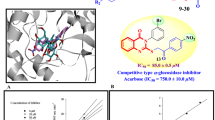
Abstract
This study is focused on the identification of thiazole-based inhibitors for the \(\alpha \)-glucosidase enzyme. For that purpose, (E)-2-(2-(arylmethylene)hydrazinyl)-4-arylthiazole derivatives were synthesized in two steps and characterized by various spectroscopic techniques. All derivatives and intermediates were evaluated for their in vitro \(\alpha \)-glucosidase inhibitory activity. Thiosemicarbazones 20 and 35, and cyclized thiazole derivatives 2, 5–11, 13, 15, 21–24, 27–31, and 36–37 showed significant inhibitory potential in the range of \(\hbox {IC}_{50}=6.2\pm 0.19\)–\(43.6\pm 0.23~\upmu \hbox {M}\) as compared to standard acarbose (\(\hbox {IC}_{50}=37.7\pm 0.19~\upmu \hbox {M}\)). A molecular modeling study was carried out to understand the binding interactions of compounds with the active site of enzyme.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus is a serious metabolic disorder of modern era and severe interminable health complications are associated with it, and type-2 diabetes is widely spread kind of this disorder [1]. The \(\alpha \)-glucosidase enzyme is necessary for human physiological function, but its overexpression increases glucose level in plasma [2] after a meal. This enzyme is present in the cell membrane of the small intestine [3, 4] and it is responsible for the digestion of carbohydrates (polysaccharides) into simple absorbable monosaccharides [5, 6]. The inhibition of \(\alpha \)-glucosidase restricts the production of glucose which is helpful in the treatment of diabetes [7]. Since 1980, the number of people in the world with diabetes increased from 153 to 347 million in 2008 [8]. According to WHO, it is expected that diabetes would be the seventh driving reason for death universally by 2030 [9].
Acarbose, miglitol, and voglibose are clinically used drugs and these all are \(\alpha \)-glucosidase inhibitors [10]. Unfortunately, gastrointestinal tract side effects such as diarrhea, flatulence, and abdominal discomfort are associated with them. Moreover, these are 50% less effective than other classes of antidiabetic agents such as metformin and sulfonylurea [11, 12] and frequently used in combination with other antidiabetic drugs to improve efficacy. Therefore, it is a crucial need to develop new, safe, and efficient therapeutic agents to control the optimal glycemic index for curing type-2 diabetic patients.
Thiazole, or 1,3-thiazole, is a heterocyclic compound that possesses both sulfur and nitrogen atoms [13]. Thiazole is an aromatic compound that obeys the Hückel rule [14]. The thiazole heterocycle can exist in two isomeric forms, 1,3-thiazole often considered as thiazole or 1,2-thiazole also known as isothiazole [15]. Thiazole-containing molecules are used in CNS disorders [16], and showed anticancer, antimalarial [17], as well as antiviral activities against four viruses such as polio, influenza A (H1N1), hepatitis B and hepatitis C [18]. In addition to these biological activities, thiazole derivatives such as thiamethoxam and clothianidin play a pivotal role as insecticides in many crop-protecting agrochemicals [19].
Our research group has identified a number of lead candidates based on heterocyclic nucleus for their use in medicinal chemistry research [20,21,22,23,24,25] and found thiazole-based compounds as potential \(\alpha \)-glucosidase inhibitors (Fig. 1) [13, 26].
In the current study, we intended to further evaluate this class for \(\alpha \)-glucosidase inhibitory activity. Thus, this report presents the synthesis of (E)-2-(2-(arylmethylene)hydrazinyl)-4-arylthiazole derivatives along with the thiosemicarbazone intermediates (1–38), structural characterization, their \(\alpha \)-glucosidase inhibitory activity, and in silico studies.
Results and discussion
Chemistry
(E)-2-(2-(Arylmethylene)hydrazinyl)-4-arylthiazole derivatives were synthesized by a two-step reaction route. In the first step, different aryl aldehydes were reacted with thiosemicarbazide in the presence of few drops of glacial acetic acid to form the thiosemicarbazones 1, 4, 12, 20, 28, and 35. In second step, these thiosemicarbazones were treated with a variety of phenacyl bromides in the presence of triethyl amine to afford hydrazinyl thiazoles 2, 3, 5–11, 13–19, 21–27, 29–34, and 36–38. Progress of both steps was monitored by thin layer chromatography (TLC) (Scheme 1).
The identity of all compounds was confirmed by EI-MS, HREI-MS, \({}^{1}\hbox {H-}\) and \({}^{13}\hbox {C-NMR}\) spectroscopic techniques. 2D-NMR experiments such as COSY, HSQC, and HMBC were performed on intermediate 1 and cyclized derivative 6 to further confirm the exact framework of the compounds. The stereochemistry of the iminic double bond was confirmed by the NOESY analysis on intermediate 1 and cyclized derivative 6. In both cases, NOESY interaction was observed between NH and iminic carbon of the compounds which can only be observed in an E-configuration (Fig. 2).
In vitro \(\alpha \)-glucosidase inhibitory activity
(E)-2-(2-(Arylmethylene)hydrazinyl)-4-arylthiazole derivatives along with intervening thiosemicarbazones (1–38) were subjected to in vitro \(\alpha \)-glucosidase inhibitory activity testing. Compounds 2, 5–11, 13, 15, 20–24, 27–31, and 35–37 showed inhibitory activity in the range of \(\hbox {IC}_{50}=6.2\pm 0.19\)– \(43.6\pm 0.23~\upmu \hbox {M}\) versus that of standard acarbose (\(\hbox {IC}_{50}=37.7\pm 0.19~\upmu \hbox {M}\)) (Table 1).
Structure–activity relationship (SAR)
All molecules possess biologically important pharmacophores such as hydrazine and thiazole moieties those might be participating in the inhibitory activity. But molecules also possess varying groups such as \(\hbox {R}_{1}\) and \(\hbox {R}_{2}\) (Fig. 3). Thus, a limited SAR was rationalized by examining the effects of varying features (\(\hbox {R}_{1}\) and \(\hbox {R}_{2})\) on inhibitory potential.
Thiosemicarbazone 1 did not show \(\alpha \)-glucosidase inhibition; however, its cyclized products compound 2 (\(\hbox {IC}_{50}=37.3\pm 0.17~\upmu \hbox {M}\)) with p-methoxy substitution displayed \(\alpha \)-glucosidase inhibitory activity comparable to standard acarbose (\(\hbox {IC}_{50}=37.7\pm 0.19~\upmu \hbox {M}\)). Interestingly, m-nitro containing analog 3 was found to be completely inactive. Its inactivity is might be due to not fulfilling the conformational requirement to fit well in the active site of the enzyme (Fig. 4).
1-Naphthyl-substituted thiosemicarbazone 4 was found to be inactive. The inactivity of this compound might be due to the presence of naphthyl ring which may create steric hindrance while binding into the active site of \(\alpha \)-glucosidase enzyme. All cyclized analogs 6–11 were found to be inhibitors for the \(\alpha \)-glucosidase enzyme except compound 5 with unsubstituted phenyl ring as \(\hbox {R}_{2}\). Among them, compound 8 (\(\hbox {IC}_{50}=6.2\,\pm \,0.19~\upmu \hbox {M}\)) with a biphenyl group as \(\hbox {R}_{2}\) was found to be the most potent molecule. Its activity might be due to the extended \(\pi \)-system which can interact with active site of \(\alpha \)-glucosidase enzyme. Replacement of phenyl ring with bromo group at the para position of \(\hbox {R}_{2 }\) as in compound 10 (\(\hbox {IC}_{50}=7.9\pm 0.19~\upmu \hbox {M}\)) showed slight decreased \(\alpha \)-glucosidase inhibitory activity. Nonetheless, replacement with p-methoxy and m-nitro groups as in compounds 7 (\(\hbox {IC}_{50}=12.2\pm 0.20~\upmu \hbox {M}\)) and 9 (\(\hbox {IC}_{50}=13.6\pm 0.20~\upmu \hbox {M}\)), respectively, also showed decrease inhibitory potential as compared to compound 8. Compound 11 (\(\hbox {IC}_{50}=26.1\pm 0.20~\upmu \hbox {M}\)) with m,p-dichloro substitution showed better activity when compared to standard acarbose, but activity was lower than compounds 7–10. Relatively decreased activity might be attributed due to two chloro atoms adjacent to each other which may create steric hindrance while binding into the active site of enzyme. Similarly, compound 6 (\(\hbox {IC}_{50}=38.5\pm 0.18~\upmu \hbox {M}\)) with p-methyl substitution showed comparable activity to standard acarbose. The pattern for \(\alpha \)-glucosidase inhibitory activity on the basis of \(\hbox {R}_{2}\) was observed in the order of \(p\hbox {-Ph}>\hbox {unsubstituted}>p\hbox {-Br}>p\hbox {-OMe}>p\hbox {-}\hbox {NO}_{2}>m, p\hbox {-}di\hbox {Cl}>p\hbox {-Me}\) (Fig. 5).
2-Naphthyl-substituted thiosemicarbazone 12 along with cyclized derivatives 14, and 16–19 were found to be inactive for \(\alpha \)-glucosidase inhibitory activity; however, compounds 13 (\(\hbox {IC}_{50}=28.4\pm 0.23~\upmu \hbox {M}\)) with no substitution and 15 (\(\hbox {IC}_{50}=37.2\pm 0.22~\upmu \hbox {M}\)) with p-methoxy substitutions on \(\hbox {R}_{2}\) were found to be good inhibitors for \(\alpha \)-glucosidase enzyme (Fig. 6).
Naphthol-substituted thiosemicarbazone 20 (\(\hbox {IC}_{50}=21.5\pm 0.21~\upmu \hbox {M}\)) and its cyclized hydrazinyl thiazoles 21–27 demonstrated \(\alpha \)-glucosidase inhibitory activity. Among them, compound 21 (\(\hbox {IC}_{50}=7.3\pm 0.19~\upmu \hbox {M}\)) with no substitution on \(\hbox {R}_{2}\), was found to be the most potent derivative. Incorporation of groups such as methyl, methoxy, and phenyl at the para position of \(\hbox {R}_{2}\) as in compounds 22 (\(\hbox {IC}_{50}=27.5\pm 0.21~\upmu \hbox {M}\)), 23 (\(\hbox {IC}_{50}=18.2\pm 0.21~\upmu \hbox {M}\)), and 24 (\(\hbox {IC}_{50}=35.4\pm 0.22~\upmu \hbox {M}\)) led to decreased activity. It showed that para position is not participating in the inhibitory potential. Similarly, compound 27 (\(\hbox {IC}_{50}=18.6\pm 0.24~\upmu \hbox {M}\)) with m,p-dichloro atoms was also found to be less active than unsubstituted analog 21. The pattern of \(\alpha \)-glucosidase inhibitory activity was found in the order of \(\hbox {unsubstituted}>p\hbox {-OMe}>m\),\(p\hbox {-}di\hbox {Cl}>p\hbox {-Me}>p\hbox {-Ph}>p\hbox {-Br} \sim p\hbox {-}\hbox {NO}_{2}\) (Fig. 7).
Biphenyl thiosemicarbazone 28 and its cyclized compounds 29–31 showed potential toward the inhibition of \(\alpha \)-glucosidase enzyme. Compound 30 (\(\hbox {IC}_{50}=16.3\pm 0.21~\upmu \hbox {M}\)) with p-methoxy substitution was found to be the most potent analog when compared to unsubstituted derivative 29 (\(\hbox {IC}_{50}=27.7\pm 0.21~\upmu \hbox {M}\)). Comparison of compound 30 with 31 revealed that replacing the methoxy group with the phenyl ring in compound 31 (\(\hbox {IC}_{50}=38.2\pm 0.23~\upmu \hbox {M}\)), leads to further decreased inhibitory activity which shows that methoxy group is playing an important role in the activity (Fig. 8).
4-Benzyloxy benzylidene thiosemicarbazone 35 (\(\hbox {IC}_{50}=17.9\pm 0.26~\upmu \hbox {M}\)) and its cyclized hydrazinyl thiazole derivative 36 (\(\hbox {IC}_{50}=41.6\pm 0.23~\upmu \hbox {M}\)) with p-methoxy and 37 (\(\hbox {IC}_{50}=43.6\pm 0.23~\upmu \hbox {M}\)) with p-Ph substitutions showed \(\alpha \)-glucosidase inhibitory activity comparable to standard acarbose (Fig. 9).
Overall, most of the thiosemicarbazones, except for 20 and 35, were failed to show \(\alpha \)-glucosidase enzyme. It is worth mentioning that most of the cyclized hydrazinyl thiazole derivatives showed \(\alpha \)-glucosidase inhibitory activity. However, in order to further evaluate the participation of various structural features in the interactions with the active site of enzyme, a molecular docking study was conducted as discussed below.
Molecular docking study
Preparation of the synthesized derivatives
To predict the binding mode of the synthesized (E)-2-(2-(arylmethylene)hydrazinyl)-4-arylthiazole derivatives with \(\alpha \)-glucosidase enzyme, a molecular docking study was carried out using MOE (Molecular Operating Environment) software package [29]. The three-dimensional structures of the synthesized derivatives were generated by using the builder tool in MOE. The generated compounds were 3D protonated and energy minimized using the default parameters of MOE (gradient: 0.05, Force Field: MMFF94X). All the compounds were then saved into an mdb file for further evaluation.
Preparation of \(\alpha \)-glucosidase 3D structure
The 3D structure for \(\alpha \)-glucosidase of Saccharomyces cerevisiae has not been solved yet; however, several homologies models of \(\alpha \)-glucosidase have been reported [30,31,32,33]. In this study, we used our reported 3D homology model of \(\alpha \)-glucosidase of Saccharomyces cerevisiae [34].
For docking studies, the parameters of MOE used were: Placement: Triangle Matcher, Rescoring 1: London dG, Refinement: Forcefield, Rescoring 2: GBVI/WSA. For each ligand 10 conformations were allowed to be formed and on the basis of docking score the top ranked conformations were selected for further analysis. Docking score is the binding free energy calculated by the GBVI/WSA scoring function which is the score of the last stage showing the overall stability of the predicted complex. For all scoring functions, lower scores indicate more favorable poses. The calculated docking scores for \(\alpha \)-glucosidase enzyme are listed in Table 1 and the unit for all scoring functions is kcal/mol.
Interactions detail
All synthetic derivatives 1–38 that were divided into six different categories, i.e., A, B, C, D, E and F on the basis of their geometries, were docked into the binding pocket of \(\alpha \)-glucosidase enzyme in order to find the binding interactions of the compounds within the active-site residues. On the basis of docking scores, the best conformations were analyzed for hydrogen bonding/arene-arene/arene-cation interactions, at the end of docking experiment. Similarly, various degrees of inhibitory potentials were predicted for the active derivatives of the series against \(\alpha \)-glucosidase enzyme.
Figure 10a–d presents the binding mode of some most active compounds. For example, Fig. 10a shows that compound 5 fits well into the binding cavity of \(\alpha \)-glucosidase enzyme showing three interactions with residues Phe177, Asn347, and Arg312. Phe177 participates in \(\pi \)–H interaction with the arylthiazole \(\pi \)-electron system. Asn347 forms another \(\pi \)–H interaction with the \(\pi \)-electrons of arylthiazole. Similarly, Arg312 forms a third \(\pi \)–H bond with the \(\pi \)-electrons of arylhydrazinyl group.
Compound 7 in this group is an intermediary active compound which forms two noticeable interactions with the binding site residues Phe177 and Glu304 as presented in Fig. 10b. Phe177 shows \(\pi \)–H interaction with the arylhydrazinyl ring of compound.
A side chain H-donor interaction was also perceived between Glu304 and H of the thiazole group. Figure 10c shows the binding mode of the most active compound 8, forming four important interactions with active site residues His279, Asn241, and Phe177. His279 forms a polar interaction with the S of thiazole and a \(\pi \)–H bond with hydrazinyl group of compound.
Asn347 involves a side chain H-acceptor interaction with the nitrogen of hydrazinyl group and Phe177 shows \(\pi \)–H interaction with the arylhydrazinyl group. Compound 10 is another significantly active compound in this group and shows two different interactions with the residues Glu276 and Phe157 as shown in Fig. 10d. Glu276 establishes an H-donor interaction with the Br of arylthiazole group. A second strong H-donor bond is observed between Phe157 and hydrazinyl group. The 3D binding mode also shows that Asp408 may also form another H-donor bond with the S of thiazole moiety as the distance between them was measured as 2.86 Å. In addition to the catalytic residues, molecular docking studies of (E)-2-(2-(arylmethylene)hydrazinyl)-4-arylthiazole derivatives predict that residues like Phe157, Asn347, Arg312, Glu304, and His279 have an important role in the \(\alpha \)-glucosidase inhibition.
Conclusion
Synthetic (E)-2-(2-(arylmethylene)hydrazinyl)-4-arylthiazoles along with intermediates were screened for \(\alpha \)-glucosidase inhibitory activity. A number of compounds demonstrated good inhibitory potential. Molecular modeling identified structural features that participate in binding interactions with the active sites of enzyme. This study identified a number of promising candidates that may serve as leads for the future research in search of therapeutic agents for type-2 diabetes mellitus.
