Abstract
A new series of 9(10H)-acridinone-1,2,3-triazole derivatives were designed, synthesized and evaluated for their cytotoxic activity against human breast cancer cell lines. The acridone skeleton was prepared through the Ullman condensation of 2-bromobenzoic acid and anilines. Subsequently, it was functionalized with propargyl bromide. Then, a click reaction of the latter compound and in situ prepared 1-(azidomethyl)-4-methoxybenzene derivatives led to the formation of the desired triazole products. Finally, all products were investigated for their capability to cause cytotoxicity against MCF-7, T-47D, and MDA-MB-231 cell lines. Among them, 2-methoxy-10-((1-(4-methoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)acridin-9(10H)-one 8c exhibited the most potency \((\hbox {IC}_{50}\,{=}\,11.0\,{\pm }\, 4.8\, \upmu \hbox {M})\) against MCF-7 cells, being more potent than etoposide \((\hbox {IC}_{50}\,{=}\, 12.4\,{\pm }\, 4.7 \upmu \hbox {M})\). Also, apoptosis induced by compound 8c was confirmed via acridine orange/ethidium bromide and Annexin V-FITC/propidium iodide (PI) double staining.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cancer is characterized by uncontrolled cell growth leading to 15 % of human deaths worldwide [1]. Apoptosis is a programmed cell death process by which the body eliminates damaged or unnecessary cells. This is a biologically important phenomenon playing a vital role in cancer development and tumor cell survival [2]. It has been proven that dysregulation of apoptosis leads to cancer and tumor progression [3, 4]. Killing tumor cells using chemotherapeutic methods is usually accomplished through the induction of apoptosis which does not destroy the organism. Hence, apoptosis inducers have been widely studied as a versatile platform for cancer therapy in medicinal chemistry [5–9].
Acridone is an important heterocyclic scaffold and both synthetic and naturally occurring derivatives have shown various valuable biological properties [10]. Acridones can be considered as 10-aza-analogs of anthrones [11] or xanthones [12] from a structural point of view. A wide range of acridone derivatives has been evaluated in vitro and in vivo to explore novel anticancer agents [13–16]. Recently, acridone-based anticancer agents that target DNA [17], topoisomerases [18], telomerase [19], multidrug resistance [13], and apoptotic inducer [20] have been reported in the literature. Furthermore, the 1,2,3-triazole ring has received much attention in both chemistry and biology due to its high dipole moment [21], metabolic stability, and capability to form hydrogen bonds [22]. Also, it can be considered an amide bioisosteric replacement [23]. The introduction of click chemistry by Sharpless and co-workers provided an efficient method for the construction of the 1,2,3-triazole ring and drug discovery investigations [24, 25]. Their antibacterial [26], antifungal [27], antitubercular [28], and anti-HIV [29] activities have been well documented. Also, extensive attention has been devoted to their anticancer activities [30, 31].
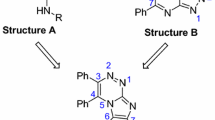
Considering the many adverse effects and development of tumor resistance to a wide variety of anticancer agents [32], there is a big demand for novel and efficient anticancer agents. Herein, in continuation of our studies on the development of new anticancer agents [33–36] and focusing on the versatile biological activity of acridone-1,2,3-triazole system [36], we designed, synthesized, and evaluated novel 9(10H)-acridinone-1,2,3-triazole hybrids (A, Fig. 1) against human breast cancer cell lines.
Results and discussion
Chemistry
Exploring novel 9(10H)-acridinone-1,2,3-triazole hybrids we focused on 10-benzyl-9(10H)-acridinone derivatives reported by Gao et al. (B, Fig. 1) [20]. Their study revealed that a benzyl group bearing methoxy substituents showed better antileukemic activitiy and, among them, 10-(3,5-dimethoxy)benzyl-9(10H)-acridinone was the most potent derivative with an \(\hbox {IC}_{50}\) of about \(0.7 \,\upmu \hbox {M}\). Considering the anticancer activity of the 1,2,3-triazole skeleton [30, 31], we decided to combine the 9(10H)-acridinone and 1,2,3-triazole scaffolds and investigate the cytotoxicity profile of the corresponding products 8a–n (Scheme 1).
The synthesis of 9(10H)-acridinone-1,2,3-triazole hybrids 8a–n is described in Scheme 1. At first, the Ullman condensation reaction of 2-bromobenzoic acid 1 with various aniline derivatives 2 in the presence of potassium carbonate \((\hbox {K}_{2}\hbox {CO}_{3})\) and copper in EtOH under reflux conditions gave 2-arylamino benzoic acids 3 [37]. Then, the cyclization of compound 3 in the presence of polyphosphoric acid (PPA) at \(100\,^{\circ }\hbox {C}\) afforded acridones 4 [38] which were reacted with propargyl bromide using potassium tert-butoxide in DMSO at room temperature to obtain 10-(prop-2-yn-1-yl)acridin-9-one derivatives 5. Using the click methodology described by Sharpless et al. [24], the reaction of compound 5 and in situ prepared 1-(azidomethyl)-4-methoxybenzene derivative 7 led to the formation of 9(10H)-acridinone-1,2,3-triazole derivatives 8a–n. To obtain compound 7, 4-methoxy-benzyl chloride derivative 6 and sodium azide reacted in the presence of \(\hbox {Et}_{3}\hbox {N}\) in \(\hbox {H}_{2}\hbox {O}/t\)-BuOH (1:1) at ambient temperature for 1 h. Then, the mixture of compound 5 and CuI was added to the mixture containing 7 and the reaction was conducted at room temperature for 24–56 h to give the 9(10H)-acridinone-1,2,3-triazole 8a–n in good yields.
Biological study
Cytotoxicity assay
The cytotoxicity of products 8a–n was evaluated in vitro against three human breast cancer cell lines comprising MCF-7, T-47D, and MDA-MB-231 using the MTT tetrazolium salt assay [39]. The calculated \(\hbox {IC}_{50}\) values of compounds 8a–n as well as etoposide (standard drug) are listed in Table 1. Compounds 8c, being the most potent compound against MCF-7 \((\hbox {IC}_{50 }= 11.0\, \upmu \hbox {M})\), showed slightly better activity than etoposide \((\hbox {IC}_{50}= 12.4\pm 4.8\,\upmu \hbox {M})\). However, \(\hbox {IC}_{50}\) values against MDA-MB-231 and T-47D were \(16.6 \pm 5.9\) and \(14.5\pm 5.22 \,\upmu \hbox {M}\), respectively.
Compounds 8a–n could be categorized into two groups considering the number of methoxy substituents on the pendant benzyl group of 1,2,3-triazol ring: (i) mono-methoxy (compounds 8a–g) and (ii) tri-methoxy (compounds 8h–n). According to our results in Table 1, the first group (8a–g) exhibited \(\hbox {IC}_{50} = 11.0-60.0\,\upmu \hbox {M}\) while the second group (8h–n) did not show activity against breast cancer cell lines \((\hbox {IC}_{50} > 100\,\upmu \hbox {M})\). It may relate to the compounds which were not able to enter the cell.
