Abstract
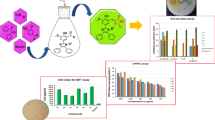
A convenient, one-pot multi-component synthesis of new 2,4-disubstituted hydrazinyl-thiazoles was accomplished using different aldehydes/ketones, thiosemicarbazide, and 4-methoxy phenacyl bromide in the presence of a catalytic amount of AcOH in EtOH. Products were obtained in reasonable yields and high purity. The in vitro antioxidant activity of hydrazinyl-thiazoles was evaluated by DPPH radical scavenging activity in comparison to ascorbic acid. Synthesized thiazoles 14c and 14g possessed the lowest \(\hbox {IC}_{50}\) values. Also, hydrazinyl-thiazoles were screened for their in vitro antibacterial activity against six strains of bacteria including S. aureus, M. luteus, E. coli, Ps. aeruginosa, B. subtilis, and A. hydrophila where some products showed good antibacterial activity. Moreover, compound 14a showed anticancer activity against melanoma cancerous cell lines A375 with \(\hbox {LC}_{50}= 0.55\hbox { mg}/\hbox {mL}\), slightly selective versus normal cell lines (Hu-2) with \(\hbox {LC}_{50}= 1.19\hbox { mg}/\hbox {mL}\).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thiazoles have attracted a great deal of attention because of their diverse biological activities. Thiamine (vitamin \(\hbox {B}_{1}\), 1) is one of the B-complex vitamins that contains a thiazole ring, which is vitally important to keep a living organism operating properly. Commercial drugs, such as sulfathiazole 2 (antimicrobial drug), ritonavir 3 (antiretroviral drug), and abafungin 4 (antifungal drug) contain a thiazole moiety (Fig. 1).
Synthesized compounds containing a thiazole moiety have been used for the treatment of allergies [1], hypertension [2], inflammation [3], human immunodeficiency virus (HIV) infections [4], and schizophrenia [5]. Thiazoles also exhibit other biological activities, such as antibacterial [6, 7], antitumor [8], analgesic [9], anticonvulsant [10], antimelanogenesis [11], antipsychotic [12], and antioxidant [13]. Also, thiazoles can act as cyclin-dependent kinase (CDK) inhibitors [14] and \(\upbeta \)-glucuronidase inhibitors [15].
Analogs such as 2-substituted-6-fluorobenzo[d]thiazoles [16], (thiazol-2-yl)hydrazine derivatives [17]. 3-Allyl-2-(substituted imino)-4-phenyl-3H-thiazoles, and 2,2\(^\prime \)-(1,3-phenylene)bis(3-substituted-2-imino-4-phenyl-3H-thiazole) derivatives [18] showed anti-candida, antibacterial, and acetylcholinesterase activities. Moreover, thiazoles have been used as semiconducting materials [19], thiazole-iridium complexes as phosphorescent organic light-emitting diodes [20], and naphthol-substituted thiazoles as ratiometric fluorescent chemosensors [21].
Recently, our research group reported the one-pot synthesis of new thiazolyl-pyrazoline derivatives 5–6 [22], bis-thiazoles 7–8 [23], and thiazolylpyridazinones 9 [24] exhibiting antibacterial activity, and new 1,4-dihydropyridines 10 bearing a thiazole moiety exhibiting high antioxidant activity [25] (Fig. 2). In continuation of our previous investigation [26], we report herein the one-pot MCR synthesis of new hydrazinyl-thiazole analogs and their biological activities.
Results and discussion
Chemistry
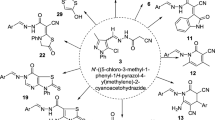
New 2,4-disubstituted hydrazinyl-thiazoles 14a–g were synthesized via the one-pot reaction of different aldehydes/ketones 11a–g, thiosemicarbazide 12, and 4-methoxy phenacyl bromide 13 in the presence of AcOH in EtOH under reflux conditions (Scheme 1). A literature survey indicated that hydrazone-thiazoles are generally synthesized in a two-step procedure. To the best of our knowledge, this is the first one-pot synthesis report for 14 analogs [27–30].
At the onset of our research, we explored the one-pot, multi-component preparation of 14a in EtOH at room temperature in the absence of a catalyst (entry 1) which after 6h afforded the desired product in 56 % yield. In the presence of AcOH, the yield of 14a increased from 56 to 60 % (entry 2). Then, the scope of the reaction was investigated under reflux conditions. As shown in Table 1, 14a was obtained after 2h in EtOH or DMF in high yield (entries 4 & 8). Since DMF is a toxic solvent, EtOH was chosen as it is an eco-friendly and green solvent.
Our results show that the products are obtained in high yields and are easily purified using non-chromatographic methods. The advantages of this method are simple set-up and product isolation, reproducibility, using cheap and eco-friendly solvent.
In most cases, upon addition of 13 to a solution of 12 in EtOH after 2 h under reflux condition with aldehydes/ketones 11a–g in the presence of few drop of AcOH, the desired product precipitated. This is a good sign for the start of the reaction. Hydrazinyl-thiazoles 14a, 14b, and 14c were obtained as pure solids without the need for further purification.
The structures of hydrazinyl-thiazoles 14a–g were determined by IR, \(^{1}\hbox {H}\) NMR, and \(^{13}\hbox {C}\) NMR spectra. In the IR spectra, the disappearance of the carbonyl and thiosemicarbazone N–H stretching vibration bands indicates the participation of thioamide moiety in the cyclization reaction formation of desired products. In \(^{1}\hbox {H}\) NMR spectra, the presence of a singlet at 7.09–6.06 ppm confirms the thiazole ring closure. Also, the \(\hbox {OCH}_{3}\) signal appeared as a singlet at 3.87–3.67 ppm. Other aromatic protons appeared in the expected region around 8.94–6.68 ppm. The N–H signal appeared as a broad singlet at 14.50–11.45 ppm. In addition, the N–H signal can be endo- or exocyclic. However, according to literature [31], the signal at 14.50–11.45 ppm is related to an exocyclic N-H. The \(^{13}\hbox {C}\) NMR spectra of 14a–g provided the expected number and types of carbons. The C–H of the thiazole ring appeared at 100.4–98.7 ppm. The signal at 55.9–55.3 is attributed to \(\hbox {OCH}_{3}\) and other aromatic carbons appeared at 112.6–171.5 ppm.
