Abstract
Throughout the ages the world has witnessed the outbreak of many infectious diseases. Emerging microbial diseases pose a serious threat to public health. Increasing resistance of microorganisms towards the existing drugs makes them ineffective. In fact, anti-microbial resistance is declared as one of the top public health threats by WHO. Hence, there is an urge for the discovery of novel antimicrobial drugs to combat with this challenge. Structural diversity and unique pharmacological effects make natural products a prime source of novel drugs. Staggeringly, in spite of its extensive biodiversity, a prominent portion of microorganism species remains unexplored for the identification of bioactives. Microorganisms are a predominant source of new chemical entities and there are remarkable number of antimicrobial drugs developed from it. In this review, we discuss the contributions of microorganism based natural products as effective antibacterial agents, studied during the period of 2010–2020. The review encompasses over 140 structures which are either natural products or semi-synthetic derivatives of microbial natural products. 65 of them are identified as newly discovered natural products. All the compounds discussed herein, have exhibited promising efficacy against various bacterial strains.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Infectious diseases continue to pose a major threat to public health, representing one of the major causes of death worldwide. It is caused by pathogenic microorganisms such as bacteria, fungi, virus, protozoa etc. According to the World Health Organisation (WHO) 2019 report, two infectious diseases, lower respiratory infections and diarrhoeal diseases, were among the top ten causes of death worldwide. A total of 2.6 million people had died in the year due to lower respiratory infections. Around 1.5 million people had lost their lives from diarrhoeal diseases alone. In low-income countries, among the top 10 leading causes of death, six were due to communicable diseases. Malaria, tuberculosis, HIV/AIDS etc. are some among the contagious diseases that has caused increased death rate in those countries during 2019 [1]. Apart from these bacterial and viral diseases, there is a stark rise in the number of invasive diseases caused by fungi too. Aspergillus, Candida and Cryptococcus are the species which are responsible for over 90% mycotic deaths [2].
Out of the total antimicrobial drugs approved between 1981 and 2019, 27% are either natural products or natural product derivatives and 22% of it are of pure synthetic origin. Antibiotics make upto 60% of the novel antibacterial agents [3]. A number of significant anti-infective drugs of synthetic origin have been successfully introduced into the market. The sulfa drugs (eg. sulfamethoxazole 1), trimethoprim (2), quinolones (eg. levofloxacin 3), oxazolidinones (eg. linezolid 4) etc. are a few examples of such synthetic antibiotics [4]. Azoles are a predominant class of antifungal drugs of synthetic origin. Posaconazole (5), itraconazole (6) fluconazole (7), and voriconazole (8) are a few triazoles which are licensed for various fungal infections (Fig. 1) [5].
Natural products remain as the major mainstay as a source of novel drugs. Natural products are structurally diverse and possess unique pharmacological or biological activities due to natural selection and evolutionary processes [6,7,8,9,10,11,12,13,14]. This makes them interesting as a source for the identification of novel drug leads [15,16,17,18]. Microbial source is one of the earliest natural product sources explored for the development of antimicrobial agents. Drug discovery from microbial source began with the serendipitous discovery of penicillin from Penicillium notatum by Alexander Fleming in 1929. The ‘Golden Age of Antibiotics’ ushered in after the discovery of penicillin between 1940 and 1962, a period during which most of the significant antibiotics in market were discovered [19, 20]. In fact, the first successful antibiotic class ever introduced was the β-lactam antibiotics, which includes penicillin, and acts by the inhibition of cell wall biosynthesis in bacteria. Penicillins, cephalosporins, tetracyclines, aminoglycosides, chloramphenicol, macrolides and glycopeptides include the major class of antibiotics originated from microorganisms. Omadacycline (9), eravacycline (10), and sarecycline (11) are a few representatives of natural product derivatives, tetracycline-based antibacterial agents, approved by FDA in the late 2018 [3] (Fig. 2).
Despite its large biodiversity, a major portion of microorganism species still remains uninvestigated [21] for its biological activities which paves the way for the isolation of new strains and its activity. It is also important to identify the source of microorganisms that were not previously explored for the potential secondary metabolites from it. The extensive diversity of microorganisms is a major factor that must be taken into consideration for future discovery of novel drugs and clinical lead candidates [22]. Drug resistant microorganisms pose a serious challenge to humankind as it makes existing antimicrobials ineffective [23]. WHO has declared antimicrobial resistance as one of the top ten global public health threats to humankind [24]. Metabolic engineering, synthetic chemistry, and genomic or metagenomic approaches are the strategies that could be adopted to develop new antimicrobial agents in order to meet the urgent need of microbial resistance.
Discussion
Antibacterials from bacterial source
Gram-positive bacteria
Out of the total microbial produced bioactive compounds, nearly three quarters are produced by actinomycetes alone. Streptomycetes are the most significant group producing a wide range of biologically relevant compounds. 3% of the total antibacterials are produced by streptomycetes alone [25].
Actinobacterial symbionts associated with social and solitary Hymenoptera are one theoretically rich source of novel natural products. Currie and colleagues studied two species of solitary mud dauber wasps to see if this was the case. They identified eleven structurally diverse secondary metabolites (12–22) including a novel polyunsaturated and polyoxygenated macrocyclic lactam, named as sceliphrolactam (12), from fifteen Streptomyces actinobacteria isolates. They tested the antifungal and antibacterial efficacy of the fifteen Streptomyces strains by pairing them with a number of fungi and bacteria. The proliferation of these bacteria and their antimicrobial properties point to their possible position as antibiotic-producing symbionts (Fig. 3) [26].
Bacillus, a Gram-positive bacteria genus, has been studied for antimicrobial activity for many decades. Antibiotic polyketides such as macrolactins, difficidins, and oxidifficidin, as well as lipopeptides, have been found in several Bacillus spp. Li et al. conducted a bioassay-guided fractionation of the organic extracts of a Bacillus amyloliquefaciens strain (AP183) which contributed to the discovery of bacillusin A (23), a new macrocyclic polyene antibiotic. The structure was identified as a novel macrodiolide composed of dimeric 4-hydroxy-2-methoxy-6-alkenylbenzoic acid lactones with conjugated pentaene-hexahydroxy polyketide chains by interpreting NMR and MS spectroscopic results. Compound 23 exhibited potent antibacterial activity in vitro against a variety of drug-resistant and drug-sensitive S. aureus and Enterococcus spp., with MICs ranging from 0.6 to 1.2 μg/mL. Its efficacy was tested against vancomycin, ciprofloxacin, and methicillin, with a MIC of 0.6 μg/mL against E. faecium ATCC 700,221 that was resistant to all three antibiotics tested at 100 μg/mL. At 20 μg/mL, it had no antifungal action against Candida albicans ATCC 90,028 (Fig. 4) [27].