As can be seen in Table 1, the kind of substituents as well as their positions on the acridone moiety of compounds 8a–g play important role in the obtained cytotoxicity. Compound 8a without substituents on the acridone ring showed no activity against MCF-7 \((\hbox {IC}_{50} > 100 \,\upmu \hbox {M})\); however, it showed moderate activity against T-47D \((\hbox {IC}_{50} = 48.4\,\pm \,4.3 \,\upmu \hbox {M})\) and \(\hbox {MB-}231(\hbox {IC}_{50} = 18.8\,\pm \,5.7 \,\upmu \hbox {M})\), respectively. Our results revealed that the presence of a halogen group (F, Cl, and Br) at any position on the acridone moiety led to loss of cytotoxic activity as 8b, 8d, and 8f were not active against all cell lines. From the mono-methoxy group (compounds 8a–g), compound 8c (methoxy group at 2-postion) showed the best activity in all three cell lines especially MCF-7 \((\hbox {IC}_{50 }= 11.0\,\pm \, 4.8 \,\upmu \hbox {M})\). However, introducing the methoxy group at the 4-position of acridone and also presence of an ethyl substituent at the 2-position of the acridone decreased anticancer activity (Table 1, 8g and 8e, respectively).
Compounds 8c and 8e having the methoxy and ethyl group at the 2-position of acridone ring, respectively, exhibited selective activity on MCF-7 cell. Compounds 8a and 8g having no substitution at the 2-position of acridone moiety showed selective inhibition of MDA-MB-231 cell growth.
Acridine orange/ethidium bromide double staining
The most active compound 8c was selected to determine its potential to induce apoptosis in MCF-7 cells morphologically by acridine orange/ethidiumbromide (AO/EB) double staining test [40] (Fig. 2). Living cells have a normal green nucleus, but apoptotic cells show orange-stained nuclei with chromatin condensation or fragmentation. Analysis of the AO/EB staining revealed that compound 8c clearly can be inducer of apoptosis because the appearance of chromatin condensation and nuclear fragmentation are evident in Fig. 2.
Annexin V-FITC and propidium iodide (PI) double staining
Apoptosis induction for compound 8c was further confirmed by flow cytometry analysis [41] (Fig. 3). As shown in Fig. 3, compound 8c induced 12.44 % apoptosis in the cancer cells. Therefore, it is evident that the cytotoxicity of this compound is related to inducing apoptosis in cancer cell lines.
Flow cytometric analysis of MCF-7 cells treated with synthetic compound 8c. Cells were stained with Annexin V-FITC/PI and quantitated by flow cytometry. The cells treated with a DMSO 1 % (negative control), b \(\hbox {IC}_{50}\) values of compound 8c for 12 h, c \(\hbox {IC}_{50}\) values of etoposide as positive control for 12 h
Conclusion
In conclusion, novel 9(10H)-acridinone-1,2,3-triazole hybrids were designed, synthesized, and evaluated as cytotoxic and apoptosis-inducing agents. The preliminary in vitro anti-proliferative activity test was done against MCF-7, T-47D, and MDA-MB-231 cells using MTT assay. Among them, 2-methoxy-10-((1-(4-methoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)acridin-9(10H)-one 8c with \(\hbox {IC}_{50}\) value of \(11.0\,\pm \,4.8 \,\upmu \hbox {M}\) was more potent than etoposide \((\hbox {IC}_{50} = 12.4\,\pm \,4.7\,\upmu \hbox {M})\) against MCF-7 cells. Also, AO/EB staining and flow cytometry analysis using Annexin V- FITC/PI double staining showed that compound 8c can induce apoptosis.
Experimental
Chemistry
Melting points are uncorrected and were measured using a Kofler hot stage apparatus. \(^{1}\hbox {H}\) and \(^{13}\hbox {C}\) NMR spectra were recorded on a Bruker FT-500, using TMS as an internal standard and DMSO as a solvent. Chemical shifts are expressed as \(\delta \) (ppm). IR spectra were recorded on a Nicolet Magna FTIR 550 spectrophotometer using KBr disks. Mass spectra were recorded on an Agilent Technology (HP) mass spectrometer operating at an ionization potential of 70 eV. Elemental analysis for C, H, and N was performed with an Elementar Analysensystem GmbH VarioEL CHNS mode.
2-Arylamino benzoic acids 3, acridone derivatives 4, and 10-(prop-2-yn-1-yl)acridin-9-ones 5 were prepared according to our recent report [36].
General procedure for the synthesis of 9(10H)-acridinone-1,2,3-triazole derivatives 8
A mixture 4-methoxy benzyl chloride derivative 6 (1.1 mmol), sodium azide (0.9 mmol), and \(\hbox {Et}_{3}\hbox {N}\) (1.3 mmol) in water (4 mL) and t-BuOH (4 mL) was stirred at room temperature for 1 h. Then, a mixture of 10-(prop-2-yn-1-yl)acridin-9-one derivative 5 (1 mmol) and CuI (7 mol%) was added to prepared azide derivative 7 and the reaction mixture was stirred at room temperature for 24–56 h. After completion of the reaction (monitored by TLC), the reaction mixture was diluted with water, poured onto ice, and the precipitate was filtered off, washed with cold water, and purified by flash chromatography on silica gel using petroleum ether/ ethyl acetate (4:1) to afford desire product.
10-((1-(4-Methoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl) acridin-9(10H)-one (8a)
Yellow crystals; yield: 84 %, mp 164–166\(\,^{\circ }\hbox {C}\). IR (KBr): \(3080, 2956, 2853, 1743, 1632, 1598 \hbox {cm}^{-1}\). \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6})\): 8.37 (d, \(J= 8.0\) Hz, 2H, \(\hbox {H}_{1}\), \(\hbox {H}_{8})\), 8.17 (s, 1H, triazole), 7.78 (t, \(J = 8.0\) Hz, 2H, \(\hbox {H}_{3}\), \(\hbox {H}_{6})\), 7.69 (d, \(J= 8.0\) Hz, 2H, \(\hbox {H}_{4}\), \(\hbox {H}_{5})\), 7.35 (t, \(J= 8.0\) Hz, 2H, \(\hbox {H}_{2}\), \(\hbox {H}_{7})\), 7.14 (d, \(J= 8.4\) Hz, 2H, \(\hbox {H}_{2^{\prime }}\), \(\hbox {H}_{6^{\prime }})\), 6.91 (d, \(J = 8.4\) Hz, 2H, \(\hbox {H}_{3^{\prime }}\), \(\hbox {H}_{5^{\prime }})\), 5.75 (s, 2H, \(\hbox {CH}_{2})\), 5.55 (s, 2H, \(\hbox {CH}_{2})\), 3.73 (s, 3H, \(\hbox {OCH}_{3})\). Anal. Calcd for \(\hbox {C}_{24}\hbox {H}_{20} \hbox {N}_{4}\hbox {O}_{2}\): C, 72.71; H, 5.09; N, 14.13. Found: C, 72.93; H, 4.96; N, 13.96.
