Abstract
Twenty eight (28) derivatives 2–29 were synthesized and four analogs were found to exhibit single-digit \(\hbox {IC}_{50}\) values as \(\upbeta \)-glucuronidase inhibitors. Molecular modeling indicates that three factors: substituent R, lone pair on the nitrogen of azomethine part, and the interactions made by the main skeleton of the molecule, determined the enzyme inhibitory potential of these compounds. The planar conformation of the molecules allows them to fit deep inside the pocket while blocking the entry of other physiological substrates seems to play an important role in their activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Computer-aided rational drug design has made significant contributions in the development of novel therapeutics leading to FDA approval, such as the anti-flu drug Relenza, a neuraminidase inhibitor [1], and the anti-AIDS cyclic urea DMP-450 [2]. During the 1990s high throughput screening (HTS) was introduced in mainstream drug discovery; however, it failed to deliver its high expectations for drug discovery. The foremost concern was the lack of target macromolecular structures of receptors. Experts in computer-aided drug design (CADD) promptly recognized the importance of HTS and developed computer-driven equivalents such as virtual screening. The principle was simple: (1) construct a 3D model of the target site and dock into it as many molecular candidates as possible to estimate how well they might bind in the active site, (2) prioritize and select the most suitable structures, and (3) synthesize the most promising candidates for in vitro screening [3, 4].
\(\upbeta \)-Glucuronidase plays a pivotal role in the hydrolysis of \(\upbeta \)-glucuronides. Glucuronides are formed in the body during the xenobiotic detoxification process. A large number of toxic compounds are eliminated safely from the body as glucuronides. Since \(\upbeta \)-glucuronidase hydrolyzes these conjugates, the inhibition of this enzyme may protect the body from the reintroduction of the original xenobiotics [5]. Evidence suggests that inhibiting the \(\upbeta \)-glucuronidase enzyme has a possible role in controlling different stages in cancer induction [6, 7]. \(\upbeta \)-Glucuronidase (EC 3.2.1.31) is an inducible enzyme elaborated by anaerobic E. coli, Peptostreptococcus, Bacteroides, and Clostridia. Studies have associated bacterial \(\upbeta \)-glucuronidase primarily to E. coli and Enterobacteriaceae. Enhanced activity of this enzyme increases the enterohepatic recirculation of toxins, hormones, drugs, and carcinogens. Recent studies showed that the Gram-positive bacteria in the gastrointestinal tract are also partially involved in \(\upbeta \)-glucuronidase activity [8]. Initiation of colon cancer is believed to be associated with \(\upbeta \)-Glucuronidase while its higher levels in intestines are connected with increased risk of colon cancer. These reports emphasize the pharmacological significance for the development of new and specific inhibitors of this enzyme [9, 10].
Thiazoles have attracted a lot of interest over the years due to their broad biological activity profiles [11–13]. These molecules have found applications in drug development for the treatment of allergies [14], hypertension [15], schizophrenia [16], inflammation [17], HIV infections [18], and they are also used as cardiotonics [19], sedatives [20], and anesthetics [21]. Additionally, while some new thiazoles possess anti-inflammatory and analgesic properties [22, 23], others are potent and selective acetyl CoA carboxylase 2 inhibitors with high promise in Alzheimer’s treatment [24, 25].
In continuation to our research interests in the development of new and simple leads, thiazole Schiff bases 2–29 were synthesized and screened for their \(\upbeta \)-glucuronidase inhibitory potentials. Synthetic compounds 2–29 demonstrated varying degrees of \(\upbeta \)-glucuronidase inhibitory potentials. d-Saccharic acid 1,4-lactone is used as a standard substrate whose \(\hbox {IC}_{50}\) value is 48.4 \(\pm \) 1.25 \(\upmu \)M. In addition, molecular modeling was performed to explore the potential binding mode of our newly synthesized thiazole derivatives.
Results and discussion
4-Phenyl-1,3-thiazol-2-amine (1), an important intermediate for pharmaceutical industry, was prepared by the condensation of thiourea and acetophenone in the presence of thionyl chloride as an oxidating agent (Scheme 1) [26]. Schiff bases 2–29 were prepared by condensing 1 with a diverse range of aldehydes (Scheme 2). All final products were confirmed by 1H NMR, EI MS, IR, and UV spectroscopy (Table 1).
SAR studies
Thiazoles 2–29 were screened against \(\upbeta \)-glucuronidase following a published protocol [27, 28] exhibiting \(\hbox {IC}_{50}\) values in the range of 152 \(\pm \, 3.35-4.88\pm \, 0.12\, \upmu \)M versus that of the standard substrate d-saccharic acid 1,4-lactone (\(48.4\, \pm \, 1.25\, \upmu \)M). The results are shown in Table 2.
Remarkably, compounds 3, 12, 17, and 18 exhibited single-digit micromolar \(\upbeta \)-glucuronidase inhibition activity, while compounds 2, 5, 8, 9, 11, 13, 14, 16, 20, 21, 22, 23, 24, 25, 26, 27, 28, and 29 also showed good \(\upbeta \)-glucuronidase inhibition activities superior to the standard. Compounds 4, 10, 15, and 19 demonstrated less activity against \(\upbeta \)-glucuronidase, and compounds 6 and 7 demonstrated no activity at all. In a broader sense, ligands are classified into four groups based on their \(\hbox {IC}_{50}\) values as shown in Table 3.
Results shown in the above table are prioritized and summarized as follows:
It has been envisioned that the aromatic side chain directly attached to azomethine moiety determined the inhibitory potential of compounds 2–29. Compound 18, with two hydroxyl groups on the aromatic ring of side chain, proved to be the most potent inhibitor (\(\hbox {IC}_{50 }= 4.88 \pm 0.12\, \upmu \)M) among the current series as well as much superior than the standard inhibitor d-saccharic acid 1,4-lactone. Similarly, compound 17 having a hydroxyl group at ortho and a chloro at meta-position of the phenyl ring also showed an excellent inhibitory potential with an \(\hbox {IC}_{50}\) value \(5.63 \pm 0.16\,\upmu \)M. Compounds 7 and 9 were proved to be exceptions to this generalization since the side chain of these compounds is aliphatic. Compound 12, without any substitution on the phenyl ring, showed an \(\hbox {IC}_{50}\) value 9.77 \(\pm \) 0.11\(\,\upmu \)M. Compound 3, with mono hydroxy substitution at para position of the phenyl ring, also showed an excellent activity (\(\hbox {IC}_{50 }= 9.84 \pm 0.33\, \upmu \)M) but less than compounds 17 and 18. Compound 8, with a mono hydroxy substitution at ortho position of the phenyl ring, also exhibited an outstanding activity (\(\hbox {IC}_{50 }= 19.1 \pm 1.20\, \upmu \)M). Introduction of meta-methoxy and meta-ethoxy substitutions to compound 8 resulted in compounds 27 and 16, respectively. Consequent outcomes of their inhibition potential against \(\upbeta \)-glucuronidase enzyme yielded \(\hbox {IC}_{50}\) values in the range of 21.82 \(\pm \) 0.40 and 21.98 \(\pm \) 0.32\(\,\upmu \)M, respectively. However, in compound 17, where meta-position of the aromatic side chain is occupied by a chloro group resulted in much lower \(\hbox {IC}_{50}\) value (5.63 \(\pm \) 0.16\(\,\upmu \)M). These findings suggest that decrease in activities of compounds 27 and 16 may be due to internal hydrogen bonding of ortho-hydroxyl and meta-methoxy/ethoxy groups. However, in case of meta-chloro analog 17, hydrogen bonding element is somewhat diminished (Fig. 1).
