Abstract
Gummy stem blight (GSB), a common disease of all major cucurbits, is caused by the fungus Didymella bryoniae. It results in serious losses in fruit production, which in cucumber can be up to 80% or more. Because the severity of the disease varies from season to season and also because of the harm to the environment caused by using pesticides to control the disease, the best method for overcoming GSB in cucumber is to develop more resistant cultivars by molecular breeding. There are no reports on molecular markers for use in breeding GSB resistance and no studies on chromosomal mapping of resistance. In this paper, a set of 160 F9 recombinant inbred lines (RILs) were derived from the cross between the wild-type GSB-resistant cucumber accession PI 183967 and the cultivated GSB-susceptible accession 931. A total of 2112 pairs of SSR primers were used to study the inheritance of GSB resistance and to detect quantitative trait loci (QTLs) conferring resistance in the cucumber stem. Genetic analysis indicated that resistance to GSB in PI 183967 was quantitative and mainly governed by three pairs of additive epistatic major genes. Five QTLs, gsb-s1.1, gsb-s2.1, gsb-s6.1, gsb-s6.2, and gsb-s6.3, for resistance to GSB in cucumber stems were detected. The loci gsb-s1.1 and gsb-s2.1 with phenotypic variations of 8.7 and 6.7% were mapped to chromosomes (Chr.) 1 and 2, respectively. The loci gsb-s6.1, gsb-s6.2, and gsb-s6.3 were linked on Chr.6. Locus gsb-s6.2 accounted for the highest phenotypic variation of 22.7% and was flanked by markers SSR04083 and SSR02940 with genetic distances of 5.0 and 1.8 cM, respectively. There were 117 candidate genes predicted between SSR04083 and SSR02940, of which 14 were related to disease resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gummy stem blight (GSB) is a destructive disease of cucurbits. All above ground and vegetative parts are affected, including leaves, petioles, vines, stems, tendrils, pedicels, flowers, peduncles, fruit, and seed. Initially, the asexual reproductive stage (anamorph) of the causal ascomycete was called Ascochyta cucumis Fautrey & Roum., and the sexual reproductive stage (teleomorph) was Didymella bryoniae (Auersw.) Rehm (Chiu and Walker 1949; Sherf and Mac Nab 1986). The anamorph form was renamed to Stagonosporopsis cucurbitacearum and now is known to be three genetically distinct species: S. cucurbitacearum (syn. Didymella bryoniae), S. citrulli, and S. caricae (Stewart et al. 2015), each able to cause the disease in cucurbits. In cucumber, Cucumis sativus L., GSB normally causes 15 to 30% losses in production, but severe infections can result in losses of 80% or greater (Wehner and Shetty 2000).
Reports on the inheritance of GSB resistance in cucurbitaceae are limited and somewhat controversial. Norton (1979) generated genetic populations of watermelon (Citrullus lanatus (Thunb.) Matsum. & Nakai) using GSB-resistant PI 189225 and GSB-susceptible ‘Charleston Gray’ and discovered that resistance is controlled by a single recessive gene (db/db). Amand and Wehner (2001) reasoned that the major effect conferred by db/db is complemented by the expression of other modifier genes for GSB resistance in stems and leaves. They found that genetic factors are weaker than environmental factors. In melons (Cucumis melo L.), five relatively independent single genes conferring resistance to GSB were identified from different cultigens, among which four were dominant and one was recessive (Zuniga et al. 1999; Wako et al. 2002; Frantz and Jahn 2004).
So far, there are no reports on molecular markers and genetic mapping of GSB resistance in the stems of cucumber. In melon, Joseph (2009) identified four amplified fragment length polymorphism (AFLP) markers linked to resistance using the GSB-resistant line PI 420145. These had genetic distances of 2.0, 6.0, 5.4, and 6.0 cM. Molecular markers CMCT505, CMTC160a+b220 and ISSR-57560, ISSR-100900, and CMTA170a have been closely linked with the melon GSB-resistant genes Gsb-1, Gsb-2, Gsb-3, and Gsb-4. Among these markers, CMTA170a and Gsb-4 had the shortest genetic distance of 5.14 cM. The Sb-x GSB-resistant gene in melon was identified and mapped to the LG4 linkage group using simple sequence repeats (SSR) markers and a double haploid (DH) melon population. Ha et al. (2010) mapped Sb-1, a gene for resistance, to the melon LG1 linkage group. These results provide valuable references for the identification of GSB-resistant genetic markers in cucumbers.