2-Chloro-10-((1-(4-methoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)acridin-9(10H)-one (8b)
Yellow crystals; yield: 79 %, mp 234–235\(\,^{\circ }\hbox {C}\). IR (KBr): 3080, 2959, 2843, 1742, 1629, 1595, 1489 \(\hbox {cm}^{-1}\). \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6})\): 8.34 (d, \(J = 7.5\) Hz, 1H, \(\hbox {H}_{8})\), 8.25 (s, 1H, \(\hbox {H}_{1})\), 8.17 (s, 1H, triazole), 8.00 (d, \(J = 8.7\) Hz, 1H, \(\hbox {H}_{3})\), 7.94 (d, \(J = 8.7\) Hz, 1H, \(\hbox {H}_{4})\), 7.83–7.82 (m, 2H, \(\hbox {H}_{5}\), \(\hbox {H}_{6})\), 7.36 (t, \(J = 7.5\) Hz, 1H, \(\hbox {H}_{7})\), 7.22 (d, \(J = 7.8\) Hz, 2H, \(\hbox {H}_{2^{\prime }}\), \(\hbox {H}_{6^{\prime }})\), 6.89 (d, \(J = 7.8\) Hz, 2H, \(\hbox {H}_{3^{\prime }}\), \(\hbox {H}_{5^{\prime }})\), 5.79 (s, 2H, \(\hbox {CH}_{2})\), 5.45 (s, 2H, \(\hbox {CH}_{2})\), 3.72 (s, 3H, \(\hbox {OCH}_{3})\). \(^{13}\)C NMR (125 MHz, DMSO-\(d_{6})\): 175.6, 159.1, 142.4, 141.6, 140.4, 134.5, 133.8, 129.5, 127.7, 126.6, 126.1, 125.2, 123.1, 122.5, 121.9, 121.5, 119.1, 116.4, 114.1, 55.1, 52.4, 41.8. MS m/z (%) 432 \(([\hbox {M}^{+.} + 2], 2)\), 430 \((\hbox {M}^{+.} 6)\), 228 (9), 200 (10), 174 (12), 121 (100), 78 (11). Anal. Calcd for \(\hbox {C}_{24}\hbox {H}_{19}\hbox {Cl} \hbox {N}_{4}\hbox {O}_{2}\): C, 66.90; H, 4.44; N, 13.00. Found: C, 67.11; H, 4.23; N, 12.91.
2-Methoxy-10-((1-(4-methoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)acridin-9(10H)-one (8c)
Yellow crystals; yield: 88 %, mp 177–179\(\,^{\circ }\hbox {C}\). IR (KBr): 2934, 2834, 1742, 1617, 1595, 1498 \(\hbox {cm}^{-1}\). \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6})\): 8.36 (dd, \(J = 8.0\), 1.1 Hz, 1H, \(\hbox {H}_{8})\), 8.14 (s, 1H, triazole), 7.93–7.91 (m, 2H, \(\hbox {H}_{4}\), \(\hbox {H}_{5})\), 7.78–7.75 (m, 2H, \(\hbox {H}_{1}\), \(\hbox {H}_{6})\), 7.45 (dd, J = 9.4, 3.3 Hz, 1H, \(\hbox {H}_{3})\), 7.31 (t, \(J = 8.0\) Hz, 1H, \(\hbox {H}_{7})\), 7.24 (d, \(J = 8.6\) Hz, 2H, \(\hbox {H}_{2^{\prime }},\hbox {H}_{6^{\prime }})\), 6.88(d, \(J = 8.6\) Hz, 2H, \(\hbox {H}_{3^{\prime }}\), \(\hbox {H}_{5^{\prime }})\), 5.77 (s, 2H, \(\hbox {CH}_{2})\), 5.44 (s, 2H, \(\hbox {CH}_{2})\), 3.86 (s, 3H, \(\hbox {OCH}_{3})\), 3.71 (s, 3H, \(\hbox {OCH}_{3})\).\(^{13}\)C NMR (125 MHz, DMSO-\(d_{6})\): 176.0, 159.1, 154.1, 142.8, 141.6, 136.5, 133.8, 129.5, 127.8, 126.6, 123.9, 123.0, 122.4, 121.1, 120.9, 118.2, 116.0, 114.0, 106.1, 55.4, 55.0, 52.3, 41.6. Anal. Calcd for \(\hbox {C}_{25}\hbox {H}_{22}\hbox {N}_{4}\hbox {O}_{3}\): C, 70.41; H, 5.21; N, 13.14. Found: C, 70.59; H, 5.08; N, 13.02.
2-Bromo-10-((1-(4-methoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)acridin-9(10H)-one (8d)
Yellow crystals; yield: 76 %, mp 237–239\(\,^{\circ }\hbox {C}\). IR (KBr): 2922, 2849, 1735, 1632, 1598 \(\hbox {cm}^{-1}\). \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6})\): 8.41 (d, \(J = 2.0\) Hz, 1H, \(\hbox {H}_{1})\), 8.35 (d, \(J = 7.5\) Hz, 1H, \(\hbox {H}_{8})\), 8.26 (s, 1H, triazole), 8.92 (dd, \(J = 9.0\), 2.0 Hz, 1H, \(\hbox {H}_{3})\), 7.81 (t, \(J = 7.5\) Hz, 1H, \(\hbox {H}_{6})\), 7.72 (d, \(J = 9.0\) Hz, 1H, \(\hbox {H}_{4})\), 7.68 (d, \(J = 7.5\) Hz, 1H, \(\hbox {H}_{5})\), 7.37 (t, \(J = 7.5\) Hz, 1H, \(\hbox {H}_{7})\), 7.22 (d, \(J = 8.4\) Hz, 2H, \(\hbox {H}_{2^{\prime }}\), \(\hbox {H}_{6^{\prime }})\), 6.89 (d, \(J = 8.4\) Hz, 2H, \(\hbox {H}_{3^{\prime }}\), \(\hbox {H}_{5^{\prime }})\), 5.77 (s, 2H, \(\hbox {CH}_{2})\), 5.54 (s, 2H, \(\hbox {CH}_{2})\), 3.73 (s, 3H, \(\hbox {OCH}_{3})\). \(^{13}\hbox {C}\) NMR (125 MHz, DMSO-\(d_{6})\): 175.6, 159.0, 142.1, 141.3, 140.3, 134.4, 133.8, 128.9, 128.4, 127.0, 126.6, 125.5, 123.4, 123.0, 122.0, 121.6, 119.4, 116.5, 114.1, 55.1, 52.7, 42.8. Anal. Calcd for \(\hbox {C}_{24}\hbox {H}_{19}\hbox {BrN}_{4}\hbox {O}_{2}\): C, 60.64; H, 4.03; N, 11.79. Found: C, 60.81; H, 4.14; N, 11.63.