In order to apply this procedure to carbonyl compounds with steric hindrance, we investigated the 3MCRs of camphor and 2-adamantanone 15 with thiosemicarbazide 12, and 4-methoxy phenacyl bromide 13. The reaction of camphor was unsuccessful and produced several by-products. However, 15 led to mixture of products 16A–16D in moderate yield (Scheme 2).
The \(^{1}\hbox {H}\) NMR spectra did not show a single product; rather, it showed a mixture of two sets of stereoisomer 16A/B and C/D. The protons of the adamantane moiety appeared at 2.20–1.25 ppm, \(\hbox {OCH}_{3}\) of isomers 16C/D appeared at expected region at 3.96–3.72 ppm, and the proton of the thiazole moiety as well as other aromatic protons appeared at 7.80–6.71 ppm. Several signals at 3.25–3.01 and 2.74–2.58 ppm corresponded to CH–N=N isomers C and D.
Biology
Antioxidant assay
Using DPPH (diphenylpicrylhydrazyl) is one of the simplest methods to evaluate antioxidant activity. DPPH is a stable, free radical of violet color. When a compound donates a radical hydrogen atom to a DPPH molecule, it reduces DPPH and so the absorbance of DPPH decreases. In brief, the higher the antioxidant activity, the lighter the violet hue. The antioxidant activity of hydrazinyl-thiazoles 14a–g was evaluated by the DPPH radical scavenging activity method. In this method, a decrease in absorption band at 517 nm indicates that the test compound possesses antioxidant activity. The radical scavenging activity of 14a–g was screened at a concentration of 4000–62.5 \(\upmu \hbox {g}/\hbox {mL}\) and monitored at 517 nm. Also, \(\hbox {IC}_{50}\) values (the concentration of 14a–g to scavenge 50 % of DPPH radical concentration) were calculated from line equation which can be obtained from Fig. 3. Ascorbic acid was used as standard. All hydrazinyl-thiazoles 14a–g showed dose-dependent antioxidant activity (Fig. 3).
The \(\hbox {IC}_{50}\) values of 14a–g are in the range 2.90–0.23 \(\upmu \hbox {M}\) (Table 2). It is worth noting that hydrazinyl-thiazole 14c has the lowest \(\hbox {IC}_{50}\) value \((0.23\,\upmu \hbox {M})\) while compound 14a has the highest \(\hbox {IC}_{50}\) value \((2.90\,\upmu \hbox {M})\) compared to the \(\hbox {IC}_{50}\) value of ascorbic acid (antioxidant compound) \((1.30\,\upmu \hbox {M})\). The higher antioxidant activity is reflected in a lower \(\hbox {IC}_{50}\). Compounds 14a–g were determined to exhibit potent radical scavenging activity in a DPPH assay with \(\hbox {IC}_{50}\) values in the following order14c, 14g, 14b, 14e, ascorbic acid, 14f, 14d, and 14a respectively.
According to the literature [32] and our recent report [25], the high antioxidant activity of 14a–g is attributed to the presence of the thiazole ring. The N–H at the \(\hbox {C}_{2}\) position of the thiazole ring can readily donate a hydrogen radical to DPPH radical and form a radical of the test compound. Therefore, the radical is highly resonance-stabilized through the thiazole ring and =C–N–NH–C moiety (Scheme 3).
In addition to the presence of the thiazole moiety, the high antioxidant activity of 14g can be due to the presence of benzylic hydrogens. These benzylic hydrogens are easily converted to the stable benzyl radical by DPPH. Furthermore, the antioxidant activity of 14b and 14c can be due to the presence of aliphatic hydrogens which can form tertiary, secondary, and primary carbon radicals. As it is mentioned in Table 2, these compounds exhibit better activity than ascorbic acid. Therefore, the presence of benzylic and aliphatic radicals as well thiazole and =C–N–NH–C moieties possibly play an effective role in antioxidant activity (Scheme 3).
Antibacterial assay
Products 14a–g were screened for their in vitro antibacterial activity against Gram-positive and Gram-negative bacteria strains including Staphylococcus aureus (S. aureus), Micrococcus luteus (M. luteus), Escherichia coli (E. coli), Pseudomonas aeruginosa (Ps. aeruginosa), Bacillus subtilis (B. subtilis), Aeromonas hydrophila (A. hydrophila) using a well-diffusion method. DMSO was used as negative control and showed no activity against the above bacterial strains. Penicillin G and Cefixime were used as positive controls (Table 3).
Hydrazinyl-thiazoles 14a–g were evaluated for their antibacterial activity at a concentration of \(4000\,\upmu \hbox {g}/\hbox {mL}\) in DMSO. The experiments were performed in triplicate. The results are presented as \(\hbox {mean}\pm \hbox {standard}\) deviation in millimeter. According to the results, only 14d was active against all six bacterial strains, and 14f had the highest antibacterial activity against S. aureus and M. luteus. Compound 14d was more active against E. coli and also showed significant inhibition activity against B. subtilis. In addition, 14e showed significant inhibition activity against Ps. aeruginosa. Only 14b showed good antibacterial activity against A. hydrophila.
Some reports showed moderate antibacterial activity of hydrazinyl-thiazoles. For example, Bharti and co-workers [33] studied the antibacterial activity of several hydrazinyl-thiazole derivatives, finding that their thiazoles showed no antibacterial activity against E. coli and only a few were active against Ps. aeruginosa. However, in our study, 14c–e had moderate antibacterial activity against E. coli, while 14e good antibacterial activity against Ps. aeruginosa. Moreover, Lee et al. [34] studied the antibacterial activity of hydrazinyl-thiazoles on several bacterial strains. According to their report, none of their synthesized compounds had antibacterial activity against Ps. aeruginosa, and only a few of them had good activity against S. aureus, B. subtilis, and E. coli. Also, the hydrazinyl-thiazoles showed no antibacterial activity against A. hydrophila.