Experimental
Materials and methods
Reagents were purchased from Sigma-Aldrich (USA) and were of analytical grade. Thin-layer chromatography was performed on pre-coated silica gel, GF-254. Spots were visualized under ultraviolet light at 254 and 366 nm. Mass spectra were recorded under electron impact (EI) condition on Varian Massen Spectrometers MAT 312 and MAT 113D. The \({}^{1}\hbox {H-NMR}\) and \({}^{13}\hbox {C-NMR}\) were recorded on Bruker AM machines operating at 300, 400 and 500 MHz. Chemical shift values are presented in ppm (\(\delta \)) relative to tetramethylsilane (TMS) as an internal standard and the coupling constant (J) are in Hz. Multiplicities are reported as singlet (s), doublet (d), triplet (t), doublet of doublets (dd), doublet of triplets (dt), quartet (q) or multiplet (m).
General procedure for the synthesis of thiosemicarbazone intermediates (1, 4, 12, 20, 28, 35)
Different aryl aldehydes (10 mmol) and thiosemicarbazide (10 mmol) were taken in ethanol (50 mL) into a 250-mL round-bottomed flask with few drops of glacial acetic acid. The reaction mixture was refluxed for 4 h with constant stirring. Progress of reaction was monitored by thin-layer chromatography (TLC). After completion, the resulting precipitate was filtered and washed with 10 mL cold ethanol to afford the pure product in good yields. All compounds 1, 4, 12, 20, 28, 35 were characterized by the spectroscopic techniques. To the best of our knowledge, structures of all intermediates are known [35,36,37,38,39,40].
(E)-2-(2,6-Dimethoxybenzylidene) hydrazinecarbothioamide (1) [35]
Solid; Light orange; Yield: 73%; M.P.: 182–184 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (400 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 11.33 (s, 1H, NH), 8.29 (s, 1H, H–C=N), 8.09 (s, 1H, NH), 7.33 (t, \(J_{{ 4(3,5)}}=8.4\) Hz, 1H, H-4), 7.19 (s, 1H, NH), 6.69 (d, \(J_{{ 3,4}}=J_{{ 5,4}}=8.4\) Hz, 2H, H-3, H-5), 3.78 (s, 6H, \(2\hbox {OCH}_{3})\); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}){:}\delta \) 177.7 (\(\hbox {C}{=}\hbox {S}\)), 158.8 (C-2), 158.8 (C-6), 138.2 (\(\hbox {HC}{=}\hbox {N}\)), 131.2 (CH-4), 110.3 (C-1), 104.3 (CH-3), 104.3 (CH-5), 56.0 (\(\hbox {OCH}_{3})\), 56.0 (\(\hbox {OCH}_{3}\)); \(\hbox {EI-MS }m/z \) (% rel. abund.): 239 (\(\hbox {M}^{+}\), 95), 164 (83), 163 (89), 149 (100), 121 (51), 106 (67), 91(95), 51(88); HREI-MS Calcd for \(\hbox {C}_{10}\hbox {H}_{13}\hbox {N}_{3}\hbox {O}_{2}\hbox {S}{:} \,m/z=239.0728\), found 239.0730; Anal. Calcd for \(\hbox {C}_{10}\hbox {H}_{13}\hbox {N}_{3}\hbox {O}_{2}\hbox {S}:\hbox {C}=50.19\); \(\hbox {H}=5.48\); \(\hbox {N}=17.56\); Found: \(\hbox {C}=50.21\); \(\hbox {H}=5.50\); \(\hbox {N}=17.59\).
(E)-2-(Naphthalen-1-ylmethylene)hydrazinecarbothioamide (4) [36]
Solid; White; Yield: 78%; M.P.: \(119\hbox {-}121~^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (400 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta ~ 8.90\) (s, 1H, H–C=N), 8.36 (d, \(J_{{ 2,3}}=8.4\) Hz, 1H, H-2), 8.25 (s, 1H, NH), 8.21 (d, \(J_{{ 4,3}}=6.8\) Hz, 1H, H-4), 8.00 (d, \(J_{{ 5,6}}=J_{{ 8,7}}=8.4\) Hz, 2H, H-5, H-8), 7.95 (s, 1H, NH), 7.66 (t, \(J_{{ 3(2,4)}}=8.0\) Hz, 1H, H-3), 7.59 (overlapping multiplet, 2H, H-6, H-7); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 177.8 (\(\hbox {C}{=}\hbox {S}\)), 142.6 (\(\hbox {HC}{=}\hbox {N}\)), 133.7 (C-10), 130.5 (CH-4), 130.3 (C-9), 128.7 (CH-5), 128.2 (C-1), 127.8 (CH-3), 126.4 (CH-6), 126.4 (CH-7), 125.7 (CH-2), 123.2 (CH-8); \(\hbox {EI-MS }m/z\) (% rel. abund.): 229 (\(\hbox {M}^{+}\), 77), 195 (17), 169 (44), 154 (86), 153 (100), 127 (47); HREI-MS Calcd for \(\hbox {C}_{12}\hbox {H}_{11}\hbox {N}_{3}\hbox {S}{:} m/z=229.0674\), found 229.0670; Anal. Calcd for \(\hbox {C}_{12}\hbox {H}_{11}\hbox {N}_{3}\hbox {S}{:}\hbox {C}=62.86\); \(\hbox {H}=4.84\); \(\hbox {N}=18.33\); Found: \(\hbox {C}=62.84\); \(\hbox {H}=4.83\); \(\hbox {N}=18.31\).
(E)-2-(Naphthalen-2-ylmethylene) hydrazinecarbothioamide (12) [37]
Solid; Off white; Yield: 67%; M.P.: 132–134 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (300 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 8.22 (s, 1H, H-1), 8.20 (s, 1H, H–C=N), 8.17 (d, \(J_{{ 3,4}}=8.7\) Hz, 1H, H-3), 8.10 (s, 1H, H-NH), 8.07 (bd s, 1H, NH), 7.96 (overlapping multiplet, 3H, H-4, H-5, H-8), 7.55 (dd, \(J_{{ 6,8}}=J_{{ 7,5}}=3.0\) Hz, \(J_{{ 6,5}}=J_{{ 7,8}}=6\) Hz, 2H, H-6, H-7); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}){:}\delta ~177.6\) (C=S), 145.9 (HC=N), 136.2 (C-10), 132.7 (C-9), 129.3 (C-2), 128.3 (CH-4), 128.0 (CH-1), 127.7 (CH-8), 127.5 (CH-5), 127.3 (CH-3), 126.3 (CH-6), 126.1 (CH-7); \(\hbox {EI-MS }m/z\) (% rel. abund.): 229 (\(\hbox {M}^{+}\), 68), 212 (10), 195 (21), 169 (23), 153 (100), 127 (44), 115 (19); HREI-MS Calcd for \(\hbox {C}_{12}\hbox {H}_{11}\hbox {N}_{3}\hbox {S}\): \(m/{z} = 229.0674\), found 229.0671; Anal. Calcd for \(\hbox {C}_{12}\hbox {H}_{11}\hbox {N}_{3}\hbox {S}\): \(\hbox {C}=62.86\); \(\hbox {H}=4.84\); \(\hbox {N}=18.33\); Found: \(\hbox {C}=62.87\); \(\hbox {H}=4.86\); \(\hbox {N}=18.35\).
(E)-2-((2-Hydroxynaphthalen-1-yl) methylene) hydrazinecarbothioamide (20) [38]
Solid; Light yellow; Yield: 75%; M.P.: 271–273\(~^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (300 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 11.35 (s, 1H, NH), 9.03 (s, 1H, H–OH), 8.50 (d, \(J_{{ 8,7}}=8.1\) Hz, 1H, H-8), 8.19 (s, 1H, H–C=N), 7.88 (overlapping multiplet, 4H, H-4, H-5, 2NH), 7.57 (m, 1H, H-7), 7.39 (t, \(J_{{ 6(5,7)}}=7.2\) Hz, 1H, H-6), 7.19 (d, \(J_{{ 3,4}}=8.7\) Hz, 1H, H-3); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta ~ 177.5\) (\(\hbox {C}{=}\hbox {S}\)), 170.2 (C-2), 144.2 (HC=N), 133.2 (C-10), 132.6 (CH-4), 130.0 (C-9), 128.4 (CH-5), 127.2 (CH-7), 124.3 (CH-6), 120.4 (CH-3), 118.9 (CH-8), 107.7 (C-1); \(\hbox {EI-MS }m/z\) (% rel. abund.): 245 (\(\hbox {M}^{+}\), 35), 169 (100), 141 (18), 128 (12), 115 (25); HREI-MS Calcd for \(\hbox {C}_{12}\hbox {H}_{11}\hbox {N}_{3}\hbox {OS}\): \(m/z=245.0623\), found 245.0616; Anal. Calcd for \(\hbox {C}_{12}\hbox {H}_{11}\hbox {N}_{3}\hbox {OS}: \hbox {C}=58.76\); \(\hbox {H}=4.52\); \(\hbox {N}=17.13\); Found: \(\hbox {C}=58.74\); \(\hbox {H}=4.55\); \(\hbox {N}=17.15\).
(E)-2-(Biphenyl-4-ylmethylene) hydrazinecarbothioamide (28) [39]
Solid; Off white; Yield: 68%; M.P.: 205–207 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (300 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 11.45 (s, 1H, NH), 8.07 (s, 1H, NH), 8.03 (s, 1H, H–C=N), 7.98 (d, \(J_{{ 2,3}}=J_{{ 6,5}}=8.4\) Hz, 2H, H-2, H-6), 7.72 (m, 4H, H-3, H-5, \(\hbox {H-2}^{\prime }\), \(\hbox {H-6}^{\prime }\)), 7.49 (t, \(J_{3^{\prime } (2^{\prime } ,4^{\prime } )}=J_{5^{\prime } (4^{\prime } ,6^{\prime } )}=7.2\) Hz, 2H, H-3\(^{\prime }\), \(\hbox {H-5}^{\prime }\)), 7.39 (t, \(J_{4^{\prime } (3^{\prime } ,5^{\prime } )}=7.2\) Hz, 1H, H-4\(^{\prime })\); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 177.9 (\(\hbox {C}{=}\hbox {S}\)), 146.2 (\(\hbox {HC}{=}\hbox {N}\)), 142.9 (C-4), 140.7 (C-7), 133.0 (C-1), 129.8 (CH-2), 129.8 (CH-6), 129.0 (CH-9), 129.0 (CH-11), 127.7 (CH-3), 127.7 (CH-5), 127.6 (CH-8), 127.6 (CH-12), 127.3 (CH-10); \(\hbox {EI-MS }m/z\) (% rel. abund.): 255 (\(\hbox {M}^{+}\), 76), 238 (27), 221 (30), 179 (100), 152 (39); HREI-MS Calcd for \(\hbox {C}_{14}\hbox {H}_{13}\hbox {N}_{3}\hbox {S}\): \(m/z=255.0830\), found 255.0822; Anal. Calcd for \(\hbox {C}_{14}\hbox {H}_{13}\hbox {N}_{3}\hbox {S}: \hbox {C}=65.86\); \(\hbox {H}=5.13\); \(\hbox {N}=16.46\); Found: \(\hbox {C}=65.88\); \(\hbox {H}=5.15\); \(\hbox {N}=16.49\).
(E)-2-(4-(Benzyloxy) benzylidene) hydrazinecarbothioamide (35) [40]
Solid; Off white; Yield: 72%; M.P.: 188–190 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (300 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 11.29 (s, 1H, NH), 8.04 (s, 1H, H–C=N), 7.97 (s, 1H, H–NH), 7.89 (bd s, 1H, NH), 7.73 (d, \(J_{{ 2,3}}=J_{{ 6,5}}=8.7\) Hz, 2H, H-2, H-6), 7.45 (t, \(J_{{ 9(8,10)}}=J_{{ 10(9,11)}}=J_{{ 11(10,12)}}=6.9\) Hz, 3H, H-9, H-10, H-11), 7.38 (overlapping multiplet, 2H, H-8, H-12), 7.04 (d, \( J_{3,2}=J_{{ 5,6}}=8.7\) Hz, 2H, H-3, H-5), 5.14 (s, 2H, \(\hbox {H-CH}_{2})\); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 177.8 (C=S), 160.2 (C-4), 146.3 (HC=N), 136.4 (C-7), 130.2 (CH-2), 130.2 (CH-6), 128.7 (CH-9), 128.7 (CH-11), 127.5 (CH-10), 127.0 (CH-8), 127.0 (CH-12), 126.2 (C-1), 114.5 (CH-3), 114.5 (CH-5), 70.6 (\(\hbox {CH}_{2})\); \(\hbox {EI-MS }m/z\) (% rel. abund.): 285 (\(\hbox {M}^{+}\), 51), 268 (27), 135 (9), 91 (100), 75 (6), 65 (20); HREI-MS Calcd for \(\hbox {C}_{15}\hbox {H}_{15}\hbox {N}_{3}\hbox {OS}\): \(m/z=285.0936\), found 285.0930; Anal. Calcd for \(\hbox {C}_{15}\hbox {H}_{15}\hbox {N}_{3}\hbox {OS}: \hbox {C}=63.13\); \(\hbox {H}=5.30\); \(\hbox {N}=14.73\); Found: \(\hbox {C}=63.15\); \(\hbox {H}=5.32\); \(\hbox {N}=14.76\).
General procedure for the synthesis of (E)-2-(2-(arylmethylene)hydrazinyl)-4-arylthiazole derivatives (2, 3, 5–11, 13–19, 21–27, 29–34, 36–38)
Thiosemicarbazone intermediates (0.5 mmol), different substituted phenacyl bromides (0.5 mmol), and triethyl amine (0.5 mmol) were taken in ethanol into a 100-mL round-bottomed flask and refluxed for 3 h with constant stirring. Progress of the reaction was monitored by the thin-layer chromatography (TLC). After completion, the resulting precipitate was filtered and washed with 5 mL cold ethanol to afford the pure products. Compounds 2, 3, 5–11, 13–19, 21–27, 29–34, 36–38 were characterized by spectroscopic analysis. To the best of our knowledge, compounds 2, 3, 11, 14, 16–19, 23, 27, and 29–34 are new compounds while other compounds are structurally known [41, 42].
(E)-2-(2-(2,6-Dimethoxybenzylidene)hydrazinyl)-4-(4-methoxyphenyl)thiazole (2)
Solid; Orange; Yield: 78%; M.P.: 145–147 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (400 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 11.80 (s, 1H, NH), 8.21 (s, 1H, H–C=N), 7.77 (d, \(J_{{ 2'',3''}}=J_{{ 6'',5''}}=\)8.8 Hz, 2H, \(\hbox {H-}2''\), \(\hbox {H-}6''\)), 7.31 (t, \(J_{{ 4(3,5)}}=8.4\) Hz, 1H, H-3), 7.06 (s, 1H, \(\hbox {H-}5'\)), 6.95 (d, \(J_{{ 3'',2''}}= J_{{ 5'',6''}}=\)8.8 Hz, 2H, \(\hbox {H-}3''\), \(\hbox {H-}5''\)), 6.71 (d, \(J_{{ 3,4}}=J_{{ 5,4}}=8.4\) Hz, 2H, H-3, H-5), 3.81 (s, 6H, 2H, \(\hbox {OCH}_{3})\), 3.77 (s, 3H, \(\hbox {OCH}_{3})\); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 168.4 (N=C–S), 158.6 (\(\hbox {C-}4''\)), 158.3 (C-2), 158.3 (C-6), 147.3 (\(\hbox {C-}4'\)), 136.1 (\(\hbox {HC}{=}\hbox {N}\)), 130.3 (CH-4), 126.7 (\(\hbox {CH-}2''\)), 126.7 (\(\hbox {CH-}6''\)), 123.7 (\(\hbox {C-}1''\)), 113.8 (\(\hbox {CH-}3''\)), 113.8 (\(\hbox {CH-}5''\)), 110.0 (C-1), 104.5 (CH-3), 104.5 (CH-5), 101.2 (\(\hbox {CH-}5'\)), 56.0 (\(\hbox {OCH}_{3})\), 56.0 (\(\hbox {OCH}_{3})\), 55.0 (\(\hbox {OCH}_{3})\); \(\hbox {EI-MS }m/z\) (% rel. abund.): 369 (\(\hbox {M}^{+}\), 64), 219 (15), 206 (100), 191 (24), 164 (34), 149 (22); HREI-MS Calcd for \(\hbox {C}_{19}\hbox {H}_{19}\hbox {N}_{3}\hbox {O}_{3}\hbox {S}\): \(m/z=369.1147\), found 369.1133; Anal. Calcd for \(\hbox {C}_{19}\hbox {H}_{19}\hbox {N}_{3}\hbox {O}_{3}\hbox {S}: \hbox {C}=61.77\); \(\hbox {H}=5.18\); \(\hbox {N}=11.37\); Found: \(\hbox {C}=61.75\); \(\hbox {H}=5.17\); \(\hbox {N}=11.35\).