The known substrate molecule—\(p\)-nitrophenyl \(\upbeta \)-d-glucuronide (a) and the standard inhibitor—d-saccharic acid 1,4-lactone (b) of \(\upbeta \)-glucuronidase are shown along with our compound 2-amino-4-phenyl-1,3-thiazole R (c). The selected derivatives (R) are shown in order of increasing \(\hbox {IC}_{50}\) (d). Color scheme: Carbons in (a), (b), (c), and (d) are colored gold, sea green, light green, and light blue. Hydrogen, oxygen, nitrogen, and sulfur molecules are colored light gray, red, blue, and yellow, respectively. Chlorine in 17 and Fluorine in 15 (d) are colored green and bright blue
Compounds 2 (\(\hbox {IC}_{50 }= 12.49 \pm 0.18\,\upmu \)M) and 28 (\(\hbox {IC}_{50 }= 10.73 \pm 0.17\,\upmu \)M) containing nitro groups at para- and meta-positions of the phenyl ring, respectively, showed similar activities. These results are comparable to those explained vide supra and suggests that the nature of atoms/groups (electron donating or withdrawing) has minimal or no effect on \(\upbeta \)-glucuronidase inhibition. Compound 29 having a bulky pyrene side chain (\(\hbox {IC}_{50 }= 16.15 \pm 0.83\,\upmu \)M) and compound 14 (\(\hbox {IC}_{50 }= 37.98 \pm 1.05\,\upmu \)M) having a 1-naphthalene ring instead of phenyl group showed a slight decline in activity. Compound 9 (\(\hbox {IC}_{50 }= 21.80 \pm 0.42\,\upmu \)M) having a vinyl-aromatic and likewise compound 7 (\(\hbox {IC}_{50 }= 152 \pm 3.35\,\upmu \)M) with an aliphatic substitution instead of phenyl residue also showed less activity. These observations suggest that the phenyl residue governs the \(\upbeta \)-glucuronidase inhibition potential of these compounds. Similarly, compound 11 (\(\hbox {IC}_{50 }= 16.64 \pm 1.37\, \upmu \)M) having a pyridyl group and compound 21 (\(\hbox {IC}_{50 }\)= 12.48 \(\pm \) 0.56 \(\upmu \)M) with a para-cumenyl group showed an appreciable amount of activity suggesting that an aromatic residue directly attached to azomethine group greatly influenced the activity.
Compounds 13 (\(\hbox {IC}_{50 }\)= 12.27 \(\pm \) 0.43 \(\upmu \)M), 23 (\(\hbox {IC}_{50 }\)= 14.25 \(\pm \) 0.72 \(\upmu \)M), and 20 (\(\hbox {IC}_{50 }\)= 22.45 \(\pm \) 0.7 \(\upmu \)M) are differently mono- and di-substituted chloro compounds and their activities suggest that an ortho-substitution is better than a para-substitution; however, di-ortho-chloro substitution decreases the \(\upbeta \)-glucuronidase inhibitory activity to a great extent. Surprisingly, compound 15 (\(\hbox {IC}_{50 }\)= 51.48 \(\pm \) 2.46 \(\,\upmu \)M) with an ortho-fluoro residue was found to be the least active compound in halogen-containing compounds. Compound 22 (\(\hbox {IC}_{50 }\)= 18.43 \(\pm \) 0.19 \(\upmu \)M) having a methyl substituted furan showed better activity as compared to indole-containing compound 24 (\(\hbox {IC}_{50 }\)= 44.14 \(\pm \) 0.25 \(\upmu \)M).
Conclusively, these observations propose that SAR of compounds is largely dependent upon aromatic ring residue and substitutions over it. Further, internal hydrogen bonding in compounds also proved one factor that resulted in lowering of inhibitory potentials. Compound 18, with two hydroxyl groups on aromatic ring of side chain, proved to be the most potent inhibitor among the current series.
Molecular modeling studies
Compounds 2–29 were docked into the active site of the enzyme using GOLD 4.1 version [29].
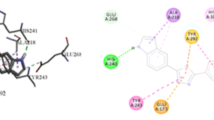
Orientation of the key catalytic residues GLU451, GLU540, and TYR504 and the predicted binding pose of the standard substrate \(p\)-nitrophenyl \(\upbeta \)-glucuronic acid are shown in Fig. 2 while the predicted binding pose of the inhibitor is shown in Fig. 3. d-Saccharic acid 1,4-lactone fails to completely mask the catalytic residues thereby allowing \(p\)-nitrophenyl \(\upbeta \)-d-glucuronide to gain access to the catalytic residues. The location of the active site pocket and its topology indicates that ligands may gain access to it from the left side.
Contrarily, compounds 2–29 fit deep in the pocket, but unlike d-saccharic acid 1,4-lactone, the standard inhibitor, they extend toward the entry way and masked the catalytic residues of enzyme (Fig. 4). Striking analogy among the predicted binding poses for our compounds suggested that the main body of the molecule, i.e., phenylthiazolyl part of the molecules is the first to gain access to the hydrophobic regions of the pocket and has the least impact on the \(\hbox {IC}_{50}\) variation. Therefore, it seems that the following three factors: (i) the substituent R on the compound, (ii) the direction of the lone pair on the nitrogen of azomethine part, and (iii) the distance between thiazole nitrogen and GLU451, determined the \(\hbox {IC}_{50}\) of these compounds. Compound 18 with two hydroxyl groups succeeds in making contacts with catalytic residues, resulting in a better accommodation of the ligand inside the pocket (Fig. 4). These extra interactions of polar groups such as hydroxyl on the side-chain residue resulted in the lowest \(\hbox {IC}_{50}\) value for compound 18. On the other hand, compounds 12, 14, 21, 25, and 29 containing non-polar aromatic groups as their side chains lack such dipolar interactions and, therefore, cause reduced affinity (Figs. 5, 6, 7).
Molecular modeling studies showed that the presence of two hydroxyl groups on an aromatic ring in the side chain of compound 18 provided extra interactions for hydrogen bonding inside the active site pocket of enzyme.
Another important aspect is the long planar conformation, as for compound 29 that allowed it to properly fit deep inside the pocket, yet blocking the entry path. Moreover, the single covalent bond between thiazole and benzene ring in the main skeleton of the molecule provided more conformational flexibility, while allowing it to create interactions that stabilize the molecule within the pocket (Fig. 8).
Conclusions
In conclusion, a combined effort of chemistry and modeling studies identify a new class of \(\upbeta \)-glucuronidase inhibitors; and compound 18 was found to be the most interesting inhibitor with \(\hbox {IC}_{50}\) value of 4.8 \(\pm \) 0.12 \(\upmu \)M. Two hydroxyl groups on benzene ring provided extra interactions within the pocket of enzyme by means of hydrogen bonding. Compounds 17, 12, 3, 28, 13, 21, and 2 also exhibited excellent inhibitory potentials; however, the compounds with non-polar displayed a relatively higher inhibitory concentrations due to lack of additional non-covalent interactions. These compounds may serve as viable lead molecules against \(\upbeta \)-glucuronidase inhibition for future research.
Experimental general
Melting points were determined on a Büchi 434 melting point apparatus and were uncorrected. Chemicals were purchased from Alfa Aesar and used without purification. Solvents were distilled through standard procedures. Solvents NMR experiments were performed on Avance Bruker AM 300, 400, and 500 MHz using deuterated solvents such as \(\hbox {CD}_{3}\)OD and \(\hbox {CDCl}_{3}\). Splitting patterns were as follows: s, singlet; d, doublet; dd, double doublets; t, triplet; and m, multiplet. Chemical shifts are reported in \(\delta \) (ppm) and coupling constants are given in Hz. Ultraviolet (UV) spectra were recorded on Perkin-Elmer Lambda-5 UV/Vis spectrometer in MeOH. Infrared (IR) spectra were recorded on JASCO IR-A-302 spectrometer as KBr (disc). Electron impact mass spectra (EI MS) were recorded on a Finnigan MAT-311A spectrometer, Germany. Thin-layer chromatography (TLC) was performed on pre-coated silica gel aluminum plates (Kieselgel 60, 254, E. Merck, Germany). Chromatograms were visualized by UV at 254 and 365 nm.