Anticancer assay
Melanoma is a cancer that develops in melanocytes (the pigment cells present in the skin). It is known that melanoma can spread to other parts of the body causing serious illness and death. Melanoma can be more serious than two other forms of skin cancers, e.g., basal-cell cancer (BCC) and squamous-cell cancer (SCC). The primary treatment for melanoma is surgery. There are a few drugs used in chemotherapy of melanoma cancer; however, none of them contain hydrazinyl-thiazole moiety, e.g., dacarbazine and temozolomide contain imidazole and carboxamide moieties, respectively. An in vitro cytotoxicity study of 14a was carried out on human melanoma cancer cell lines A375 and Hu-02 (normal human skin cell lines) obtained from the National Centre for Cell Science, Tehran, Iran. Cell viability of 14a was evaluated using the colorimetric MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay protocol at concentrations of 0.5, 0.25, 0.125, 0.075, 0.037 mg/mL in DMSO. DMSO was used as negative control. The MTT method is based on the reduction of soluble MTT by mitochondrial reductase of the viable cells to insoluble purple crystals of formazan ((E, Z)-5-(4,5-dimethylthiazol-2-yl)-1,3-diphenylformazan). The time of exposure was 24 h and 48 h (Fig. 4). Results are reported as the percent of cell viability \(\pm \) standard deviation. Statistical analysis demonstrated that 14a had cytotoxic activity on Hu-02 (69.4 %) and A375 (58.8 %) cell lines at 24 h time of exposure and on Hu-02 (64.3 %) and A375 (51.2 %) at 48 h time of exposure at a concentration of 0.5 mg/mL \((\hbox {P}\le 0.05)\).
The \(\hbox {LC}_{50}\) values of 14a are shown in Table 4. The \(\hbox {LC}_{50}\) values are between 1.19 and 0.55 mg/mL. As it is shown, the \(\hbox {LC}_{50}\) values on melanoma cancer cells (A375) are lower than on skin cells (Hu-02). However, increasing the time of exposure from 24 h to 48 h did not have a significant effect on the \(\hbox {LC}_{50}\) values.
Conclusions
Several new hydrazinyl-thiazoles derivatives 14a–g were synthesized via one-pot reaction of aldehydes/ketones 11a–g, thiosemicarbazide 12, and 4-methoxy phenacyl bromide 13 through a reliable procedure for high yields and high purity. Hydrazinyl-thiazoles 14a, 14b, and 14c were obtained as pure solids without further purification. All products showed high antioxidant activity in comparison to ascorbic acid. Compounds 14b and 14e had the highest antioxidant activity; their \(\hbox {IC}_{50}\) was less than that of ascorbic acid. Compounds 14a–g showed low to moderate antibacterial activity. Compound 14d possessed the highest antibacterial activity against B. subtilis. Compound 14a had cytotoxic activity at a concentration of 0.5 mg/mL upon 24 h and 48 h time of exposure on melanoma cancer cell lines.
Experimental section
Materials and instruments
Starting materials, reagents, biological cultures, and solvents were obtained from commercial suppliers Fluka, Merck, Sd-fine, Quelab and were used without further purification. All reactions were monitored by silica gel-coated TLC plates (\(60\hbox { F}_{254}\) Merck). IR spectra were recorded on a Shimadzu IR-470 spectrophotometer in anhydrous KBr. \(^{1}\hbox {H}\) NMR and \(^{13}\hbox {C}\) NMR spectra were recorded on a 400 MHz and 500 MHz Bruker spectrometers using DMSO-\(\hbox {d}_{6}\) and \(\hbox {CDCl}_{3}\) as solvents. Chemical shifts are expressed relative to TMS as singlet (s), doublet (d), triplet (t), multiple (m), doublet of doublet (dd), doublet of triplet (dt), triplet of triplet (tt), doublet of doublet of doublet (ddd), and quintet (quin). Coupling constants are expressed in Hertz (Hz). Elemental analyses were carried out on a Carlo–Erba EA1110 CNNO-S analyzer. Melting points were determined with a Mettler Fp5 apparatus, and were uncorrected. Absorbance in the antioxidant assay was recorded on an Unico 2100 spectrophotometer.
General procedure for the synthesis of hydrazinyl-thiazoles 14a–g
To a solution of thiosemicarbazide 12 (1 mmol) in EtOH, aldehydes/ketones 11a–g (1 mmol) and a few drops of AcOH were added and this mixture was refluxed and stirred. After a few minutes (1–15 min), 4-methoxy phenacyl bromide 13 (1 mmol) was added and the reaction was refluxed and stirred until completion (1.5–2 h). Thin layer chromatography (TLC) was used to monitor the progress of the reaction (EtOAc:n-hexane 3:6). After completion of the reaction, the reaction mixture was cooled down to room temperature. The resulting solid was filtered and washed with or recrystallized in EtOH.
2-(2-(6-Methoxy-3,4-dihydronaphthalen-1(2H)-ylidene)hydrazinyl)-4-(4-methoxyphenyl)thiazole (14a):
Cream solid (0.3 g, 80 %); mp 233–236\(\,^{\circ }\hbox {C}\) (from EtOH); IR (KBr) \(\upnu _\mathrm{max}\) 3210 (stretch N–H), 3100 (stretch C–H aromatic), 2920, 2830 (stretch C–H aliphatic), 1610 (stretch C=N), 1570, 1500 (stretch C=C), 1250, 1240, 1045 (stretch C–O), 825, 760, 700 (OOP. C–H) \(\hbox {cm}^{-1}\); \(\hbox {R}_{f }\)(83 %, hexane: EtOAc 6:3); \(^{1}\hbox {H}\) NMR (400 MHz, \(\hbox {CDCl}_{3}\)) \({\updelta }\) 12.46 (s, 1H, NH), 8.02 (d, \(J = 8.8\hbox {Hz}\), 1H), 7.67 (d, \(J = 8.0\hbox { Hz}\), 2H), 6.99 (d, \(J = 8.8\hbox { Hz}\), 2H), 6.82 (dd, \(J = 8.8\), 2.8 Hz, 1H), 6.68 (d, \(J = 2.4\hbox { Hz}\), 1H), 6.06 (s, 1H), 3.84 (s, 3H), 3.83 (s, 3H), 2.90 (t, \(J = 6.4\hbox { Hz}\), 2H), 2.79 (t, \(J = 6.0\hbox { Hz}\), 2H), 2.02 (quin, \(J = 6.3\hbox { Hz}\), 2H) ppm; \(^{13}\hbox {C}\) NMR (100 MHz, \(\hbox {CDCl}_{3}\)) \({\updelta }\) 169.3, 161.2, 160.7, 154.0, 142.5, 127.1, 126.9, 123.7, 121.5, 114.7, 113.5, 112.6, 99.1, 55.4, 55.3, 29.7, 27.2, 21.6 ppm; Anal. calcd. for \(\hbox {C}_{21}\hbox {H}_{21}\hbox {N}_{3}\hbox {O}_{2}\hbox {S}\): C, 66.49, H, 5.56, N, 11.10. Found: C, 66.51; H, 5.59; N, 11.06 %.