The natural product actinomadurol (24) and the known compound JBIR-65 (25) were isolated together from the actinomycete Actinomadura strain KC 191. Compound 24 has been identified as an unusual member of the bacterial C-19 norditerpenoid class. With MIC values ranging from 0.39 to 0.78 g/mL, it showed potent antibacterial activity against pathogenic strains such as Staphylococcus aureus, Kocuria rhizophila and Proteus hauser. It was on par with, if not higher than, the positive regulation ampicillin (Fig. 5) [28].
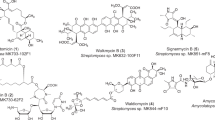
Lanen et al. isolated four new Y-type actinomycin analogues, Y6-Y9 (26–29) and actinomycin Zp (30), from the scale up fermentation of the Streptomyces sp. strain Gö-GS12. They were isolated as a natural substance for the first time. NMR spectroscopy and HRMS were used to determine the structures of the new compounds. The 4-hydroxythreonine on the β-ring of 26 is the only one that undergoes a twofold acyl shift as well as an additional ring closure. Compounds 27–29 demonstrated potent antibacterial activity against Gram-positive bacteria, which was consistent with cytotoxicity against human cell lines. In comparison to the non-rearranged comparator actinomycin Y5 and other actinomycins, the combination of a -ring rearrangement and additional ring closure in 26 made this actinomycin substantially less potent. The antimicrobial studies revealed that among the isolated compounds, actinomycin Y9 (29) is the most promising antibacterial agent with MIC between 0.012 to 0.96 μg/mL against various pathogenic bacterial strains (Fig. 6) [29].
Four new Y-type actinomycin analogues, Y6-Y9 isolated from Streptomyces sp. strain Gö-GS12. (Val: valine; MeVal: N-methylvaline; MeAla: N-methyl-L-alanine; Sar: sarcosine, MPro: cis-5-methylproline; HMPro: trans-3-hydroxy-cis-5-methylproline, Hyp: trans-4-hydroxyproline; OPro: 4-oxoproline, MOPro: cis-5-methyl-4-oxoproline; aHyp: cis-4-hydroxyproline; Thr: threonine; HThr: 4-hydroxythreonine, ClThr: 4-chlorothreonine; crHThr: cyclic rearranged hydroxythreonine)
Oh and colleagues isolated nicrophorusamides A and B (31 and 32) from an unusual actinomycete, Microbacterium sp., from the gut of the carrion beetle Nicrophorous concolor. Based on 1D and 2D NMR spectroscopic analysis, the structures were identified as new chlorinated cyclic hexapeptides with unusual amino acid units. On evaluating the antimicrobial activity of the compounds, it was found that nicrophorusamides B which bears D-asparagine was eightfold less potent than the D-threo-β-hydroxyasparagine bearing nicrophorusamides A. Nicrophorusamide A showed antibacterial activity against Staphylococcus aureus ATCC 25,923, Enterococcus faecalis ATCC 19,433, Enterococcus faecium ATCC 19,434, and Salmonella enterica ATCC 14,028 with minimum inhibitory concentration (MIC) values of 8 − 16 μg/mL. This suggests the significance of the D-threo-β-hydroxyasparagine in the antibacterial properties of the compound 31 (Fig. 7) [30].
Korean researchers discovered three cyclic lipopeptides with the unusual enamide linkage group from Streptomyces sp. KCB14A132, including one recognised (33) and two additional (34 and 35) compounds. The amino acid residues in the peptide backbone varied according to NMR and MS characterization. These three compounds were tested for antibacterial activity against a panel of pathogenic bacteria including MRSA, QRSA, Enterococcus faecalis and Bacillus subtilis. The antibacterial activity of chemically prepared deacetonide derivatives of enamidonins was found to be inactive, showing that the dimethylimidazolidinone residue is needed for antibacterial activity. Enamidonin (33), with a MIC of 4 μg/mL, was active only against Bacillus subtilis. Enamidonin B and enamidonin C (34 and 35), with MICs of 8–64 μg/mL, were active against MRSA, QRSA, Enterococcus faecalis, and Bacillus subtilis (Fig. 8) [31].
Microorganisms, some of which play protective roles in colony defence, form complex relationships with social insects. Pupo et al. investigated the microbiota associated symbiotically to the nurse bees Melipona scutellaris in order to explore it as a source of novel bioactives. New cyclic hexadepsipeptides namely meliponamycin A and meliponamycin B (36 and 37) isolated from Streptomyces sp. ICBG1318, present in M. scutellaris, demonstrated good activity against the entomopathogen Paenibacillus larvae and human pathogens Staphylococcus aureus and Leishmania infantum (Fig. 9) [32].
Gram-negative bacteria
The myxobacteria-derived cystobactamids (38–40), which were isolated from Cystobacter sp. Cbv34, were found to be highly potent novel antibacterial compounds by Müller and colleagues. At concentrations as low as 1 μg/mL, all of the derivatives inhibited E. coli development. Hexapeptide (39) was effective against the Gram-negative bacteria Acinetobacter baumannii, a member of the ESKAPE panel. Hexapeptide (39) also inhibited Gram-positive bacteria which includes E. faecalis and S. pneumonia, at concentrations of approximately 0.1 μg/mL. This result was very much comparable or even more effective than the activity of ciprofloxacin. Bacterial type IIa topoisomerases were discovered to be the molecular targets. The cystobactamids provide exciting alternatives to produce novel antibiotics using medicinal chemistry and biosynthetic engineering, considers the researchers, as quinolones are largely exhausted as a template for new type II topoisomerase inhibitors (Fig. 10) [33].