2-Ethyl-10-((1-(4-methoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)acridin-9(10H)-one (8e)
Pale yellow crystals; yield: 79 %, mp 193–195\(\,^{\circ }\hbox {C}\). IR (KBr): 3071, 2928, 1735, 1635, 1598 \(\hbox {cm}^{-1}\). \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6})\): 8.35 (d, \(J = 7.5\) Hz, 1H, \(\hbox {H}_{8})\), 8.17 (m, 2H, \(\hbox {H}_{1}\), triazole), 7.91 (d, \(J = 7.5\) Hz, 1H, \(\hbox {H}_{5})\), 7.86 (d, \(J = 8.8\) Hz, 1H, \(\hbox {H}_{4})\), 7.78 (t, \(J = 7.5\) Hz, 1H, \(\hbox {H}_{6})\), 7.67 (dd, \(J = 8.8\), 1.8 Hz, 1H, \(\hbox {H}_{3})\), 7.32 (t, \(J = 7.5\) Hz, 1H, \(\hbox {H}_{7})\), 7.25 (d, \(J = 7.7\) Hz, 2H, \(\hbox {H}_{2^{\prime }}\), \(\hbox {H}_{6^{\prime }})\), 6.86 (d, \(J = 7.7\) Hz, 2H, \(\hbox {H}_{3^{\prime }}\), \(\hbox {H}_{5^{\prime }})\), 5.75 (s, 2H, \(\hbox {CH}_{2})\), 5.45 (s, 2H, \(\hbox {CH}_{2})\), 3.72 (s, 3H, \(\hbox {OCH}_{3})\), 2.73 (q, \(J = 7.5\) Hz, 2H, \(\hbox {CH}_{2})\), 1.30 (t, \(J = 7.5\) Hz, 3H, \(\hbox {CH}_{3})\).\(^{13}\hbox {C}\) NMR (125 MHz, DMSO-\(d_{6})\): 176.4, 159.1, 142.8, 141.6, 140.1, 136.9, 134.4, 133.9, 131.2, 129.5, 127.8, 126.6, 124.5, 123.0, 121.6, 121.2, 116.4, 116.1, 114.0, 55.1, 52.3, 41.5, 27.3, 15.5. Anal. Calcd for \(\hbox {C}_{26}\hbox {H}_{24}\hbox {N}_{4}\hbox {O}_{2}\): C, 73.56; H, 5.70; N, 13.20. Found: C, 73.74; H, 5.58; N, 13.03.
4-Fluoro-10-((1-(4-methoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)acridin-9(10H)-one (8f)
White crystals; yield: 71 %, mp 132–133\(\,^{\circ }\hbox {C}\). IR (KBr): 3150, 2937, 1738, 1638, 1608 \(\hbox {cm}^{-1}\). \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6})\): 8.29 (d, \(J = 7.3\) Hz, 1H, \(\hbox {H}_{8})\), 8.18–8.16 (m, 2H, \(\hbox {H}_{1}\), triazole), 7.81–7.76 (m, 2H, \(\hbox {H}_{5}\), \(\hbox {H}_{6})\), 7.67 (ddd, \(J= 15.2\), 7.6, 1.3‘Hz, 1H, \(\hbox {H}_{3})\), 7.36–7.29 (m, 2H, \(\hbox {H}_{2}\), \(\hbox {H}_{7})\), 7.24 (d, \(J= 8.6\) Hz, 2H, \(\hbox {H}_{2^{\prime }}\), \(\hbox {H}_{6^{\prime }})\), 6.89 (d, \(J = 8.6\) Hz, 2H, \(\hbox {H}_{3^{\prime }}\), \(\hbox {H}_{5^{\prime }})\), 5.69 (s, 2H, \(\hbox {CH}_{2})\), 5.47(s, 2H, \(\hbox {CH}_{2})\), 3.66 (s, 3H, \(\hbox {OCH}_{3})\). \(^{13}\)C NMR (125 MHz, DMSO-\(d_{6})\): 176.0, 159.0, 151.3 (d, \(J_{C-F}\) = 243.9 Hz), 144.2, 143.5, 134.5, 132.2, 129.4, 127.8, 126.4, 124.9, 122.9, 122.7, 122.2, 121.9 (d, \(J_{C-F}\) = 7.6 Hz), 121.8, 121.1 (d, \(J_{C-F}\) = 23.4 Hz), 116.6, 114.0, 55.1, 52.3, 47.1 (d, \(J_{C-F}\) = 14.8 Hz). Anal. Calcd for \(\hbox {C}_{24}\hbox {H}_{19}\hbox {F}\, \hbox {N}_{4}\hbox {O}_{2}\): C, 69.55; H, 4.62; N, 13.52. Found: C, 69.73; H, 4.51; N, 13.35.
4-Methoxy-10-((1-(4-methoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)acridin-9(10H)-one (8g)
Pale yellow crystals; yield: 69 %, mp 134–135\(\,^{\circ }\hbox {C}\). IR (KBr): 3120, 2922, 1735, 1629, 1598 \(\hbox {cm}^{-1}\). \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6})\): 8.24 (dd, \(J = 8.0\), 1.4 Hz, 1H, \(\hbox {H}_{8})\), 8.03 (s, 1H, triazole), 7.92 (dd, \(J = 7.6\), 1.0 Hz, 1H, \(\hbox {H}_{1})\), 7.79–7.72 (m, 2H, \(\hbox {H}_{5}\), \(\hbox {H}_{6})\), 7.43 (dd, \(J = 7.6\), 1.0 Hz, 1H, \(\hbox {H}_{3})\), 7.32–7.29 (m, 2H, \(\hbox {H}_{2}\), \(\hbox {H}_{7})\), 7.18 (d, \(J = 7.8\) Hz, 2H, \(\hbox {H}_{2^{\prime }}\), \(\hbox {H}_{6^{\prime }})\), 6.80 (d, \(J = 7.8\) Hz, 2H, \(\hbox {H}_{3^{\prime }}\), \(\hbox {H}_{5^{\prime }})\), 5.73 (s, 2H, \(\hbox {CH}_{2})\), 5.51 (s, 2H, \(\hbox {CH}_{2})\), 3.75 (s, 3H, \(\hbox {OCH}_{3})\), 3.69 (s, 3H, \(\hbox {OCH}_{3})\). Anal. Calcd for \(\hbox {C}_{25}\hbox {H}_{22}\hbox {N}_{4}\hbox {O}_{3}\): C, 70.41; H, 5.20; N, 13.14. Found: C, 70.68; H, 5.04; N, 12.94.
10-((1-(3,4,5-Trimethoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)acridin-9(10H)-one (8h)
White crystals; yield: 68 %, mp 232–234\(\,^{\circ }\hbox {C}\) . IR (KBr): 3114, 2937, 1745, 1631, 1603 \(\hbox {cm}^{-1}\). \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6})\): 8.24 (dd, \(J = 7.5\) Hz, 1.3 Hz, 2H, \(\hbox {H}_{1}\), \(\hbox {H}_{8})\), 8.22 (s, 1H, triazole), 7.94 (d, \(J = 7.5\) Hz, 2H, \(\hbox {H}_{4}\), \(\hbox {H}_{5})\), 7.82–7.78 (t, \(J = 7.5\) Hz, 2H, 2H, \(\hbox {H}_{3}\), \(\hbox {H}_{6})\), 7.35 (t, \(J= 7.5\) Hz, 2H, \(\hbox {H}_{2}\), \(\hbox {H}_{7})\), 6.56 (s, 2H, \(\hbox {H}_{2^{\prime }}\), \(\hbox {H}_{6^{\prime }})\), 5.81 (s, 2H, \(\hbox {CH}_{2})\), 5.45 (s, 2H, \(\hbox {CH}_{2})\), 3.70 (s, 6H, \(\hbox {OCH}_{3})\), 3.69 (s, 3H, \(\hbox {OCH}_{3})\). Anal. Calcd for \(\hbox {C}_{26}\hbox {H}_{24}\hbox {N}_{4}\hbox {O}_{4}\): C, 68.41; H, 5.30; N, 12.27. Found: C, 68.59; H, 5.18; N, 12.13.