4-(4-Methoxyphenyl)-2-(2-(4-phenylcyclohexylidene)hydrazinyl)thiazole (14b)
Orange solid (0.3 g, 85 %); mp 186–189\(\,^{\circ }\hbox {C}\) (from EtOH); IR (KBr) \(\upnu _\mathrm{max}\) 3210 (stretch N–H), 3020 (stretch C–H aromatic), 2920 (stretch C–H aliphatic), 1610 (stretch C=N), 1560, 1540, 1510 (stretch C=C), 1250, 1040 (stretch C–O), 820, 740, 690 (OOP. C–H) \(\hbox {cm}^{-1}\); \(\hbox {R}_{f}\) (80 %, hexane: EtOAc 6:3); \(^{1}\hbox {H}\) NMR (400 MHz, \(\hbox {CDCl}_{3}\)) \({\updelta }\) 12.45 (s, 1H, NH), 7.67 (d, \(J = 8.8\hbox { Hz}\), 2H), 7.33 (t, \(J = 7.2\hbox { Hz}\), 2H), 7.26–7.22 (m, 3H), 7.01 (d, \(J = 8.8\hbox { Hz}\), 2H), 6.56 (s, 1H), 3.86 (s, 3H), 3.32 (d, \(J = 14.8\hbox { Hz}\), 1H), 2.88 (tt, \(J= 12\), 3.3 Hz, 1H), 2.72 (d, \(J = 14.4\hbox { Hz}\), 1H), 2.47 (dt, \(J = 14\), 4.9 Hz, 1H), 2.32 (dt, \(J = 14.1\), 5.3 Hz, 1H), 2.27–2.17 (m, 2H), 1.79 (ddd, \(J= 25.5\), 13.1, 4.3 Hz, 2H) ppm; \(^{13}\hbox {C}\) NMR (125 MHz, \(\hbox {CDCl}_{3}\)) \({\updelta }\) 170.1, 164.2, 161.5, 145.1, 140.8, 129.0, 127.5, 127.1, 127.0, 120.3, 115.4, 98.8, 55.8, 43.5, 35.2, 34.6, 33.3, 29.4 ppm; Anal. calcd. for \(\hbox {C}_{22}\hbox {H}_{23}\hbox {N}_{3}\hbox {OS}\): C, 70.03; H, 6.11; N, 11.09. Found: C, 69.98; H, 6.15; N, 11.12 %.
2-(2-(Heptan-4-ylidene)hydrazinyl)-4-(4-methoxyphenyl)thiazole (14c)
Orange solid (0.26 g, 83 %); mp 127–131\(\,^{\circ }\hbox {C}\) (from EtOH); IR (KBr) \(\upnu _\mathrm{max}\) 3210 (stretch N–H), 3100 (stretch C–H aromatic), 2950, 2850 (stretch C–H aliphatic), 1610 (stretch C=N), 1560, 1540, 1505 (stretch C=C), 1250, 1020 (stretch C–O), 830 (OOP. C–H) \(\hbox {cm}^{-1}\); \(\hbox {R}_{f}\) (82 %, hexane: EtOAc 6:3); \(^{1}\hbox {H}\) NMR (400 MHz, \(\hbox {CDCl}_{3}\)) \({\updelta }\) 12.39 (s, 1H, NH), 7.66 (d, \(J = 8.2\hbox { Hz}\), 2H), 6.99 (d, \(J = 7.8\hbox { Hz}\), 2H), 6.53 (s, 1H), 3.85 (s, 3H), 2.49 (t, \(J = 9\hbox { Hz}\), J = 8 Hz, 2H), 2.34 (t, \(J = 7.6\hbox { Hz}\), 2H), 1.70–1.61 (m, 4H), 1.11 (t, \(J = 7.2\hbox { Hz}\), 3H), 0.98 (t, \(J = 7.4\hbox { Hz}\), 3H) ppm; \(^{13}\hbox {C}\) NMR (125 MHz, \(\hbox {DMSO}-d_{6}\)) \({\updelta }\) 170.2, 166.1, 161.5, 140.7, 127.5, 120.4, 115.3, 98.7, 55.8, 38.9, 33.8, 19.8, 19.7, 14.6, 14.1 ppm; Anal. calcd. for \(\hbox {C}_{17}\hbox {H}_{23}\hbox {N}_{3}\hbox {OS}\): C, 64.35; H, 7.28; N, 13.21. Found: C, 64.30; H, 7.31; N, 13.17 %.