Three new pyoluteorin analogues, mindapyrroles A − C (41–43) were purified by Concepcion et al. from Pseudomonas aeruginosa strain 1682U.R.0a.27, a gill-associated bacterium isolated from giant shipworm Kuphus polythalamius. Different pathogenic bacteria were inhibited by mindapyrroles B and C. Mindapyrrole B (42) has the best antimicrobial activity, against Gram positive and Gram-negative bacteria with MIC values ranging from 2–8 μg/mL, and the broadest selectivity index among mammalian cells. The extra pyoluteorin (44) structure in compound 43 reduces activity against Gram-negative pathogens significantly. The addition of a hydroxyphenyl thiazole unit to the dimeric mindapyrrole position A's -CH2 bridge boosts antimicrobial activity and controls cytotoxicity. The compound showed low cytotoxicity in mammals, with an IC50 of 67 μg/mL against MDCK cells (Fig. 11) [34].
Clark et al. looked at a Chinese strain of Pseudomonas aurantiaca that produced a new benzoquinone (48) and furanone (49) along with the known dialkyl resorcinols (45 and 46). Degradation studies on treatment with acids, the major dialkyl resorcinol 45 led to the discovery of the furanone derivative (50), a hydroxyquinone (51), and an unusual resorcinol 52. The degradation of the dialkyl resorcinol (45) under basic condition also produced the same compounds (50–52), in addition to a new dimer (53). The antibacterial activity of compounds 45 and 46 was moderate against a panel of Gram-positive pathogens. However, the degraded products of the dialkyl resorcinol (45) could not show any effect on the tested pathogens. These studies show how artifacts can be used as a source of additional chemical diversity (Fig. 12) [35].
Antibacterials from fungal source
Fungi are proved to be a rich source of biologically active secondary metabolites. These eukaryotic, heterotrophic microorganisms often live symbiotically. In fact, fungi are most commonly observed as plant endophytes than bacteria [36].
Antioxidant and antibacterial function of organic extracts from eight fungal species were examined by Karaman et al. S. aureusan was the most susceptible bacterium, with a minimum inhibition concentration (MIC) of 0.28 mg/mL in the G. applanatum extract. The extracts of C. versicolor and G. applanatum had the highest activity. The extract of L. sulphureus was the least active among the tested ones. The studies revealed that Gram-positive bacteria are inhibited by coriolin, a sesquiterpene derived from Coriolus species. Triterpenoids and ganomycins present in Ganoderma species hinder the development of methicillin-resistant S. aureus and other bacteria such as E. coli and Pseudomonas aeruginosa. Animal bacteria strains were more susceptible than ATTC and human bacterial strains. These fungal extracts activity against S. aureus suggests that they may be used to treat animals, particularly because these bacterial strains are multi-resistant to traditional antibiotics [37].
Verrucamides A-D (54–57), novel cyclic tetradecapeptides, were isolated by Che and co-workers from the ascomycete fungi Myrothecium verrucaria. The isolated compounds were tested for antibacterial activity against the Gram-positive bacteria Staphylococcus aureus Col (CGMCC 1.2465) and displayed IC50 and MIC values of 3.59 to 9.09 and 10.0 to 40.0 μg/mL respectively (Fig. 13) [38].
Watnick et al. developed a sensitive and stable high throughput metabolic filter for novel antibiotics that is low-tech and inexpensive. They tested over 39,000 crude extracts obtained from organisms that grow in the diverse ecosystems of Costa Rica and found forty-nine with consistent antibacterial activity. One of the endophytic fungal extracts on further characterization led to the discovery of three new natural products (58–60). Among them a hydroquinone named as mirandamycin (58) has broad antibacterial activity against E. coli, Pseudomonas aeruginosa, Vibrio cholerae, methicillin-resistant Staphylococcus aureus, and Mycobacterium tuberculosis (Fig. 14) [39].
Urnucratins A-C (61–63) was isolated from Urnula craterium, a North American cup fungus, by Zhang and his colleagues. Urnucratins possesses bisnaphthospiroether skeleton with one oxygen and one C–C bridge, which is odd. The absolute configuration of the steriogenic spirane carbons of the urnucratins was determined by quantum chemical CD calculations and assigned as R. The presence of the carbonyl group in the structure of urnucratins A and B is likely to be responsible for its significant higher potency than urnucratin C. Urnucratin A was found to be more active than urnucratin B against Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus faecium, E. faecalis, Streptococcus pyogenes with MIC values of 2, 2, 1, 0.5 and 0.5 μg/mL, respectively. None of the urnucratins showed activity against the Gram negative bacteria that were tested (E. coli and P. aeruginosa) (Fig. 15) [40].
A new tetramic acid, methiosetin (64), was discovered by Singh et al. in a tropical sooty mould, Capnodium sp. Epicorazine A (65), a well-known antibiotic, was also developed by the fungus. The antibacterial activity exhibited by both compounds against S. aureus and Haemophilus influenzae was mild to moderate. The isolation, structure elucidation and antibacterial activity of both the compounds were defined. Methiosetin had a higher MIC of 256 μg/mL against S. aureus EP167 and a marginally better MIC of 32 μg/mL against H. influenzae. Epicorazine A was eight times more effective against S. aureus (MIC 32 μg/mL) and evidently more effective against H. influenzae (MIC 0.5 μg/mL) (Fig. 16) [41].
Purohit et al. tested thirty-five fungi for antimicrobial behaviour from extreme environment of effluent treatment plants. Bikaverin (66), produced by one of the fungal isolates, HKF15 identified as Fusarium sp., substantially inhibited E. coli development, according to the researchers. According to the colony count, a 25 ppm concentration was able to slow down bacterial development. In certain cells treated with 25 ppm bikaverin, the CFU count was reduced to zero after 6 h. After 1 h, there was a major inhibition of development in the presence of 50 ppm bikaverin. This showed that, in addition to its other bioactivities, bikaverin has antibacterial properties (Fig. 17) [42].