In vitro \(\upbeta \)-glucuronidase assay protocol
\(\upbeta \)-Glucuronidase activity was determined by measuring the absorbance at 405 nm of \(p\)-nitrophenol, formed from the substrate, by the spectrophotometric method. The total reaction volume was 250 \(\upmu \)L. The reaction mixture containing 185 \(\upmu \)L of 0.1 M acetate buffer, 5 \(\upmu \)L of test compound solution, and 10 \(\upmu \)L of enzyme solution was incubated at 37 \(^{\circ }\)C for 30 min. The plates were read on a multiplate reader (SpectraMax plus 384, Applied Biosystem, CA, USA) at 405 nm after the addition of 50 \(\upmu \)L of 0.4 mM \(p\)-nitrophenyl-\(\upbeta \)-d-glucuronide [27, 28]. All the assays were run in triplicate.
Procedure for the synthesis of 4-phenyl-1,3-thiazol-2-amine
To a mixture of acetophenone (0.2 mol) and thiourea (0.4 mol), thionyl chloride (0.2 mol) as oxidizing agent was added and the reaction mixture was heated on a steam bath for 24 h. The progress of reaction was monitored by TLC; after the completion of reaction, the crude product was washed by \(n\)-hexane and crystallized from ethanol.
General procedure for the synthesis
To a mixture of 2-amino-4-phenyl thiazole (0.52 g, 3 mmol) and aldehydes (3 mmol), ethanol (15 mL) was added. The progress of reaction was monitored by TLC; after the completion of reaction, solvent was removed under vacuum on a rotary evaporator. The resulting product was crystallized from ethanol.
N-(4-Nitrobenzylidene)-4-phenylthiazol-2-amine (2)
Yield: 2.51 g (55 %); \(\hbox {m.p.} = 135.1^{\circ }\hbox {C}; \hbox {R}_{f }= 0.64\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 258 (log \(\upvarepsilon = 2.76\)) nm; IR (KBr): \(\upnu _{\mathrm{max}}\,2851, 1627, 1605, 1346\,\hbox {cm}^{-1},{}^{1}\)H NMR (300 MHz, \(\hbox {CD}_{3}\hbox {OD}\)): \(\delta \) 9.18 (s, 1H, =CH–S), 8.42 (s, 1H, N=CH), 8.39 (d, \(J_{3^{\prime },2^{\prime }}=J_{5^{\prime },6^{\prime }} = 7.8 \hbox {Hz}, 2\hbox {H}, \hbox {H}\)-\(3^{{\prime }}, \hbox {H}\)-\(5^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 8.14 (d, \(J_{2^{\prime },3^{\prime }}=J_{6^{\prime },5^{\prime }} = 7.6\) Hz, 2H, \(\hbox {H}\)-\(2^{{\prime }}, \hbox {H}\)-\(6^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.60 (m, 5H, H-2, H-3, H-4, H-5, H-6, Ar–H); EI MS: \(m/z\) (rel. abund. %), 309 (47.8), 262 (12.15), 176 (45.23).
4-(((4-Phenylthiazol-2-yl)imino)methyl)phenol (3)
Yield: 1.31 g (73 %); \(\hbox {m.p.} = 111.4^{\circ }\hbox {C}; \hbox {R}_{f }= 0.91\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 225 (log \(\upvarepsilon = 2.37\)) nm; IR (KBr): \(\upnu _{\mathrm{max}}\) 3635, 3051, 1665, 1326 \(\hbox {cm}^{-1}, ^{1}\)H NMR (400 MHz, \(\hbox {CD}_{3}\hbox {OD}\)): \(\delta \) 8.8 (s, 1H, =CH–S), 7.78 (s, 1H, N=CH), 6.92 (d, \(J_{3^{\prime },2^{\prime }}=J_{5^{\prime }, 6^{\prime }} = 8.0 \hbox {Hz}, 2\hbox {H}, \hbox {H}\)-\(3^{{\prime }}, \hbox {H}\)-\(5^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 6.77 (d, \(J_{2^{\prime },3^{\prime }}=J_{6^{\prime },5^{\prime }} = 7.8 \hbox {Hz}, 2\hbox {H}, \hbox {H}\)-\(2^{{\prime }}, \hbox {H}\)-\(6^{{\prime }}, Ar\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.59 (m, 5H, H-2, H-3, H-4, H-5, H-6, Ar–H); EI MS: \(m/z\) (rel. abund. %), 280 (36.4), 263 (6.8), 176 (35.0).
N-(4-Ethoxybenzylidene)-4-phenylthiazol-2-amine (4)
Yield: 1.19 g (26 %); \(\hbox {m.p.} = 117.4 ^{\circ }\hbox {C}; \hbox {R}_{f }= 0.88\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 236 (log \(\upvarepsilon = 2.79\)) nm; IR (KBr): \(\upnu _{\mathrm{max}}2980, 1671, 1331, 1245, \hbox {cm}^{-1}, {}^{1}\)H NMR (300 MHz, \(\hbox {CD}_{3}\hbox {OD}\)): \(\delta \) 8.5 (s, 1H, =CH–S), 7.78 (s, 1H, N=CH), 7.02 (d, \(J_{3^{\prime },2^{\prime }}=J_{5^{\prime },6^{\prime }} = 7.7\hbox {Hz}, 2\hbox {H}, \hbox {H}\)-\(3^{{\prime }}, \hbox {H}\)-\(5^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.48 (d, \(J_{2^{\prime },3^{\prime }}=J_{6^{\prime },5^{\prime }} = 7.8 \hbox {Hz}, 2\hbox {H}, \hbox {H}\)-\(2^{{\prime }}, \hbox {H}\)-\(6^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.64 (m, 5H, H-2, H-3, H-4, H-5, H-6, Ar–H), 3.98 (q, 2H, –\(\hbox {CH}_{2})\), 1.35 (s, 3H, –\(\hbox {CH}_{3})\); EI MS: \(m/z\) (rel. abund. %), 308 (75.3), 263 (43.3), 208 (20.8).
N-(4-Methoxybenzylidene)-4-phenylthiazol-2-amine (5)
Yield: 0.59 g (69 %); \(\hbox {m.p.} = 116.4 ^{\circ }\hbox {C}; \hbox {R}_{f }= 0.76\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 232 (log \(\upvarepsilon = 2.35\)) nm.; IR (KBr): \(\upnu _{\mathrm{max}} 1652, 1605, 1305, 1250, \hbox {cm}^{-1},{}^{1}\)H NMR (300 MHz, \(\hbox {CD}_{3}\)OD): \(\delta \) 8.87 (s, 1H, =CH–S), 7.97 (s, 1H, N=CH), 7.33 (d, \(J_{2^{\prime },3^{\prime }}=J_{6^{\prime },5^{\prime }} = 8.0 \hbox {Hz}, 2\hbox {H}, \hbox {H}\)-\(2', \hbox {H}\)-\(6', \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 6.88 (d, \(J_{3^{\prime },2^{\prime }}=J_{5^{\prime },6^{\prime } }= 7.8 \hbox {Hz}, 2\hbox {H}, \hbox {H}\)-\(3^{{\prime }}, \hbox {H}\)-\(5^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.53 (m, 5H, H-2, H-3, H-4, H-5, H-6, Ar–H); EI MS: \(m/z\) (rel. abund. %), 294 (100), 263 (6.8), 279 (7.20).
N-(4-(Dimethylamino)benzylidene)-4-phenylthiazol-2-amine (6)
Yield: 2.7 g (60 %); \(\hbox {m.p.} = 164.7 ^{\circ }\hbox {C}; \hbox {R}_{f }= 0.71\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 340 (log \(\upvarepsilon = 2.21\)) nm; IR (KBr): \(\upnu _{\mathrm{max}} 2920, 1662, 1594, 1328\,\hbox {cm}^{-1}, {}^{1}\)H NMR (300 MHz, \(\hbox {CD}_{3}\hbox {OD}\)): 9.06 (s, 1H, =CH–S), \(\delta \) 7.21 (s, 1H, N=CH), 7.24 (d, \(J_{3^{\prime },2^{\prime }}=J_{5^{\prime },6^{\prime }} = 8.0 \hbox {Hz}, 2\hbox {H}, \hbox {H}\)-\(3^{{\prime }}, \hbox {H}\)-\(5^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.73 (d, \(J_{2^{\prime },3^{\prime }}=J_{6^{\prime }, 5^{\prime }} = 7.8 \hbox {Hz}, 2\hbox {H}, \hbox {H}\)-\(2^{{\prime }}, \hbox {H}\)-\(6^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.55 (m, 5H, H-2, H-3, H-4, H-5, H-6, Ar–H), 3.29 (s, 6H, –C\(H_{3})\); EI MS: \(m/z\) (rel. abund. %), 307 (46.4), 134 (18.5), 176 (6.2).