In this study, we used wild-type cucumber and recombinant inbred lines (RILs) with distinct resistance to GSB to analyze cucumber stem GSB inheritance. Genetic analysis and mapping of quantitative trait loci (QTLs) were performed to provide a basis for fine mapping and molecular cloning of genes for GSB resistance and for future marker-assisted selection (MAS) to develop new GSB-resistant cultivars.
Materials and methods
Plant materials
A wild-type GSB-resistant cucumber accession PI 183967 (Cucumis sativus var. hardiwickii (Royle) Alef.) was crossed with a cultivated GSB-susceptible cucumber accession designated 931 (C. sativus var. sativus). After single seed descent (SSD) reproduction, a population was developed that consisted of 160 F9 RILs.
Disease resistance screening and symptom assessment
In autumn 2012 and spring 2013, the parents, F1, and RILs were planted. The experiments were repeated three times, and a total of 18 plants were grown out for each line. All of the field and greenhouse experiments were conducted at the Nankou Farm, Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences. The distances between plants and rows were 25 and 55 cm, respectively. The plants were grown using standard cultivation practices. Well-grown and healthy stems from the parents, F1, and RILs were harvested, trimmed to 15 cm, and arranged randomly with three replications of six stems each.
Didymella bryoniae was grown on cucumber fruits for 7–10 days to allow pycnidia formation, and the spores were collected by scraping the infested fruits and suspended in 10 mL of sterile water. Spore concentration was measured with a hemocytometer and adjusted to 106 spores/mL. The ends of the trimmed cucumber stems were soaked in spore suspension for 30 min and then placed in a humidified chamber with the soaked ends facing the same direction. Disease resistance was rated 3–5 days after inoculation, and a disease index (DI) was calculated as a weighted mean according to the formula:
The disease rating scale for each stem was as follows: Grade 0: no symptoms, Grade 1: infected part of the stem was less than one fourth of the total stem, Grade 2: infected stem was one fourth to one half of the total stem, Grade 3: infected stem was one half to three fourths of the total stem, and Grade 4: infected stem was greater than three fourths of the total stem. The disease index was calculated as follows: high resistance (HR) 0 < DI ≤ 15, resistance (R) 15 < DI ≤ 35, medium resistance (MR) 35 < DI ≤ 55, susceptible (S) 55 < DI ≤ 80, and highly susceptible (HS) DI > 80.
Genetic analysis software and statistical analysis
The DI of each stem was recorded and summarized using Microsoft Excel 2003 and SAS 9.0. The mean DI of each line and the genetic parameters of the parents, F1, and RILs were calculated. A joint analysis assuming major genes plus the polygene model of RILs (Gai et al. 2003) was used for the GSB resistance analysis. The steps were to establish the genetic models, estimate the iterated expectation and conditional maximization algorithm, select the best genetic model from the value for AIC (Akaike information criterion), and evaluate goodness-of-fit through the least squares method. The optimized genetic model was then used for estimation of other genetic parameters.
SSR marker analysis
DNA was extracted from young leaf tissue of the parents, the F1, and each plant in the RILs population using a modified CTAB extraction procedure (Wang et al. 2006). The concentration and quality was determined after electrophoresis on 1% (w/v) agarose gels and then diluted with distilled water to 10 ng/uL. Each 15 μL of the PCR reaction mix contained 8.02 μL of double distilled water, 1.5 μL of 10 × buffer, 0.2 μL of dNTPs (10 mM), 0.08 μL of Taq DNA polymerase (10 U/μL), 0.6 μL of forward and reverse primers (50 ng/μL), and 4.0 μL of DNA (10 ng/μL). The PCR amplifications were performed by using a GeneAmp PCR system 9700 (Applied BiosystemsFoster City, CA) as follows: denaturation at 94 °C for 4 min, 35 cycles of denaturation at 94 °C for 15 s, annealing at 55 °C for 15 s, and extension at 72 °C for 30 s, with a final extension at 72 °C for 5 min. Amplified products were separated on 6.0% nondenaturing polyacrylamide gels at 150 V for 1 h, and the bands were visualized and photographed after silver staining.
A total of 2112 pairs of SSR primers were screened to identify polymorphisms between the parental lines (PI 183967 and 931) of the RILs populations. The development of the SSR primers used in this study was described by Ren et al. (2009). Polymorphic SSR primers were identified from individual plants of the RILs for linkage construction and QTL analysis.