(E)-2-(2-(2,6-Dimethoxybenzylidene) hydrazinyl)-4-(3-nitrophenyl)thiazole (3)
Solid; Yellow; Yield: 75%; M.P.: 178–180 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (400 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 11.98 (s, 1H, NH), 8.65 (s, 1H, H–C=N), 8.29 (d, \(J_{{ 4'',5''}}=7.6\) Hz, 1H, \(\hbox {H-}4''\)), 8.24 (s, 1H, \(\hbox {H-}2''\)), 8.14 (dd, \(J_{{ 6'',4''}}=1.6\) Hz, \( J_{{ 6'',5''}}=8.0\) Hz, 1H, \(\hbox {H-}6''\)), 7.71 (t, \(J_{{ 5''(4'',6'')}} = 8.0\) Hz, 1H, \(\hbox {H-}6''\)), 7.58 (s, 1H, \(\hbox {H-5}^{\prime }\)), 7.32 (t, \(J_{{ 4(3,5)}}=8.4\) Hz, 1H, H-4), 6.72 (d, \(J_{{ 3,4}}=J_{{ 5,4}}=8.4\) Hz), 3.82 (s, 6H, \(2\hbox {OCH}_{3})\); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 170.6 (N=C–S), 158.4 (C-2), 158.4 (C-6), 148.1 (\(\hbox {C-}4'\)), 147.2 (\(\hbox {C-}3''\)), 136.7 (\(\hbox {HC}{=}\hbox {N}\)), 131.8 (\(\hbox {C-}1''\)), 131.5 (\(\hbox {CH-}6''\)), 130.5 (CH-4), 130.1 (\(\hbox {CH-}2''\)), 122.8 (\(\hbox {CH-}4''\)), 119.8 (\(\hbox {CH-}5''\)), 110.1 (C-1), 106.2 (\(\hbox {CH-}5'\)), 104.5 (CH-3), 104.5 (CH-5), 56.0 (\(\hbox {OCH}_{3})\), 56.0 (\(\hbox {OCH}_{3})\); \(\hbox {EI-MS }m/z\) (% rel. abund.): 384 (\(\hbox {M}^{+}\), 31), 221 (26), 175 (56), 149 (23), 121 (11), 89 (10); HREI-MS Calcd for \(\hbox {C}_{18}\hbox {H}_{16}\hbox {N}_{4}\hbox {O}_{4}\hbox {S}\): \(m/z=384.0892\), found 384.0967; Anal. Calcd for \(\hbox {C}_{18}\hbox {H}_{16}\hbox {N}_{4}\hbox {O}_{4}\hbox {S}: \hbox {C}=56.24\); \(\hbox {H}=4.20\); \(\hbox {N}=14.58\); Found: \(\hbox {C}=56.27\); \(\hbox {H}=4.23\); \(\hbox {N}=14.60\).
(E)-2-(2-(Naphthalen-1-ylmethylene) hydrazinyl)-4-phenylthiazole (5) [CAS # 464200-44-0]
Solid; Brick brown; Yield: 58%; M.P.: 183–185 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (600 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.29 (s, 1H, NH), 8.76 (d, \(J_{{ 2,3}}=8.4\) Hz, 1H, H-2), 8.66 (s, 1H, H–C=N), 8.01 (d, \(J_{{ 4,3}}=7.8\) Hz, 1H, H-4), 7.98 (d, \(J_{{ 5,6}}=\)7.8 Hz, 1H, H-5), 7.88 (d, \(J_{{ 2'',6''}}=J_{{ 6'',5''}}=7.2\) Hz, 2H, \(\hbox {H-}2''\), \(\hbox {H-}6''\)), 7.85 (d, \(J_{{ 8,7}}=7.2\) Hz, 1H, H-8), 7.68 (t, \(J_{{ 7(6,8)}}=7.8\) Hz, 1H, H-7), 7.60 (overlapping multiplet, 2H, H-3, H-6), 7.42 (t, \(J_{3''(2,4)}=J_{{ 5''(4'',6'')}}=7.8\) Hz, 2H, \(\hbox {H-}3''\), \(\hbox {H-}5''\)), 7.37 (s, 1H, \(\hbox {H-5}^{\prime }\)), 7.31 (t, \(J_{{ 4''(3'',5'')}}=7.2\) Hz, 1H, \(\hbox {H-}4''\)); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 168.2 (\(\hbox {N}{=}\hbox {C-S}\)), 150.1 (\(\hbox {C-}4'\)), 141.4 (\(\hbox {HC}{=}\hbox {N}\)), 133.2 (C-10), 133.1 (\(\hbox {C-}1''\)), 130.5 (CH-4), 130.3 (C-9), 129.0 (CH-5), 129.0 (C-1), 128.6 (CH-3), 128.5 (CH-6), 128.2 (CH-7), 127.5 (CH-2), 127.3 (\(\hbox {CH-}3''\)), 127.3 (\(\hbox {CH-}5''\)), 126.2 (\(\hbox {CH-}4''\)), 125.5 (\(\hbox {CH-}2''\)), 125.5 (\(\hbox {CH-}6''\)), 123.2 (CH-8), 105.6 (\(\hbox {CH-}5'\)); \(\hbox {EI-MS }m/z\) (% rel. abund.): 329 (\(\hbox {M}^{+}\), 68.2), 176 (100), 153 (36), 134 (48), 127 (18); HREI-MS Calcd for \(\hbox {C}_{20}\hbox {H}_{15}\hbox {N}_{3}\hbox {S}\): \(m/z=329.0987\), found 329.0965; Anal. Calcd for \(\hbox {C}_{20}\hbox {H}_{15}\hbox {N}_{3}\hbox {S}: \hbox {C}=72.92\); \(\hbox {H}=4.59\); \(\hbox {N}=12.76\); Found: \(\hbox {C}=72.94\); \(\hbox {H}=4.61\); \(\hbox {N}=12.78\).
(E)-2-(2-(Naphthalen-1-ylmethylene) hydrazinyl)-4-p-tolylthiazole (6) [41]
Solid; Light brown; Yield: 50%; M.P.: 209–211 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (600 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.26 (s, 1H, NH), 8.76 (d, \(J_{{ 2,3}}=8.4\) Hz, 1H, H-2), 8.65 (s, 1H, H–C=N), 8.01 (d, \(J_{{ 4,3}}=\)7.8 Hz, 1H, H-4), 7.97 (d, \(J_{{ 5,6}}=7.8\) Hz, 1H, H-5), 7.85 (d, \(J_{{ 8,7}}=\,7.5\) Hz, 1H, H-8), 7.76 (d, \(J_{{ 2'',3''}}=J_{{ 6'',5''}}=7.8~\)Hz, 2H, \(\hbox {H-}2''\), \(\hbox {H-}6''\)), 7.68 (t, \(J_{{ 7(6,8)}}=7.8\) Hz, 1H, H-7), 7.60 (overlapping multiplet, 2H, H-3, H-6), 7.28 (s, 1H, \(\hbox {H-5}^{\prime }\)), 7.22 (d, \(J_{{ 3'',2''}}=J_{{ 5'',6''}}=7.8\) Hz, 2H, \(\hbox {H-}3''\), \(\hbox {H-}5''\)), 2.32 (s, 3H, \(\hbox {H-CH}_{3})\); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 168.0 (N=C–S), 150.8 (\(\hbox {C-}4'\)), 141.1 (\(\hbox {HC}{=}\hbox {N}\)), 136.8 (\(\hbox {C-}4''\)), 133.6 (C-10), 132.0 (\(\hbox {C-}1''\)), 129.8 (CH-4), 129.5 (C-9), 129.2 (\(\hbox {CH-}3''\)), 129.2 (\(\hbox {CH-}5''\)), 128.9 (CH-5), 127.2 (C-1), 127.0 (CH-3), 126.2 (CH-6), 126.1 (CH-7), 125.6 (CH-2), 125.5 (\(\hbox {CH-}2''\)), 125.5 (\(\hbox {CH-}6''\)), 124.0 (CH-8), 102.8 (\(\hbox {CH-}5'\)), 20.8 (\(\hbox {CH}_{3})\); \(\hbox {EI-MS }m/z\) (% rel. abund.): 343 (\(\hbox {M}^{+}\), 97), 189 (100), 148 (39), 127 (17); HREI-MS Calcd for \(\hbox {C}_{21}\hbox {H}_{17}\hbox {N}_{3}\hbox {S}\): \(m/z=343.1143\), found 343.1124; Anal. Calcd for \(\hbox {C}_{21}\hbox {H}_{17}\hbox {N}_{3}\hbox {S}: \hbox {C}=73.44\); \(\hbox {H}=4.99\); \(\hbox {N}=12.24\); Found: \(\hbox {C}=73.47\); \(\hbox {H}=4.98\); \(\hbox {N}=12.26\).
(E)-4-(4-Methoxyphenyl)-2-(2-(naphthalen-1-ylmethylene)hydrazinyl)thiazole (7) [41]
Solid; Dark orange; Yield: 55%; M.P.: 207–209 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (600 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.26 (s, 1H, NH), 8.76 (d, \(J_{{ 2,3}}=8.4\) Hz, 1H, H-2), 8.65 (s, 1H, H–C=N), 8.01 (d, \(J_{{ 4,3}}=8.4\) Hz, 1H, H-4), 7.97 (d, \(J_{{ 5,6}}=7.8\) Hz, 1H, H-5), 7.85 (d, \(J_{{ 8,7}}=7.2\) Hz, 1H, H-8), 7.80 (d, \(J_{{ 2'',3''}}=8.4\) Hz, 1H, \(\hbox {H-}2''\)), 7.68 (t, \(J_{{ 7(6,8)}}=8.4\) Hz, 1H, H-7), 7.60 (overlapping multiplet, 2H, H-3, H-6), 7.18 (s, 1H, \(\hbox {H-5}^{\prime }\)), 6.97 (d, \(J_{{ 3'',2''}}=J_{{ 5'',6''}}=8.4\) Hz, 2H, \(\hbox {H-}3''\), \(\hbox {H-}5''\)), 3.78 (s, 3H, \(\hbox {OCH}_{3})\); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 168.7 (N=C–S), 159.4 (\(\hbox {C-}4''\)), 150.5 (\(\hbox {C-}4'\)), 141.7(\(\hbox {HC}{=}\hbox {N}\)), 133.2 (C-10), 130.7 (CH-4), 130.5 (C-9), 128.8 (CH-5), 128.4 (C-1), 128.3 (\(\hbox {CH-}2''\)), 128.3 (\(\hbox {CH-}6''\)), 127.6 (CH-3), 126.4 (CH-6), 125.6 (CH-7), 125.5(\(\hbox {C-}1''\)), 125.5 (CH-2), 123.4 (CH-8), 114.7 (\(\hbox {CH-}3''\)), 114.7 (\(\hbox {CH-}5''\)), 105.7 (\(\hbox {CH-}5'\)), 55.6 (\(\hbox {OCH}_{3})\); \(\hbox {EI-MS }m/z\) (% rel. abund.): 359 (\(\hbox {M}^{+}\), 84), 205 (100), 190 (23), 163 (34), 148 (17), 127 (13); HREI-MS Calcd for \(\hbox {C}_{21}\hbox {H}_{17}\hbox {N}_{3}\hbox {OS}\): \(m/z=359.1092\), found 359.1074; Anal. Calcd for \(\hbox {C}_{21}\hbox {H}_{17}\hbox {N}_{3}\hbox {OS}: \hbox {C}=70.17\); \(\hbox {H}=4.77\); \(\hbox {N}=11.69\); Found: \(\hbox {C}=70.19\); \(\hbox {H}=4.75\); \(\hbox {N}=11.71\).
(E)-4-(Biphenyl-4-yl)-2-(2-(naphthalen-1-ylmethylene)hydrazinyl)thiazole (8) [CAS # 468750-89-2]
Solid; Orange; Yield: 52%; M.P.: 177–179 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (600 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.27 (s, 1H, NH), 8.76 (d, \(J_{{ 2,3}}=8.4\) Hz, 1H, H-2), 8.66 (s, 1H, H–C=N), 8.00 (overlapping multiplet, 4H, H-4, H-5, \(\hbox {H-}2'''\), \(\hbox {H-}6'''\)), 7.85 (d, \(J_{{ 8,7}}=7.2\) Hz, 1H, H-8), 7.72 (overlapping multiplet, 4H, \(\hbox {H-}2''\), \(\hbox {H-}3''\), \(\hbox {H-}5''\), \(\hbox {H-}6''\)), 7.66 (t, \(J_{{ 7(6,8)}}=7.2\) Hz, 1H, H-7), 7.59 (t, \(J_{{ 3(2,4)}}=J_{{ 5(4,6)}}=7.2\) Hz, 2H, H-3, H-5), 7.48 (t, \(J_{{ 3'''(2''',4''')}}=J_{{ 5'''(4''',6''')}}=7.6\) Hz, 2H, \(3'''\), \(\hbox {H-}5'''\)), 7.42 (s, 1H, \(\hbox {H-5}^{\prime }\)), 7.37 (t, \(J_{{ 4'''(3''',5''')}}=7.2\) Hz, 1H, \(\hbox {H-}4'''\)); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 168.4 (N=C–S), 150.5 (\(\hbox {C-}4'\)), 141.6 (\(\hbox {HC}{=}\hbox {N}\)), 140.8 (\(\hbox {C-}4''\)), 140.7 (\(\hbox {C-}7''\)), 133.2 (C-10), 131.8 (\(\hbox {C-}1''\)), 130.5 (CH-4), 130.1 (C-9), 129.1 (\(\hbox {CH-}9''\)), 129.1 (\(\hbox {CH-}11''\)), 128.5 (CH-5), 128.4 (C-1), 128.1 (\(\hbox {CH-}2''\)), 128.1 (\(\hbox {CH-}6''\)), 127.8 (\(\hbox {CH-}8''\)), 127.8 (\(\hbox {CH-}12''\)), 127.6 (\(\hbox {CH-}10''\)), 127.1 (CH-3), 127.0 (\(\hbox {CH-}3''\)), 127.0 (\(\hbox {CH-}5''\)), 126.4 (CH-6), 125.6 (CH-7), 125.6 (CH-2), 123.4 (CH-8), 105.8 (\(\hbox {CH-}5'\)); \(\hbox {EI-MS }m/z\) (% rel. abund.): 405 (\(\hbox {M}^{+}\), 95), 251 (100), 210 (56), 154 (16), 127 (11); HREI-MS Calcd for \(\hbox {C}_{26}\hbox {H}_{19}\hbox {N}_{3}\hbox {S}\): \(m/z=405.1300\), found 405.1324; Anal. Calcd for \(\hbox {C}_{26}\hbox {H}_{19}\hbox {N}_{3}\hbox {S}: \hbox {C}=77.01\); \(\hbox {H}=4.72\); \(\hbox {N}=10.36\); Found: \(\hbox {C}=77.03\); \(\hbox {H}=4.70\); \(\hbox {N}=10.38\).
(E)-2-(2-(Naphthalen-1-ylmethylene) hydrazinyl)-4-(3-nitrophenyl)thiazole (9) [CAS # 464200-43-9]
Solid; Yellow; Yield: 47%; M.P.: 228–230 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (600 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.42 (s, 1H, NH), 8.77 (d, \(J_{{ 2,3}}=8.4\) Hz, 1H, H-2), 8.69 (d, \(J_{{ 2'',6''}}=1.8\) Hz, 1H, \(\hbox {H-}2''\)), 8.67 (s, 1H, H–C=N), 8.33 (d, \(J_{{ 6'',5''}}=7.8\) Hz, 1H, \(\hbox {H-}6''\)), 8.16 (dd, \( J_{4'',2''}=1.8\) Hz, \(J_{4'',3''}=8.4\) Hz, 1H, \(\hbox {H-}4''\)), 8.01 (d, \(J_{{ 4,3}}=7.8\) Hz, 1H, H-4), 7.99 (d, \(J_{{ 5,6}}=8.4\) Hz, 1H, H-5), 7.87 (d, \(J_{{ 8,7}}=7.2\) Hz, 1H, H-8), 7.73 (t, \(J_{{ 5''(4'',6'')}}=7.8\) Hz, 1H, \(\hbox {H-}5''\)), 7.70 (s, 1H, \(\hbox {H-5}^{\prime }\)), 7.69 (t, \(J_{{ 7(6,8)}}=7.2\) Hz, 1H, H-7), 7.61 (overlapping multiplet, 2H, H-3, H-6); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 169.8 (N=C–S), 151.3 (\(\hbox {C-}4'\)), 148.5 (\(\hbox {C-}3''\)), 142.3 (\(\hbox {HC}{=}\hbox {N}\)), 133.8 (\(\hbox {C-}1''\)), 133.7 (\(\hbox {CH-}6''\)), 133.4 (C-10), 130.8 (\(\hbox {CH-}2''\)), 130.7 (CH-4), 130.4 (C-9), 128.9 (CH-5), 128.5 (C-1), 127.9 (CH-3), 126.5 (CH-6), 125.7 (CH-7), 125.7 (CH-2), 123.7 (CH-8), 123.5 (\(\hbox {CH-}4''\)), 122.6 (\(\hbox {CH-}5''\)), 106.6 (\(\hbox {CH-}5'\)); \(\hbox {EI-MS }m/z\) (% rel. abund.): 374 (\(\hbox {M}^{+}\), 34), 220 (100), 175 (30), 154 (15), 127(19); HREI-MS Calcd for \(\hbox {C}_{20}\hbox {H}_{14}\hbox {N}_{4}\hbox {O}_{2}\hbox {S}\): \(m/z=374.0837\), found 374.0832; Anal. Calcd for \(\hbox {C}_{20}\hbox {H}_{14}\hbox {N}_{4}\hbox {O}_{2}\hbox {S}: \hbox {C}=64.16\); \(\hbox {H}=3.77\); \(\hbox {N}=14.96\); Found: \(\hbox {C}=64.19\); \(\hbox {H}=3.78\); \(\hbox {N}=14.94\).