Mutagenesis in Aspergillus oryzae is believed to lead to the formation of antibacterial compounds. Hayman and his team used a screen to find individual mutated strains that inhibit Methicillin-resistant Staphylococcus aureus (MRSA). The mutated A. oryzae strains were created by treating the spores with ethyl methanesulfonate (EMS). On screening over 3000 EMS-treated A. oryzae cultures it was observed that the CAL220 isolate showed altered morphology and antibacterial activity. Mutations inhibited MRSA and Pseudomonas aeruginosa but not Klebsiella pneumoniae or Proteus vulgaris. A. Oryzae's genome has been sequenced, targeted and spontaneous mutations could be added to aid in the detection of novel antibacterial compounds [43].
Spiteller and colleagues isolated a new lanostanoid, 19-nor-lanosta-5(10),6,8,24-tetraene-1α,3β,12β,22S-tetraol (67) from Diaporthe sp. LG23, an endophytic fungus that lives on the leaves of the Chinese medicinal plant Mahonia fortunei. In addition, six recognised biosynthetically based steroids (68–73) were isolated in parallel. According to the researchers, the findings show that endophytes have the ability to help conventional host plant therapies as well as confer host health. Compound 67 was shown to have significant antibacterial activity against Gram-positive and Gram-negative bacteria. Compounds 68 and 71 were both effective against B. subtilis, which is comparable to streptomycin, the gold standard (Fig. 18) [44].
From the endophytic fungus Lasiodiplodia theobromae ZJ-HQ1, She and colleagues identified the first chlorinated preussomerins, chloropreussomerins A and B (74 and 75), as well as their nine recognised analogues (76–84). Their compositions were elucidated using a variety of spectroscopic analyses. The evaluation of the absolute configurations of 74 and 75 was done using single-crystal X-ray diffraction with Cu Kα radiation. The compounds 74, 75, 78–80 and 84 were discovered to be biologically active against Staphylococcus aureus (MIC values ranging from 1.6 to 13 μg/mL). With a MIC of 1.6 μg/mL, compound 78 was found to be the most promising amongst of all (Fig. 19) [45].
Bioassay-guided extraction of metabolites from cultures of the plant-derived fungus Emericella sp. TJ29 by Zhang et al. developed three new terpene-polyketide hybrid meroterpenoids, emervaridones A − C (85 − 87), two new polyketides, varioxiranediols A and B (89 and 90), and three known analogues (88, 91, and 92). Compounds 85 and 89 were shown to be effective against five drug-resistant bacteria, and displayed bacteriostatic and bactericidal activity, respectively. Both were of low toxicity to mammalian cells. These two compounds could be used to develop new chemical scaffolds for the development of antibacterial agents against drug-resistant microbial pathogens. The inhibitory action of emervaridone A (85) against ESBL-producing E. coli was equivalent to that of amikacin, a commonly used antibiotic with a MIC of 2 μ/mL. These compounds may be used to produce new antibiotics for the treatment of antibiotic-resistant infections, according to the researchers (Fig. 20) [46].
Spiteller and colleagues isolated three new and seven recognised calopins from Caloboletus radicans (93–102). NMR and MS data are used to elucidate the properties of the new cyclocalopins, 8-deacetylcyclocalopin B (93), cyclocalopin A-15-ol (94), and 12,15-dimethoxycyclocalopin A (95). Though 93–102 were ineffective against two cancer cell lines, they showed anti-staphylococcal activity against MRSA strains with MIC values of 16–256 μg/mL. Compound 93 was shown to be more active than norfloxacin, which served as a positive control with MIC value of 16 μg/mL against strains ATCC 25,923 and SA-1199B. Some of the calopins were active against the fish pathogen Enterococcus faecalis F1B1 too (Fig. 21) [47].
Bacterial biofilms play a role in 65 percent of bacterial infections in humans. Biofilms are linked to oral candidiasis in 7% of children, 31% of AIDS patients, and 20% of cancer patients. Microporenic acids A-G (103–109) are seven previously unknown acyclic diterpenes connected to isocitric acid by an ether linkage, isolated from the cultures of a species of the genus Microporus, as stated by Stadler and colleagues. Microporenic acids D and E (106 and 107) displayed antimicrobial activity against a many pathogenic of gram-positive bacteria and a yeast, Candida tenuis. Compound 103 has had strong antifungal and antibacterial efficacy against Candida albicans and Staphylococcus aureus preformed biofilms. Antimicrobial resistance continues to be a global health concern, necessitating the development of effective compounds (Fig. 22) [48].
Three novel cytochalasan alkaloids, termed armochaetoglosins A–C (110–112), were isolated by feeding 1-methyl-L-tryptophan into cultures of the arthropod-associated fungus Chaetomium globosum TW1-1. With MIC values of 4.0 μg/mL and 16.0 μg/mL, compound 112 had the best antibacterial activity against K. pneumoniae and ESBL-E. coli, respectively. In fact, armochaetoglosin C had a greater inhibitory activity against K. pneumoniae than the commonly used antibiotic meropenem with MIC value of 8 μg/mL. Zhang et al., put forward new chemical models for the production of new antibacterial agents against drug-resistant microbial pathogens (Fig. 23) [49].
Stadler and colleagues studied the ex-type strain of Sparticola junci to study the chemical diversity of metabolites from new Dothideomycetes species. From submerged cultures of the fungus, seven highly oxygenated and functionalized spirodioxynaphthalene natural products, sparticolins A − G (113–119) were isolated. Spectroscopic and X-ray crystallographic analyses were used to determine their chemical compositions, including relative and absolute configurations. Using the broth microdilution process, antimicrobial activities of the compounds were evaluated in serial dilution assays against different fungal and bacterial strains. Sparticolin B (114) was active against the Gram-positive bacteria Bacillus subtilis, Micrococcus luteus, and Staphylococcus aureus with MIC values 4.2 μg/mL, 8.3 μg/mL and 4.2 μg/mL respectively. Sparticolin G (119) were active against the fungi Schizosaccharomyces pombe and Mucor hiemalis. Compounds 114 and 119 have had mild cytotoxic activity against seven mammalian cell lines (Fig. 24) [50].