Linkage construction and QTL mapping
JoinMap 4.0 (Van Ooijen 2006) was used to develop linkage groups. Chromosomal assignment of the linkage groups (LGs) was based on common markers between the present map and the high-resolution cucumber genetic map produced by Ren et al. (2009). Marker data were assigned to LGs by using a minimum logarithm of odds (LOD) likelihood score of 2.5. The Kosambi map function (Kosambi 1944) was used to calculate the genetic distance between markers. An interval mapping analysis was conducted by using MapQTL 4.0 (Van Ooijen et al. 2000) to detect QTLs. Permutation tests were conducted to assess LOD threshold at α = 0.05 level. Each locus was named by an abbreviation of the trait followed by the chromosome (Chr.) number and a locus number.
Sequence annotation and gene prediction in genomic regions harboring the major QTLs
Genomic regions for the locations of the major QTLs were annotated based on the whole genome sequence of the cucumber inbred line 9930 (Huang et al. 2009). The DNA sequences were aligned to the cucumber genome sequences using BLASTN at an E value cutoff of 1 × 10 to 1 × 20. Only matches with an identity of ≥95% were retained. Gene prediction was estimated using the computer programs BGF (http://bgf.genomics.org.cn) and veriWed by FGENESH (http://sunl.softberry.com/) (Salamov and Solovyev 2000). Gene annotation was carried out with InterProScan (http://www.ebi.ac.uk/InterProScan) (Zdobnov and Apweiler 2001).
Results
GSB resistance in cucumber stems of the progeny from the cross between PI 183967 × 931
In autumn 2012, the average DI of the GSB-resistant parent, PI 183967, was 27.09 (R), while the average DI of the GSB-susceptible parent, 931, was 77.38 (S). The average DI of the F1 was 96.43 (HS), indicating a transgressive segregation phenotype. In spring 2013, the average DI of the GSB-resistant parent was 21.88 (R), while that of the GSB-susceptible parent was 89.60 (HS) (Supplementary Fig. 1). The average DI of the F1 was 66.29 (S), intermediate between the two parents with a trend toward susceptibility. The mean DI of the RIL populations was intermediate between the two parents, but skewed toward the susceptible parent (Table 1). Genetic parameter analysis produced negative skewness values for the RIL population, further indicating that the DI of the RIL populations was inclined toward the 931 parent. There was a continuous distribution of DI values in the RIL populations, suggesting that the GSB-resistant phenotype was not conferred by a single major gene. These results suggest that the GSB-resistant phenotype of the parental line, PI 183967, has quantitative trait heritability characteristics.
Inheritance of GSB resistance in the cucumber stem
Genetic models were generated from the RILs populations and major gene plus polygene mixed genetic model analysis, together with the DI measurements in cucumber stems during autumn 2012 and spring 2013. The maximum likelihood (ML) and AIC of 35 of these models were estimated. Based on the principle that the smallest AIC value is the best-fitting genetic model, three models (F-4, F-1, and G-2) were prioritized based on the 2012 autumn data, and the lowest AIC was selected and further tested for goodness-of-fit (Table 2 and Table 3). The number of statistically different parameters was 8, 4, and 6 for F-4, F-1, and G-2, respectively. Since model F-1 had the lowest number, it was considered the best fit for the autumn 2012 GSB stem resistance inheritance analysis. The AIC values of F-1, F-4, and G-2 for the spring 2013 data were relatively lower than other models, and therefore, they were selected for goodness-of-fit testing (Table 3). The number of statistically different parameters was 3, 8, and 6 for the F-1, F-4, and G-2 models, respectively. Since model F-1 had the lowest number, it was considered the best-fit model for analysis of the 2013 spring GSB stem resistance inheritance. Taken together, the F-1 model (three pairs of additive epistatic major genes, no polygenes) appeared to be the best model for both seasons. The first order genetic parameters are shown in Supplementary Table 1. The test of the best-fit model indicated that the three major genes controlling the cucumber GSB-resistant phenotype all had additive and epistatic effects. The inheritance of these major genes was 98.63 and 97.34%, respectively, for autumn 2012 and spring 2013.
QTL mapping of GSB resistance in cucumber stems
A total of 2112 pairs of SSR primers were tested on cucumber lines PI 183967 and 931 resulting in 1125 that generated polymorphic amplicons (53.2%). From these, 350 that were evenly distributed over the seven cucumber chromosomes were selected for further analysis of the RIL population, and 307 were used to develop a genetic linkage map. The map included the seven linkage groups (corresponding to the seven chromosomes) and covered a genomic length of 993.3 cM, with an average distance of 3.23 cM (Supplementary Fig.2).