(E)-4-(4-Bromophenyl)-2-(2-(naphthalen-1-ylmethylene)hydrazinyl)thiazole (10) [CAS # 464211-54-9]
Solid; Pale yellow; Yield: 60%; M.P.: 192–194 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (600 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.30 (s, 1H, NH), 8.75 (d, \(J_{{ 2,3}}=8.4\) Hz, 1H, H-2), 8.66 (s, 1H, H–C=N), 8.01 (d, \(J_{{ 4,3}}=7.8\) Hz, 1H, H-4), 7.98 (d, \(J_{{ 5,6}}=7.8\) Hz, 1H, H-5), 7.85 (d, \(J_{{ 8,7}}=7.2\) Hz, 1H, H-8), 7.83 (d, \(J_{{ 2'',3''}}=J_{{ 6'',5''}}=8.4\) Hz, 2H, \(\hbox {H-}2''\), \(H6''\)), 7.68 (t, \(J_{7(8,6)}=7.2\) Hz, 1H, H-7), 7.61 (overlapping multiplet, 4H, H-3, H-6, \(\hbox {H-}3''\), \(\hbox {H-}5''\)), 7.45 (s, 1H, \(\hbox {H-5}^{\prime }\)), 3.78 (s, 3H, \(\hbox {OCH}_{3})\); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 168.1 (N=C–S), 150.3 (\(\hbox {C-}4'\)), 141.5 (\(\hbox {HC}{=}\hbox {N}\)), 133.0 (C-10), 132.1 (\(\hbox {CH-}3''\)), 132.1 (\(\hbox {CH-}5''\)), 132.0 (\(\hbox {C-}1''\)), 130.5 (CH-4), 130.2 (C-9), 128.5 (CH-5), 128.5 (C-1), 128.4 (\(\hbox {CH-}2''\)), 128.4 (\(\hbox {CH-}6''\)), 127.6 (CH-3), 126.5 (CH-6), 125.6 (CH-7), 125.6 (CH-2), 123.4 (CH-8), 123.3 (\(\hbox {C-}4''\)), 105.7 (\(\hbox {CH-}5'\)); \(\hbox {EI-MS }m/z\) (% rel. abund.): 407 (\(\hbox {M}^{+}\), 39), 409 (M+2, 36), 256 (100), 214 (15), 174 (22), 154 (16), 127 (20); HREI-MS Calcd for \(\hbox {C}_{20}\hbox {H}_{14}\hbox {BrN}_{3}\hbox {S}\): \(m/z=407.0092\), found 407.0083; Anal. Calcd for \(\hbox {C}_{20}\hbox {H}_{14}\hbox {BrN}_{3}\hbox {S}: \hbox {C}=58.83\); \(\hbox {H}=3.46\); \(\hbox {N}=10.29\); Found: \(\hbox {C}=58.85\); \(\hbox {H}=3.48\); \(\hbox {N}=10.31\).
(E)-4-(3,4-Dichlorophenyl)-2 -(2-(naphthalen-1-ylmethylene) hydrazinyl) thiazole (11)
Solid; Brick red; Yield: 48%; M.P.: 160–162 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (600 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.33 (s, 1H, NH), 8.75 (d, \(J_{{ 2,3}}=8.4\) Hz, 1H, H-2), 8.66 (s, 1H, H–C=N), 8.10 (d, \(J_{{ 2'',6''}}=1.8\) Hz, 1H, \(\hbox {H-}2''\)), 8.01 (d, \(J_{{ 4,3}}=7.8\) Hz, 1H, H-4), 7.99 (d, \(J_{{ 5,6}}=8.4\) Hz, 1H, H-5), 7.86 (overlapping multiplet, 2H, H-8, \(\hbox {H-}6''\)), 7.68 (overlapping multiplet, 2H, H-7, \(\hbox {H-}5''\)), 7.61 (m, 3H, H-3, H-6, \(\hbox {H-5}^{\prime }\)); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 168.3 (N=C–S), 150.4 (\(\hbox {C-}4'\)), 141.6 (\(\hbox {HC}{=}\hbox {N}\)), 133.6 (\(\hbox {C-}4''\)), 133.1 (C-10), 132.7 (\(\hbox {C-}3''\)), 132.5 (\(\hbox {C-}1''\)), 130.6 (CH-4), 130.5 (\(\hbox {CH-}5''\)), 130.2 (C-9), 128.6 (\(\hbox {CH-}2''\)), 128.4 (CH-5), 128.3 (C-1), 127.7 (CH-3), 127.3 (\(\hbox {CH-}6''\)), 126.3 (CH-6), 125.5 (CH-7), 125.5 (CH-2), 123.3 (CH-8), 105.9 (\(\hbox {CH-}5'\)); \(\hbox {EI-MS }m/z\) (% rel. abund.): 397 (\(\hbox {M}^{+}\), 24), 399 (M+2, 18), 401 (M+4, 7), 244 (100), 202 (17), 154 (15), 127 (18); HREI-MS Calcd for \(\hbox {C}_{20}\hbox {H}_{13}\hbox {Cl}_{2}\hbox {N}_{3}\hbox {S}\): \(m/z=397.0207\), found 397.0190; Anal. Calcd for \(\hbox {C}_{20}\hbox {H}_{13}\hbox {Cl}_{2}\hbox {N}_{3}\hbox {S}: \hbox {C}=60.31\); \(\hbox {H}=3.29\); \(\hbox {N}=10.55\); Found: \(\hbox {C}=60.33\); \(\hbox {H}=3.27\); \(\hbox {N}=10.52\).
(E)-2-(2-(Naphthalen-2-ylmethylene) hydrazinyl)-4-phenylthiazole (13) [CAS # 1860007-95-9]
Solid; Yellow; Yield: 75%; M.P.: 229–231 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (500 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.28 (s, 1H, NH), 8.18 (s, 1H, H-1), 8.04 (s, 1H, H–C=N), 7.96 (overlapping multiplet, 4H, H-3, H-4, H-5, H-8), 7.86 (d, \(J_{{ 2'',3''}}=J_{{ 6'',5''}}=\,7.5\) Hz, 2H, \(\hbox {H-}2''\), \(\hbox {H-}6''\)), 7.54 (dd, \(J_{{ 6,8}}=J_{{ 7,5}}=1.5\) Hz, \(J_{{ 6,5}}=J_{{ 7,8}}=9.0\) Hz, 2H, H-6, H-7), 7.42 (t, \(J_{{ 3''(2'',4'')}}=J_{{ 5''(4'',6'')}}=7.5\) Hz, 2H, \(\hbox {H-}3''\), \(\hbox {H-}5''\)), 7.35 (s, 1H, \(\hbox {H-5}^{\prime }\)), 7.31 (t, \(J_{{ 4''(3'',5'')}}=7.5\) Hz, 1H, \(\hbox {H-}4''\)); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 171.5 (N=C–S), 150.4 (\(\hbox {C-}4'\)), 143.3 (\(\hbox {HC}{=}\hbox {N}\)), 135.9 (C-10), 132.4 (C-9), 132.2 (\(\hbox {C-}1''\)), 129.4 (\(\hbox {CH-}3''\)), 129.4 (\(\hbox {CH-}5''\)), 129.0 (C-2), 128.4 (\(\hbox {CH-}4''\)), 128.2 (CH-4), 127.8 (CH-1), 127.6 (CH-8), 127.4 (CH-5), 127.2 (CH-3), 126.3 (\(\hbox {CH-}2''\)), 126.3 (\(\hbox {CH-}6''\)), 126.1 (CH-6), 126.0 (CH-7), 105.3 (\(\hbox {CH-}5'\)); \(\hbox {EI-MS }m/z\) (% rel. abund.): 329 (\(\hbox {M}^{+}\), 56), 176 (100), 154 (9), 134 (34), 127 (13); HREI-MS Calcd for \(\hbox {C}_{20}\hbox {H}_{15}\hbox {N}_{3}\hbox {S}\): \(m/z=329.0987\), found 329.0969; Anal. Calcd for \(\hbox {C}_{20}\hbox {H}_{15}\hbox {N}_{3}\hbox {S}: \hbox {C}=72.92\); \(\hbox {H}=4.59\); \(\hbox {N}=12.76\); Found: \(\hbox {C}=72.94\); \(\hbox {H}=4.61\); \(\hbox {N}=12.78\).
(E)-2-(2-(Naphthalen-2-ylmethylene) hydrazinyl)-4-p-tolylthiazole (14)
Solid; Yellow; Yield: 62%; M.P.: 240–242 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (500 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.24 (s, 1H, H-NH), 8.18 (s, 1H, H-1), 8.03 (s, 1H, H–C=N), 7.96 (overlapping multiplet, 4H, H-3, H-4, H-5, H-8), 7.75 (d, \(J_{{ 2'',3''}}=J_{{ 6'',5''}}=4.8\) Hz, 2H, \(\hbox {H-}2''\), \(\hbox {H-}6''\)), 7.54 (dd, \(J_{{ 6,8}}=J_{{ 7,5}}=2.4\) Hz, \(J_{{ 6,5}}=J_{{ 7,8}}=4.5\) Hz, 2H, H-6, H-7), 7.27 (s, 1H, \(\hbox {H-}5'\)), 7.21 (d, \(J_{{ 3'',2''}}=J_{{ 5'',6''}}=5.1\) Hz, 2H, \(\hbox {H-}3''\), \(\hbox {H-}5''\)), 2.31 (s, 3H, \(\hbox {CH}_{3})\); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 171.3 (N=C–S), 150.0 (\(\hbox {C-}4'\)), 143.1 (\(\hbox {HC}{=}\hbox {N}\)), 135.6 (C-10), 132.4 (C-9), 130.0 (\(\hbox {C-}4''\)), 129.1 (\(\hbox {CH-}3''\)), 129.1 (\(\hbox {CH-}5''\)), 128.5 (\(\hbox {C-}1''\)), 128.1 (C-2), 127.9 (CH-4), 127.4 (\(\hbox {CH-}2''\)), 127.4 (\(\hbox {CH-}6''\)), 127.3 (CH-1), 126.1 (CH-8), 127.1 (CH-5), 127.0 (CH-3), 126.4 (CH-6), 125.9 (CH-7), 104.7 (\(\hbox {CH-}5'\)), 21.1 (\(\hbox {CH}_{3})\); \(\hbox {EI-MS }m/z\) (% rel. abund.): 343 (\(\hbox {M}^{+}\), 64), 190 (100), 153 (10), 148 (33), 127 (14); HREI-MS Calcd for \(\hbox {C}_{21}\hbox {H}_{17}\hbox {N}_{3}\hbox {S}\): \(m/z=343.1143\), found 343.1157; Anal. Calcd for \(\hbox {C}_{21}\hbox {H}_{17}\hbox {N}_{3}\hbox {S}: \hbox {C}=73.44\); \(\hbox {H}=4.99\); \(\hbox {N}=12.24\); Found: \(\hbox {C}=73.42\); \(\hbox {H}=4.97\); \(\hbox {N}=12.26\).
(E)-4-(4-Methoxyphenyl)-2-(2-(naphthalen-2-ylmethylene) hydrazinyl)thiazole (15) [CAS # 1808939-62-9]
Solid; Dark yellow; Yield: 65%; M.P.: 238–240 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (300 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.21 (s, 1H, NH), 8.17 (s, 1H, H-1), 8.03 (s, 1H, H–C=N), 7.96 (overlapping multiplet, 4H, H-3, H-4, H-5, H-8), 7.80 (d, \(J_{{ 2'',3''}}=J_{{ 6'',5''}}=8.7\) Hz, 2H, \(\hbox {H-}2''\), \(\hbox {H-}6''\)), 7.55 (overlapping multiplet, 2H, H-6, H-7), 7.17 (s, 1H, \(\hbox {H-5}^{\prime }\)), 6.97 (d, \(J_{{ 3'',2''}}=J_{{ 5'',6''}}=8.7\) Hz, 2H, \(\hbox {H-}3''\), \(\hbox {H-}5''\)), 3.77 (s, 3H, \(\hbox {OCH}_{3})\); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 171.2 (N=C–S), 159.4 (\(\hbox {C-}4''\)), 149.8 (\(\hbox {C-}4'\)), 143.0 (\(\hbox {HC}{=}\hbox {N}\)), 135.8 (C-10), 132.3 (C-9), 129.0 (C-2), 128.4 (CH-4), 128.3 (\(\hbox {CH-}2''\)), 128.3 (\(\hbox {CH-}6''\)), 128.2 (CH-1), 127.7 (CH-8), 127.6 (CH-5), 127.4 (CH-3), 126.3 (CH-6), 126.1 (CH-7), 125.4 (\(\hbox {C-}1''\)), 114.6 (\(\hbox {CH-}3''\)), 114.6 (\(\hbox {CH-}5''\)), 104.5 (\(\hbox {CH-}5'\)), 55.5 (\(\hbox {OCH}_{3})\); \(\hbox {EI-MS }m/z\) (% rel. abund.): 359 (\(\hbox {M}^{+}\), 83), 206 (100), 191 (16), 164 (32), 149 (14), 127 (16); HREI-MS Calcd for \(\hbox {C}_{21}\hbox {H}_{17}\hbox {N}_{3}\hbox {OS}\): \(m/z=359.1092\), found 359.1082; Anal. Calcd for \(\hbox {C}_{21}\hbox {H}_{17}\hbox {N}_{3}\hbox {OS}: \hbox {C}=70.17\); \(\hbox {H}=4.77\); \(\hbox {N}=11.69\); Found: \(\hbox {C}=70.19\); \(\hbox {H}=4.75\); \(\hbox {N}=11.67\).
(E)-4-(Biphenyl-4-yl)-2-(2-(naphthalen-2-ylmethylene)hydrazinyl)thiazole (16)
Solid; Yellow; Yield: 66%; M.P.: 279–281 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (300 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.28 (s, 1H, NH), 8.20 (s, 1H, H-1), 8.04 (s, 1H, H–C=N), 7.97 (overlapping multiplet, 6H, H-3, H-4, H-5, H-8, \(\hbox {H-}2''\), \(\hbox {H-}6''\)), 7.73 (overlapping multiplet, 4H, \(\hbox {H-}3''\), \(\hbox {H-}5''\), \(\hbox {H-}8''\), \(\hbox {H-}12''\)), 7.55 (m, 2H, H-6, H-7), 7.49 (t, \(J_{9''(8'',10'')}=J_{11''(10'',12'')}=\)7.5 Hz, 2H, \(\hbox {H-9}''\), \(\hbox {H-}11''\)), 7.42 (s, 1H, \(\hbox {H-5}^{\prime }\)), 7.38 (t, \(J_{10''(9'',11'')}=7.2\) Hz, 1H, \(\hbox {H-}10''\)); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 171.5 (N=C–S), 150.2 (\(\hbox {C-}4'\)), 143.8 (\(\hbox {HC}{=}\hbox {N}\)), 140.8 (\(\hbox {C-}4''\)), 140.7 (\(\hbox {C-}7''\)), 136.0 (C-10), 132.5 (C-9), 131.4 (\(\hbox {C-}1''\)), 129.3 (\(\hbox {CH-}9''\)), 129.3 (\(\hbox {CH-}11''\)), 129.2 (C-2), 128.5 (CH-4), 128.4 (CH-1), 127.9 (CH-8), 127.8 (\(\hbox {C-}10''\)), 127.7 (\(\hbox {CH-}2''\)), 127.7 (\(\hbox {CH-}6''\)), 127.6 (\(\hbox {CH-}8''\)), 127.6 (\(\hbox {CH-}12''\)), 127.5 (CH-5), 127.4 (\(\hbox {CH-}3''\)), 127.4 (\(\hbox {CH-}5''\)), 127.2 (CH-3), 126.5 (CH-6), 126.3 (CH-7), 105.4 (\(\hbox {CH-}5'\)); \(\hbox {EI-MS }m/z\) (% rel. abund.): 405 (\(\hbox {M}^{+}\), 44), 252 (100), 210 (28), 180 (4), 153 (9), 127 (9); HREI-MS Calcd for \(\hbox {C}_{26}\hbox {H}_{19}\hbox {N}_{3}\hbox {S}\): \(m/z=405.1300\), found 405.1311; Anal. Calcd for \(\hbox {C}_{26}\hbox {H}_{19}\hbox {N}_{3}\hbox {S}: \hbox {C}=77.01\); \(\hbox {H}=4.72\); \(\hbox {N}=10.36\); Found: \(\hbox {C}=77.04\); \(\hbox {H}=4.70\); \(\hbox {N}=10.34\).