2-(2-((4-Chlorophenyl)(phenyl)methylene)hydrazinyl)-4-(4-methoxyphenyl)thiazole (14d)
Orange crystal (0.32 g, 78 %); mp 239–242\(\,^{\circ }\hbox {C}\) (from EtOH); IR (KBr) \(\upnu _\mathrm{max}\) 3120 (stretch C–H aromatic), 1610 (stretch C=N), 1580, 1560, 1540 (stretch C=C), 1250, 1030 (stretch C–O), 830, 740, 690 (OOP. C–H) \(\hbox {cm}^{-1}\); \(\hbox {R}_{f}\) (73 %, hexane: EtOAc 6:3); \(^{1}\hbox {H}\) NMR (400 MHz, \(\hbox {CDCl}_{3}\)) \({\updelta }\) 12.07 (s, 1H, NH), 7.69 (d, \(J = 8.8\hbox { Hz}\), 2H), 7.66 (d, \(J = 8.4\hbox { Hz}\), 2H), 7.60 (dd, \(J = 7.8\), 1.3 Hz, 2H), 7.49 (tt, \(J = 6.6\), 1.8 Hz, 1H), 7.42 (tt, \(J = 8.8\), 1.2, 2H), 7.35 (d, \(J = 8.8\hbox { Hz}\), 2H), 6.85 (d, \(J = 8.8\hbox { Hz}\), 2H), 6.63 (s, 1H) 3.85 (s, 3H) ppm; \(^{13}\hbox {C}\) NMR (125 MHz, \(\hbox {CDCl}_{3}/\hbox {DMSO}-d_{6}\)) \({\updelta }\) 170.0, 161.3, 137.2, 135.7, 131.3, 130.7, 130.6, 130.4, 129.7, 129.6, 128.9, 128.5, 128.3, 127.6, 115.1, 114.6, 100.4, 55.7 ppm; Anal. calcd. for \(\hbox {C}_{23}\hbox {H}_{18}\hbox {C}\hbox {lN}_{3}\hbox {OS}\): C, 65.81; H, 4.29; N, 9.98. Found: C, 65.85; H, 4.33; N, 10.03 %.
4-(4-Methoxyphenyl)-2-(phenyl(pyridin-2-yl)methylene)hydrazinyl)thiazole (14e)
Red solid (0.27 g, 71 %); mp 215–220\(\,^{\circ }\hbox {C}\) (from EtOH); IR (KBr) \(\upnu _\mathrm{max}\) 1605 (stretch C=N), 1550, 1510, 1480 (stretch C=C), 1240, 1050 (stretch C–O), 830, 760, 730, 700 (OOP. C–H) \(\hbox {cm}^{-1}\); \(\hbox {R}_{f}\) (74 %, hexane: EtOAc 6:3); \(^{1}\hbox {H}\) NMR (400 MHz, \(\hbox {CDCl}_{3}\)) \({\updelta }\) 14.51 (s, 1H, NH), 8.93 (d, \(J = 3.6\hbox { Hz}\), 1H), 7.83–7.77 (m, 3H), 7.64–7.60 (m, 2H), 7.52–7.39 (m, 5H), 6.97 (d, \(J = 8.8\hbox { Hz}\), 2H), 6.78 (s, 1H), 3.87 (s, 3H) ppm; \(^{13}\hbox {C}\) NMR (125 MHz, \(\hbox {CDCl}_{3}\)) \({\updelta }\) 171.5, 160.6, 150.2, 148.6, 138.7, 136.0, 129.9, 129.6, 129.3, 128.7, 128.6, 127.6, 127.3, 126.0, 124.7, 114.6, 100.3, 55.3 ppm; Anal. calcd. for \(\hbox {C}_{22}\hbox {H}_{18}\hbox {N}_{4}\hbox {OS}\): C, 68.41; H, 4.72; N, 14.53. Found: C, 68.36; H, 4.67; N, 14.48 %.
2-Methoxy-4-((2-(4-(4-methoxyphenyl)thiazol-2-yl)hydrazono)methyl)-6-nitrophenol (14f)
Red solid (0.3 g, 75 %); mp 207–209\(\,^{\circ }\hbox {C}\) (from EtOH); IR (KBr) \(\upnu _\mathrm{max}\) 3250 (stretch N–H), 3100 (stretch C–H aromatic), 2980, 2830 (stretch C–H aliphatic), 1610 (stretch C=N), 1570, 1480 (stretch C=C), 1540, 1340 (stretch \(\hbox {NO}_{2}\)), 1250, 1050 (stretch C–O), 840, 760, 740, 690 (OOP. C–H) \(\hbox {cm}^{-1}\); \(\hbox {R}_{f}\) (60 %, hexane: EtOAc 6:3); \(^{1}\hbox {H}\) NMR (500 MHz, \(\hbox {DMSO}-d_{6}\)) \({\updelta }\,\sim 11.45\) (s, 1H, NH), 7.95 (s, 1H), 7.74 (d, \(J = 8.76\hbox { Hz}\), 2H), 7.67 (dd, \(J = 1.52\hbox { Hz}\), 1H), 7.49 (dd, \(J = 1.48\hbox { Hz}\), 1H), 7.09 (s, 1H), 6.92 (d, \(J = 8.7\hbox { Hz}\), 2H), 3.90 (s, 3H), 3.74 (s, 3H) ppm; \(^{13}\hbox {C}\) NMR (125 MHz, \(\hbox {DMSO}-d_{6}\)) \({\updelta }\) 168.8, 159.6, 151.1, 150.6, 144.2, 140.2, 138.1, 128.3, 127.6, 126.2, 115.1, 114.8, 113.1, 102.3, 57.4, 55.9 ppm; Anal. calcd. for \(\hbox {C}_{18}\hbox {H}_{16}\hbox {N}_{4}\hbox {O}_{5}\hbox {S}\): C, 54.03; H, 4.06; N, 13.97. Found: C, 53.97; H, 4.02; N, 14.04 %.