2-Chloro-10-((1-(3,4,5-trimethoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)acridin-9(10H)-one (8i)
Yellow crystals; yield: 63 %, mp 244–245\(\,^{\circ }\hbox {C}\). IR (KBr): 3114, 2953, 1787, 16031, 1594 \(\hbox {cm}^{-1}\). \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6})\): 8.35 (d, \(J = 7.5\) Hz, 1H, \(\hbox {H}_{8})\), 8.26 (d, \(J = 2.3\) Hz, 1H, \(\hbox {H}_{1})\), 8.22 (s, 1H, triazole), 8.01 (d, \(J = 9.0\) Hz, 1H, \(\hbox {H}_{3})\), 7.96 (d, \(J = 9.0\) Hz, 1H, \(\hbox {H}_{4})\), 7.83 (m, 2H, \(\hbox {H}_{5}\), \(\hbox {H}_{6})\), 7.37 (t, \(J = 7.5\) Hz, 1H, \(\hbox {H}_{7})\), 6.56 (s, 2H, \(\hbox {H}_{2^{\prime }}\), \(\hbox {H}_{6^{\prime }})\), 5.82 (s, 2H, \(\hbox {CH}_{2})\), 5.45 (s, 2H, \(\hbox {CH}_{2})\), 3.66 (s, 6H, \(\hbox {OCH}_{3})\), 3.61(s, 3H, \(\hbox {OCH}_{3})\). \(^{13}\)C NMR (125 MHz, DMSO-\(d_{6})\): 175.6, 152.9, 142.5, 141.6, 140.4, 137.2, 134.5, 133.8, 131.3, 126.5, 126.1, 125.2, 123.5, 122.6, 121.9, 121.6, 119.1, 116.4, 105.3, 59.9, 55.7, 53.0, 41.7. Anal. Calcd for \(\hbox {C}_{26}\hbox {H}_{23}\hbox {ClN}_{4}\hbox {O}_{4}\): C, 63.61; H, 4.72; N, 11.41. Found: C, 63.77; H, 4.58; N, 11.58.
2-Methoxy-10-((1-(3,4,5-trimethoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)acridin-9(10H)-one (8j)
Yellow crystals; yield: 64 %, mp 193–194\(\,^{\circ }\hbox {C}\). IR (KBr): 3112, 2931, 1735, 1631, 1594 \(\hbox {cm}^{-1}\). \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6})\): 8.36 (d, \(J = 7.5\) Hz, 1H, \(\hbox {H}_{8})\), 8.19 (s, 1H, triazole), 7.96–7.93 (m, 2H, \(\hbox {H}_{4}\), \(\hbox {H}_{5})\), 7.79–7.77 (m, 2H, \(\hbox {H}_{1}\), \(\hbox {H}_{6})\), 7.46 (dd, \(J= 9.2\), 3.0 Hz, 1H, \(\hbox {H}_{3})\), 7.32 (t, \(J = 7.5\) Hz, 1H, \(\hbox {H}_{7})\), 6.59 (s, 2H, \(\hbox {H}_{2^{\prime }}\), \(\hbox {H}_{6^{\prime }})\), 5.82 (s, 2H, \(\hbox {CH}_{2})\), 5.44 (s, 2H, \(\hbox {CH}_{2})\), 3.88 (s, 3H, \(\hbox {OCH}_{3})\), 3.65 (s, 6H, \(\hbox {OCH}_{3})\), 3.61 (s, 3H, \(\hbox {OCH}_{3})\). \(^{13}\)C NMR (125 MHz, DMSO-\(d_{6})\): 176.0, 154.1, 152.9, 142.8, 141.3, 137.1, 136.5, 133.8, 131.4, 126.6, 123.9, 123.4, 122.4, 121.1, 120.9, 118.3, 116.1, 106.2, 105.2, 59.9, 55.7, 55.4, 52.9, 41.6. Anal. Calcd for \(\hbox {C}_{27}\hbox {H}_{26}\hbox {N}_{4}\hbox {O}_{5}\): C, 66.66; H, 5.39; N, 11.52. Found: C, 66.83; H, 5.21; N, 11.35.
2-Bromo-10-((1-(3,4,5-trimethoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)acridin-9(10H)-one (8k)
Pale yellow crystals; yield: 62 %, mp 246–248\(\,^{\circ }\hbox {C}\) . IR (KBr): 3115, 2931, 1751, 1627, 1595 \(\hbox {cm}^{-1}\). \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6})\): 8.41 (s, 1H, \(\hbox {H}_{1})\), 8.35 (d, \(J = 7.8\) Hz, 1H, \(\hbox {H}_{8})\), 8.22 (s, 1H, triazole), 7.97–7.95 (m, 3H, \(\hbox {H}_{3}\), \(\hbox {H}_{4}\), \(\hbox {H}_{5})\), 7.84–7.81 (t, \(J = 7.8\) Hz, 1H, \(\hbox {H}_{6})\), 7.38 (t, \(J = 7.8\) Hz, 1H, \(\hbox {H}_{7})\), 6.56 (s, 2H, \(\hbox {H}_{2^{\prime }}\), \(\hbox {H}_{6^{\prime }})\), 5.82 (s, 2H, \(\hbox {CH}_{2})\), 5.44 (s, 2H, \(\hbox {CH}_{2})\), 3.66 (s, 6H, \(\hbox {OCH}_{3})\), 3.61 (s, 3H, \(\hbox {OCH}_{3})\). MS m/z (%) 537 \(([\hbox {M}^{+.} + 2], 10)\), 535 \((\hbox {M}^{+.} 10)\), 534 (29), 274 (13), 244 (25), 228 (32), 203 (19), 181 (100), 165 (27), 148 (29), 135 (26), 121 (50), 106 (94), 91 (51), 77 (46), 51 (23). Anal. Calcd for \(\hbox {C}_{26}\hbox {H}_{23}\) Br \(\hbox {N}_{4}\hbox {O}_{4}\): C, 58.33; H, 4.33; N, 10.46. Found: C, 58.51; H, 4.18; N, 10.29.
2-Ethyl-10-((1-(3,4,5-trimethoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)acridin-9(10H)-one (8l)
Yellow crystals; yield: 67 %, mp 197–198\(\,^{\circ }\hbox {C}\). IR (KBr): 3111, 2925, 1735, 1639, 1594 \(\hbox {cm}^{-1}\). \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6})\): 8.36 (dd, \(J = 8.0\), 1.8 Hz, 1H, \(\hbox {H}_{8})\), 8.18–8.17 (m, 2H, \(\hbox {H}_{1}\), triazole), 7.93 (d,\( J = 8.0\) Hz, 1H, \(\hbox {H}_{5})\), 7.88 (d,\( J= 9.0\) Hz, 1H, \(\hbox {H}_{4})\), 7.80–7.76 (td, J = 8.0, 1.8 Hz, 1H, \(\hbox {H}_{6})\), 7.67 (dd, \(J = 9.0\), 2.2 Hz, 1H, \(\hbox {H}_{3})\), 7.33 (t, \(J = 8.0\) Hz, 1H, \(\hbox {H}_{7})\), 6.55 (s, 2H, \(\hbox {H}_{2^{\prime }}\), \(\hbox {H}_{6^{\prime }})\), 5.80 (s, 2H, \(\hbox {CH}_{2})\), 5.44 (s, 2H, \(\hbox {CH}_{2})\), 3.65 (s, 6H, \(\hbox {OCH}_{3})\), 3.60 (s, 3H, \(\hbox {OCH}_{3})\), 2.73 (q, \(J = 7.5\) Hz, 2H, \(\hbox {CH}_{2})\), 1.24 (t, \(J = 7.5\) Hz, 3H, \(\hbox {CH}_{3})\). \(^{13}\)C NMR (125 MHz, DMSO-\(d_{6})\): 176.7, 152.9, 142.8, 141.6, 140.1, 136.9, 134.4, 133.9, 131.4, 126.6, 125.4, 124.5, 123.4, 122.8, 121.6, 121.2, 116.4, 116.1, 105.2, 59.9, 55.7, 52.9, 41.5, 27.3, 15.5. Anal. Calcd for \(\hbox {C}_{28}\hbox {H}_{28}\hbox {N}_{4}\hbox {O}_{4}\): C, 69.41; H, 5.82; N, 11.56. Found: C, 69.31; H, 5.91; N, 11.32.