N-Butylidene-4-phenylthiazol-2-amine (7)
Yield: 0.38 g (60.3 %); \(\hbox {m.p.} = 203 ^{\circ }\hbox {C}; \hbox {R}_{f}= 0.83\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 241 (log \(\upvarepsilon = 2.91\)) nm; IR (KBr): \(\upnu _{\mathrm{max}} 2964,, 1662,, 1592\, 1351\,\hbox {cm}^{-1}, {}^{1}\)H NMR (300 MHz, \(\hbox {CD}_{3}\)OD): \(\delta \) 9.13 (s, 1H,=CH–S), 7.78 (s, 1H, N=CH), 1.83 (m, 2H, –C\(H_{2})\), 1.03 (t, –C\(H_{3})\), 7.67 (m, 5H, H-2, H-3, H-4, H-5, H-6, Ar–H); EI MS: \(m/z\) (rel. abund. %), 216 (17.0), 201 (12.3), 156 (20.6).
2-(((4-Phenylthiazol-2-yl)imino)methyl)phenol (8)
Yield: 0.59 g (72 %); \(\hbox {m.p.} = 133 ^{\circ }\hbox {C}; \hbox {R}_{f }= 0.87\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 241 (log \(\upvarepsilon = 2.64\)) nm; IR (KBr): \(\upnu _{\mathrm{max}} 2993, 1690, 1603, 1328, \hbox {cm}^{-1},{}^{1}\)H NMR (400 MHz, \(\hbox {CD}_{3}\)OD): \(\delta \) 8.70 (s, 1H, =CH–S), 7.78 (s, 1H, N=CH), 7.28 (m, 5H, H-2, H-3, H-4, H-5, H-6, Ar–H), 7.16 (m, \(\hbox {H}\)-\(3^{{\prime }}, \hbox {H}\)-\(4^{{\prime }}, \hbox {H}\)-\(5^{{\prime }}, \hbox {H}\)-\(6^{{\prime }}, 4\hbox {H}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\); EI MS: \(m/z\) (rel. abund. %), 280 (20.8), 263 (7.5), 176 (35.0).
N-((E)-3-(4-Methoxyphenyl)allylidene)-4-phenylthiazol-2-amine (9)
Yield: 0.90 g (99 %); \(\hbox {m.p.} = 197.3 ^{\circ }\hbox {C}; \hbox {R}_{f }= 0.81\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 244 (log \(\upvarepsilon = 2.07\)) nm; IR (KBr): \(\upnu _{\mathrm{max}} 1726, 1664, 1348, 1110\,\hbox {cm}^{-1},{}^{1}\)H NMR (300 MHz, \(\hbox {CD}_{3}\hbox {OD}\)): \(\delta \) 9.12 (s, =CH–S), 7.64 (s, 1H, N=CH), 7.04 (d, \(J_{3^{\prime },2^{\prime }}=J_{5^{\prime },6^{\prime }} = 7.8\, \hbox {Hz}, 2\hbox {H}, \hbox {H}\)-\(3^{{\prime }}, \hbox {H}\)-\(5^{{\prime }} \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.95 (d, \(J_{2^{\prime },3^{\prime }}=J_{6^{\prime },5^{\prime }} = 7.9 \hbox {Hz}, 2\hbox {H}, \hbox {H}\)-\(2^{{\prime }}, \hbox {H}\)-\(6^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.42 (m, H-2, H-3, H-4, H-5, H-6, 5H, Ar–H); EI MS: \(m/z\) (rel. abund. %), 322 (28.1), 323 (7.2), 291 (14.9).
N-(4-(Methylthio)benzylidene)-4-phenylthiazol-2-amine (10)
Yield: 0.47 g (46.2 %); \(\hbox {m.p.} = 176.2 ^{\circ }\hbox {C}; \hbox {R}_{f }= 0.81\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 241.6 (log \(\upvarepsilon = 2.67\)) nm; IR (KBr): \(\upnu _{\mathrm{max}} 2958, 1663, 1590, 1349, \hbox {cm}^{-1}, {}^{1}\)H NMR (300 MHz, \(\hbox {CD}_{3}\hbox {OD}\)): \(\delta \) 9.17 (s, 1H, =CH–S), 7.89 (s, 1H, N=CH), 7.57 (d, \(J_{2^{\prime },3^{\prime }}=J_{6^{\prime },5^{\prime }} = 8.0 \hbox {Hz}, 2\hbox {H}, \hbox {H}\)-\(2^{{\prime }}, \hbox {H}\)-\(6^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.38 (d, \(J_{3^{\prime },2^{\prime }}=J_{5^{\prime },6^{\prime }} = 7.8 \hbox {Hz}, 2\hbox {H}, \hbox {H}\)-\(3^{{\prime }}, \hbox {H}\)-\(5^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.43 (m, H-2, H-3, H-4, H-5, H-6, 5H, Ar–H); EI MS: \(m/z\) (rel. abund. %), 310 (9.0), 311 (3.5), 263 (5.4).
4-Phenyl-N-(pyridin-4-ylmethylene)thiazol-2-amine (11)
Yield: 0.46 g (61.3 %); \(\hbox {m.p.} = 171.8 ^{\circ }\hbox {C}; \hbox {R}_{f }= 0.85\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 239 (log \(\upvarepsilon = 2.62\)) nm; IR (KBr): \(\upnu _{\mathrm{max}} 2924, 1659, 1604, 1286, \hbox {cm}^{-1}, {}^{1}\)H NMR (500 MHz, \(\hbox {CD}_{3}\hbox {OD}\)): \(\delta \) 8.73 (s, 1H, =CH–S), 7.96 (s, 1H, N=CH) 7.36 (d, \(J_{3^{\prime },2^{\prime }}=J_{5^{\prime },6^{\prime }} = 7.9 \hbox {Hz}, 2\hbox {H}, \hbox {H}\)-\(3^{{\prime }}, \hbox {H}\)-\(5^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.84 (d, \(J_{2^{\prime },3^{\prime }}=J_{6^{\prime },5^{\prime }} = 7.6 \hbox {Hz}, 2\hbox {H} , \hbox {H}\)-\(2^{{\prime }}, \hbox {H}\)-\(6^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.59 (m, 4H, H-2, H-3, H-5, H-6, Ar–H), 7.18 (t, 1H, \(\hbox {H}-4^{{\prime }}, \hbox {Ar}\underline{\hbox {H}}^{{\prime }})\); EI MS: \(m/z\) (rel. abund. %), 265 (42.0), 267 (67.5), 253 (7.0).
N-Benzylidene-4-phenylthiazol-2-amine (12)
Yield: 0.21 g (27 %); \(\hbox {m.p.} = 192.4 ^{\circ }\hbox {C}; \hbox {R}_{f }= 0.84\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 240 (log \(\upvarepsilon = 2.42\)) nm; IR (KBr): \(\upnu _{\mathrm{max}} 2857, 1662, 1605, 1250, \hbox {cm}^{-1}, ^{1}\)H NMR (400 MHz, \(\hbox {CD}_{3}\hbox {OD}\)): \(\delta \) 8.80 (s, 1H, =CH–S), 7.78 (s, 1H, N=CH), 7.58 (m, 5H, \(\hbox {H}\)-\(2^{{\prime }}, \hbox {H}\)-\(3^{{\prime }}, \hbox {H}\)-\(4^{{\prime }}, \hbox {H}\)-\(5^{{\prime }}, \hbox {H}\)-\(6^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.41 (m, 5H, H-2, H-3, H-4, H-5, H-6, Ar–H); EI MS: \(m/z\) (rel. abund. %), 264 (100), 186 (4.6), 108 (6.3).
