, a major QTL associated with rice grain width and weight. Cell Res 18(12):1199–1209. https://doi.org/10.1038/cr.2008.307
Xing Y, Zhang Q (2010) Genetic and molecular bases of rice yield. Annu Rev Plant Biol 61:421–442. https://doi.org/10.1146/annurev-arplant-042809-112209
Xu JL, Wang Y, Zhang F, Wu Y, Zheng TQ, Wang YH, Zhao XQ, Cui YR, Chen K, Zhang Q, Lin HX, Li JY, Li ZK (2015) SS1 (NAL1)- and SS2-mediated genetic networks underlying source-sink and yield traits in rice (Oryza sativa L.). PloS One 10(7):e0132060. https://doi.org/10.1371/journal.pone.0132060
Yang W, Guo Z, Huang C, Duan L, Chen G, Jiang N, Fang W, Feng H, Xie W, Lian X, Wang G, Luo Q, Zhang Q, Liu Q, Xiong L (2014) Combining high-throughput phenotyping and genome-wide association studies to reveal natural genetic variation in rice. Nat Commun 5:5087. https://doi.org/10.1038/ncomms6087
Yang W, Wu K, Wang B, Liu H, Guo S, Guo X, Luo W, Sun S, Ouyang Y, Fu X, Chong K, Zhang Q, Xu Y (2021) The RING E3 ligase CLG1 targets GS3 for degradation via the endosome pathway to determine grain size in rice. Mol Plant 14(10):1699–1713. https://doi.org/10.1016/j.molp.2021.06.027
Yu J, Hu S, Wang J, Wong GK, Li S, Liu B, Deng Y, Dai L, Zhou Y, Zhang X, Cao M, Liu J, Sun J, Tang J, Chen Y, Huang X, Lin W, Ye C, Tong W, Cong L, Geng J, Han Y, Li L, Li W, Hu G, Huang X, Li W, Li J, Liu Z, Li L, Liu J, Qi Q, Liu J, Li L, Li T, Wang X, Lu H, Wu T, Zhu M, Ni P, Han H, Dong W, Ren X, Feng X, Cui P, Li X, Wang H, Xu X, Zhai W, Xu Z, Zhang J, He S, Zhang J, Xu J, Zhang K, Zheng X, Dong J, Zeng W, Tao L, Ye J, Tan J, Ren X, Chen X, He J, Liu D, Tian W, Tian C, Xia H, Bao Q, Li G, Gao H, Cao T, Wang J, Zhao W, Li P, Chen W, Wang X, Zhang Y, Hu J, Wang J, Liu S, Yang J, Zhang G, Xiong Y, Li Z, Mao L, Zhou C, Zhu Z, Chen R, Hao B, Zheng W, Chen S, Guo W, Li G, Liu S, Tao M, Wang J, Zhu L, Yuan L, Yang H (2002) A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science 296(5565):79–92. https://doi.org/10.1126/science.1068037
Yuan H, Qin P, Hu L, Zhan S, Wang S, Gao P, Li J, Jin M, Xu Z, Gao Q, Du A, Tu B, Chen W, Ma B, Wang Y, Li S (2019) OsSPL18 controls grain weight and grain number in rice. J Genet Genomics 46(1):41–51. https://doi.org/10.1016/j.jgg.2019.01.003
Zhai L, Yan A, Shao K, Wang S, Wang Y, Chen ZH, Xu J (2023) Large Vascular Bundle Phloem Area 4 enhances grain yield and quality in rice via source-sink-flow. Plant Physiol 191(1):317–334. https://doi.org/10.1093/plphys/kiac461
Zhang X, Wang J, Huang J, Lan H, Wang C, Yin C, Wu Y, Tang H, Qian Q, Li J, Zhang H (2012) Rare allele of OsPPKL1 associated with grain length causes extra-large grain and a significant yield increase in rice. Proc Natl Acad Sci U S A 109(52):21534–21539. https://doi.org/10.1073/pnas.1219776110
Zhang GH, Li SY, Wang L, Ye WJ, Zeng DL, Rao YC, Peng YL, Hu J, Yang YL, Xu J, Ren DY, Gao ZY, Zhu L, Dong GJ, Hu XM, Yan MX, Guo LB, Li CY, Qian Q (2014) LSCHL4 from Japonica Cultivar, which is allelic to NAL1, increases yield of indica super rice 93–11. Mol Plant 7(8):1350–1364. https://doi.org/10.1093/mp/ssu055
Zhang HW, Fan YY, Zhu YJ, Chen JY, Yu SB, Zhuang JY (2016) Dissection of the qTGW1.1 region into two tightly-linked minor QTLs having stable effects for grain weight in rice. BMC Genet 17(1):98. https://doi.org/10.1186/s12863-016-0410-5
Zhao DS, Li QF, Zhang CQ, Zhang C, Yang QQ, Pan LX, Ren XY, Lu J, Gu MH, Liu QQ (2018) GS9 acts as a transcriptional activator to regulate rice grain shape and appearance quality. Nat Commun 9(1):1240. https://doi.org/10.1038/s41467-018-03616-y
Zhou Y, Miao J, Gu H, Peng X, Leburu M, Yuan F, Gu H, Gao Y, Tao Y, Zhu J, Gong Z, Yi C, Gu M, Yang Z, Liang G (2015) Natural variations in SLG7 regulate grain shape in rice. Genetics 201(4):1591–1599. https://doi.org/10.1534/genetics.115.181115
Acknowledgements
We would like to express our gratitude to Dr. Hao Zhou from Sichuan Agricultural University for helping us analyze the GBS data.
Funding
This work was supported by grants from the National Key R&D Program of China (2021YFD1200500), the Natural Science Foundation of Anhui Province (2008085QC120), the National Natural Science Foundation (31901529), the Major Scientific Technological Project of Anhui Province (202003a06020005 and 2021d06050002), and the National Rice Industry Technology System (CARS-01–063).
Author information
Authors and Affiliations
Contributions
PY and ZFL designed the research; PY and CJZ performed most of the experiments; TCM developed the RILs; JFX, KNZ, and YLW participated in part of agronomic trait statistics and field experiments. PY, CJZ, and ZFL performed data analysis and manuscript writing; all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All authors approved the submission.
Consent to participate
N/A.
Consent for publication
Yes.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peng Yun and Caijuan Zhang are co-first authors of the article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yun, P., Zhang, C., Ma, T. et al. Identification of qGL4.1 and qGL4.2, two closely linked QTL controlling grain length in rice. Mol Breeding 44, 11 (2024). https://doi.org/10.1007/s11032-024-01447-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-024-01447-y