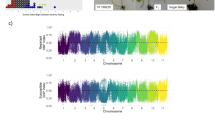
QTL mapping was conducted using this linkage map. In autumn 2012, two QTLs for GSB resistance in the cucumber stem, gsb-s1.1 and gsb-s6.1, were detected. They were located on Chr.1 and Chr.6, respectively. QTL gsb-s1.1 was between SSR12157 and SSR31116, with a LOD of 2.50, phenotypic variation of 8.7%, and additive effect of −9.54. QTL gsb-s6.1 was between SSR01012 and SSR03527, with genetic distances of 0.4 and 0.3 cM, respectively, with a LOD of 2.56, and phenotypic variation of 10.3%, (Table 4, Fig. 1). Both QTLs had negative effects, while the genes with positive effects were inherited from the GSB-susceptible parent, 931.
In spring 2013, three QTLs for GSB resistance were detected, which were gsb-s2.1, gsb-s6.2, and gsb-s6.3. Among these, gsb-s2.1 was located on Chr.2 between SSR13275 and SSR10064, with a LOD value of 2.53 and phenotypic variation of 6.7%. QTL gsb-s2.1 was inherited from the GSB-resistant parent PI 183967 and promoted the GSB-resistant phenotype. QTLs gsb-s6.2 and gsb-s6.3 were located on Chr.6 at 30.7 and 179.0 cM, between SSR04083-SSR02940 and SSR13251-SSR15516, respectively. QTLs gsb-s6.2 and gsb-s6.3 provided additive effects of −15.92 and −8.70 and contributed to the GSB-resistant phenotype by 22.7% (LOD = 7.30) and 8.7% (LOD = 3.24), respectively. Both gsb-s6.2 and gsb-s6.3 were inherited from the GSB-susceptible parent and can inhibit the GSB-resistant phenotype. Based on its contribution level and LOD, gsb-s6.2 was considered a major QTL, with genetic distances to nearby markers SSR04083 and SSR02940 of 5.0 and 1.8 cM, respectively.
Sequence annotation and gene prediction in genomic regions harboring QTL
QTLs gsb-s6.1 and gsb-s6.2 had both greater contributions and shorter genetic distances than the other QTLs and were detected in both seasons (Fig. 1) suggesting that GSB resistance exists in these two regions. BLAST analysis of the sequence in the SSR01012-SSR02940 region showed that this region has a physical distance of 1.27 Mbp and contains 117 annotated genes, including 14 disease resistance-related genes (Supplementary Table 2), ten kinase domains (Csa6G052030, Csa6G052080, Csa6G052130, Csa6G052820, Csa6G055410, Csa6G058190, Csa6G067340, Csa6G061230, Csa6G074020, Csa6G076750), three Zn-finger motifs (Csa6G055930, Csa6G067930, Csa6G074560), and one leucine-rich domain (Csa6G062270). The large number of genes in this region makes it difficult to determine the specific cucumber gene(s) that have large effects on GSB resistance. However, these predicted GSB resistance-related genes will be key candidates for further study.
Discussion
In this study, resistance to gummy stem blight (GSB) in cucumber was analyzed using a cross between the wild-type GSB-resistant cucumber accession PI 183967 (Cucumis sativus var. hardiwickii (Royle) Alef.) and the cultivated GSB-susceptible cucumber accession 931 (C. sativus var. sativus). Plants were grown in the field during autumn 2012 and spring 2013. Based on the disease index analyses for the genetic populations, the results suggest that resistance to GSB in PI 183967 is quantitative and controlled by three pairs of additive epistatic major genes. On the other hand, Norton (1979) reported that resistance in watermelon is controlled by a single recessive gene. These different patterns of inheritance may be explained by several factors. First, different species, cultivars, and breeding lines have been used as resistance sources. Second, different disease identification methods and rating scales make it difficult to compare studies. Third, as shown in this paper, the development of GSB in cucumber is strongly affected by environmental conditions making it difficult to maintain uniform environmental conditions when plants are grown in the field during testing. Multiple tests with several replications and years are required.
The best method for controlling GSB in cucumber is the use of resistant cultivars because of the effect on the environment by the use of pesticides to control the disease. As a result, GSB resistance is one of the main objectives in cucumber breeding programs (van der Meer et al. 1978; Wehner et al. 1996). However, there are no reports on the molecular biology of GSB resistance in cucumber.