N-(2-Chlorobenzylidene)-4-phenylthiazol-2-amine (13)
Yield: 0.47 g (60 %); \(\hbox {m.p.} = 146.4 ^{\circ }\hbox {C}; \hbox {R}_{f }= 0.85\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 238 (log \(\upvarepsilon = 2.43\)) nm; IR (KBr): \(\upnu _{\mathrm{max}}1660, 1607, 1328, 754 \hbox {cm}^{-1}, {}^{1}\)H NMR (300 MHz, \(\hbox {CD}_{3}\)OD): \(\delta \) 8.83 (s, 1H, =CH–S), 7.81 (s, 1H, N=CH), 7.54 (m, 2H, \(\hbox {H}\)-\(3^{{\prime }}, \hbox {H}\)-\(6^{{\prime }}\), Ar–H’), 7.43 (m, 2H, \(\hbox {H}\)-\(4^{{\prime }}, \hbox {H}\)-\(5^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.32 (m, 5H, H-2, H-3, H-4, H-5, H-6, Ar–H); EI MS: \(m/z\) (rel. abund. %), 298 (35.5), 301 (8.3), 221 (10.1).
N-(Naphthalen-1-ylmethylene)-4-phenylthiazol-2-amine (14)
Yield: 0.33 g (36 %); \(\hbox {m.p.} = 148.2 ^{\circ }\hbox {C}; \hbox {R}_{f }= 0.78\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 222 (log \(\upvarepsilon = 2.54\)) nm.; IR (KBr): \(\upnu _{\mathrm{max}} 3062, 1654, 1617, 1213, \hbox {cm}^{-1}, {}^{1}\)H NMR (300 MHz, \(\hbox {CD}_{3}\hbox {OD}\)): \(\delta \) 9.06 (s, 1H, =CH–S), 7.99 (s, 1H, N=CH), 7.37 (m, 4H, H-2, H-3, H-5, H-6, Ar–H), 7.60 (m, 1H, H-4, Ar–H), 7.75 (m, 1H, \(\hbox {H}\)-\(6^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }}),^{ }7.33 (d, J_{2^{\prime },3^{\prime }} = 8.0 \hbox {Hz}, 1\hbox {H}, \hbox {H}-2^{{\prime }}, \hbox {Ar}-\underline{\hbox {H}}^{{\prime }})\), 7.78 (d, 1H, \(4^{{\prime }} \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.57 (t, 1H, \(\hbox {H}\)-\(3^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 8.12 (m, 1H, \(\hbox {H}\)-\(8^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 8.06 (t,1H, \(\hbox {H}\)-\(7^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.93 (d, \(J_{5^{\prime }, 6^{\prime }} = 7.9 \hbox {Hz}, 1\hbox {H}, \hbox {H}\)-\(5^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\); EI MS: \(m/z\) (rel. abund. %), 314 (23.1), 315 (6.2), 273 (8.8).
N-(2-Fluorobenzylidene)-4-phenylthiazol-2-amine (15)
Yield: 0.31 g (37.3 %); \(\hbox {m.p.} = 217.5 ^{\circ }\hbox {C}; \hbox {R}_{f }= 0.91\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 24 (log \(\upvarepsilon = 2.92\)) nm; IR (KBr): \(\upnu _{\mathrm{max}} 1663, 1613, 1335, 1087 \hbox {cm}^{-1},_{ }^{1}\)H NMR (300 MHz, \(\hbox {CD}_{3}\hbox {OD}\)): \(\delta \) 8.74 (s, 1H, =CH–S), 7.63 (s, 1H, N=CH), 7.55 (m, 2H, \(\hbox {H}\)-\(4^{{\prime }}, \hbox {H}\)-\(6^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.52 (m, 1H, H-4, Ar–H), 7.38 (m, 2H, \(\hbox {H}\)-\(3^{{\prime }}, \hbox {H}\)-\(5^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.38 (m, 4H, H-2, H-3, H-5, H-6, Ar–H); EI MS: \(m/z\) (rel. abund. %), 282 (44.6), 284 (29.2), 241 (17.6).
2-Ethoxy-6-(((4-phenylthiazol-2-yl)imino)methyl)phenol (16)
Yield: 0.45 g (47.3 %); \(\hbox {m.p.} = 132 ^{\circ }\hbox {C}; \hbox {R}_{f }= 0.80\); (Hex: EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 238 (log \(\upvarepsilon = 2.94\)) nm; IR (KBr): \(\upnu _{\mathrm{max}} 1693, 1217, 1271, 1615 \hbox {cm}^{-1}, {}^{1}\)H NMR (300 MHz, \(\hbox {CD}_{3}\)OD): \(\delta \) 9.02 (s, 1H, =CH–S), 7.16 (s, 1H, N=CH), 6.98 (d or dd \(J_{4{\prime },5^{\prime }}=J_{6^{\prime },5^{\prime }} = 8.0 \hbox {Hz}, 2\hbox {H}, \hbox {H}\)-\(4^{{\prime }}, H\)-\(6^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.50 (m, 1H, \(\hbox {H}\)-\(5^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.45 (m, 5H, H-2, H-3, H-4, H-5, H-6, Ar–H); EI MS: \(m/z\) (rel. abund. %), 324 (77.4), 309 (47.6), 263 (8.8).
4-Chloro-2-(((4-phenylthiazol-2-yl)imino)methyl)phenol (17)
Yield: 0.68 g (70.6 %); \(\hbox {m.p.} = 115.7 ^{\circ }\hbox {C}; \hbox {R}_{f }= 0.89\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 240 (log \(\upvarepsilon = 2.91\)) nm; IR (KBr): \(\upnu _{\mathrm{max}} 2875, 1616, 1275, 540\,\hbox {cm}^{-1},{}^{ 1}\)H NMR (300 MHz, \(\hbox {CD}_{3}\hbox {OD}\)): \(\delta \) 9.13 (s, 1H, =CH–S), 7.78 (s, 1H, N=CH), 6.83 (d, \(J_{3^{\prime },4^{\prime }} = 7.9 \hbox {Hz}, 1\hbox {H}, \hbox {H}\)-\(3^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.04 (s, 1H, \(\hbox {H}\)-\(6^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.48 (m = 1H, \(\hbox {H}\)-\(4^{{\prime }} \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.48 (m = 1H, H-4, Ar–H), 7.42 (m, 4H, H-2, H-3, H-5, H-6, Ar–H); EI MS: \(m/z\) (rel. abund. %), 314 (77.8), 315 (18.1), 297 (34.6).
3-(((4-Phenylthiazol-2-yl)imino)methyl)benzene-1,2-diol (18)
Yield: 0.15 g (38 %); \(\hbox {m.p.} = 142 ^{\circ }\hbox {C}; \hbox {R}_{f }= 0.77\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 239 (log \(\upvarepsilon = 2.64\)) nm; IR (KBr): \(\upnu _{\mathrm{max}} 3285, 1695, 1617, 1282, \hbox {cm}^{-1}, {}^{1}\)H NMR (300 MHz, \(\hbox {CD}_{3}\hbox {OD}\)): \(\delta \) 8.91 (s, 1H, =CH–S), 7.78 (s, 1H, N=CH), 7.43 (m = 3H, \(\hbox {H}\)-\(4^{{\prime }}, \hbox {H}\)-\(5^{{\prime }}, \hbox {H}\)-\(6^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.32 (m, 5H, H-2, H-3, H-4, H-5, H-6, Ar–H); EI MS: \(m/z\) (rel. abund. %), 296 (43.3), 218 (4.12), 176 (34.7).