4-Fluoro-10-((1-(3,4,5-trimethoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)acridin-9(10H)-one (8m)
Yellow crystals; yield: 62 %, mp >250\(\,^{\circ }\hbox {C}\) . IR (KBr): 3120, 2938, 1742, 1639, 1597 \(\hbox {cm}^{-1}\). \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-d\(_{6})\): 8.31 (d, \(J = 7.7\) Hz, 1H, \(\hbox {H}_{8})\), 8.23 (s, 1H, triazole), 8.19 (d, \(J = 7.6\) Hz, 1H, \(\hbox {H}_{1})\), 7.79–7.78 (m, 2H, \(\hbox {H}_{5}\), \(\hbox {H}_{6})\), 7.68 (ddd, \(J= 15.3, 7.6, 1.1\) Hz, 1H, \(\hbox {H}_{3})\), 7.38–7.31 (m, 2H, \(\hbox {H}_{2}\), \(\hbox {H}_{7})\), 6.52 (s, 2H, \(\hbox {H}_{2^{\prime }}\), \(\hbox {H}_{6^{\prime }})\), 5.73 (s, 2H, \(\hbox {CH}_{2})\), 5.47(s, 2H, \(\hbox {CH}_{2})\), 3.64 (s, 6H, \(\hbox {OCH}_{3})\), 3.61 (s, 3H, \(\hbox {OCH}_{3})\). \(^{13}\)C NMR (125 MHz, DMSO-\(d_{6})\): 179.1, 152.9, 151.4 (d, \(J_{C-F}\) = 254.2 Hz), 144.4, 143.5, 134.5, 132.2, 131.5, 126.4, 124.9, 123.3, 122.7, 122.2, 121.9 (d, \(J_{C-F}\) = 18.9 Hz), 121.8, 121.2, 121.0, 116.6, 105.1, 59.9, 55.7, 52.9, 47.1 (d, \(J_{C-F}\) = 14.8 Hz). Anal. Calcd for \(\hbox {C}_{26}\hbox {H}_{23}\hbox {F}\, \hbox {N}_{4}\hbox {O}_{4}\): C, 65.81; H, 4.89; N, 11.81. Found: C, 65.64; H, 5.07; N, 11.98.
4-Methoxy-10-((1-(3,4,5-trimethoxybenzyl)-1H-1,2,3-triazol-4-yl)methyl)acridin-9(10H)-one (8n)
Yellow crystals; yield: 61 %, mp 182–184\(\,^{\circ }\hbox {C}\) . IR (KBr): 3113, 2945, 1732, 1623, 1598 \(\hbox {cm}^{-1}\). \(^{1}\hbox {H}\) NMR (500 MHz, DMSO-\(d_{6})\): 8.23 (d, \(J = 7.5\) Hz, 1H, \(\hbox {H}_{8})\), 8.05 (s, 1H, triazole), 7.92 (d, \(J = 7.5\) Hz, 1H, \(\hbox {H}_{1})\), 7.79–7.72 (m, 2H, \(\hbox {H}_{5}\), \(\hbox {H}_{6})\), 7.42 (d, J = 7.5 Hz, 1H, \(\hbox {H}_{3})\), 7.29 (t, \(J = 7.5\) Hz, 2H, \(\hbox {H}_{2}\), \(\hbox {H}_{7})\), 6.57 (s, 2H, \(\hbox {H}_{2^{\prime }}\), \(\hbox {H}_{6^{\prime }})\), 5.74 (s, 2H, \(\hbox {CH}_{2})\), 5.45 (s, 2H, \(\hbox {CH}_{2})\), 3.78 (s, 3H, \(\hbox {OCH}_{3})\), 3.68 (s, 6H, \(\hbox {OCH}_{3})\), 3.62 (s, 3H, \(\hbox {OCH}_{3})\). \(^{13}\)C NMR (125 MHz, DMSO-\(d_{6})\): 176.9, 152.9, 149.7, 145.5, 144.8, 137.2, 134.4, 133.9, 131.6, 126.1, 124.8, 123.4, 122.4, 122.2, 121.7, 118.1, 117.3, 116.3, 105.2, 59.94, 56.4, 55.8, 52.9, 48.4. Anal. Calcd for \(\hbox {C}_{27}\hbox {H}_{26}\hbox {N}_{4}\hbox {O}_{5}\): C, 66.65; H, 5.39; N, 11.52. Found: C, 66.76; H, 5.22; N, 11.39.
Biological assays
Cell culture
MCF-7, T-47D and MDA-MB-231 were purchased from the National Cell Bank of Iran (NCBI). The cells were cultured in RPMI 1640 medium supplemented with 10 % heat-inactivated fetal calf serum (from GibcoeBRL, UK) and 100 mg/mL streptomycin and 100 U/mL penicillin at \(37\,^{\circ }\hbox {C}\) in 5 % \(\hbox {CO}_{2}\)-humidified atmosphere.
In vitro cytotoxicity assay
Three different breast cancer cell lines (MCF-7, T-47D and MDA-MB-23) \((5\times 10^{4}\) cells/mL in 96-well culture plates) were incubated for 48 h with different concentrations of compounds 8a–n dissolved in DMSO (the final volume of DMSO/medium was less than 1 % in all experiments). Etoposide and DMSO were used as positive and negative controls, respectively. After treatment, the medium was removed and \(200 \,\upmu \hbox {L}\) of a phenol red-free medium containing MTT (1 mg/mL, final concentration) was added to all wells. After 4 h of incubation, the culture medium was replaced with \(100\,\upmu \hbox {L}\) of DMSO to each well. The absorbance was measured at 492 nm with a multi-well plate reader (Gen5, Power wave xs2, BioTek, America). All experiments were performed at least three times and the \(\hbox {IC}_{50}\) values for all compounds were calculated by nonlinear regression analysis and expressed in mean \(\pm \) SD compared with the control [39].
AO/EB staining method
MCF-7 cell grown in 6-well plates \((3\times 10^{5}\) cells/well) were treated with and without compound 8c for 24 h. Plates were washed three times by phosphate buffered saline (PBS) and \(9 \,\upmu \hbox {L}\) of cell suspension was stained with \(1\,\upmu \hbox {L}\) of dye mixture (100 mg/mL AO and 100 mg/mL EB in PBS). \(10\,\upmu \hbox {L}\) of stained cell suspension were placed on a clean microscope slide and covered with a coverslip and examined by fluorescence microscope (Axoscope 2 plus, Zeiss, Germany) [40].