In this study, 2112 pairs of SSR primers were used to map resistance genes for GSB in the cucumber stem resulting in the detection of five QTLs with ten SSR markers: gsb-s1.1, located between SSR12157 and SSR31116 with a genetic distance of 7.3 cM; gsb-s6.1, located between SSR01012 and SSR03527 with a genetic distance of 0.7 cM; gsb-s2.1, located between SSR13275 and SSR10064 with a genetic distance of 10.4 cM; gsb-s6.2, located between SSR04083 and SSR02940 with a genetic distance of 6.8 cM; and gsb-s6.3, located between SSR13251 and SSR15516 with a genetic distance of 3.1 cM.
Many genes conferring resistance (R genes) to a diverse array of pathogens, including bacteria, fungi, oomycetes, viruses, and nematodes, have been isolated in plants (Dangl and Jones 2001; Hulbert et al. 2001; Meyers et al. 2003). In the cucumber 9930 draft genome, 61 nucleotide binding site (NBS) resistance gene analogs (RGAs) were identified and were distributed mostly in 11 clusters (Huang et al. 2009). In this study, BLAST analysis of the genomic regions bearing the major QTLs of gsb-s6.1 and gsb-s6.2 showed 117 genes, of which 14 were disease resistance-related and one (Csa6G062270) belonged to the NBS series. The one NBS-type RGA will be studied for GSB-resistant candidate genes. The ten SSR markers above will be used in future studies to clarify resistance to GSB and to develop marker-assisted selection (MAS) for GSB resistance in cucumber.
References
Amand PC, Wehner TC (2001) Generation means analysis of leaf and stem resistance to gummy stem blight in cucumber. J Am Soc Hortic Sci 126:95–99
Chiu WF, Walker JC (1949) Physiology and pathogenicity of cucurbit black-rot fungus. Agri Res 78:589–615
Dangl JL, Jones JD (2001) Plant pathogens and integrated defence responses to infection. Nature 411:826–833
Frantz JD, Jahn MM (2004) Five independent loci each control monogenic resistance to gummy stem blight in melon (Cucumis melo L.). Theor Appl Genet 108:1033–1038
Gai JY, Zhang YM, Wang JK (2003) Genetic system of quantitative traits in plants. Science Press, Beijing
Ha KW, Zhang HL, Liu JL, Liu P, Wang JS (2010) Molecular location of Didymella bryoniae resistance gene carried by 4G21 on Cucumis melo L. Acta Hort Sin 37:1079–1084
Huang SW, Li RQ, Zhang ZH, Li L, Gu XF, Fan W, Lucas WJ, Wang XW, Xie BY, Ni PX, Ren YY, Zhu HM, Li J, Lin K, Jin WW, Fei ZJ, Li GC, Staub JE, Kilian A, Van der Vossen EAG, Wu Y, Guo J, He J, Jia ZQ, Ren Y, Tian G, Lu Y, Ruan J, Qian WB, Wang MW, Huang QF, Li B, Xuan ZL, Cao JJ, San A, Wu ZG, Zhang JB, Cai QL, Bai YQ, Zhao BW, Han YH, Li Y, Li XF, Wang SH, Shi QX, Liu SQ, Cho WK, Kim J-Y, Xu Y, Heller-Uszynska K, Miao H, Cheng ZC, Zhang SP, Wu J, Yang YH, Kang HX, Li M, Liang HQ, Ren XL, Shi ZB, Wen M, Jian M, Yang HL, Zhang GJ, Yang ZT, Chen R, Liu SF, Li JW, Ma LJ, Liu H, Zhou Y, Zhao J, Fang XD, Li GQ, Fang L, Li YR, Liu DY, Zheng HK, Zhang Y, Qin N, Li Z, Yang GH, Yang S, Bolund L, Kristiansen K, Zheng HC, Li SC, Zhang XQ, Yang HM, Wang J, Sun RF, Zhang BX, Jiang SZ, Wang J, Du YC, Li SG (2009) The genome of the cucumber, Cucumis sativus L. Nat Genet 41:1275–1281
Hulbert SH, Webb CA, Smith SM, Sun Q (2001) Resistance gene complexes: evolution and utilization. Annu Rev Phytopathol 39:285–312
Joseph NW (2009) Identification of amplified fragment length polymorphism markers linked to gummy stem blight resistance in melon (Cucumis melo L.). Hortscience 44:32–34
Kosambi DD (1944) The estimation of map distance from recombination values. Ann Eugenics 12:172–175
Meyers BC, Kozik A, Griego A, Kuang H, Michelmore RW (2003) Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell Online 15:809