N-(3,4-Dimethoxybenzylidene)-4-phenylthiazol-2-amine (19)
Yield: 0.68 g (71.5 %); \(\hbox {m.p.} = 119 ^{\circ }\hbox {C}; \hbox {R}_{f }= 0.85\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 234.2 (log \(\upvarepsilon = 2.99\)) nm; IR (KBr): \(\upnu _{\mathrm{max}}, 1680, 1271, 1135, 1013\,\hbox {cm}^{-1}, {}^{1}\)H NMR (300 MHz, \(\hbox {CD}_{3}\)OD): \(\delta \) 9.02 (s, 1H, =CH–S), 7.83 (s, 1H, N=CH), 7.13 (s, 1H, \(\hbox {H}\)-\(2^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 6.84 (d, \(J_{5^{\prime },6^{\prime }} = 7.9 \hbox {Hz}, 1\hbox {H}, \hbox {H}\)-\(5^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.37 (d, \(J_{6^{\prime },5^{\prime }} = 8.0 \hbox {Hz}, 1\hbox {H}, \hbox {H}\)-\(6^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.54 (m, 5H, H-2, H-3, H-4, H-5, H-6, Ar–H); EI MS: \(m/z\) (rel. abund. %), 324 (13.6), 325 (2.12), 224 (11.1).
N-(2,6-Dichlorobenzylidene)-4-phenylthiazol-2-amine (20)
Yield: 0.13 g (26 %); \(\hbox {m.p.} = 162.7^{\circ }\hbox {C}; \hbox {R}_{f }= 0.80\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 239.4 (log \(\upvarepsilon = 2.55\)) nm; IR (KBr): \(\upnu _{\mathrm{max}}\) 1622, 1202, 779, 696, \(\hbox {cm}^{-1}, {}^{1}\)H NMR (300 MHz, \(\hbox {CD}_{3}\hbox {OD}\)): \(\delta \) 9.14 (s, 1H, =CH–S), 7.78 (s, 1H, N=CH), 7.30 (m, 3H, \(\hbox {H}\)-\(3^{{\prime }}, \hbox {H}\)-\(4^{{\prime }}, \hbox {H}\)-\(5^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.66 (m, 5H, H-2, H-3, H-4, H-5, H-6, Ar–H); EI MS: \(m/z\) (rel. abund. %), 333 (3.42), 297 (38.6), 255 (3.64).
N-(4-Isopropylbenzylidene)-4-phenylthiazol-2-amine (21)
Yield: 0.16 g (38 %); \(\hbox {m.p.} = 158.2 ^{\circ }\hbox {C}; \hbox {R}_{f }= 0.80\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 239.8 (log \(\upvarepsilon = 2.53\)) nm; IR (KBr): \(\upnu _{\mathrm{max}} 2960, 1659, 1617, 1282, \hbox {cm}^{-1},{}^{ 1}\)H NMR (500 MHz, \(\hbox {CD}_{3}\hbox {OD}\)): \(\delta \) 8.89 (s, 1H, =CH–S), 7.93 (s, 1H, N=CH), 7.33 (d, \(J_{3^{\prime },2^{\prime }}=J_{5^{\prime },6^{\prime }}=8.0 \hbox {Hz}, = 8.0 \hbox {Hz}, \hbox {2H}, \hbox {H}\)-\(3^{{\prime }}, \hbox {H}\)-\(5^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.52 (d, \(J_{2^{\prime },3^{\prime }}=J_{6^{\prime },5^{\prime }} = 7.8 \hbox {Hz}, = 7.8 \hbox {Hz}, 2\hbox {H}, \hbox {H}\)-\(2^{{\prime }}, \hbox {H}\)-\(6^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 2.97 (d, \(J_{1^{{\prime }{\prime }}, 2^{{\prime }{\prime }}} = 7.7\) Hz, 6H, CH(C\(H_{3})_{2})\), 2.89 (m, 1H, CH(C\(H_{3})_{2})\) 7.52 (m, 5H, H-2, H-3, H-4, H-5, H-6, Ar–H); EI MS: \(m/z\) (rel. abund %), 306 (72.8), 263 (34.2), 291 (5.4).
N-((5-Methylfuran-2-yl)methylene)-4-phenylthiazol-2-amine (22)
Yield: 0.02 g (90 %); \(\hbox {m.p.} = 150 ^{\circ }\hbox {C}; \hbox {R}_{f }= 0.74\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 240 (log \(\upvarepsilon = 2.60\)) nm; IR (KBr): \(\upnu _{\mathrm{max}} 1659, 1618, 1335, 1266 \hbox {cm}^{-1},{}^{1}\)H NMR (300 MHz, \(\hbox {CD}_{3}\hbox {OD}\)): \(\delta \) 8.82 (s, 1H, =CH–S), 7.69 (s, 1H, N=CH), 7.17 (d, \(J_{2^{\prime },3^{\prime }} = 7.9 \hbox {Hz}, 1\hbox {H}, \hbox {H}\)-\(2^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 6.23 (d, \(J_{3^{\prime },2^{\prime }} = 7.8 \hbox {Hz}, 1\hbox {H}, \hbox {H}\)-\(3^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime })}\), 1.28 (s, 3H,–C\(H_{3})\), 7.46 (m, 5H, H-2, H-3, H-4, H-5, H-6, Ar–H); EI MS: \(m/z\) (rel. abund. %), 268 (14.1), 253 (4.2), 190 (4.0).
N-(4-Chlorobenzylidene)-4-phenylthiazol-2-amine (23)
Yield: 0.16 g (40 %); \(\hbox {m.p.} = 216.8 ^{\circ }\hbox {C}; \hbox {R}_{f }= 0.71\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 240.4 (log \(\upvarepsilon = 2.67\)) nm; IR (KBr): \(\upnu _{\mathrm{max}} 1620, 1663, 1347, 773\,\hbox {cm}^{-1},{}^{1}\)H NMR (300 MHz, \(\hbox {CD}_{3}\hbox {OD}\)): \(\delta \) 8.91 (s, 1H, =CH–S), 7.98 (s, 1H, N=CH), 7.52 (d, \(J_{3^{\prime },2^{\prime }}=J_{5^{\prime },6^{\prime }}=8.0\,\hbox {Hz}, 2\hbox {H}, \hbox {H}\)-\(3^{{\prime }}, \hbox {H}\)-\(5^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.18 (d, \(J_{2^{\prime },3^{\prime }}=J_{6^{\prime },5^{\prime }} = 7.8\, \hbox {Hz}, 2\hbox {H}, \hbox {H}\)-\(2^{{\prime }}, \hbox {H}\)-\(6^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.52 (m, 5H, H-2, H-3, H-4, H-5, H-6, Ar–H); EI MS: \(m/z\) (rel. abund. %), 298 (57.7), 263 (19.0), 220 (3.4).
N-((1H-Indol-3-yl)methylene)-4-phenylthiazol-2-amine (24)
Yield: 0.55g (62 %); \(\hbox {m.p.} = 139.4 ^{\circ }\hbox {C}; \hbox {R}_{f }= 0.78\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 293.8 (log \(\upvarepsilon = 3.07\)) nm; IR(KBr): \(\upnu _{\mathrm{max}} 3171, 1635, 1577, 1335 \hbox {cm}^{-1},{}^{ 1}\)H NMR (300 MHz, \(\hbox {CD}_{3}\hbox {OD}\)): \(\delta \) 9.08 (s, 1H, =CH–S), 8.08 (s, 1H, N=CH), 8.13 (m, 4H, H-4, H-5, H-6, H-7), 7.46 (m, 5H, H-2, H-3, H-4, H-5, H-6, Ar–H); EI MS:\(m/z\) (rel. abund. %), 303 (94.4), 225 (4.5), 262 (20.2).