(E)-2-(2-(Naphthalen-2-ylmethylene) hydrazinyl)-4-(3-nitrophenyl)thiazole (17)
Solid; Yellow; Yield: 67%; M.P.: 232–234 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (300 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.39 (s, 1H, NH), 8.64 (s, 1H, \(\hbox {H-}2''\)), 8.32 (d, \(J_{{ 4,3}}=7.8\) Hz, 1H, \(\hbox {H-}4''\)), 8.20 (s, 1H, H-1), 8.16 (dd, \(J_{{ 6'',4''}}=2.1\) Hz, \(J_{{ 6'',5''}}=8.1\) Hz, 1H, \(\hbox {H-}6''\)), 8.05 (s, 1H, H–C=N), 7.97 (overlapping multiplet, 4H, H-3, H-4, H-5, H-8), 7.73 (overlapping multiplet, 2H, H-5, \(\hbox {H-5}^{\prime }\)), 7.55 (overlapping multiplet, 2H, H-6, H-7); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 172.4 (N=C–S), 150.9 (\(\hbox {C-}3''\)), 150.7 (\(\hbox {C-}4'\)), 149.1 (\(\hbox {C-}1''\)), 144.5 (\(\hbox {HC}{=}\hbox {N}\)), 136.4 (C-10), 134.0 (\(\hbox {CH-}6''\)), 133.0 (C-9), 132.1 (\(\hbox {CH-}2''\)), 129.6 (C-2), 128.7 (CH-4), 128.6 (CH-1), 128.0 (CH-8), 127.9 (CH-5), 127.7 (CH-3), 126.8 (CH-6), 126.3 (CH-7), 124.2 (\(\hbox {CH-}4''\)), 123.2 (\(\hbox {CH-}5''\)), 106.0 (\(\hbox {CH-}5'\)); \(\hbox {EI-MS }m/z\) (% rel. abund.): 374 (\(\hbox {M}^{+}\), 25), 221 (100), 175 (26), 153 (24), 127 (17); HREI-MS Calcd for \(\hbox {C}_{20}\hbox {H}_{14}\hbox {N}_{4}\hbox {O}_{2}\hbox {S}\): \(m/z=374.0837\), found 374.0868; Anal. Calcd for \(\hbox {C}_{20}\hbox {H}_{14}\hbox {N}_{4}\hbox {O}_{2}\hbox {S}: \hbox {C}=64.16\); \(\hbox {H}=3.77\); \(\hbox {N}=14.96\); Found: \(\hbox {C}=64.18\); \(\hbox {H}=3.75\); \(\hbox {N}=14.98\).
(E)-4-(4-Bromophenyl)-2-(2-(naphthalen-2-ylmethylene)hydrazinyl)thiazole (18)
Solid; Brown; Yield: 68%; M.P.: 263–265 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (500 MHz, \(\hbox {DMSO-}d_{{ 6}})\) 12.28 (s, 1H, H-NH), 8.19 (s, 1H, H-1), 8.04 (s, 1H, H–C=N), 7.96 (overlapping multiplet, 4H, H-3, H-4, H-5, H-8), 7.82 (d, \(J_{{ 2'',3''}}=J_{{ 6'',5''}}=8.5\) Hz, 2H, \(\hbox {H-}2''\), \(\hbox {H-}6''\)), 7.60 (d, \(J_{{ 3'',2''}}=J_{{ 5'',6''}}=8.5\) Hz, 2H, \(\hbox {H-}3''\), \(\hbox {H-}5''\)), 7.54 (m, 2H, H-7, H-8), 7.43 (s, 1H, \(\hbox {H-5}^{\prime }\)); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 171.3 (N=C–S), 149.9 (\(\hbox {C-}4'\)), 143.1 (\(\hbox {HC}{=}\hbox {N}\)), 135.8 (C-10), 132.4 (C-9), 132.2 (\(\hbox {CH-}3''\)), 132.2 (\(\hbox {CH-}5''\)), 132.1 (\(\hbox {C-}1''\)), 129.2 (C-2), 128.5 (CH-4), 128.4 (\(\hbox {CH-}2''\)), 128.4 (\(\hbox {CH-}6''\)), 128.3 (CH-1), 127.8 (CH-8), 127.7 (CH-5), 127.5 (CH-3), 126.4 (CH-6), 126.2 (CH-7), 123.4 (\(\hbox {C-}4''\)), 104.6 (\(\hbox {CH-}5'\)); \(\hbox {EI-MS }m/z\) (% rel. abund.): 407 (\(\hbox {M}^{+}\), 39), 409 (M+2, 44), 256 (98), 254 (100), 214 (16), 21 (16), 174 (36), 153 (18), 127 (25); HREI-MS Calcd for \(\hbox {C}_{20}\hbox {H}_{14}\)BrN3S: \(m/z=407.0092\), found 407.0088; Anal. Calcd for \(\hbox {C}_{20}\hbox {H}_{14}\hbox {BrN}_{3}\hbox {S}: \hbox {C}=58.83\); \(\hbox {H}=3.46\); \(\hbox {N}=10.29\); Found: \(\hbox {C}=58.85\); \(\hbox {H}=3.49\); \(\hbox {N}=10.31\).
(E)-4-(3,4-Dichlorophenyl)-2-(2-(naphthalen-2-ylmethylene) hydrazinyl)thiazole (19)
Solid; Off white; Yield: 58%; M.P.: 235–237 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (300 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.29 (s, 1H, NH), 8.19 (s, 1H, H-1), 8.09 (d, \(J_{{ 2'',6''}}=1.8\) Hz, 1H, \(\hbox {H-}2''\)), 8.05 (s, 1H, H–C=N), 7.96 (overlapping multiplet, 4H, H-3, H-4, H-5, H-8), 7.86 (dd, \(J_{{ 6'',2''}}=2.1\) Hz, \(J_{{ 6'',5''}}=8.4\) Hz, 1H, \(\hbox {H-}6''\)), 7.68 (d, \(J_{{ 5'',6''}}=8.4\) Hz, 1H, \(\hbox {H-}5''\)), 7.58 (s, 1H, \(\hbox {H-5}^{\prime }\)), 7.55 (dd, \(J_{{ 6,8}}=J_{{ 7,5}}=2.4\) Hz, \(J_{{ 6,5}}=J_{{ 7,8}}=4.5\) Hz, 2H, H-6, H-7); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 171.5 (N=C–S), 150.0 (\(\hbox {C-}4'\)), 143.3 (\(\hbox {HC}{=}\hbox {N}\)), 136.0 (C-10), 133.3 (\(\hbox {C-}4''\)), 133.0 (\(\hbox {C-}3''\)), 132.8 (\(\hbox {C-}1''\)), 132.6 (C-9), 131.2 (\(\hbox {CH-}5''\)), 129.3 (C-2), 129.1 (CH-4), 128.5 (\(\hbox {CH-}2''\)), 128.3 (CH-1), 127.9 (CH-8), 127.7 (CH-5), 127.5 (CH-3), 127.2 (\(\hbox {CH-}6''\)), 126.5 (CH-6), 126.3 (CH-7), 105.2 (\(\hbox {CH-}5'\)); \(\hbox {EI-MS }m/z\) (% rel. abund.): 397 (\(\hbox {M}^{+}\), 49), 399 (M + 2, 33), 246 (80), 244 (100), 208 (15), 202 (20), 127 (21); HREI-MS Calcd for \(\hbox {C}_{20}\hbox {H}_{13}\hbox {Cl}_{2}\hbox {N}_{3}\hbox {S}\): \(m/z=397.0207\), found 397.0212; Anal. Calcd for \(\hbox {C}_{20}\hbox {H}_{13}\hbox {Cl}_{2}\hbox {N}_{3}\hbox {S}: \hbox {C}=60.31\); \(\hbox {H}=3.29\); \(\hbox {N}=10.55\); Found: \(\hbox {C}=60.34\); \(\hbox {H}=3.30\); \(\hbox {N}=10.57\).
(E)-1-((2-(4-Phenylthiazol-2-yl) hydrazono)methyl)naphthalen-2-ol (21) [42]
Solid; Green; Yield: 55%; M.P.: 248–250 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (300 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 11.01 (s, 1H, NH), 8.96 (s, 1H, H–C=N), 8.70 (d, \(J_{{ 4,3}}=8.7\) Hz, 1H, H-4), 7.87 (overlapping multiplet, 4H, \(\hbox {H-}2''\), \(\hbox {H-}3''\), \(\hbox {H-}5''\), \(\hbox {H-}6''\)), 7.60 (t,\( J_{{ 4(3,5)}}=7.2\) Hz, 1H, \(\hbox {H-}4''\)), 7.44 (m, 3H, H-5, H-6, H-7), 7.35 (m, 2H, \(\hbox {H-5}^{\prime }\), H-6), 7.23 (d, \(J_{{ 3,4}}=9.0\) Hz, 1H, H-3); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 171.5 (N=C–S), 170.2 (C-2), 150.4 (\(\hbox {C-}4'\)), 143.1 (\(\hbox {HC}{=}\hbox {N}\)), 133.3 (C-10), 132.8 (CH-4), 132.5 (\(\hbox {C-}1''\)), 130.2 (C-9), 129.0 (\(\hbox {CH-}3''\)), 129.0 (\(\hbox {CH-}5''\)), 128.5 (\(\hbox {CH-}4''\)), 128.4 (CH-5), 127.4 (CH-7), 127.2 (\(\hbox {CH-}2''\)), 127.2 (\(\hbox {CH-}6''\)), 124.5 (CH-6), 120.3 (CH-3), 118.7 (CH-8), 107.8 (C-1), 105.3 (\(\hbox {CH-}5'\)); \(\hbox {EI-MS }m/z\) (% rel. abund.): 345 (\(\hbox {M}^{+}\), 79), 328 (100), 176 (92), 170 (35), 134 (39), 115 (17); HREI-MS Calcd for \(\hbox {C}_{20}\hbox {H}_{15}\hbox {N}_{3}\hbox {OS}\): \(m/z=345.0936\), found 345.0917; Anal. Calcd for \(\hbox {C}_{20}\hbox {H}_{15}\hbox {N}_{3}\hbox {OS}: \hbox {C}=69.54\); \(\hbox {H}=4.38\); \(\hbox {N}=12.17\); Found: \(\hbox {C}=69.57\); \(\hbox {H}=4.40\); \(\hbox {N}=12.19\).
(E)-1-((2-(4-p -Tolylthiazol-2-yl) hydrazono)methyl)naphthalen-2-ol (22) [CAS # 357650-77-2]
Solid; Light green; Yield: 78%; M.P.: 268–270 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (300 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 11.03 (s, 1H, NH), 8.96 (s, 1H, H–C=N), 8.69 (d, \(J_{{ 4,3}}=8.4\) Hz, 1H, H-4), 7.87 (d, \(J_{{ 5,6}}=J_{{ 8,7}}=8.7\) Hz, 2H, H-5, H-8), 7.76 (d, \(J_{{ 2'',3''}}=J_{{ 6'',5''}}=8.1\) Hz, 2H, \(\hbox {H-}2''\), \(\hbox {H-}6''\)), 7.59 (t, \(J_{{ 7(6,8)}}=7.8\) Hz, 1H, H-7), 7.40 (t, \(J_{{ 6(5,7)}}=7.5\) Hz, 1H, H-6), 7.25 (m, 4H, H-3, \(\hbox {H-5}^{\prime }\), \(\hbox {H-}3''\), \(\hbox {H-}4''\)), 2.31 (s, 3H, \(\hbox {H-CH}_{3})\); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 171.3 (N=C–S), 170.0 (C-2), 150.2 (\(\hbox {C-}4'\)), 143.0 (\(\hbox {HC}{=}\hbox {N}\)), 133.5 (C-10), 132.4 (CH-4), 131.4 (\(\hbox {C-}4''\)), 130.2 (\(\hbox {C-}1''\)), 130.1 (C-9), 129.3 (\(\hbox {CH-}3''\)), 129.3 (\(\hbox {CH-}5''\)), 128.6 (CH-5), 127.5 (CH-7), 125.4 (\(\hbox {CH-}2''\)), 125.4 (\(\hbox {CH-}6''\)), 124.3 (CH-6), 120.1 (CH-3), 118.5 (CH-8), 107.7 (C-1), 105.1 (\(\hbox {CH-}5'\)), 21.0 (\(\hbox {CH}_{3})\); \(\hbox {EI-MS }m/z\) (% rel. abund.): 359 (\(\hbox {M}^{+}\), 77), 342 (100), 190 (89), 170 (32), 148 (27), 115 (20); HREI-MS Calcd for \(\hbox {C}_{21}\hbox {H}_{17}\hbox {N}_{3}\hbox {OS}\): \(m/z=359.1092\), found 359.1081; Anal. Calcd for \(\hbox {C}_{21}\hbox {H}_{17}\hbox {N}_{3}\hbox {OS}: \hbox {C}=70.17\); \(\hbox {H}=4.77\); \(\hbox {N}=11.69\); Found: \(\hbox {C}=70.15\); \(\hbox {H}=4.75\); \(\hbox {N}=11.70\).
(E)-1-((2-(4-(4-Methoxyphenyl)thiazol-2-yl)hydrazono)methyl)naphthalen-2-ol (23)
Solid; Yellow; Yield: 87%; M.P.: 235–237 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (300 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 11.05 (s, 1H, NH), 8.97 (s, 1H, OH), 8.97 (s, 1H, H–C=N), 8.67 (d, \(J_{{ 4,3}}=8.7\) Hz, 1H, H-4), 7.87 (d, \(J_{{ 2'',3''}}=J_{{ 6'',5''}}=8.7\) Hz, 2H, \(\hbox {H-}2''\), \(\hbox {H-}6''\)), 7.80 (d, \(J_{{ 5,6}}=J_{{ 8,7}}=8.7\) Hz, 2H, H-5, H-8), 7.59 (t, \(J_{{ 7(6,8)}}=7.5\) Hz, 1H, H-7), 7.40 (t, \(J_{{ 6(5,7)}}=7.5\) Hz, 1H, H-6), 7.23 (d, \(J_{{ 3,4}}=9.0\) Hz, 1H, H-3), 7.15 (s, 1H, \(\hbox {H-5}^{\prime }\)), 6.99 (d, \(J_{{ 3'',2''}}=J_{{ 5'',6''}}=8.7\) Hz, 2H, \(\hbox {H-}3''\), \(\hbox {H-}5''\)), (s, 3H, \(\hbox {OCH}_{3})\); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 171.4 (N=C–S), 170.1 (C-2), 158.7 (\(\hbox {C-}4''\)), 150.3 (\(\hbox {C-}4'\)), 143.0 (\(\hbox {HC}{=}\hbox {N}\)), 133.1 (C-10), 132.4 (CH-4), 130.2 (C-9), 128.4 (CH-5), 128.3 (\(\hbox {CH-}2''\)), 128.3 (\(\hbox {CH-}6''\)), 127.3 (CH-7), 125.4 (\(\hbox {C-}1''\)), 124.4 (CH-6), 120.2 (CH-3), 118.6 (CH-8), 114.6 (\(\hbox {CH-}3''\)), 114.6 (\(\hbox {CH-}5''\)), 107.7 (C-1), 105.2 (\(\hbox {CH-}5'\)), 54.6 (\(\hbox {OCH}_{3})\); \(\hbox {EI-MS }m/z\) (% rel. abund.): 375 (\(\hbox {M}^{+}\), 57), 358 (85), 206 (100), 191 (37), 170 (44), 149 (30), 115 (19); HREI-MS Calcd for \(\hbox {C}_{21}\hbox {H}_{17}\hbox {N}_{3}\hbox {O}_{2}\hbox {S}\): \(m/z=375.1041\), found 375.1028; Anal. Calcd for \(\hbox {C}_{21}\hbox {H}_{17}\hbox {N}_{3}\hbox {O}_{2}\hbox {S}: \hbox {C}=67.18\); \(\hbox {H}=4.56\); \(\hbox {N}=11.19\); Found: \(\hbox {C}=67.20\); \(\hbox {H}=4.58\); \(\hbox {N}=11.20\).