4-(4-Methoxyphenyl)-2-(2-(2,4,6-trimethylbenzylidene)hydrozinyl)thiazole (14g)
Cream solid (0.26 g, 75 %); mp 229–233\(\,^{\circ }\hbox {C}\) (from EtOH); IR (KBr) \(\upnu _\mathrm{max}\) 3400 (stretch N–H), 3100 (stretch C–H aromatic), 2950, 2820 (stretch C–H aliphatic), 1610 (stretch C=N), 1510 (stretch C=C), 1250, 1020 (stretch C–O), 850, 750, 740, (OOP. C–H) \(\hbox {cm}^{-1}\); \(\hbox {R}_{f}\) (75 %, hexane: EtOAc 6:3); \(^{1}\hbox {H}\) NMR (500 MHz, \(\hbox {DMSO}-d_{6}\)) \({\updelta }\sim 13.00\) (s, 1H, NH), 8.65 (s, 1H), 7.52 (d, \(J = 8.6\hbox { Hz}\), 2H), 6.81 (d, \(J = 6.9\hbox { Hz}\), 2H), 6.75 (s, 2H), 6.56 (s, 1H), 3.67 (s, 3H), 2.31 (s, 6H), 2.13 (s, 3H) ppm; \(^{13}\hbox {C}\) NMR (125 MHz, \(\hbox {DMS0}-d_{6}\)) \({\updelta }\,\hbox {DMSO}-d_{6}\)) \({\updelta }\sim 170\), 161.3, 151.2, 141.1, 140.9, 139.1, 130.4, 127.9, 126.8, 120.6, 115.0, 100.2, 55.7, 22.2, 21.5 ppm. Anal. calcd. for \(\hbox {C}_{20}\hbox {H}_{21}\hbox {N}_{3}\hbox {OS}\): C, 68.37; H, 6.05; N, 11.94. Found: C, 68.32; H, 5.98; N, 11.98 %.
2-(Adamantan-2-ylidenehydraono)-4-(4-methoxyphenyl)-2,3-dihydrothiaole (16)
Orange solid (0.17g, 50 %); mp 140–148\(\,^{\circ }\hbox {C}\) (from EtOH); IR (KBr) \(\upnu _\mathrm{max}\) 2917, 2849 (stretch C-H aliphatic), 1605 (stretch C=N), 1549, 1503 (stretch C=C) \(\hbox {cm}^{-1}\); \(\hbox {R}_{f}\) (35 %, hexane: EtOAc 6:3); \(^{1}\hbox {H}\) NMR (500 MHz, \(\hbox {CDCl}_{3}\)) \({\updelta }\) 7.80–6.71 (m, Ar, NH, H-thiazole), 3.96–3.72 (OCH\(_{3})\), 3.01–2.55 (CH–C=N of C and D), 2.20–1.25 (CH and \(\hbox {CH}_{2}\) adamantan) ppm.
Biological methods
DPPH radical scavenging assay
The DPPH radical scavenging activity of 14a–d was evaluated according to the literature [25]. A 2,2-diphenyl-2-picrylhydrazyl (DPPH) solution was prepared by dissolving an appropriate amount of DPPH in MeOH to give a concentration of \(6.25 \times 10^{-5}\hbox { M}\). Compounds 14a–g and DPPH with different concentrations (4000, 2000, 1000, 500, 250, 125, \(62.5\,\upmu \hbox {g}/\hbox {mL}\)) in MeOH were prepared. Then, 0.1 mL of each hydrazinyl-thiazole solution was added to 3.9 mL of DPPH solution and was shaken vigorously. Samples were kept in darkness for 30 min and then their absorbance was measured at 517 nm. MeOH was used as blank. Radical scavenging activity was calculated as follows:

where \(A_\mathrm{control}\) is the absorbance of the negative control (containing all reagents except test compounds), \(A_\mathrm{sample}\) the absorbance of the test compounds. \(\hbox {IC}_{50}\) values of the test compounds were determined by plotting the radical scavenging activity percentage against concentration of the test compound.
Antibacterial assay
The antibacterial activity of hydrazinyl-thiazoles was evaluated biologically using a well-diffusion method. First, nutrient agar and nutrient broth cultures were prepared according to manufacturer’s instructions and were incubated at \(37\,^{\circ }\hbox {C}\). After incubation for the appropriate time, a suspension of \(30\,\upmu \hbox {L}\) of each bacterium was added to the nutrient agar plates. Cups (5 mm in diameter) were cut in the agar using sterilized glass tube. Each well received \(30\,\upmu \hbox {L}\) of the test compounds at concentration of \(4000\,\upmu \hbox {g}/\hbox {ml}\) in DMSO. Then, plates were incubated at \(37\,^{\circ }\hbox {C}\) for 24 h, after this time the zone of inhibition was measured. The values are expressed in millimeters (mm). The experiments were performed in triplicate. The results are reported as \(\hbox {mean}\pm \hbox {SD}\) of zone of inhibition in millimeter. Antibacterial activity of each hydrazinyl-thiazole was compared with Penicillin G and Cefixime as standards. DMSO was used as negative control.
In vitro anticancer assay
The anticancer activity of the hydrazinyl-thiazole 14a was determined against melanoma cancerous cell lines Hu-02 and A375 using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) method. The melanoma cancerous cell lines were provided by the Iranian Biological Resource Center, Tehran. The MTT method is based on the reductive cleavage of the tetrazolium ring of MTT into insoluble purple formazan. \(100\,\upmu \hbox {L}\) of the cell suspension were added to each well of 96-well microplate and then incubated for 12–24 h at \(37\,^{\circ }\hbox {C}\) in a \(\hbox {CO}_{2}\) incubator. Compound 14a was diluted in concentrations of 0.037–0.5 mg/mL in DMSO by two-fold serial dilution. DMSO was used as negative control. Different concentrations of a test compound were added to each well and then incubated for 24 and 48 h at \(37\,^{\circ }\hbox {C}\) in a \(\hbox {CO}_{2}\) incubator. Then, \(10\,\upmu \hbox {L}\) of MTT (5 mg/mL) stock solution in PBS were added to each well and incubated for 4 h, the upper solution was removed, and \(100\,\upmu \hbox {L}\) of DMSO were added to the media. The plates were shaken slowly until the insoluble crystals of the produced formazan dissolved. The plates were kept for 2–4 h in the dark. After that, the plates were read at 360 nm on an ELISA reader. The experiments were performed in triplicate.
The percentage of cell viability was calculated using the following formula:

The \(\hbox {LC}_{50}\) values (lethal concentration of the test compound required to kill 50 % of sample population) were determined by plotting cell viability percentage against concentration of the sample.