N-(Naphthalen-2-ylmethylene)-4-phenylthiazol-2-amine (25)
Yield: 0.24 g (26 %); \(\hbox {m.p.} = 141.7 ^{\circ }\hbox {C}; \hbox {R}_{f }= 0.76\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 282.6 (log \(\upvarepsilon = 2.85\)) nm; IR (KBr): \(\upnu _{\mathrm{max}}, 3051, 1681, 1621, 1338\,\hbox {cm}^{-1},{}^{ 1}\)H NMR (300 MHz, \(\hbox {CD}_{3}\hbox {OD}\)): \(\delta \) 9.16 (s, 1H, =CH–S), 8.92 (s, 1H, N=CH), 7.37 (m, 4H, H-2, H-3, H-5, H-6, Ar-H), 7.60 (m, 1H, H-4, Ar-H), 7.75 (m, 1H, \(\hbox {H}\)-\(6^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }}),7.73 (d, J_{2^{\prime },3^{\prime }} = 8.0\, \hbox {Hz}, 1\hbox {H}, \hbox {H}\)-\(2^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.87 (d, \(J_{4{\prime },3^{\prime }} = 8.0\,\hbox {Hz}, 1\hbox {H}, 4^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.57 (t, 1H, \(\hbox {H}\)-\(3^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 8.12 (m, 1H, \(\hbox {H}\)-\(8^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.95 (d, \(J_{5^{\prime }, 6^{\prime }} = 7.9 \hbox {Hz}, 1\hbox {H}, \hbox {H}\)-\(5^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }}), 7.93 (\hbox {t}, 1\hbox {H}, \hbox {H}\)-\(7^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\); EI MS: \(m/z\) (rel. abund. %), 314 (57.1), 236 (10.4), 186 (5.0).
4-Phenyl-N-(2,3,4-trimethoxybenzylidene)thiazol-2-amine (26)
Yield: 0.06 g (12 %); \(\hbox {m.p.} = 119 ^{\circ }\hbox {C}; \hbox {R}_{f }= 0.81\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 237.2 (log \(\upvarepsilon = 2.53\)) nm; IR (KBr): \(\upnu _{\mathrm{max}} 1664, 1351, 1283, 1202,, \hbox {cm}^{-1}, {}^{1}\)H NMR (300 MHz, \(\hbox {CD}_{3}\hbox {OD}\)): \(\delta \) 9.11 (s, 1H, =CH–S), 8.56 (s, 1H, N=CH), 7.13 (d, \(J_{6^{\prime }, 5^{\prime }} = 8.0 \hbox {Hz}, 1\hbox {H}, \hbox {H}\)-\(6^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 6.78 (d, \(J_{5^{\prime }, 6^{\prime }} = 8.0 \hbox {Hz}, 1\hbox {H}, \hbox {H}\)-\(5^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }}), 3.83 (s, 6\hbox {H}, \hbox {O--C}\underline{\hbox {H}}_{3})\), 7.46 (m, 5H, H-2, H-3, H-4, H-5, H-6, Ar–H); EI MS: \(m/z\) (rel. abund. %), 354 (43.6), 323 (2.5), 279 (13.1).
2-Methoxy-6-(((4-phenylthiazol-2-yl)imino)methyl)phenol (27)
Yield: 0.07 g (17 %); \(\hbox {m.p.} = 128.1^{\circ }\hbox {C}; \hbox {R}_{f }= 0.77\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 240.8 (log \(\upvarepsilon = 2.88\)) nm; IR (KBr): \(\upnu _{\mathrm{max}} 3276, 1694, 1272, 1220\,\hbox {cm}^{-1}, {}^{1}\)H NMR (300 MHz, \(\hbox {CD}_{3}\hbox {OD}\)): \(\delta \) 9.26 (s, 1H, =CH–S), 8.56 (s, 1H, N=CH), 7.38 (m, 3H, \(\hbox {H}\)-\(4^{{\prime }}, \hbox {H}\)-\(5^{{\prime }}, \hbox {H}\)-\(6^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime })}\), 3.72 (s, 3H, \(\hbox {O--C}\underline{\hbox {H}}_{3})\), 7.53 (m, 5H, H-2, H-3, H-4, H-5, H-6, Ar–H); EI MS: \(m/z\) (rel. abund. %), 310 (71.7), 279 (10.4), 293 (8.8), 232 (4.5).
N-(3-Nitrobenzylidene)-4-phenylthiazol-2-amine (28)
Yield: 0.04 g (10 %); \(\hbox {m.p.} = 126.6^{\circ }\hbox {C}; \hbox {R}_{f }= 0.87\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 243.6 (log \(\upvarepsilon = 2.69\)) nm; IR (KBr): \(\upnu _{\mathrm{max}} 1698, 1611, 1350, 1203, \hbox {cm}^{-1}, {}^{1}\)H NMR (300 MHz, DMSO-\(d_{6})\): \(\delta \) 9.03 (s, 1H, =CH–S), 8.81 (s, 1H, N=CH), 8.68 (s, 1H, \(\hbox {H}\)-\(2^{{\prime }}\), Ar–\(\underline{\hbox {H}}^{{\prime }})\), 8.34 (d, \(J_{6^{\prime },5^{\prime }}= 7.8 \hbox {Hz}, 1\hbox {H}, \hbox {H}\)-\(6^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.98 (d, \(J_{4{\prime },5^{\prime }}= 7.8 \hbox {Hz}, 1\hbox {H}, \hbox {H}\)-\(4^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }}), 7.77 (\hbox {m}, 2\hbox {H}, \hbox {H}\)-\(5^{{\prime }}, \hbox {H}\)-\(6^{{\prime }},\hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.67 (m, 5H, H-2, H-3, H-4, H-5, H-6, Ar–H); EI MS: \(m/z\) (rel. abund. %), 309 (41.3), 262 (9.7), 176 (16.2).
4-Phenyl-N-(pyren-2-ylmethylene)thiazol-2-amine (29)
Yield: 0.08 g (13 %); \(\hbox {m.p.} = 148 ^{\circ }\hbox {C}; \hbox {R}_{f }= 0.88\); (Hex:EtOAc, 7:3); UV (MeOH): \(\lambda _{\mathrm{max}}\) 241.6 (log \(\upvarepsilon = 2.52\)) nm; IR (KBr): \(\upnu _{\mathrm{max}}, 2695, 1643, 1614, 1086 \hbox {cm}^{-1},{}^{ 1}\)H NMR (300 MHz, DMSO-\(d_{6})\): \(\delta \) 9.13 (s, 1H, =CH–S), 8.36 (s, 1H, N=CH), 7.51 (m, 9H, \(\hbox {H}\)-\(2^{{\prime }}, \hbox {H}\)-\(3^{{\prime }}, \hbox {H}\)-\(4^{{\prime }}, \hbox {H}\)-\(5^{{\prime }}, \hbox {H}\)-\(6^{{\prime }}, \hbox {H}\)-\(7^{{\prime }}, \hbox {H}\)-\(8^{{\prime }}, \hbox {H}\)-\(9^{{\prime }}, \hbox {H}\)-\(10^{{\prime }}, \hbox {Ar}\)–\(\underline{\hbox {H}}^{{\prime }})\), 7.40 (m, 5H, H-2, H-3, H-4, H-5, H-6, Ar–H); EI MS: \(m/z\) (rel. abund. %), 390 (29.4), 313 (4.8), 388 (37.2).