A broad family of oxygenated heterocyclic compounds known as xanthones, are privileged enough to communicate with a wide range of biological targets. Penicillium purpurogenum SC0070 solid cultures yielded eight new polyhydroxanthones namely penicixanthones A − H (120 − 127). As measured against human carcinoma A549, HeLa, and HepG2 cells, penicixanthone F (125) demonstrated the highest cytotoxicity (IC50 = 0.3–0.6 μM), while it was nontoxic to the normal Vero cells (IC50 > 50 μM). It was also the most effective antibacterial against Staphylococcus aureus and the methicillin-resistant strain MRSA with MIC value 0.4 μg/mL (Fig. 25) [51].
Antibacterials from semi-synthetic modifications
Synthetic modification of the existing lead natural products is one of the simplest approach to optimise its therapeutic properties. Simple functional group transformations or remodelling of the existing scaffold can in turn result in the overall improvement of its physico-chemical and pharmacokinetic properties. Here, we have discussed various cases of semi-synthetic modifications of microbial derived natural products, which has led to the improvement of its overall properties.
Evidente et al. studied the antibacterial efficacy of Diplodia cupressi's major phytotoxin sphaeropsidin A (128), its two natural analogues sphaeropsidins B and C (129 and 130) as well as fourteen other derivatives obtained by chemical modifications against Xanthomonas oryzae pv. oryzae (Xoo), Pseudomonas fuscovaginae, and Burkholderia glumae. Sphaeropsidin A displayed strong activity against Xoo. Only the monoacetyl derivative (131) of sphaeropsidin A had the same high activity as compound 128, while the diacetyl (132) and dihydro (133) derivatives of sphaeropsidin A had a decreased activity. All the other natural and hemisynthesized compounds were essentially inactive. This antibacterial activity is believed to be due to the involvement of the C-7 carbonyl group and the hemiketalic lactone functionality. This results may help in the production of novel compounds for agricultural applications (Fig. 26) [52].
Caprazamycins (CPZs) are a category of novel liponucleoside antibiotics with a variety of alkyl side chains that are divided into seven components CPZ-A to CPZ-G (134 a-g) based on the difference in the containing fatty acid. They were discovered by Takahashi and co-workers during a search for new antimycobacterial drugs. Caprazene (135) was synthesised by the acidic treatment of a mixture of caprazamycins isolated from a screen of novel antimycobacterial agents (Fig. 27).
Various derivatives were produced by chemical modification of the resulting caprazene. Antibacterial activity was observed in derivatives against mycobacterial species and pathogenic Gram positive and negative bacteria. Compounds 138 b (CPZEN-45), 138 d (CPZEN-48), 138 f, and 138 g (CPZEN-51) were found to have higher potency than CPZ-B (134 b) against Mycobacterium tuberculosis and Mycobacterium avium complex strains. The most powerful of the derivatives was discovered to be CPZEN-45 and could be created as a new potent anti-TB drug as well as an anti-MAC agent. The presence of an NH proton adjacent to the carbonyl group at the 1"'-position could play a role in the antibacterial activity (Fig. 28) [53].
Salinomycin (SAL) (140), a natural polyether ionophores antibiotic, was extracted from Streptomyces albus. Huczyński and colleagues synthesised eleven new SAL amides, characterised them using X-ray and spectroscopic techniques, and tested them for antiproliferative and antibacterial activity. The amides containing dopamine (141 d) and 4-fluorobenzyl (141 a) have a relatively strong antiproliferative function (e.g., against multidrug resistant and their parental cancer cell lines). The compounds are less toxic to normal murine fibroblasts than anticancer medications like cisplatin and doxorubicin. Amides 141 b, 141 e, 141 c and 141 f have strong potency in inhibiting MRSE formation, with MICs ranging from 16 to 64 μ/ml, which are slightly less active than unmodified SAL (MIC = 8 to16 μg/mL). Amides with a saturated alkyl chain and an extra heteroatom have been shown to have antibacterial action against human pathogenic bacteria, including drug-resistant Staphylococcus epidermidis strains (Fig. 29) [54].
Nisin (142) is an antimicrobial produced by Lactococcus lactis strains. The capacity of Nisin's N-terminal A/B ring system to bind lipid II, an integral cell-wall precursor in bacteria, is responsible for its antibacterial action [55,56,57] Martin et al. identified the optimised chemoenzymatic degradation of nisin to produce the A/B ring fragment, which was then modified at the C-terminus with a series of lipids. A subset of semisynthetic analogues with antibiotic activity comparable to nisin and improved stability was discovered among the semisynthetic analogues produced. By coupling compound 143 with a significant excess of the lipid-amine of choice in the presence of BOP/DIPEA for a short time, the lipidated constructs 144–147 (Fig. 30) is easily synthesised. Click reaction was used to ligate compound 147 with a single equivalent of the required alkyne-modified lipid, yielding compound 148. (Fig. 31). Compounds 144, 145, 146, and 148 have the most active antibacterial activity among the analogues prepared against drug-susceptible and drug-resistant Gram-positive bacteria, including clinically important MRSA and VRE strains. The inclusion of five lanthionine rings (A-E) confers a predefined conformation, which is critical for nisin's mode of action and potency [58].
Wollamide B (149), a cyclic hexapeptide natural product, has been known previously to show anti-Mycobacterium bovis activity. 27 peptides, including wollamides A/B and desotamide B were synthesised by Sun et al. and tested against a panel of clinically important bacterial pathogens. Wollamide A (150), and B (149) along with their corresponding II (L-Leu) analog (151) showed the best antituberculosis efficacy, with the lowest minimum inhibitory concentration (MIC = 1.56 μg/mL) against virulent M. tuberculosis H37Rv. The antimicrobial properties of these peptides are not due to destruction of the bacterial membrane, so further research into their mode of action is required (Fig. 32) [59].
Conclusion
Natural products play a pivotal role in drug discovery and are the basis of many of the early developed drugs. Emergence of combinatorial chemistry has led to the abandonment of the traditional method of drug discovery from natural sources, by various pharmaceutical industries. Combinatorial chemical techniques along with high throughput screening have paved a new way for the drug discovery process in the identification of active lead compounds, against various biological targets. Though there are claims that combinatorial chemistry produces large collection of novel drug leads, the stark decline in the number of novel chemical entities by this method proves that some of the libraries of compounds which were generated earlier were “poorly designed, impractically large and structurally simplistic” [60].