References
Hargrave KD, Hess FK, Oliver JT (1983) \(N\)-(4-substituted-thiazolyl)oxamic acid derivatives, a new series of potent, orally active antiallergy agents. J Med Chem 26:1158–1163. doi:10.1021/jm00362a014
Patt WC, Hamilton HW, Taylor MD, Ryan MJ, Taylor DG Jr, Connolly CJC, Doherty AM, Klutchko SR, Sircar I, Steinbaugh BA, Batley BL, Painchaud CA, Rapundalo ST, Michniewicz BM, Olson SCJ (1992) Structure-activity relationships of a series of 2-amino-4-thiazole-containing renin inhibitors. J Med Chem 35:2562–2572. doi:10.1021/jm00092a006
Aggarwal R, Kumar S, Kaushik P, Dhirender K, Girish Kumar G (2013) Synthesis and pharmacological evaluation of some novel 2-(5-hydroxy-5-trifluoromethyl-4,5-dihydropyrazol-1-yl)-4-(coumarin-3-yl)thiazoles. Eur J Med Chem 62:508–514. doi:10.1016/j.ejmech.2012.11.046
Cantrell AS, Engelhardt P, Högberg M, Jaskunas SR, Gunnar Johansson N, Jordan CL, Kangasmetsä J, Kinnick MD, Lind P, Morin JM, Muesing MA Jr, Noreén R, Öberg B, Pranc P, Sahlberg C, Ternansky RJ, Vasileff RT, Vrang L, West SJ, Zhang H (1996) Phenethythiazolethiourea (PETT) compounds; a new class of HIV-1 reverse transcriptase inhibitors. Synthesis and basic structure activity relationship studies of PETT analogs. J Med Chem 39:4261–4274. doi:10.1021/jm950639r
Jaen JC, Wise LD, Caprathe BW, Tecle H, Bergmeier S, Humblet CC, Heffner TG, Meltzner LT, Pugsley TA (1990) 4–1,2,5,6-Tetrahydro-l-alkyl-3-pyridinyl)-2-thizolamines: A novel class of compounds with central dopamine agonist properties. J Med Chem 33:311–317. doi:10.1021/jm00163a051
Tsuji K, Ishikawa H (1994) Synthesis and anti-pseudomonal activity of new 2-isocephems with a dihydroxypyridone moiety at C-7. Bioorg Med Chem Lett 4:1601–1606. doi:10.1016/S0960-894X(01)80574-6
Jukič M, Đorđević A, Lazarević J, Gobec M, Šmelcerović A, Anderluh A (2013) Antimicrobial activity and cytotoxicity of some 2-amino-5-alkylidene-thiazol-4-ones. Mol Divers 17:773–780. doi:10.1007/s11030-013-9474-6
Li M, Sim Y, Wook Ham S (2010) Discovery of 2-aminothiazole derivatives as antitumor agents. Bull Korean Chem Soc 31:1463–1464. doi:10.5012/bkcs.2010.31.6.1463
Regnier G, Canevar L, Le RJ, Douarec JC, Halstop S, Daussy J (1972) Triphenylpropylpiperazine derivatives as new potent analgetic substances. J Med Chem 15:295–301. doi:10.1021/jm00273a600
Siddiqui N, Waquar A (2010) Triazole incorporated thiazoles as a new class of anticonvulsants: Design, synthesis and in vivo screening. Eur J Med Chem 45:1536–1543. doi:10.1016/j.ejmech.2009.12.062
Ha YM, Uehara Y, Park D, Jeong HO, Park JY, Park YJ, Lee JY, Lee HJ, Song YM, Moon HR, Chung HY (2012) Synthesis and preliminary in vitro biological evaluation of 5-chloro-2-(substituted Phenyl)benzo[d]thiazole derivatives designed as novel antimelanogenesis agents. Appl Biochem Biotechnol 168:1416–1433. doi:10.1007/s12010-012-9867-5
Chandra Sekhar KVG, Rao VS, Deuther-Conrad W, Sridhar D, Nagesh HN, Kumar VS, Brust P, Kumar MMK (2013) Design, synthesis, and preliminary in vitro and in vivo pharmacological evaluation of 4-{4-[2-(4-(2-substitutedquinoxalin-3-yl)piperazin-1-yl)ethyl]phenyl}thiazoles as atypical antipsychotic agents. Med Chem Res 22:1660–1673. doi:10.1007/s00044-012-0164-1
Anbazhagan R, Sankaran KR (2013) Syntheses, spectral characterization, single crystal X-ray diffraction and DFT computational studies of novel thiazole derivatives. J Mol Struct 1050:73–80. doi:10.1016/j.molstruc.2013.07.019
McIntyre NA, McInnes C, Griffiths G, Barnett AL, Kontopidis G, Slawin AM, Jackson W, Thomas M, Zheleva DI, Wang S, Blake DG, Westwood NJ, Fischer PM (2010) Design, synthesis, and evaluation of 2-methyl- and 2-amino-\(N\)-aryl-4,5-dihydrothiazolo[4,5-h]quinazolin-8-amines as ring-constrained 2-anilino-4-(thiazol-5-yl)pyrimidine cyclin-dependent kinase inhibitors. J Med Chem 53:2136–2145. doi:10.1021/jm901660c
Mohammed Khan K, Karim A, Saied S, Ambreen N, Rustamova X, Naureen S, Mansoor S, Ali M, Perveen S, Choudhary MI, Antonio Morales G (2014) Evaluation of the thiazole Schiff bases as \(\beta \) -glucuronidase inhibitors and their in silico studies. Mol Divers 18:295–306. doi:10.1007/s11030-013-9500-8
Imramovsky A, Pejchal V, Štepánková Š, Vorcáková K, Jampílek J, Vancod J, Šimunek P, Královec K, Brucková L, Mandíková J, Trejtnar F (2013) Synthesis and in vitro evaluation of new derivatives of 2-substituted-6-fluorobenzo[d]thiazoles as cholinesterase inhibitors. Bioorg Med Chem 21:1735–1748. doi:10.1016/j.bmc.2013.01.052
Carradori S, Secci D, Bolasco A, Rivanera D, Mari E, Zicari A, Lotti LV, Bizzarri B (2013) Synthesis and cytotoxicity of novel (thiazol-2-yl)hydrazine derivatives as promising anti-candida agents. Eur J Med Chem 65:102–111. doi:10.1016/j.ejmech.2013.04.042