References
Moscona A (2005) Neuraminidase inhibitors for influenza. New Engl J Med 353:1363–1373. doi:10.1056/NEJMra050740
Hodge CN, Aldrich PE, Bacheler LT, Chang CH, Eyermann CJ, Garber S, Grubb M, Jackson DA, Jadhav PK, Korant B, Lam PYS, Maurin MB, Meek JL, Otto MJ, Rayner MM, Reid C, Sharpe TR, Shum L, Winslow DL, Viitanen SE (1996) Improved cyclic urea inhibitors of the HIV-1 protease: synthesis, potency, resistance profile, human pharmacokinetics and X-ray crystal structure of DMP 450. Chem Biol 3:301–314. doi:10.1016/S1074-5521(96)90110-6
Richards WG (1994) Computer-aided drug design. Pure Appl Chem 66:1589–1596. doi:10.1351/pac199466081589
Davie P (2010) Cloud computing: a drug discovery game changer? Innov Pharm Technol 33:34–36
Maruti SS, Li L, Chang JL, Prunty J, Schwarz Y, Li SS, King IB, Potter JD, Lampe JW (2010) Dietary and demographic correlates of serum \(\beta \)-glucuronidase activity. Nutr Cancer 62:208–219. doi: 10.1080/01635580903305375
Hanausek M, Walaszek Z, Slaga TJ (2003) Detoxifying cancer causing agents to prevent cancer. Integr Cancer Ther 2:139–44. doi:10.1177/1534735403002002005
Walaszek Z (1990) Potential use of d-glucaric acid derivatives in cancer prevention. Cancer Lett 54:1–8
Krahulec J, Szemes T, Krahulcová J (2010) Bioinformatics characterization of potential new \(\beta \)-glucuronidase from Streptococcus equi subsp. Zooepidemicus. Mol Biotechnol 44:232–241. doi: 10.1007/s12033-009-9234-0
Khan KM, Khan M, Ambreen N, Rahim F, Naureen S, Perveen S, Choudhary MI, Voelter W (2012) Synthesis and \(\beta \)-glucuronidase inhibitory potential of benzimidazole derivatives. Med Chem 8:421–427. doi:10.2174/157340641120803042111
Khan KM, Rahim F, Halim SA, Taha M, Khan M, Perveen S, Haq Z, Mesaik MA, Choudhary MI (2011) Synthesis of novel inhibitors of \(\beta \)-glucuronidase based on benzothiazole skeleton and study of their binding affinity by molecular docking. Bioorg Med Chem 19:4286–4294. doi: 10.1016/j.bbr.2011.03.031
Tsuji K, Ogino T, Seki N, Sawada M, Sudo Y, Nishigaki F, Manda T, Matsuo M (1998) Synthesis and effects of novel thiazole derivatives against thrombocytopenia. Bioorg Med Chem Lett 8:2473–2478. doi:10.1016/S0960-894X(98)00404-1
Quiroga J, Hernández P, Insuasty B, Abonía R, Cobo J, Sánchez A, Nogueras M, Low JN (2002) Control of the reaction between 2-aminobenzothiazoles and Mannich bases. Synthesis of pyrido[2,1-\(b\)][1,3]benzothiazoles versus [1,3]benzothiazolo[2,3-\(b\)]quinazolines. J Chem Soc Perkin Trans I:555–559. doi: 10.1039/B109676A
Hutchinson I, Jennings SA, Vishnuvajjala BR, Westwell AD, Stevens MF (2002) Antitumor benzothiazoles. 16. Synthesis and pharmaceutical properties of antitumor 2-(4-aminophenyl)benzothiazole amino acid prodrugs. J Med Chem 45:744–747. doi:10.1021/jm011025r
Hargrave KD, Hess FK, Oliver JT (1983) N-(4-Substituted-thiazolyl)oxamic acid derivatives, new series of potent, orally active antiallergy agents. J Med Chem 26:1158–1163. doi:10.1021/jm00362a014
Pat WC, Hamilton HW, Taylor MD, Ryan MJ, Taylor DG Jr, Connolly CJC, Doherty AM, Klutchko SR, Sircar I (1992) Structure–activity relationships of a series of 2-amino-4-thiazole-containing renin inhibitors. J Med Chem 35:2562–2572. doi:10.1021/jm00092a006
Jaen JC, Wise LD, Caprathe BW, Tecle H, Bergmeier S, Humblet CC, Heffner TG, Meltzer LT, Pugsley TA (1990) 4-(1,2,5,6-Tetrahydro-1-alkyl-3-pyridinyl)-2-thiazolamines: a novel class of compounds with central dopamine agonist properties. J Med Chem 33:311–317. doi:10.1021/jm00163a051
Haviv F, Ratajczyk JD, DeNet RW, Kerdesky FA, Walters RL, Schmidt SP, Holms JH, Young PR, Carter GW (1998) 3-[1-(2-Benzoxazolyl)hydrazino]propanenitrile derivatives: inhibitors of immune complex induced inflammation. J Med Chem 31:1719–1728. doi:10.1021/jm00117a010
Bell FW, Cantrell AS, Hoegberg M, Jaskunas SR, Johansson NG, Jordon CL, Kinnick MD, Lind P, Morin JM Jr (1995) Phenethylthiazolethiourea (PETT) compounds, a new class of HIV-1 reverse transcriptase inhibitors. 1. Synthesis and basic structure-activity relationship studies of PETT analogs. J Med Chem 38:4929–4936. doi:10.1021/jm00025a010
Suri KA, Suri OP, Atal CK (1986) Genus crotalaria-XLVI-synthesis of 4-aryl-2-(pyrrolizidinylidenenydrazino) thiazoles as potential cardiotonic agents. Ind Drug 23:207–20
Louis L (1998) Eur Pat, 263, 020. Chem Abstr 109:128995q
Geronikaki A, Theophilidis G (1992) Synthesis of 2-(amino\(\alpha \) cetylamino)thiazole derivatives and comparison of their local anaesthetic activity by method of action potential. Eur J Med Chem 27:709–716. doi: 10.1016/0223-5234(92)90091-E
Kumar A, Rajput CS, Bhati SK (2007) Synthesis of 3-[\(4^\prime \)-(\(p\)-chlorophenyl)-thiazol-\(2^\prime \)-yl]-2-[(substituted azetidinone/thi azolidinone)-aminomethyl]-6-bromoquin- azolin-4-ones as anti-inflammatory agents. Bioorg Med Chem 15:3089–3096. doi:10.1016/j.bmc.2007.01.042
Sharma PK, Sawhney SN (1997) Potent antiinflammatory 3-thiazole-4(5)-acetic acids of 1,2-benzisothiazole. Bioorg Med Chem Lett 7:2427–2230. doi:10.1016/S0960-894X(97)00449-6
Russo F, Guccione S, Romeo G, Barretta GU, Pucci S, Caruso A, Roxas MA, Cutuli V (1993) Pyrazolothiazolopyrimidine derivatives as a novel class of anti-inflammatory or antinociceptive agents: synthesis, structural characterization and pharmacological evaluation. Eur J Med Chem 28:363–376. doi:10.1016/0223-5234(93)90123-V
Clark RF, Zhang T, Wang X, Wang R, Zhang X, Camp HS, Beutel BA, Sham HL, Gu GY (2007) Phenoxy thiazole derivatives as potent and selective acetyl-CoA carboxylase 2 inhibitors: modulation of isozyme selectivity by incorporation of phenyl ring substituents. Bioorg Med Chem Lett 17:1961–1965. doi:10.1016/j.bmcl.2007.01.022
Dodson RM, King LC (1946) The reaction of acetophenone with thiourea and oxidizing agents. J Am Chem Soc 68:871–871. doi:10.1021/ja01209a049
Sperker B, Backman JT, Kromer HK (1997) The role of \(\beta \)-glucuronidase in drug disposition and drug targeting in humans. Clin Pharmacokinet 33:18–31
Collins RA, Ng TB, Fong WP, Wan CC, Yeung HW (1997) Inhibition of glycohydrolase enzymes by aqueous extracts of Chinese medicinal herbs in a microplate format. Biochem Mol Biol Int 42:1163–1169
Cole JC, Nissink JWM, Taylor R (2005) Protein–ligand docking and virtual screening with GOLD. In: Shoichet B, Alvarez J (eds) Virtual screening in drug discovery. Taylor & Francis CRC Press, Boca Raton
Acknowledgments
The authors are thankful to Higher Education Commission (HEC) Pakistan for financial support under “National Research Support Program for Universities” (Project No. 1910).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Khan, K.M., Karim, A., Saied, S. et al. Evaluation of the thiazole Schiff bases as \(\upbeta \)-glucuronidase inhibitors and their in silico studies. Mol Divers 18, 295–306 (2014). https://doi.org/10.1007/s11030-013-9500-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-013-9500-8