Flow cytometric analysis of apoptosis
Annexin V-FITC/propidium iodide (PI) double staining test was performed using an Annexin-V- FITC kit (Biovision) as described in protocol. The MCF-7 cells were treated with \(\hbox {IC}_{50}\) concentrations of the compound 8c, etoposide and DMSO 1 %. After 24 h incubation, the cells (\(5 \times 10^{5}\) cells) were collected and washed twice with cold PBS and resuspended in \(500\,\upmu \hbox {l}\) of 1X binding buffer. Then, the cells were double stained with \(5\,\upmu \hbox {L}\) of Annexin V-FITC and \(5\,\upmu \hbox {L}\) of PI solution. Finally, the samples were incubated for 5 min at room temperature and then analyzed by flow cytometry [41].
References
Reddy L, Odhav B, Bhoola KD (2003) Natural products for cancer prevention: a global perspective. Pharmacol Ther 99:1–13. doi:10.1016/S0163-7258(03)00042-1
Fabregat I, Roncero C, Fernandez M (2007) Survival and apoptosis: a dysregulated balance in liver cancer. Liver Int 27:155–162. doi:10.1111/j.1478-3231.2006.01409.x
Solary E, Dubrez L, Eymin B (1996) The role of apoptosis in the pathogenesis and treatment of diseases. Eur Respir J 9:1293–1305. doi:10.1183/09031936.96.09061293
Gottesman MM (2002) Mechanisms of cancer drug resistance. Annu Rev Med 53:615–627. doi:10.1146/annurev.med.53.082901.103929
Kuno T, Tsukamoto T, Hara A, Tanaka T (2012) Cancer chemoprevention through the induction of apoptosis by natural compounds. J Biophys Chem 3:156–173. doi:10.4236/jbpc.2012.32018
Chou CC, Yang JS, Lu HF, Ip SW, Lo C, Wu CC, Lin JP, Tang NY, Chung JG, Chou MJ, Teng YH, Chen DR (2010) Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondria pathway in human breast cancer MCF-7 cells. Arch Pharm Res 33:1181–1191. doi:10.1007/s12272-010-0808-y
Kemnitzer W, Sirisoma N, Nguyen B, Jiang S, Kasibhatla S, Crogan-Grundy C, Tseng B, Drewe J, Cai SX (2008) Discovery of 1-benzoyl-3-cyanopyrrolo[1,2-\(a\)]quinolines as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. Part 1: Structure-activity relationships of the 1- and 3-positions. Bioorg Med Chem Lett 18:6259–6264. doi:10.1016/j.bmcl.2008.09.110
Kemnitzer W, Sirisoma N, Nguyen B, Jiang S, Kasibhatla S, Crogan-Grundy C, Tseng B, Drewe J, Cai SX (2009) Discovery of \(N\)-aryl-9-oxo-9\(H\)-fluorene-1-carboxamides as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. 1. Structure-activity relationships of the carboxamide group. Bioorg Med Chem Lett 19:3045–3049. doi:10.1016/j.bmcl.2009.04.009
Kerr JF, Winterford CM, Harmon BV (1994) Apoptosis. Its significance in cancer and cancer therapy. Cancer 73:2013–2026. doi:10.1038/nrc2663
Cholewiński G, Dzierzbicka K, Kołodziejczyk AM (2011) Natural and synthetic acridines/acridones as antitumor agents: their biological activities and methods of synthesis. Pharmacol Rep 63:305–336. doi:10.1002/chin.201213236
Cao R, Gao CM, Meier H (2005) A facile synthesis of homotriptycenes from anthranol derivatives. Synlett 20:3166–3168. doi:10.1055/s-2005-921929
Liu Y, Zou L, Ma L, Chen WH, Wang B, Xu ZL (2006) Synthesis and pharmacological activities of xanthone derivatives as \(\alpha \)-glucosidase inhibitors. Bioorg Med Chem 14:5683–5690. doi:10.1016/j.bmc.2006.04.014
Boumendjel A, Macalou S, Ahmed-Belkacem A, Blanc M, Di Pietro A (2007) Design, synthesis, and inhibition of breast cancer resistance protein ABCG2. Bioorg Med Chem 15:2892–2897. doi:10.1016/j.bmc.2007.02.017
Harrison RJ, Reszka AP, Haider SM, Romagnoli B, Morrell J, Read MA, Gowan SM, Incles CM, Kelland LR, Neidle S (2004) Evaluation of by disubstituted acridone derivatives as telomerase inhibitors: the importance of G-quadruplex binding. Bioorg Med Chem Lett 14:5845–5849. doi:10.1016/j.bmcl.2004.09.037
Dzierzbicka K, Kolodziejczyk AM, Wysocka-Skrzela B, Mysliwski A, Sosnowska D (2001) Synthesis and antitumor activity of conjugates of muramyldipeptide, normuramyldipeptide, and desmuramylpeptides with acridine/acridone derivatives. J Med Chem 44:3606–3615. doi:10.1021/jm001115g
Braga PAC, Santos DAPD, Da Silva MFDGF, Vieira PC, Fernandes JB, Houghton PJ, Fang R (2007) In vitro cytotoxicity activity on several cancer cell lines of acridone and \(N\)-phenylethyl-benzamide derivatives from Swingles glutinosa (Bl.) Merr. Nat Prod Res 21:47–55
Dheyongera JP, Geldenhuys WJ, Dekker TG, Van der Schyf CJ (2005) Synthesis, biological evaluation, and molecular modeling of novel thioacridone derivatives related to the anticancer alkaloid acronycine. Bioorg Med Chem 13:689–698. doi:10.1016/j.bmc.2004.10.051
Cho HJ, Jung MJ, Kwonb Y, Na Y (2009) Oxiranylmethyloxy or thiiranylmethyloxy-azaxanthones and -acridone analogues as potential topoisomerase I inhibitors. Bioorg. Med Chem Lett 19:6766–6769. doi:10.1016/j.bmcl.2009.09.091
Cuenca F, Moore MJB, Johnson K, Guyen B, De Cian A, Neidle S (2009) Design, synthesis and evaluation of 4,5-di-substituted acridone ligands with high G-quadruplex affinity and selectivity, together with low toxicity to normal cells. Bioorg Med Chem Lett 19:5109–5113. doi:10.1016/j.bmcl.2009.07.033
Gao C, Jiang Y, Tan C, Zu X, Liu H, Cao D (2008) Synthesis and potent antileukemic activities of 10-benzyl-9(10\(H)\)-acridinones. Bioorg Med Chem 16:8670–8675. doi:10.1016/j.bmc.2008.07.086
Abboud JLM, Foces-Foces C, Notario R, Trifonov RE, Volovodenko AP, Ostrovskii VA, Alkorta I, Elguero J (2001) Basicity of N-H- and N-Methyl-1,2,3-triazoles in the gas phase, in solution, and in the solid state—an experimental and theoretical study. Eur J Org Chem 16:3013–3024. doi:10.1002/1099-0690(200108)2001:16<3013::AID-EJOC3013>3.0.CO;2-Y