Abbasi Shiran J, Yahyazadeh A, Mamaghani M, Rassa M (2013) Regioselective synthesis of novel 3-allyl-2-(substituted imino)-4-phenyl-3H-thiazole and \(2,2^\prime \)-(1,3-phenylene)bis(3-substituted-2-imino-4-phenyl-3H-thiazole) derivatives as antibacterial agents. J Mol Struct 1039:113–118. doi:10.1016/j.molstruc.2013.02.003
Kudrjasova J, Herckens R, Penxten H, Adriaensens P, Lutsen L, Vanderzandea D, Maes W (2014) Direct arylation as a versatile tool towards thiazolo[5,4-d]thiazole-based semiconducting materials. Org Biomol Chem 12:4663–4672. doi:10.1039/C4OB00360H
Giridhar T, Cho W, Park J, Park J-S, Gal Y-S, Kang S, Leec JY, Jin S-H (2013) Facile synthesis and characterization of iridium(III) complexes containing an N-ethylcarbazole-thiazole main ligand using a tandem reaction for solution processed phosphorescent organic light-emitting diodes. J Mater Chem C 1:2368–2378. doi:10.1039/C3TC00323J
Hela A, Harun M, Choi C-H, Kima H-S (2012) New regioisomeric naphthol-substituted thiazole based ratiometric fluorescence sensor for \(\text{ Zn }^{2+}\) with a remarkable red shift in emission spectra. Tetrahedron 68:647–653. doi:10.1016/j.tet.2011.10.106
Sharifzadeh B, Mahmoodi NO, Mamaghani M, Tabatabaeian K, Salimi Chirani A, Nikokar I (2013) Facile regioselective synthesis of novel bioactive thiazolyl-pyrazoline derivatives via a three-component reaction and their antimicrobial activity. Bioorg Med Chem Lett 23:548–551. doi:10.1016/j.bmcl.2012.11.024
Mahmoodi NO, Parvizi J, Sharifzadeh B, Rassa M (2013) Facile regioselective synthesis of novel bis-thiazole derivatives and their antimicrobial activity. Arch Pharm Chem Life Sci 346:860–864. doi:10.1002/ardp.201300187
Mahmoodi NO, Safari N, Sharifzadeh B (2014) One-Pot synthesis of novel 2-(thiazol-2-yl)-4,5-dihydropyridazin-\(3(2H)\)-one derivatives catalyzed by activated KSF. Synth Commun 44:245–250. doi:10.1080/00397911.2013.801077
Mahmoodi NO, Ramzanpour S, Ghanbari Pirbasti F (2015) One-pot multi-component synthesis of 1,4-dihydropyridines using \(\text{ Zn }^{2+}\)@KSF and evaluation their antibacterial and antioxidant activities. Arch der Pharm 348:275–282. doi:10.1002/ardp.201400414
Mahmoodi NO, Mamaghani M, Behzadi T (2012) Synthesis and structure-behavior relationships of tetra-substituted imidazole derivatives of 1, 3-diazabicyclo [3, 1, 0] hex-3-ene. Mol Divers 16:737–747. doi:10.1007/s11030-012-9409-7
Caputto ME, Ciccarelli A, Frank F, Moglioni AG, Moltrasio GY, Vega D, Lombardo E, Finkielsztein LM (2012) Synthesis and biological evaluation of some novel 1-indanone thiazolylhydrazone derivatives as anti-trypanosoma cruzi agents. Eur J Med Chem 55:155–163. doi:10.1016/j.ejmech.2012.07.013
Secci D, Bolasco A, Carradori S, D’Ascenzio M, Nescatelli R, Yáñez M (2012) Recent advances in the development of selective human MAO-B inhibitors: (Hetero)arylidene-(4-substituted-thiazol-2-yl)hydrazines. Eur J Med Chem 58:405–417. doi:10.1016/j.ejmech.2012.10.032
Gaikwad ND, Patil SV, Bobade VD (2012) Hybrids of ravuconazole: synthesis and biological evaluation. Eur J Med Chem 54:295–302. doi:10.1016/j.ejmech.2012.05.010
Arshad A, Osman H, Bagley MC, Kit Lam C, Mohamad S, Safirah A, Zahariluddin M (2011) Synthesis and antimicrobial properties of some new thiazolyl coumarin derivatives. Eur J Med Chem 46:3788–3794. doi:10.1016/j.ejmech.2011.05.044
Hassan AA, Ibrahim YR, El-Sheref EM, Abdel-Aziz M, Bräse S, Nieger M (2013) Synthesis and antibacterial activity of 4-aryl-2-(1-substituted ethylidene)thiazoles. Arch Pharm Chem Life Sci 346:562–570. doi:10.1002/ardp.201300099
Osman H, Arshad A, Kit Lam C, Bagley MC (2012) Microwave-assisted synthesis and antioxidant properties of hydrazinyl thiazolyl coumarin derivatives. Chem Cent J 6:32. doi:10.1186/1752-153X-6-32
Bharti SK, Nath G, Tilak R, Singh SK (2010) Synthesis, anti-bacterial and anti-fungal activities of some novel Schiff bases containing 2,4-disubstituted thiazole ring. J Med Chem 45:651–660. doi:10.1016/j.ejmech.2009.11.008
Alam MS, Liu L, Lee YE, Lee DU (2011) Cytotoxicity of new 5-phenyl-4,5-dihydro-1,3,4-thiadiazole analogues. Chem Pharm Bull 59:568–573. doi:10.1002/chin.201215131
Acknowledgments
The authors are greatly thankful to Dr. Zohreh Ramezanpour and Miss Somaie Rasouli Dogaheh for their helpful assistance in running biological assays. The authors appreciate the valuable and helpful comments of Professor Dr. Guillermo A. Morales, Editor-In-Chief of Molecular Diversity.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghanbari Pirbasti, F., Mahmoodi, N.O. Facile synthesis and biological assays of novel 2,4-disubstituted hydrazinyl-thiazoles analogs. Mol Divers 20, 497–506 (2016). https://doi.org/10.1007/s11030-015-9654-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-015-9654-7