(E)-1-((2-(4-(Biphenyl-4-yl)thiazol-2-yl)hydrazono)methyl)naphthalen-2-ol (24) [CAS # 468751-46-4]
Solid; Brown; Yield: 80%; M.P.: 248–250 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (300 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 11.01 (s, 1H, H-NH), 8.97 (s, 1H, H–C=N), 8.72 (d, \(J_{{ 5,6}}=J_{{ 8,7}}=8.4\) Hz, 2H, H-5, H-8), 7.88 (d, \(J_{{ 2'',3''}}=J_{{ 6'',5''}}=8.7\) Hz, 2H, \(\hbox {H-}2''\), \(\hbox {H-}6''\)), 7.74 (overlapping multiplet, 4H, \(\hbox {H-}2'''\), \(\hbox {H-}3''\), \(\hbox {H-}5''\), \(\hbox {H-}6'''\)), 7.60 (t, \(J_{{ 7(6,8)}}=7.2\) Hz, 1H, H-7), 7.50 (t, \(J_{{ 3'''(2''',4''')}}=J_{{ 5''(4'',6'')}}=7.2\) Hz, 2H, \(\hbox {H-}3'''\), \(\hbox {H-}5'''\)), 7.41 (overlapping multiplet, 3H, H-6, \(\hbox {H-5}^{\prime }\), \(\hbox {H-}4'''\)), 7.24 (d, \(J_{{ 3,4}}=9.0\) Hz, 1H, H-3); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 171.5 (N=C–S), 170.3 (C-2), 150.5 (\(\hbox {C-}4'\)), 143.2 (\(\hbox {HC}{=}\hbox {N}\)), 140.8 (\(\hbox {C-}4''\)), 140.7 (\(\hbox {C-}7''\)), 133.4 (C-10), 132.6 (CH-4), 131.8 (\(\hbox {C-}1''\)), 130.3 (C-9), 129.1 (\(\hbox {CH-}9''\)), 129.1 (\(\hbox {CH-}11''\)), 128.6 (CH-5), 128.2 (\(\hbox {CH-}2''\)), 128.2 (\(\hbox {CH-}6''\)), 127.8 (\(\hbox {CH-}8''\)), 127.8 (\(\hbox {CH-}12''\)), 127.4 (\(\hbox {CH-}4''\)), 127.0 (\(\hbox {CH-}3''\)), 127.0 (\(\hbox {CH-}5''\)), 126.9 (CH-7), 124.6 (CH-6), 120.4 (CH-3), 118.8 (CH-8), 107.9 (C-1), 105.4 (\(\hbox {CH-}5'\)); \(\hbox {EI-MS }m/z\) (% rel. abund.): 421 (\(\hbox {M}^{+}\), 53), 404 (66), 252 (100), 210 (36), 170 (31), 115 (11); HREI-MS Calcd for \(\hbox {C}_{26}\hbox {H}_{19}\hbox {N}_{3}\hbox {OS}\): \(m/z=421.1249\), found 421.1233; Anal. Calcd for \(\hbox {C}_{26}\hbox {H}_{19}\hbox {N}_{3}\hbox {OS}: \hbox {C}=74.09\); \(\hbox {H}=4.54\); \(\hbox {N}=9.97\); Found: \(\hbox {C}=74.12\); \(\hbox {H}=4.55\); \(\hbox {N}=9.99\).
(E)-1-((2-(4-(3-Nitrophenyl)thiazol-2-yl)hydrazono)methyl)naphthalen-2-ol (25) [CAS # 307533-15-9]
Solid; Yellow; Yield: 85%; M.P.: 210–212 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (300 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.32 (s, 1H, NH), 10.88 (s, 1H, OH), 8.94 (s, 1H, H–C=N), 8.78 (d, \(J_{{ 4,3}}=8.7\) Hz, 1H, H-4), 8.69 (d, \(J_{{ 2'',6''}}=1.8\) Hz, 1H, \(\hbox {H-}2''\)), 8.33 (d, \(J_{{ 4'',5''}}=8.1\) Hz, 1H, \(\hbox {H-}4''\)), 8.17 (dd, \(J_{{ 6'',4''}}=1.8\) Hz, \(J_{{ 6'',5''}}=7.8\) Hz, 1H, \(\hbox {H-}6''\)), 7.87 (d, \(J_{{ 5,6}}=J_{{ 8,7}}=8.7\) Hz, 2H, H-5, H-8), 7.74 (overlapping multiplet, 2H, \(\hbox {H-5}^{\prime }\), \(\hbox {H-}5''\)), 7.60 (t, \(J_{{ 7(6,8)}}=8.1\) Hz, 1H, H-7), 7.40 (t, \(J_{{ 6(5,7)}}=7.8\) Hz, 1H, H-6), 7.23 (d, \(J_{{ 3,4}}=8.7\) Hz, 1H, H-3); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 172.0 (N=C–S), 170.5 (C-2), 150.9 (\(\hbox {C-}4'\)), 148.8 (\(\hbox {C-}3''\)), 143.7 (\(\hbox {HC}{=}\hbox {N}\)), 133.8 (\(\hbox {C-}1''\)), 133.7 (\(\hbox {CH-}6''\)), 133.5 (C-10), 132.7 (CH-4), 130.5 (\(\hbox {CH-}2''\)), 130.4 (C-9), 128.8 (CH-5), 127.6 (CH-7), 124.8 (CH-6), 123.6 (\(\hbox {CH-}4''\)), 122.6 (\(\hbox {CH-}5''\)), 120.5 (CH-3), 118.9 (CH-8), 108.0 (C-1), 106.1 (\(\hbox {CH-}5'\)); \(\hbox {EI-MS }m/z\) (% rel. abund.): 390 (\(\hbox {M}^{+}\), 50), 373 (50), 221 (100), 170 (35), 115 (19); HREI-MS Calcd for \(\hbox {C}_{20}\hbox {H}_{14}\hbox {N}_{4}\hbox {O}_{3}\hbox {S}\): \(m/z=390.0787\), found 390.0769; Anal. Calcd for \(\hbox {C}_{20}\hbox {H}_{14}\hbox {N}_{4}\hbox {O}_{3}\hbox {S}: \hbox {C}=61.53\); \(\hbox {H}=3.61\); \(\hbox {N}=14.35\); Found: \(\hbox {C}=61.55\); \(\hbox {H}=3.63\); \(\hbox {N}=14.37\).
(E)-1-((2-(4-(4-Bromophenyl)thiazol-2-yl)hydrazono)methyl)naphthalen-2-ol (26) [CAS # 404016-81-5]
Solid; Green; Yield: 78%; M.P.: 248–250 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (300 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.23 (s, 1H, NH), 10.92 (s, 1H, OH), 8.95 (s, 1H, H–C=N), 8.73 (d, \(J_{{ 4,3}}=8.4\) Hz, 1H, H-4), 7.87 (overlapping multiplet, 4H, \(\hbox {H-}2''\), \(\hbox {H-}3''\), \(\hbox {H-}5''\), \(\hbox {H-}6''\)), 7.62 (overlapping multiplet, 3H, H-5, H-8, \(\hbox {H-5}^{\prime }\)), 7.43 (overlapping multiplet, 2H, H-6, H-7), 7.23 (d, \(J_{{ 3,4}}=9.0\) Hz, 1H, H-3); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 171.4 (N=C–S), 170.0 (C-2), 150.3 (\(\hbox {C-}4'\)), 143.0 (\(\hbox {HC}{=}\hbox {N}\)), 133.2 (C-10), 132.5 (CH-4), 132.1 (\(\hbox {C-}1''\)), 132.0 (\(\hbox {CH-}3''\)), 132.0 (\(\hbox {CH-}5''\)), 130.2 (C-9), 128.5 (CH-5), 128.4 (\(\hbox {CH-}2''\)), 128.4 (\(\hbox {CH-}6''\)), 127.3 (CH-7), 124.2 (CH-6), 123.5 (\(\hbox {C-}4''\)), 120.1 (CH-3), 118.6 (CH-8), 107.7 (C-1), 105.1 (\(\hbox {CH-}5'\)); \(\hbox {EI-MS }m/z\) (% rel. abund.): 423 (\(\hbox {M}^{+}\), 63), 425 (M+2, 61), 406 (62), 256 (100), 213 (21), 170 (48), 115 (38); HREI-MS Calcd for \(\hbox {C}_{20}\hbox {H}_{14}\hbox {BrN}_{3}\hbox {OS}\): \(m/z=423.0041\), found 423.0026; Anal. Calcd for \(\hbox {C}_{20}\hbox {H}_{14}\hbox {BrN}_{3}\hbox {OS}: \hbox {C}=56.61\); \(\hbox {H}=3.33\); \(\hbox {N}=9.90\); Found: \(\hbox {C}=56.59\); \(\hbox {H}=3.35\); \(\hbox {N}=9.92\).
(E)-1-((2-(4-(3,4-Dichlorophenyl)thiazol-2-yl)hydrazono)methyl)naphthalen-2-ol (27)
Solid; Green; Yield: 88%; M.P.: 248–250 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (300 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.24 (s, 1H, NH), 10.90 (s, 1H, OH), 8.95 (s, 1H, H–C=N), 8.76 (d, \(J_{{ 4,3}}=8.7\) Hz, 1H, H-4), 8.10 (d, \(J_{{ 2'',6''}}=1.8\) Hz, 1H, \(\hbox {H-}2''\)), 7.87 (d, \(J_{{ 5,6}}=J_{{ 8,7}}=J_{{ 6,5}}=8.7\) Hz, 3H, H-5, H-8, \(\hbox {H-}6''\)), 7.69 (d, \(J_{{ 5,6}}=8.4\) Hz, 1H, \(\hbox {H-}5''\)), 7.57 (overlapping multiplet, 2H, H7, \(\hbox {H-5}^{\prime }\)), 7.40 (t, \(J_{{ 6(5,7)}}=7.8\) Hz, 1H, H-6), 7.23 (d, \(J_{{ 3,4}}=9.0\) Hz, 1H, H-3); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 171.5 (N=C–S), 170.3 (C-2), 150.4 (\(\hbox {C-}4'\)), 143.2 (\(\hbox {HC}{=}\hbox {N}\)), 133.3 (\(\hbox {C-}4''\)), 133.2 (C-10), 132.6 (\(\hbox {C-}3''\)), 132.5 (\(\hbox {C-}1''\)), 132.4 (CH-4), 130.7 (\(\hbox {CH-}5''\)), 130.4 (C-9), 128.9 (\(\hbox {CH-}2''\)), 128.7 (CH-5), 127.5 (\(\hbox {CH-}6''\)), 127.1 (CH-7), 124.6 (CH-6), 120.4 (CH-3), 118.8 (CH-8), 107.9 (C-1), 105.4 (\(\hbox {CH-}5'\)); \(\hbox {EI-MS }m/z\) (% rel. abund.): 411 (\(\hbox {M}^{+}\), 22), 413 (M+2, 48),415 (M+4, 26) 396 (45), 340 (17), 244 (100), 202 (27), 170 (68), 115 (21); HREI-MS Calcd for \(\hbox {C}_{20}\hbox {H}_{13}\hbox {Cl}_{2}\hbox {N}_{3}\hbox {OS}\): \(m/z=413.0156\), found 413.0149; Anal. Calcd for \(\hbox {C}_{20}\hbox {H}_{13}\hbox {Cl}_{2}\hbox {N}_{3}\hbox {OS}: \hbox {C}=57.98\); \(\hbox {H}=3.16\); \(\hbox {N}=10.14\); Found: \(\hbox {C}=57.96\); \(\hbox {H}=3.15\); \(\hbox {N}=10.12\).
(E)-2-(2-(Biphenyl-4-ylmethylene) hydrazinyl)-4-phenylthiazole (29)
Solid; Yellow; Yield: 70%; M.P.: 234–236 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (300 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.21 (s, 1H, NH), 8.06 (s, 1H, H–C=N), 7.86 (d, \(J_{{ 2'',3''}}=J_{{ 6'',5''}}=7.5\) Hz, 2H, \(\hbox {H-}2''\), \(\hbox {H-}6''\)), 7.74 (overlapping multiplet, 6H, H-2, H-3, H-5, H-6, \(\hbox {H-}2'''\), \(\hbox {H-}6'''\)), 7.50 (t, \(J_{{ 3''(2'',4'')}}=J_{{ 5''(4'',6'')}}=7.2\) Hz, 2H, \(\hbox {H-}3''\), \(\hbox {H-}5''\)), 7.42 (t, \(J_{{ 3'''(2''',4''')}}=J_{{ 5'''(4''',6''')}}=J_{4''(3'',2'')}=7.5\) Hz, 3H, \(\hbox {H-}3'''\), \(\hbox {H-}5'''\), \(\hbox {H-}4''\)), 7.33 (s, 1H, \(\hbox {H-5}^{\prime }\)), 7.31 (t, \(J_{{ 4'''(3''',5''')}}=7.5\) Hz, 1H, \(\hbox {H-}4'''\)); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 171.5 (N=C–S), 150.0 (\(\hbox {C-}4'\)), 143.4 (\(\hbox {HC}{=}\hbox {N}\)), 142.8 (C-4), 140.6 (C-7), 133.2 (C-1), 132.7 (\(\hbox {C-}1''\)), 129.7 (CH-2), 129.7 (CH-6), 129.2 (\(\hbox {CH-}3''\)), 129.2 (\(\hbox {CH-}5''\)), 129.0 (CH-9), 129.0 (CH-11), 128.5 (\(\hbox {C-}4''\)), 127.8 (CH-3), 127.8 (CH-5), 127.5 (CH-8), 127.5 (CH-12), 127.4 (\(\hbox {CH-}2''\)), 127.4 (\(\hbox {CH-}6''\)), 127.3 (CH-10), 105.2 (\(\hbox {CH-}5'\)); \(\hbox {EI-MS }m/z\) (% rel. abund.): 355 (\(\hbox {M}^{+}\), 61), 176 (100), 152 (14), 134 (34); HREI-MS Calcd for \(\hbox {C}_{22}\hbox {H}_{17}\hbox {N}_{3}\hbox {S}\): \(m/z=355.1143\), found 355.1144; Anal. Calcd for \(\hbox {C}_{22}\hbox {H}_{17}\hbox {N}_{3}\hbox {S}: \hbox {C}=74.34\); \(\hbox {H}=4.82\); \(\hbox {N}=11.82\); Found: \(\hbox {C}=74.36\); \(\hbox {H}=4.81\); \(\hbox {N}=11.83\).
(E)-2-(2-(Biphenyl-4-ylmethylene) hydrazinyl)-4-(4-methoxyphenyl)thiazole (30)
Solid; Yellow; Yield: 65%; M.P.: 233–235 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (300 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.17 (s, 1H, NH), 8.05 (s, 1H, H–C=N), 7.79 (d, \(J_{{ 2'',3''}}=J_{{ 6'',5''}}=8.7\) Hz, 2H, \(\hbox {H-}2''\), \(\hbox {H-}6''\)), 7.76 (overlapping multiplet, 6H, H-2, H-3, H-5, H-6, H-7, H-11), 7.50 (t, \(J_{8(7,9)}=J_{{ 10(9,11)}}=7.2\) Hz, 2H, H-8, H-10), 7.15 (s, 1H, \(\hbox {H-5}^{\prime }\)), 6.97 (d, \(J_{{ 3'',2''}}=J_{{ 5'',6''}}=8.7\) Hz, 2H, \(\hbox {H-}3''\), \(\hbox {H-}5''\)), 3.77 (s, 3H, \(\hbox {OCH}_{3})\); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 171.6 (N=C–S), 159.2 (\(\hbox {C-}4''\)), 150.2 (\(\hbox {C-}4'\)), 143.5 (\(\hbox {HC}{=}\hbox {N}\)), 142.9 (C-4), 140.7 (C-7), 133.3 (C-1), 129.8 (CH-2), 129.8 (CH-6), 129.3 (CH-9), 129.3 (CH-11), 128.3 (\(\hbox {CH-}2''\)), 128.3 (\(\hbox {CH-}6''\)), 127.8 (CH-3), 127.8 (CH-5), 127.6 (CH-8), 127.6 (CH-12), 127.4 (CH-10), 125.2 (\(\hbox {C-}1''\)), 114.7 (\(\hbox {CH-}3''\)), 114.7 (\(\hbox {CH-}5''\)), 105.3 (\(\hbox {CH-}5'\)), 55.6 (\(\hbox {OCH}_{3})\); \(\hbox {EI-MS }m/z\) (% rel. abund.): 385 (\(\hbox {M}^{+}\), 100), 206 (83), 191 (16), 180 (11), 164 (28); HREI-MS Calcd for \(\hbox {C}_{23}\hbox {H}_{19}\hbox {N}_{3}\hbox {OS}\): \(m/z=385.1249\), found 385.1251; Anal. Calcd for C23H19N3OS: \(\hbox {C}=71.66\); \(\hbox {H}=4.97\); \(\hbox {N}=10.90\); Found: \(\hbox {C}=71.68\); \(\hbox {H}=4.99\); \(\hbox {N}=10.92\).
(E)-4-(Biphenyl-4-yl)-2-(2-(biphenyl- 4-ylmethylene)hydrazinyl)thiazole (31)
Solid; Dark green; Yield: 82%; M.P.: 258–260 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (600 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.25 (s, 1H, NH), 8.07 (s, 1H, H–C=N), 7.95 (d, \(J_{{ 2'',3''}}=J_{{ 6'',5''}}=8.4\) Hz, 2H, \(\hbox {H-}2''\), \(\hbox {H-}6''\)), 7.74 (overlapping multiplet, 4H, H-2, H-3, H-5, H-6), 7.72 (m, 6H, \(\hbox {H-}3''\), \(\hbox {H-}5''\), \(\hbox {H-}7''\), \(\hbox {H-}8''\), \(\hbox {H-}10''\), \(\hbox {H-}11''\)), 7.49 (q, 4H, H-7, H-8, H-10, H-11), 7.41 (s, 1H, \(\hbox {H-5}^{\prime }\)), 7.39 (m, 2H, H-9, \(\hbox {H-}9''\)); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 171.5 (N=C–S), 150.1 (\(\hbox {C-}4'\)), 143.4 (HC=N), 142.8 (C-4), 140.7 (C-7), 140.6 (\(\hbox {C-}4''\)), 140.5 (\(\hbox {C-}7''\)), 133.3 (C-1), 131.7 (\(\hbox {C-}1''\)), 129.6 (CH-2), 129.6 (CH-6), 129.2 (\(\hbox {CH-}2''\)), 129.2 (\(\hbox {CH-}6''\)), 129.0 (CH-9), 129.0 (CH-11), 127.8 (\(\hbox {CH-}9''\)), 127.8 (\(\hbox {CH-}11''\)), 127.7 (CH-3), 127.7 (CH-5), 127.7 (\(\hbox {CH-}3''\)), 127.7 (\(\hbox {CH-}5''\)), 127.6 (CH-8), 127.6 (CH-12), 127.5 (CH-10), 127.3 (\(\hbox {CH-}10''\)), 127.0 (\(\hbox {CH-}8''\)), 127.0 (\(\hbox {CH-}12''\)), 105.3 (\(\hbox {CH-}5'\)); \(\hbox {EI-MS }m/z\) (% rel. abund.): 431 (\(\hbox {M}^{+}\), 53), 252 (100), 210 (29), 180 (11), 152 (10); HREI-MS Calcd for \(\hbox {C}_{28}\hbox {H}_{21}\hbox {N}_{3}\hbox {S}\): \(m/z=431.1456\), found 431.1311; Anal. Calcd for C28H21N3S: \(\hbox {C}=77.93\); \(\hbox {H}=4.91\); \(\hbox {N}=9.74\); Found: \(\hbox {C}=77.96\); \(\hbox {H}=4.93\); \(\hbox {N}=9.72\).