Vatmurge NS, Hazra BG, Pore VS, Shirazi F, Chavan PS, Deshpande MV (2008) Synthesis and antimicrobial activity of \(\beta \)-lactam-bile acid conjugates linked via triazole. Bioorg Med Chem Lett 18:2043–2047. doi:10.1016/j.bmcl.2008.01.102
Lee T, Cho M, Ko SY, Youn HJ, Baek DJ, Cho WJ, Kang CY, Kim S (2007) Synthesis and evaluation of 1,2,3-triazole containing analogues of the immunostimulant \(\alpha \)-GalCer. J Med Chem 50:585–589. doi:10.1021/jm061243q
Lewis WG, Green LG, Grynszpan F, Radić Z, Carlier PR, Taylor P, Finn MG, Sharpless KB (2002) Click chemistry in situ: acetylcholinesterase as a reaction vessel for the selective assembly of a femtomolar inhibitor from an array of building blocks. Angew Chem Int Ed Engl 41:1053–1057. doi:10.1002/1521-3757(20020315)114:6<1095::AID-ANGE1095>3.0.CO;2-3
Tron GC, Pirali T, Billington RA, Canoniico PL, Sorba G, Genazzani AA (2008) Click chemistry reactions in medicinal chemistry: applications of the 1,3-dipolar cycloaddition between azides and alkynes. Med Res Rev 28:278–308. doi:10.1002/med.20107
Phillips OA, Udo EE, Abdel-Hamid ME, Varghese R (2009) Synthesis and antibacterial activity of novel 5-(4-methyl-1\(H\)-1,2,3-triazole)methyl oxazolidinones. Eur J Med Chem 44:3217–3227. doi:10.1016/j.ejmech.2009.03.024
Aher NG, Pore VS, Mishra NN, Kumar A, Shukla PK, Sharma A, Bhat MK (2009) Synthesis and antifungal activity of 1,2,3-triazole containing fluconazole analogues. Bioorg Med Chem Lett 19:759–763. doi:10.1016/j.bmcl.2008.12.026
Gill C, Jadhav G, Shaikh M, Kale R, Ghawalkar A, Nagargoje D, Shiradkar M (2008) Clubbed [1,2,3] triazoles by fluorine benzimidazole: a novel approach to H37Rv inhibitors as a potential treatment for tuberculosis. Bioorg Med Chem Lett 18:6244–6247. doi:10.1016/j.bmcl.2008.09.096
Giffin MJ, Heaslet H, Brik A, Lin YC, Cauvi G, Wong CH, McRee DE, Elder JH, Stout CD, Torbett BE (2008) A copper(I)-catalyzed 1,2,3-triazole azide-alkyne click compound is a potent inhibitor of a multidrug-resistant HIV-1 protease variant. J Med Chem 51:6263–6270. doi:10.1021/jm800149m
Tian LJ, Sun YX, Li HJ, Zheng XL, Cheng YZ, Liu XL, Qian BH (2005) Synthesis, characterization and biological activity of triorganotin 2-phenyl-1,2,3-triazole-4-carboxylates. J Inorg Biochem 99:1646–1652. doi:10.1016/j.jinorgbio.2005.05.006
Kim S, Cho M, Lee T, Lee S, Min HY, Lee SK (2007) Design, synthesis, and preliminary biological evaluation of a novel triazole analogue of ceramide. Bioorg Med Chem Lett 17:4584–4587. doi:10.1016/j.bmcl.2007.05.086
Pérez-Tomás R (2006) Multidrug resistance: retrospect and prospects in anti-cancer drug treatment. Curr Med Chem 13:1859–1876. doi:10.2174/092986706777585077
Fallah-Tafti A, Foroumadi A, Tiwari R, Shirazi AN, Hangauer DG, Bu Y, Akbarzadeh T, Parang K, Shafiee A (2011) Thiazolyl \(N\)-benzyl-substituted acetamide derivatives: synthesis, Src kinase inhibitory and anticancer activities. Eur J Med Chem 46:4853–4858. doi:10.1016/j.ejmech.2011.07.050
Fallah-Tafti A, Tiwari R, Shirazi AN, Akbarzadeh T, Mandal D, Shafiee A, Parang K, Foroumadi A (2011) 4-Aryl-4\(H\)-chromene-3-carbonitrile derivatives: evaluation of Src kinase inhibitory and anticancer activities. Med Chem 7:466–472. doi:10.2174/157340611796799258
Motavallizadeh S, Fallah-Tafti A, Maleki S, Shirazi AN, Pordeli M, Safavi M, Ardestani SK, Asd S, Tiwari R, Ohc D, Shafiee A, Foroumadi A, Parang K, Akbarzadeh T (2014) Synthesis and evaluation of antiproliferative activity of substituted \(N\)-xanthen-4-yl)benzenesulfonamides. Tetrahedron Lett 55:373–375. doi:10.1016/j.tetlet.2013.11.033
Mohammadi-Khanaposhtani M, Saeedi M, Zafarghandi NS, Mahdavi M, Sabourian R, Razkenari EK, Alinezhad H, Khanavi M, Foroumadi A, Shafiee A, Akbarzadeh T (2015) Potent acetylcholinesterase inhibitors: design, synthesis, biological evaluation, and docking study of acridone linked to 1,2,3-triazole derivatives. Eur J Med Chem 92:799–806. doi:10.1016/j.ejmech.2015.01.044
Wolf C, Liu S, Mei X, August AT, Casimir M (2006) Regioselective copper-catalyzed amination of bromobenzoic acids using aliphatic and aromatic amines. J Org Chem 71:3270–3273. doi:10.1021/jo060034a
Hedge R, Thimmaiah P, Yerigeri MC, Krishnegowda G, Thimmaiah KN, Houghton PJ (2004) Anti-calmodulin acridone derivatives modulate vinblastine resistance in multidrug resistant (MDR) cancer cells. Eur J Med Chem 39:161–178. doi:10.1016/j.ejmech.2003.12.001
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. doi:10.1016/0022-1759(83)90303-4
Safavi M, Esmati N, Ardestani SK, Emami S, Ajdari S, Davoodi J, Shafiee A, Foroumadi A (2012) Halogenated flavanones as potential apoptosis-inducing agents: synthesis and biological activity evaluation. Eur J Med Chem 58:573–580. doi:10.1016/j.ejmech.2012.10.043
Itamochi H, Oishi T, Shimada M, Sato S, Uegaki K, Naniwa J, Sato S, Nonaka M, Terakawa N, Kigawa J, Harada T (2011) Inhibiting the mTOR pathway synergistically enhances cytotoxicity in ovarian cancer cells induced by etoposide through upregulation of c-Jun. Clin Cancer Res 17:4742–4750. doi:10.1158/1078-0432.CCR-11-0190
Acknowledgments
This work was supported by Grant from the Research Council of Tehran University of Medical Sciences under Grant No. 94-01-33-28706.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohammadi-Khanaposhtani, M., Safavi, M., Sabourian, R. et al. Design, synthesis, in vitro cytotoxic activity evaluation, and apoptosis-induction study of new 9(10H)-acridinone-1,2,3-triazoles. Mol Divers 19, 787–795 (2015). https://doi.org/10.1007/s11030-015-9616-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-015-9616-0