Increasing microbial infections and microbial resistance to existing drugs demand the discovery of new antimicrobial agents. Microorganisms are a prolific source for the development of novel drugs. A single microorganism can often produce nearly fifty different secondary metabolites [61]. But the fact is that only a very slight segment of microbes is harnessed for the identification of secondary metabolites. Hence, emphasis on in-depth exploration of the untapped microbial world can lead to the discovery of many significant anti-microbial drugs. In the current review, we summarise the advances made in the area of microbial based natural products as potent antimicrobials, during the period of 2010–2020. This review describes nearly 140 microbial natural products and natural products derived compounds as potential antimicrobial agents. 65 of them are newly identified natural products. The compounds discussed here belong to varied structural classes that include alkaloids, terpenoids, quinones, phenols, polypeptides, macrocyclic lactams, binaphthalenes, macrocyclic polyenes, steroids, polyketides, lipopeptides, xanthones etc. All the compounds showed medium to high anti-microbial activity. We hope, this review may aid the scientific community in their quest for novel antimicrobial drugs on further investigating the structural classes from microorganisms, that has been discussed here.
Abbreviations
- SA:
-
Staphylococcus aureus
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- EF:
-
Enterococcus faecium
- EFl:
-
E. faecalis
- SP:
-
Streptococcus pyogenes
- HI:
-
Haemophilus influenzae
- AB:
-
Acinetobacter baumannii
- PA:
-
Pseudomonas aeruginosa
- BS:
-
Bacillus subtilis
- PH:
-
Proteus hauser
- KR:
-
Kocuria rhizophila
- MA:
-
Mycobacterium aurum
- ML:
-
Micrococcus luteus
- ESBL-EC:
-
Extended-spectrum β-lactamase-producing Escherichia coli
- KP:
-
Klebsiella pneumoniae
- SH:
-
Schizosaccharomyces pombe
- MH:
-
Mucor hiemalis
- SE:
-
Staphylococcus epidermidis
- PL:
-
Paenibacillus larvae
- LT:
-
Leishmania infantum
- MT:
-
Mycobacterium tuberculosis
- Xoo :
-
Xanthomonas oryzae Pv. oryzae
- MIC:
-
Minimum inhibitory concentration
- IC50 :
-
Half-maximal inhibitory concentration
- ZOID:
-
Zone of inhibition diameter
- S. aureus :
-
Staphylococcus aureus
- M. scutellaris :
-
Melipona scutellaris
- E. coli :
-
Escherichia coli
- E. faecalis :
-
Enterococcus faecalis
- S. pneumoniae :
-
Streptococcus pneumoniae
- C. versicolor :
-
Coriolus versicolor
- G. applanatum :
-
Ganoderma applanatum
- L. sulphureus :
-
Laetiporus sulphureus
- P. aeruginosa :
-
Pseudomonas aeruginosa
- H. influenzae :
-
Haemophilus influenzae
- A. oryzae :
-
Aspergillus oryzae
- K. pneumoniae :
-
Klebsiella pneumoniae
- M. tuberculosis :
-
Mycobacterium tuberculosis
References
WHO (2018) the-Top-10-Causes-of-Death @ www.who.int. top 10 causes death Consultado 23 de marzo de 2019
Robbins N, Caplan T, Cowen LE (2017) Molecular evolution of antifungal drug resistance. Annu Rev Microbiol 71:753–775. https://doi.org/10.1146/annurev-micro-030117-020345
Newman DJ, Cragg GM (2020) Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod 83:770–803. https://doi.org/10.1021/acs.jnatprod.9b01285
Wright GD (2017) Opportunities for natural products in 21st century antibiotic discovery. Nat Prod Rep 34:694–701. https://doi.org/10.1039/c7np00019g
Ostrosky-Zeichner L, Casadevall A, Galgiani JN et al (2010) An insight into the antifungal pipeline: selected new molecules and beyond. Nat Rev Drug Discov 9:719–727. https://doi.org/10.1038/nrd3074
El-Senduny FF, Altouhamy M, Zayed G et al (2021) Azadiradione-loaded liposomes with improved bioavailability and anticancer efficacy against triple negative breast cancer. J Drug Deliv Sci Technol 65:102665. https://doi.org/10.1016/j.jddst.2021.102665
Shyni GL, Renjitha J, Somappa S, Raghu KG (2021) Zerumin A attenuates the inflammatory responses in LPS-stimulated H9c2 cardiomyoblasts. J Biochem Mol Toxicol 35:1–11. https://doi.org/10.1002/jbt.22777
Jalaja R, Leela SG, Mohan S et al (2021) Anti-hyperlipidemic potential of natural product based labdane-pyrroles via inhibition of cholesterol and triglycerides synthesis. Bioorg Chem 108:104664. https://doi.org/10.1016/j.bioorg.2021.104664
Jalaja R, Leela SG, Valmiki PK et al (2018) Discovery of natural product derived labdane appended triazoles as potent pancreatic lipase inhibitors. ACS Med Chem Lett 9:662–666. https://doi.org/10.1021/acsmedchemlett.8b00109
Shilpa G, Renjitha J, Saranga R et al (2017) Epoxyazadiradione purified from the azadirachta indica seed induced mitochondrial apoptosis and inhibition of NFκB nuclear translocation in human cervical cancer cells. Phyther Res 31:1892–1902. https://doi.org/10.1002/ptr.5932
Lakshmi S, Renjitha J, Sasidhar SB, Priya S (2021) Epoxyazadiradione induced apoptosis/anoikis in triple-negative breast cancer cells, MDA-MB-231, by modulating diverse cellular effects. J Biochem Mol Toxicol 35:1–17. https://doi.org/10.1002/jbt.22756
Chandrashekhar M, Nayak VL, Ramakrishna S, Mallavadhani UV (2016) Novel triazole hybrids of myrrhanone C, a natural polypodane triterpene: synthesis, cytotoxic activity and cell based studies. Eur J Med Chem 114:293–307. https://doi.org/10.1016/j.ejmech.2016.03.013