(E)-2-(2-(Biphenyl-4-ylmethylene) hydrazinyl)-4-(3-nitrophenyl)thiazole (32)
Solid; Orange; Yield: 63%; M.P.: 230–232 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (300 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.34 (s, 1H, NH), 8.67 (s, 1H, \(\hbox {H-}2''\)), 8.31 (d, \(J_{{ 4'',5''}}=7.8\) Hz, 1H, \(\hbox {H-}4''\)), 8.16 (dd, \(J_{{ 6'',4''}}=2.1\) Hz, \(J_{{ 6'',5''}}=8.1\) Hz, 1H, \(\hbox {H-}6''\)), 8.08 (s, 1H, H–C=N), 7.75 (overlapping multiplet, 4H, H-2, H-3, H-5, H-6), 7.73 (overlapping multiplet, 3H, H-7, H-11, \(\hbox {H-}5''\)), 7.67 (s, 1H, \(\hbox {H-5}^{\prime }\)), 7.50 (t, \(J_{8(7,9)}=J_{{ 10(9,11)}}=7.2\) Hz, 2H, H-8, H-10), 7.40 (t, \(J_{{ 9(8,10)}}=7.2\) Hz, 1H, H-9); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 171.7 (N=C–S), 150.4 (\(\hbox {C-}4'\)), 148.6 (\(\hbox {C-}3''\)), 143.7 (\(\hbox {HC}{=}\hbox {N}\)), 142.9 (C-4), 140.8 (C-7), 133.8 (\(\hbox {C-}1''\)), 133.5 (\(\hbox {CH-}6''\)), 133.4 (C-1), 130.4 (\(\hbox {CH-}2''\)), 129.9 (CH-2), 129.9 (CH-6), 129.3 (CH-9), 129.3 (CH-11), 127.8 (CH-3), 127.8 (CH-5), 127.6 (CH-8), 127.6 (CH-12), 127.5 (CH-10), 123.8 (\(\hbox {CH-}4''\)), 122.8 (\(\hbox {CH-}5''\)), 105.8 (\(\hbox {CH-}5'\)); \(\hbox {EI-MS }m/z\) (% rel. abund.): 400 (\(\hbox {M}^{+}\), 34), 221 (100), 180 (11), 175 (26), 152 (15); HREI-MS Calcd for \(\hbox {C}_{22}\hbox {H}_{16}\hbox {N}_{4}\hbox {O}_{2}\hbox {S}\): \(m/z=400.0994\), found 400.1003; Anal. Calcd for C22H16N4O2S: \(\hbox {C}=65.99\); \(\hbox {H}=4.03\); \(\hbox {N}=13.99\); Found: \(\hbox {C}=65.97\); \(\hbox {H}=4.01\); \(\hbox {N}=13.97\).
(E)-2-(2-(Biphenyl-4-ylmethylene) hydrazinyl)-4-(4-bromophenyl)thiazole (33)
Solid; Off white; Yield: 58%; M.P.: 250–252 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (300 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.20 (s, 1H, NH), 8.06 (s, 1H, H–C=N), 7.82 (d, \(J_{{ 2'',3''}}=J_{{ 5'',6''}}=8.7\) Hz, 2H, \(\hbox {H-}2''\), \(\hbox {H-}6''\)), 7.74 (overlapping multiplet, 4H, H-2, H-3, H-5, H-6), 7.72 (d, \(J_{{ 7,8}}=J_{11,10}=7.2\) Hz, 2H, H-7, H-11), 7.60 (d, \(J_{{ 3'',2''}}=J_{{ 5'',6''}}=8.7\) Hz, 2H, \(\hbox {H-}3''\), \(\hbox {H-}5''\)), 7.50 (t, \(J_{8(7,9)}=J_{{ 10(9,11)}}=7.2\) Hz, 2H, H-8, H-10), 7.42 (s, 1H, \(\hbox {H-5}^{\prime }\)), 7.40 (t, \(J_{{ 9(8,10)}}=7.2\) Hz, 1H, H-9); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 171.4 (N=C–S), 149.9 (\(\hbox {C-}4'\)), 143.2 (\(\hbox {HC}{=}\hbox {N}\)), 142.7 (C-4), 140.6 (C-7), 133.0 (C-1), 132.1 (\(\hbox {C-}1''\)), 132.0 (\(\hbox {CH-}3''\)), 132.0 (\(\hbox {CH-}5''\)), 129.6 (CH-2), 129.6 (CH-6), 129.0 (CH-9), 129.0 (CH-11), 128.2 (\(\hbox {CH-}2''\)), 128.2 (\(\hbox {CH-}6''\)), 127.7 (CH-3), 127.7 (CH-5), 127.5 (CH-8), 127.5 (CH-12), 127.3 (CH-10), 123.3 (\(\hbox {C-}4''\)), 105.0 (\(\hbox {CH-}5'\)); \(\hbox {EI-MS }m/z\) (% rel. abund.): 433 (\(\hbox {M}^{+}\), 48), 435 (M+2, 53), 256 (100), 214 (11), 174 (25), 152 (16); HREI-MS Calcd for \(\hbox {C}_{22}\hbox {H}_{16}\hbox {BrN}_{3}\hbox {S}\): \(m/z=433.0248\), found 433.0237; Anal. Calcd for \(\hbox {C}_{22}\hbox {H}_{16}\hbox {BrN}_{3}\hbox {S}: \hbox {C}=60.84\); \(\hbox {H}=3.71\); \(\hbox {N}=9.67\); Found: \(\hbox {C}=60.86\); \(\hbox {H}=3.73\); \(\hbox {N}=9.66\).
(E)-2-(2-(Biphenyl-4-ylmethylene) hydrazinyl)-4-(3,4-dichlorophenyl)thiazole (34)
Solid; Light yellow; Yield: 46%; M.P.: 220–222 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (300 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.25 (s, 1H, NH), 8.08 (overlapping multiplet, 2H, \(\hbox {H-}2''\), H–C=N), 7.85 (dd, \(J_{{ 6'',2''}}=1.8\) Hz, \(J_{{ 6'',5''}}=8.4\) Hz, 1H, \(\hbox {H-}6''\)), 7.74 (overlapping multiplet, 4H, H-2, H-3, H-5, H-6), 7.72 (m, 3H, H-7, H-11, \(\hbox {H-}5''\)), 7.56 (s, 1H, \(\hbox {H-5}^{\prime }\)), 7.50 (t, \(J_{8(7,9)}=J_{{ 10(9,11)}}=7.2\) Hz, 2H, H-8, H-10); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 171.6 (N=C–S), 150.1 (\(\hbox {C-}4'\)), 143.3 (\(\hbox {HC}{=}\hbox {N}\)), 142.8 (C-4), 140.7 (C-7), 133.5 (\(\hbox {C-}4''\)), 133.2 (C-1), 132.6 (\(\hbox {C-}3''\)), 132.8 (\(\hbox {C-}1''\)), 130.7 (\(\hbox {CH-}5''\)), 129.7 (CH-2), 129.7 (CH-6), 129.1 (CH-9), 129.1 (CH-11), 128.7 (\(\hbox {CH-}2''\)), 127.7 (CH-3), 127.7 (CH-5), 127.6 (CH-8), 127.6 (CH-12), 127.4 (CH-10), 127.2 (\(\hbox {CH-}6''\)), 105.2 (\(\hbox {CH-}5'\)); \(\hbox {EI-MS }m/z\) (% rel. abund.): 423 (\(\hbox {M}^{+}\), 56), 425 (M+2, 35), 243 (100), 202 (18), 180 (14), 152 (16); HREI-MS Calcd for \(\hbox {C}_{22}\hbox {H}_{15}\hbox {Cl}_{2}\hbox {N}_{3}\hbox {S}\): \(m/z=423.0364\), found 423.0364; Anal. Calcd for \(\hbox {C}_{22}\hbox {H}_{15}\hbox {Cl}_{2}\hbox {N}_{3}\hbox {S}: \hbox {C}=62.27\); \(\hbox {H}=3.56\); \(\hbox {N}=9.90\); Found: \(\hbox {C}=62.30\); \(\hbox {H}=3.58\); \(\hbox {N}=9.92\).
(E)-2-(2-(4-(Benzyloxy)benzylidene) hydrazinyl)-4-(4-methoxyphenyl)thiazole (36) [CAS # 1808939-63-0]
Solid; Orange; Yield: 62%; M.P.: 204–206 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (300 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 11.96 (s, 1H, NH), 7.95 (s, 1H, H–C=N), 7.78 (d, \(J_{{ 2,3}}=J_{{ 6,5}}=8.7\) Hz, 2H, H-2, H-6), 7.59 (d, \(J_{{ 2'',3''}}=J_{{ 6'',5''}}=9.0\) Hz, 2H, \(\hbox {H-}2''\), \(\hbox {H-}6''\)), 7.46 (t, \(J_{{ 9(8,10)}}=J_{{ 10(9,11)}}=J_{{ 11(10,12)}}=9.0\) Hz, 3H, H-9, H-10, H-11), 7.39, (overlapping multiplet, 2H, H-8, H-12), 7.11 (s, 1H, \(\hbox {H-5}^{\prime }\)), 7.08 (d, \(J_{3,2}=J_{{ 5,6}}=8.7\) Hz, 2H, H-3, H-5), 6.96 (d, \(J_{{ 3'',2''}}=J_{{ 5'',6''}}=9.0\) Hz, 2H, \(\hbox {H-}3''\), \(\hbox {H-}5''\)), 5.14 (s, 2H, \(\hbox {H-CH}_{2})\); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 171.4 (N=C–S), 160.2 (C-4), 158.7 (\(\hbox {C-}4''\)), 149.9 (\(\hbox {C-}4'\)), 143.5 (\(\hbox {HC}{=}\hbox {N}\)), 136.4 (C-7), 130.2 (CH-2), 130.2 (CH-6), 128.7 (CH-9), 128.7 (CH-11), 128.3 (\(\hbox {CH-}2''\)), 128.3 (\(\hbox {CH-}6''\)), 127.5 (CH-10), 127.0 (CH-8), 127.0 (CH-12), 126.2 (C-1), 125.4 (\(\hbox {C-}1''\)), 114.6 (\(\hbox {CH-}3''\)), 114.6 (\(\hbox {CH-}5''\)), 114.5 (CH-3), 114.5 (CH-5), 105.2 (\(\hbox {CH-}5'\)), 70.6 (\(\hbox {CH}_{2})\), 55.5 (\(\hbox {OCH}_{3})\); \(\hbox {EI-MS }m/z\) (% rel. abund.): 415 (\(\hbox {M}^{+}\), 51), 206 (100), 191 (14), 164 (20), 149 (19), 134 (11), 91 (84); HREI-MS Calcd for \(\hbox {C}_{24}\hbox {H}_{21}\hbox {N}_{3}\hbox {O}_{2}\hbox {S}\): \(m/z=415.1354\), found 415.1340; Anal. Calcd for \(\hbox {C}_{24}\hbox {H}_{21}\hbox {N}_{3}\hbox {O}_{2}\hbox {S}: \hbox {C}=69.38\); \(\hbox {H}=5.09\); \(\hbox {N}=10.11\); Found: \(\hbox {C}=69.40\); \(\hbox {H}=5.11\); \(\hbox {N}=10.13\).
(E)-2-(2-(4-(Benzyloxy)benzylidene) hydrazinyl)-4-(biphenyl-4-yl)thiazole (37) [CAS # 468750-68-7]
Solid; Dark brown; Yield: 77%; M.P.: 235–237 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (500 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.03 (s, 1H, NH), 7.98 (s, 1H, H–C=N), 7.94 (d, \(J_{{ 2'',3''}}=J_{{ 6'',5''}}=\)8.5 Hz, 2H, \(\hbox {H-}2''\), \(\hbox {H-}6''\)), 7.71(d, \(J_{{ 2,3}}=J_{{ 6,5}}=J_{{ 3'',2''}}=J_{{ 5'',6''}}=8.0\) Hz, 4H, H-2, H-6, \(\hbox {H-}3''\), \(\hbox {H-}5''\)), 7.60 (d, \(J_{{ 8'',9''}}=J_{{ 12'',11''}}=9.0\) Hz, 2H, \(\hbox {H-}8''\), \(\hbox {H-}12''\)), 7.48 (overlapping multiplet, 4H, H-8, H-12, \(\hbox {H-}9''\), \(\hbox {H-}11''\)), 7.41 (t, \(J_{{ 9(8,10)}}=J_{{ 11(10,12)}}=7.5\) Hz, 2H, H-9, H-11), 7.36 (overlapping multiplet, 3H, H-10, \(\hbox {H-5}^{\prime }\), \(\hbox {H-}10''\)), 7.08 (d, \(J_{3,2}=J_{{ 5,6}}=8.1\) Hz, 2H, H-3, H-5), 5.14 (s, 2H, \(\hbox {H-CH}_{2})\); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 171.5 (N=C–S), 160.3 (C-4), 150.1 (\(\hbox {C-}4'\)), 143.7 (\(\hbox {HC}{=}\hbox {N}\)), 140.7 (\(\hbox {C-}4''\)), 140.6 (\(\hbox {C-}7''\)), 136.5 (C-7), 130.6 (\(\hbox {C-}1''\)), 130.4 (CH-2), 130.4 (CH-6), 129.3 (\(\hbox {CH-}2''\)), 129.3 (\(\hbox {CH-}6''\)), 128.8 (CH-9), 128.8 (CH-11), 128.4 (\(\hbox {CH-}9''\)), 128.4 (\(\hbox {CH-}11''\)), 127.8 (\(\hbox {CH-}3''\)), 127.8 (\(\hbox {CH-}5''\)), 127.7 (CH-10), 127.2 (\(\hbox {CH-}8''\)), 127.2 (\(\hbox {CH-}12''\)), 127.0 (CH-8), 127.0 (CH-12), 126.9 (\(\hbox {CH-}10''\)), 126.3 (C-1), 114.4 (CH-3), 114.4 (CH-5), 105.2 (\(\hbox {CH-}5'\)), 70.7 (\(\hbox {CH}_{2})\); \(\hbox {EI-MS }m/z\) (% rel. abund.): 461 (\(\hbox {M}^{+}\), 59), 252 (100), 238 (11), 210 (26), 165 (8), 91 (79); HREI-MS Calcd for \(\hbox {C}_{29}\hbox {H}_{23}\hbox {N}_{3}\hbox {OS}\): \(m/z=461.1562\), found 461.1543; Anal. Calcd for \(\hbox {C}_{29}\hbox {H}_{23}\hbox {N}_{3}\hbox {OS}: \hbox {C}=75.46\); \(\hbox {H}=5.02\); \(\hbox {N}=9.10\); Found: \(\hbox {C}=75.49\); \(\hbox {H}=5.04\); \(\hbox {N}=9.12\).