Norton J (1979) Inheritance of resistance to gummy stem blight in watermelon. Hortscience 14:630–632
Ren Y, Zhang ZH, Liu JH, Staub JE, Han YH, Cheng ZC, Li XF, Lu JY, Miao H, Kang HX, Xie BY, Gu XF, Wang XW, Du YC, Jin WW, Huang SW (2009) An integrated genetic and cytogenetic map of the cucumber genome. PLoS One 4:e5795
Salamov AA, Solovyev VV (2000) Ab initio gene finding in Drosophila genomic DNA. Cold Spring Harbor Laboratory Press, New York, NY
Sherf AF, Mac Nab AA (1986) Vegetable diseases and their control, 2nd edn. Wiley, New York
Stewart JE, Turner AN, Brewer MT (2015) Evolutionary history and variation in host range of three Stagonosporopsis species causing gummy stem blight of cucurbits. Fungal Biol 119(5):370–382
Van Ooijen JW (2006) JoinMap® 4, software for the calculation of genetic linkage maps in experimental populations. Kyazma BV, Wageningen
Van Ooijen JW, Boer MP, Jansen RC, Maliepaard C (2000) MapQTL Version 4.0, software for the calculation of QTL positions on genetic maps. Wageningen: Plant Research International
Van der Meer QP, van Bennekom JL, van der Giessen AC (1978) Gummy stem blight resistance of cucumber (Cucumis sativus L.). Euphytica 27:861–864
Wako T, Sakata Y, Sugiyama M, Ohara T, Ishiuchi D, Kojima A (2002) Identification of melon accessions resistant to gummy stem blight and genetic analysis of the resistance using an efficient techniquence for seeding test. In: Nishimura S, Ezura H, Matsuda T, Tazuke A (eds) Second International Symposium on Cucurbits. Acta Hort 588:161–164
Wang HJ, Wu Y, Gu W, Sun XD, Qin ZW (2006) Extraction of DNA from cucumber by improved CTAB method. Heilongjiang Agric Sci 5:124–125 Chinese
Wehner TC, Amand PC, Lower RL (1996) ‘M17’gummy stem blight resistant picking cucumber inbred. Hortscience 31:1248–1249
Wehner TC, Shetty NV (2000) Screening the cucumber germplasm collection for resistance to gummy stem blight in North Carolina field tests. Hortscience 35:1132–1140
Zdobnov EM, Apweiler R (2001) InterProScan—an integration platform for the signature-recognition methods in InterPro. Oxford Univ. Press, Oxford
Zhang SP, Liu MM, Miao H, Zhang SQ, Yang Y, Xie BY, Wehner TC, Gu XF (2013) Chromosomal mapping and QTL analysis of resistance to downy mildew in Cucumis sativus. Plant Dis 97:245–251
Zuniga TL, Jantz JP, Zitter TA, Jahn MK (1999) Monogenic dominant resistance to gummy stem blight in two melon (Cucumis melo) accessions. Plant Dis 83:1105–1107
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 31272187), the Key Laboratory of Biology and Genetic Improvement of Horticultural Crops, Ministry of Agriculture, the People’s Republic of China, and the earmarked fund for Modern Agro-industry Technology Research System (CARS-25). The author would like to thank Dr. Graham Collins of the University of Adelaide, South Australia for proofreading.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
The experiments comply with the ethical standards in the country in which they were performed.
Additional information
Shengping Zhang and Shulin Liu have contributed equally to this work.
Electronic supplementary material
Supplementary Fig. 1
Phenotypes of the parental lines. a PI183967 with resistance to gummy stem blight; b 931with susceptibility to gummy stem blight. (JPEG 556 kb).
ESM 1
(DOCX 17 kb).
ESM 2
(DOCX 19 kb).
Supplementary Fig. 2
(JPEG 2.42 mb).
Rights and permissions
About this article
Cite this article
Zhang, S., Liu, S., Miao, H. et al. Inheritance and QTL mapping of resistance to gummy stem blight in cucumber stem. Mol Breeding 37, 49 (2017). https://doi.org/10.1007/s11032-017-0623-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-017-0623-y