Madasu C, Xu Y-M, Wijeratne EMK et al (2022) Semi-synthesis and cytotoxicity evaluation of pyrimidine, thiazole, and indole analogues of argentatins A-C from guayule (Parthenium argentatum) resin. Med Chem Res. https://doi.org/10.1007/s00044-021-02835-1
Madasu C, Karri S, Sangaraju R et al (2020) Synthesis and biological evaluation of some novel 1,2,3-triazole hybrids of myrrhanone B isolated from Commiphora mukul gum resin: Identification of potent antiproliferative leads active against prostate cancer cells (PC-3). Eur J Med Chem 188:111974. https://doi.org/10.1016/j.ejmech.2019.111974
Mohan B, Salfeena CTF, Ashitha KT et al (2018) Functionalized pyrimidines from alkynes and nitriles: application towards the synthesis of marine natural product meridianin analogs. ChemistrySelect 3:6394–6398. https://doi.org/10.1002/slct.201801126
Anaga N, Abraham B et al (2020) Advanced glycation end-products (AGE) trapping agents: design and synthesis of nature inspired indeno[2,1-c]pyridinones. Bioorg Chem 105:104375. https://doi.org/10.1016/j.bioorg.2020.104375
Praveen Kumar V, Renjitha J, Fathimath Salfeena CT et al (2017) Antibacterial and antitubercular evaluation of dihydronaphthalenone-indole hybrid analogs. Chem Biol Drug Des 90:703–708. https://doi.org/10.1111/cbdd.12990
Biradar JS, Somappa SB (2016) Synthesis of novel Indolyl benzo[b][1,4]diazepins as potent antimicrobial and antioxidant agents. Arab J Chem 9:S1063–S1068. https://doi.org/10.1016/j.arabjc.2011.11.014
Cragg GM, Newman DJ (2013) Natural products: a continuing source of novel drug leads. Biochim Biophys Acta - Gen Subj 1830:3670–3695. https://doi.org/10.1016/j.bbagen.2013.02.008
Khazir J, Mir BA, Mir SA, Cowan D (2013) Natural products as lead compounds in drug discovery. J Asian Nat Prod Res 15:764–788. https://doi.org/10.1080/10286020.2013.798314
Bérdy J (2012) Thoughts and facts about antibiotics: Where we are now and where we are heading. J Antibiot (Tokyo) 65:385–395. https://doi.org/10.1038/ja.2012.27
Demain AL (2009) Antibiotics: natural products essential to human health. Med Res Rev 29:821–842. https://doi.org/10.1002/med.20154
Rossiter SE, Fletcher MH, Wuest WM (2017) Natural products as platforms to overcome antibiotic resistance. Chem Rev 117:12415–12474. https://doi.org/10.1021/acs.chemrev.7b00283
World Health Organization (WHO) (2018) Antimicrobial-Resistance @www.who.int
Watve MG, Tickoo R, Jog MM, Bhole BD (2001) How many antibiotics are produced by the genus Streptomyces? Arch Microbiol 176:386–390. https://doi.org/10.1007/s002030100345
Poulsen M, Oh DC, Clardy J, Currie CR (2011) Chemical analyses of wasp-associated Streptomyces bacteria reveal a prolific potential for natural products discovery. PLoS ONE. https://doi.org/10.1371/journal.pone.0016763
Ravu RR, Jacob MR, Chen X et al (2015) Bacillusin A, an antibacterial macrodiolide from Bacillus amyloliquefaciens AP183. J Nat Prod 78:924–928. https://doi.org/10.1021/np500911k
Shin B, Kim BY, Cho E et al (2016) Actinomadurol, an antibacterial norditerpenoid from a rare actinomycete, actinomadura sp. KC 191. J Nat Prod 79:1886–1890. https://doi.org/10.1021/acs.jnatprod.6b00268
Cai W, Wang X, Elshahawi SI et al (2016) Antibacterial and cytotoxic actinomycins Y6–Y9 and Zp from streptomyces sp. strain Gö-GS12. J Nat Prod 79:2731–2739. https://doi.org/10.1021/acs.jnatprod.6b00742
Shin YH, Bae S, Sim J et al (2017) Nicrophorusamides a and b, antibacterial chlorinated cyclic peptides from a gut bacterium of the carrion beetle nicrophorus concolor. J Nat Prod 80:2962–2968. https://doi.org/10.1021/acs.jnatprod.7b00506
Son S, Ko SK, Kim SM et al (2018) Antibacterial cyclic lipopeptide enamidonins with an enamide-linked acyl chain from a streptomyces species. J Nat Prod 81:2462–2469. https://doi.org/10.1021/acs.jnatprod.8b00497
Menegatti C, Lourenzon VB, Rodríguez-Hernández D et al (2020) Meliponamycins: Antimicrobials from Stingless Bee-Associated Streptomyces sp. J Nat Prod 83:610–616. https://doi.org/10.1021/acs.jnatprod.9b01011
Baumann S, Herrmann J, Raju R et al (2014) Cystobactamids: Myxobacterial topoisomerase inhibitors exhibiting potent antibacterial activity. Angew Chemie - Int Ed 53:14605–14609. https://doi.org/10.1002/anie.201409964
Lacerna NM, Miller BW, Lim AL et al (2019) Mindapyrroles A-C, pyoluteorin analogues from a shipworm-associated bacterium. J Nat Prod 82:1024–1028. https://doi.org/10.1021/acs.jnatprod.8b00979
Shi Y, Zaleta-Pinet DA, Clark BR (2020) Isolation, Identification, and Decomposition of Antibacterial Dialkylresorcinols from a Chinese Pseudomonas aurantiaca Strain. J Nat Prod 83:194–201. https://doi.org/10.1021/acs.jnatprod.9b00315
Abdel-Razek AS, El-Naggar ME, Allam A et al (2020) Microbial natural products in drug discovery. Processes 8:1–19. https://doi.org/10.3390/PR8040470
Karaman M, Jovin E, Malbaša R et al (2010) Medicinal and edible lignicolous fungi as natural sources of antioxidative and antibacterial agents. Phyther Res 24:1473–1481. https://doi.org/10.1002/ptr.2969
Zou X, Niu S, Ren J et al (2011) Verrucamides A-D, antibacterial cyclopeptides from Myrothecium verrucaria. J Nat Prod 74:1111–1116. https://doi.org/10.1021/np200050r