(E)-2-(2-(4-(Benzyloxy)benzylidene) hydrazinyl)-4-(3-nitrophenyl)thiazole (38) [CAS # 469871-00-9]
Solid; Orange; Yield: 58%; M.P.: 207–209 \(^{\circ }\hbox {C}\); \({}^{1}\hbox {H-NMR}\) (300 MHz, \(\hbox {DMSO-}d_{{ 6}})~\delta \) 12.14 (s, 1H, NH), 8.66 (s, 1H, \(\hbox {H-}2''\)), 8.30 (d, \(J_{{ 4'',5''}}=7.8\) Hz, 1H, \(\hbox {H-}4''\)), 8.15 (dd, \(J_{{ 6'',2''}}=2.1\) Hz, \(J_{{ 6'',5''}}=8.1\) Hz, 1H, \(\hbox {H-}6''\)), 7.98 (s, 1H, H–C=N), 7.72 (t, \(J_{{ 5''(4'',6'')}}=8.1\) Hz, 1H, \(\hbox {H-}5''\)), 7.63 (overlapping multiplet, 3H, H-2, H-6, \(\hbox {H-5}^{\prime }\)), 7.47 (t, \(J_{{ 9(8,10)}}=J_{{ 10(9,11)}}=J_{{ 11(10,12)}}=8.7\) Hz, 3H, H-9, H-10, H-11), 7.39, (overlapping multiplet, 2H, H-8, H-12), 7.08 (d, \(J_{3,2}=J_{{ 5,6}}=8.7\) Hz, 2H, H-3, H-5), 5.14 (s, 2H, \(\hbox {H-CH}_{2})\); \({}^{13}\hbox {C-NMR}\) (125 MHz, \(\hbox {DMSO-}d_{{ 6}}):\delta \) 171.8 (N=C–S), 160.3 (C-4), 150.3 (\(\hbox {C-}4'\)), 148.9 (\(\hbox {C-}3''\)), 143.9 (\(\hbox {HC}{=}\hbox {N}\)), 136.6 (C-7), 133.7 (\(\hbox {C-}1''\)), 133.5 (\(\hbox {CH-}6''\)), 130.8 (\(\hbox {CH-}2''\)), 130.3 (CH-2), 130.3 (CH-6), 128.8 (CH-9), 128.8 (CH-11), 127.7 (CH-10), 127.2 (CH-8), 127.2 (CH-12), 126.3 (C-1), 123.8 (\(\hbox {CH-}4''\)), 122.6 (\(\hbox {CH-}5''\)), 114.6 (CH-3), 114.6 (CH-5), 105.7 (\(\hbox {CH-}5'\)), 70.8 (\(\hbox {CH}_{2})\); \(\hbox {EI-MS }m/z\) (% rel. abund.): 430 (\(\hbox {M}^{+}\), 15), 221 (81), 191 (11), 175 (9), 91 (100); HREI-MS Calcd for \(\hbox {C}_{23}\hbox {H}_{18}\hbox {N}_{4}\hbox {O}_{3}\hbox {S}\): \(m/z=430.1100\), found 430.1109; Anal. Calcd for \(\hbox {C}_{23}\hbox {H}_{18}\hbox {N}_{4}\hbox {O}_{3}\hbox {S}: \hbox {C}=64.17\); \(\hbox {H}=4.21\); \(\hbox {N}=13.02\); Found: \(\hbox {C}=64.20\); \(\hbox {H}=4.23\); \(\hbox {N}=13.00\).
In vitro \(\alpha \)-glucosidase inhibition assay
The \(\alpha \)-glucosidase inhibitory profile of all synthesized (E)-2-(2-(arylmethylene)hydrazinyl)-4-arylthiazoles and intermediates was measured by following a reported method [43]. Typically, \(\alpha \)-glucosidase activity was performed in phosphate buffer 50 mM of pH 6.8 which contains 5% v/v dimethylsulfoxide and PNP glycoside was used as a substrate. The inhibitors were pre-incubated with enzyme for half an hour at \(37~^{\circ }\hbox {C}\). Then substrate was added and the enzymatic reaction was performed for 60 s at \(37\,^{\circ }\hbox {C}\). Absorbance was measured spectrophotometrically at 400 nm. The assay was carried at five different concentrations around the \(\hbox {IC}_{50}\) values that were roughly calculated in the first turn of the experiments, and the mean values were adopted.
References
Kasturi S, Surarapu S, Uppalanchi S, Anireddy JS, Dwivedi S, Anantaraju HS, Ethiraj KS (2017) Synthesis and \(\alpha \)-glucosidase inhibition activity of dihydroxy pyrrolidines. Bioorg Med Chem Lett 27:2818–2823. https://doi.org/10.1016/j.bmcl.2017.04.078
Yue LM, Lee J, Zheng L, Park YD, Ye ZM, Yang JM (2017) Computational prediction integrating the inhibition kinetics of gallotannin on \(\alpha \)-glucosidase. Int J Biol Macromol 103:829–838. https://doi.org/10.1016/j.ijbiomac.2017.05.106
Hatano A, Kanno Y, Kondo Y, Sunaga Y, Umezawa H, Okada M, Fukui K (2017) Synthesis and characterization of novel, conjugated, fluorescent DNJ derivatives for \(\alpha \)-glucosidase recognition. Bioorg Med Chem 25:773–778. https://doi.org/10.1016/j.bmc.2016.11.053
Liu DM, Chen J, Shi YP (2017) Screening of enzyme inhibitors from traditional Chinese medicine by magnetic immobilized \(\alpha \)-glucosidase coupled with capillary electrophoresis. Talanta 164:548–555. https://doi.org/10.1016/j.talanta.2016.12.028
Chen H, Ouyang K, Jiang Y, Yang Z, Hu W, Xiong L, Wang W (2017) Constituent analysis of the ethanol extracts of Chimonanthus nitens Oliv. leaves and their inhibitory effect on \(\alpha \)-glucosidase activity. Int J Biol Macromol 98:829–836. https://doi.org/10.1016/j.ijbiomac.2017.02.044
Chaudhry F, Choudhry S, Huma R, Ashraf M, al-Rashida A, Munir R, Khan MA (2017) Hetarylcoumarins: synthesis and biological evaluation as potent \(\alpha \)-glucosidase inhibitors. Bioorg Chem 73:1–9. https://doi.org/10.1016/j.bioorg.2017.05.009
Chaudhry F, Naureen S, Huma R, Shaukat A, al-Rashida M, Asif N, Khan MA (2017) In search of new \(\alpha \)-glucosidase inhibitors: imidazolylpyrazole derivatives. Bioorg Chem 71:102–109. https://doi.org/10.1016/j.bioorg.2017.01.017
Yar M, Bajda M, Shahzad S, Ullah N, Gilani MA, Ashraf M, Shaukat A (2015) Organocatalyzed solvent free an efficient novel synthesis of 2,4,5-trisubstituted imidazoles for \(\alpha \)-glucosidase inhibition to treat diabetes. Bioorg Chem 58:65–71. https://doi.org/10.1016/j.bioorg.2014.11.006
Asano N (2003) Glycosidase inhibitors: update and perspectives on practical use. Glycobiology 13:93R–104R. https://doi.org/10.1093/glycob/cwg090
Toeller M (1994) \(\alpha \)-Glucosidase inhibitors in diabetes: efficacy in NIDDM subjects. Eur J Clin Invest 24:31–35. https://doi.org/10.1111/j.1365-2362.1994.tb02253.x
Scott LJ, Spencer CM (2000) Miglitol: a review of its therapeutic potential in type 2 diabetes mellitus. Drugs 59:521–549
Ag H (1994) Pharmacology of \(\alpha \)-glucosidase inhibition. Eur J Clin Invest 24:3–10
Khan KM, Qurban S, Salar U, Taha M, Hussain S, Perveen S, Wadood A (2016) Synthesis, in vitro \(\alpha \)-glucosidase inhibitory activity and molecular docking studies of new thiazole derivatives. Bioorg Chem 68:245–258. https://doi.org/10.1016/j.bioorg.2016.08.010
Mouri K, Saito S, Yamaguchi S (2012) Highly flexible \(\pi \)-expanded cyclooctatetraenes: cyclic thiazole tetramers with head-to-tail connection. Angew Chem Int Ed 51:5971–5975. https://doi.org/10.1002/anie.201201265
Frija LM, Pombeiro AJ, Kopylovich MN (2016) Coordination chemistry of thiazoles, isothiazoles and thiadiazoles. Coord Chem Rev 308:32–55. https://doi.org/10.1016/j.ccr.2015.10.003
Mishra CB, Kumari S, Tiwari M (2015) Thiazole: a promising heterocycle for the development of potent CNS active agents. Eur J Med Chem 92:1–34. https://doi.org/10.1016/j.ejmech.2014.12.031
Rouf A, Tanyeli C (2015) Bioactive thiazole and benzothiazole derivatives. Eur J Med Chem 97:911–927. https://doi.org/10.1016/j.ejmech.2014.10.058
Dawood KM, Eldebss TM, El-Zahabi HS, Yousef MH (2015) Synthesis and antiviral activity of some new bis-1,3-thiazole derivatives. Eur J Med Chem 102:266–276. https://doi.org/10.1016/j.ejmech.2015.08.005
Maienfisch P, Edmunds AJ (2017) Chapter three-thiazole and isothiazole ring-containing compounds in crop protection. Adv Heterocycl Chem 121:35–88. https://doi.org/10.1016/bs.aihch.2016.04.010
Salar U, Khan KM, Iqbal J, Ejaz SA, Hameed A, Al-Rashida M, Perveen S, Tahir MN (2017) Coumarin sulfonates: new alkaline phosphatase inhibitors; in vitro and in silico studies. Eur J Med Chem 131:29–47. https://doi.org/10.1016/j.ejmech.2017.03.003
Salar U, Khan KM, Taha M, Ismail NH, Ali B, Qurat-ul-Ain Perveen S, Ghufran M, Wadood A (2017) Biology-oriented drug synthesis (BIODS): in vitro \(\beta \)-glucuronidase inhibitory and in silico studies on 2-(2-methyl-5-nitro-1\(H\)-imidazol-1-yl) ethyl aryl carboxylate derivatives. Eur J Med Chem 125:1289–1299. https://doi.org/10.1016/j.ejmech.2016.11.031
Salar U, Khan KM, Syed S, Taha M, Ali F, Ismail NH, Perveen S, Wadood A, Ghufran M (2017) Synthesis, in vitro \(\beta \)-glucuronidase inhibitory activity and in silico studies of novel \((E)\)-4-Aryl-2-(2-(pyren-1-ylmethylene) hydrazinyl) thiazoles. Bioorg Chem 70:199–209. https://doi.org/10.1016/j.bioorg.2016.12.011
Arshad T, Khan KM, Rasool N, Salar U, Hussain S, Asghar H, Ashraf M, Wadood A, Riaz M, Perveen S, Taha M, Ismail NH (2017) 5-Bromo-2-aryl benzimidazole derivatives as non-cytotoxic potential dual inhibitors of \(\alpha \)-glucosidase and urease enzymes. Bioorg Chem 72:21–31. https://doi.org/10.1016/j.bioorg.2017.03.007
Salar U, Taha M, Ismail NH, Khan KM, Imran S, Perveen S, Wadood A, Riaz M (2016) Thiadiazole derivatives as new class of \(\beta \)-glucuronidase inhibitors. Bioorg Med Chem 24:1909–1918. https://doi.org/10.1016/j.bmc.2016.03.020
Ali F, Khan KM, Salar U, Iqbal S, Taha M, Ismail NH, Perveen S, Wadood A, Ghufran M, Ali B (2016) Dihydropyrimidones: as novel class of \(\beta \)-glucuronidase inhibitors. Bioorg Med Chem 24:3624–3635. https://doi.org/10.1016/j.bmc.2016.06.002
Salar U, Taha M, Khan KM, Ismail NH, Imran S, Perveen S, Gul S, Wadood A (2016) Syntheses of new 3-thiazolyl coumarin derivatives, in vitro \(\alpha \)-glucosidase inhibitory activity, and molecular modeling studies. Eur J Med Chem 122:196–204. https://doi.org/10.1016/j.ejmech.2016.06.037
Ali F, Khan KM, Salar U, Taha M, Ismail NH, Wadood A, Riaz M, Perveen S (2017) Hydrazinyl arylthiazole based pyridine scaffolds: synthesis, structural characterization, in vitro \(\alpha \)-glucosidase inhibitory activity, and in silico studies. Eur J Med Chem 138:255–272. https://doi.org/10.1016/j.ejmech.2017.06.041
Rahim F, Ullah Hayat, Javid MT, Wadood A, Taha M, Ashraf M, Shaukat A, Junaid M, Hussain S, Rehman W, Mehmood R, Sajid M, Khan MN, Khan KM (2015) Synthesis, in vitro evaluation and molecular docking studies of thiazole derivatives as new inhibitors of \(\alpha \)-glucosidase. Bioorg Chem 62:15–21. https://doi.org/10.1016/j.bioorg.2015.06.006
Molecular Operating Environment (MOE), 2016.08; Chemical Computing Group Inc., 1010 Sherbrooke St. West, Suite #910, Montreal, QC, Canada, H3A 2R7, 2016
Ferreira SB, Sodero ACR, Cardoso MFC, Lima ES, Kaiser CR, Silva FP, Ferreira VF (2010) Synthesis, biological activity, and molecular modeling studies of 1\(H\)-1,2,3-triazole derivatives of carbohydrates as \(\alpha \)-glucosidases inhibitors. J Med Chem 53:2364–2375. https://doi.org/10.1021/jm901265h
Park JH, Ko S, Park H (2008) Toward the virtual screening of \(\alpha \)-glucosidase inhibitors with the homology-modeled protein structure. Bull Korean Chem Soc 29:921–927. https://doi.org/10.5012/bkcs.2008.29.5.921
Roujeinikova A, Raasch C, Sedelnikova S, Liebl W, Rice W (2002) Crystal structure of Thermotoga maritima 4-\(\alpha \)-glucanotransferase and its acarbose complex: implications for substrate specificity and catalysis. J Mol Biol 321:149–162. https://doi.org/10.1016/S0022-2836(02)00570-3
Guerreiro LR, Carriero EP, Fernandes L, Cardote TA, Moreira R, Caldeira AT, Guedes RC, Burke AJ (2013) Five-membered iminocyclitol \(\alpha \)-glucosidase inhibitors: synthetic, biological screening and in silico studies. Bioorg Med Chem 21:1911–1917. https://doi.org/10.1016/j.bmc.2013.01.030
Taha M, Ismail NH, Imran S, Wadood A, Ali M, Rahim F, Khan AA, Riaz M (2016) Novel thiosemicarbazide-oxadiazole hybrids as unprecedented inhibitors of yeast \(\alpha \)-glucosidase and in silico binding analysis. RSC Adv 6:33733–33742. https://doi.org/10.1039/C5RA28012E
Turner S, Myers M, Gadie B, Nelson AJ, Pape R, Saville JF, Doxey JC, Berridge TL (1988) Antihypertensive thiadiazoles. 1. Synthesis of some 2-aryl-5-hydrazino-1,3,4-thiadiazoles with vasodilator activity. J Med Chem 31:902–906. https://doi.org/10.1021/jm00400a003
Osman H, Arshad A, Lam CK, Bagley MC (2012) Microwave-assisted synthesis and antioxidant properties of hydrazinyl thiazolyl coumarin derivatives. Chem Cent J 6:32. https://doi.org/10.1186/1752-153X-6-32
Hassan AA, Mohamed SK, Mohamed NK, El-Shaieb KM, Abdel-Aziz AT, Mague JT, Akkurt M (2016) Synthesis of 1,3-thiazolidin-4-ones; reactivity of the thiosemicarbazone function towards dimethyl acetylenedicarboxylate. J Chem Res 40:173–177. https://doi.org/10.3184/174751916X14552868764580
Subhashree GR, Haribabu J, Saranya S, Yuvaraj P, Anantha Krishnan D, Karvembu R, Gayathri D (2017) In vitro antioxidant, antiinflammatory and in silico molecular docking studies of thiosemicarbazones. J Mol Str 1145:160–169. https://doi.org/10.1016/j.molstruc.2017.05.054
Linciano P, Moraes CB, Alcantara LM, Franco CH, Pascoalino B, Freitas-Junior LH, Macedo S, Santarem N, Cordeiro-da-Silva A, Gul S, Witt G, Kuzikove M, Ellingere B, Ferraria S, Luciania R, Quotadamoa A, Costantinoa L, Costia MP (2018) Aryl thiosemicarbazones for the treatment of trypanosomatidic infections. Eur J Med Chem 146:423–434. https://doi.org/10.1016/j.ejmech.2018.01.043
Alagramam KN, Gopal SR, Geng R, Chen DH, Nemet I, Lee R, Tian G, Miyagi M, Malagu KF, Lock CJ, Esmieu WR (2016) A small molecule mitigates hearing loss in a mouse model of Usher syndrome III. Nat Chem Bio 12:444–451. https://doi.org/10.1038/nchembio.2069
Chimenti F, Bizzarri B, Bolasco A, Secci D, Chimenti P, Granese A, Carradori S, D’Ascenzio M, Lilli D, Rivanera D (2011) Synthesis and biological evaluation of novel 2, 4-disubstituted-1,3-thiazoles as anti-Candida spp. agents. Eur J Med Chem 46:378–382. https://doi.org/10.1016/j.ejmech.2010.10.027
Abdelhamid AO, Ismail ZH, El Gendy MS, Ghorab MM (2007) Reactions of hydrazonoyl halides \(54^{1}\): synthesis and reactivity of 3-aza-2-bromo-1-(3-oxo benzo[\(f\)]chromen-2-yl-3-(arylamino)prop-2-en-1-one. Phosphorus Sulfur Silicon Relat Elem 182:2409–2418. https://doi.org/10.1080/10426500701521548
Cai CY, Rao L, Rao Y, Guo JX, Xiao ZZ, Cao JY, Huang ZS, Wang B (2017) Analogues of xanthones-chalcones and bis-chalcones as \(\alpha \)-glucosidase inhibitors and anti-diabetes candidates. Eur J Med Chem 130:51–59. https://doi.org/10.1016/j.ejmech.2017.02.007
Acknowledgements
This work was supported by the Higher Education Commission (HEC) Pakistan under the National Research Program for Universities (Project No. 20-1910).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ali, M., Khan, K.M., Salar, U. et al. Synthesis, in vitro \(\alpha \)-glucosidase inhibitory activity, and in silico study of (E)-thiosemicarbazones and (E)-2-(2-(arylmethylene)hydrazinyl)-4-arylthiazole derivatives. Mol Divers 22, 841–861 (2018). https://doi.org/10.1007/s11030-018-9835-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-018-9835-2