Ymele-Leki P, Cao S, Sharp J et al (2012) A High-Throughput screen identifies a new natural product with Broad-Spectrum antibacterial activity. PLoS ONE. https://doi.org/10.1371/journal.pone.0031307
Liu XT, Schwan WR, Volk TJ et al (2012) Antibacterial spirobisnaphthalenes from the North American cup fungus Urnula craterium. J Nat Prod 75:1534–1538. https://doi.org/10.1021/np300221a
Herath K, Jayasuriya H, Zink DL et al (2012) Isolation, structure elucidation, and antibacterial activity of methiosetin, a tetramic acid from a tropical sooty mold (Capnodium sp.). J Nat Prod 75:420–424. https://doi.org/10.1021/np200857y
Deshmukh R, Mathew A, Purohit HJ (2014) Characterization of antibacterial activity of bikaverin from Fusarium sp. HKF15. J Biosci Bioeng 117:443–448. https://doi.org/10.1016/j.jbiosc.2013.09.017
Leonard CA, Brown SD, Hayman JR (2013) Random mutagenesis of the aspergillus oryzae genome results in fungal antibacterial activity. Int J Microbiol. https://doi.org/10.1155/2013/901697
Li G, Kusari S, Kusari P et al (2015) Endophytic diaporthe sp. LG23 produces a potent antibacterial tetracyclic triterpenoid. J Nat Prod 78:2128–2132. https://doi.org/10.1021/acs.jnatprod.5b00170
Chen S, Chen D, Cai R et al (2016) Cytotoxic and Antibacterial Preussomerins from the Mangrove Endophytic Fungus Lasiodiplodia theobromae ZJ-HQ1. J Nat Prod 79:2397–2402. https://doi.org/10.1021/acs.jnatprod.6b00639
He Y, Hu Z, Li Q et al (2017) Bioassay-Guided Isolation of Antibacterial Metabolites from Emericella sp. TJ29. J Nat Prod 80:2399–2405. https://doi.org/10.1021/acs.jnatprod.7b00077
Tareq FS, Hasan CM, Rahman MM et al (2018) Anti-Staphylococcal calopins from fruiting bodies of caloboletus radicans. J Nat Prod 81:400–404. https://doi.org/10.1021/acs.jnatprod.7b00525
Chepkirui C, Yuyama KT, Wanga LA et al (2018) Microporenic acids A-G, biofilm inhibitors, and antimicrobial agents from the basidiomycete microporus species. J Nat Prod 81:778–784. https://doi.org/10.1021/acs.jnatprod.7b00764
Gao W, He Y, Li F et al (2019) Antibacterial activity against drug-resistant microbial pathogens of cytochalasan alkaloids from the arthropod-associated fungus Chaetomium globosum TW1-1. Bioorg Chem 83:98–104. https://doi.org/10.1016/j.bioorg.2018.10.020
Phukhamsakda C, Macabeo APG, Huch V et al (2019) Sparticolins A-G, biologically active oxidized spirodioxynaphthalene derivatives from the ascomycete sparticola junci. J Nat Prod 82:2878–2885. https://doi.org/10.1021/acs.jnatprod.9b00604
Xue J, Li H, Wu P et al (2020) bioactive polyhydroxanthones from penicillium purpurogenum. J Nat Prod 83:1480–1487. https://doi.org/10.1021/acs.jnatprod.9b01071
Evidente A, Venturi V, Masi M et al (2011) In vitro antibacterial activity of sphaeropsidins and chemical derivatives toward Xanthomonas oryzae pv. oryzae, the causal agent of rice bacterial blight. J Nat Prod 74:2520–2525. https://doi.org/10.1021/np200625m
Takahashi Y, Igarashi M, Miyake T et al (2013) Novel semisynthetic antibiotics from caprazamycins A-G: caprazene derivatives and their antibacterial activity. J Antibiot (Tokyo) 66:171–178. https://doi.org/10.1038/ja.2013.9
Antoszczak M, Maj E, Stefańska J et al (2014) Synthesis, antiproliferative and antibacterial activity of new amides of salinomycin. Bioorganic Med Chem Lett 24:1724–1729. https://doi.org/10.1016/j.bmcl.2014.02.042
E. B, I. W, van KC, et al (1999) Use of the cell wall precursor lipid II by a pore-forming peptide antibiotic. Science 286:2361–2364. https://doi.org/10.1126/science.286.5448.2361
Hsu STD, Breukink E, Tischenko E et al (2004) The nisin-lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics. Nat Struct Mol Biol 11:963–967. https://doi.org/10.1038/nsmb830
Breukink E, de Kruijff B (2006) Lipid II as a target for antibiotics. Nat Rev Drug Discov 5:321–323. https://doi.org/10.1038/nrd2004
Koopmans T, Wood TM, ’T Hart P et al (2015) Semisynthetic lipopeptides derived from nisin display antibacterial activity and lipid II binding on par with that of the parent compound. J Am Chem Soc 137:9382–9389. https://doi.org/10.1021/jacs.5b04501
Tsutsumi LS, Elmore JM, Dang UT et al (2018) Solid-phase synthesis and antibacterial activity of cyclohexapeptide wollamide B analogs. ACS Comb Sci 20:172–185. https://doi.org/10.1021/acscombsci.7b00189
Cragg GM, Grothaus PG, Newman DJ (2009) Impact of natural products on developing new anti-cancer agents. Chem Rev 109:3012–3043. https://doi.org/10.1021/cr900019j
Demain AL (2014) Importance of microbial natural products and the need to revitalize their discovery. J Ind Microbiol Biotechnol 41:185–201. https://doi.org/10.1007/s10295-013-1325-z
Acknowledgements
Financial support from the CSIR Young Scientist Award Research Grant (HRDG/YSA-19/02/33(0045)/2020) and CSIR Biostimulant Network Project (MLP 0209), from Council of Scientific and Industrial Research (CSIR), Government of India, New Delhi, is gratefully acknowledged. Sangeetha Mohan thanks UGC for Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohan, S., Ajay Krishna, M.S., Chandramouli, M. et al. Antibacterial natural products from microbial and fungal sources: a decade of advances. Mol Divers 27, 517–541 (2023). https://doi.org/10.1007/s11030-022-10417-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10417-5